The Nude Mutant Gene Foxn1 is a HOXC13 Regulatory Target during Hair Follicle and Nail Differentiation (original) (raw)
. Author manuscript; available in PMC: 2011 Oct 1.
Published in final edited form as: J Invest Dermatol. 2010 Dec 30;131(4):828–837. doi: 10.1038/jid.2010.391
Abstract
Among the Hox genes, Hoxc13 has been shown to be essential for proper hair shaft differentiation as Hoxc13 gene-targeted (Hoxc13tm1Mrc) mice completely lack external hair. Because of the remarkable overt phenotypic parallels to the Foxn1nu (nude) mutant mice, we sought to determine whether Hoxc13 and Foxn1 might act in a common pathway of hair follicle (HF) differentiation. We show that the alopecia exhibited by both the Hoxc13tm1Mrc and Foxn1nu mice is due to strikingly similar defects in hair shaft differentiation and that both mutants suffer from a severe nail dystrophy. These phenotypic similarities are consistent with the extensive overlap between Hoxc13 and Foxn1 expression patterns in the HF and the nail matrix. Furthermore, DNA microarray analysis of skin from Hoxc13tm1Mrc mice identified Foxn1 as significantly down-regulated along with numerous hair keratin genes. This Foxn1 down-regulation apparently reflects the loss of direct transcriptional control by HOXC13 as indicated by our results obtained through co-transfection and chromatin immunoprecipitation (ChIP) assays. As presented in the discussion, these data support a regulatory model of keratinocyte differentiation in which HOXC13-dependent activation of Foxn1 is part of a regulatory cascade controlling the expression of terminal differentiation markers.
Keywords: Hoxc13, Foxn1, nude, mouse mutant, target gene, hair follicle, nail, differentiation, regulatory network
INTRODUCTION
The HOX transcription factors are crucial regulators of developmental processes, (Duboule, 1992; Krumlauf, 1994; Akin and Nazarali, 2005), including epidermal and HF differentiation (Awgulewitsch, 2003). A primary role of Hox genes is defining regional identities in axial and paraxial embryonic structures (Duboule, 1992; McGinnis and Krumlauf, 1992; Capecchi, 1997), and certain Hox genes apparently establish topographical specificity in skin and its appendages, including HFs (Chuong et al., 1990; Bieberich et al., 1991; Kanzler et al., 1994; Chuong and Noveen, 1999; Reid and Gaunt, 2002). However, several Hox genes are expressed globally in all follicles (Awgulewitsch, 2003). This includes Hoxc13, which is expressed primarily in the upper bulb region of the HF of all hair types in mouse (Godwin and Capecchi, 1998) and human (Jave-Suarez et al., 2002). Hoxc13 expression is initiated at early stages of HF differentiation both during HF morphogenesis and the progressive growth phase (anagen) in cycling hair, and it continues in the proximal-most region of catagen follicles during HF regression (Shang et al., 2002). In anagen follicles, the Hoxc13 expression domain encompasses distinct subpopulations of cells primarily in the matrix and the three cylindrical layers of the differentiating hair shaft, including cuticle, cortex, and medulla (Jave-Suarez et al., 2002; Potter et al., 2006). Functional studies revealed that both Hoxc13 null (Hoxc13tm1Mrc) and Hoxc13 over-expressing transgenic (FVB/NTac-Tg(Hoxc13)61B1Awg/J) mice exhibit severe hair growth defects (Godwin and Capecchi, 1998; Tkatchenko et al., 2001) resulting in structurally defective hair shafts in both cases. In the latter case, this manifests itself in the delayed formation of a thin and scruffy-looking hair coat during postnatal development and progressive alopecia during adulthood (Fig S1b; Tkatchenko et al., 2001), whereas Hoxc13 null mice fail to grow any external hair, thus resulting in a completely nude appearance (Fig S1d; Godwin and Capecchi, 1998); in addition to alopecia, loss of Hoxc13 function results in defective nail development (ibid). These hair and nail defects are remarkably similar to the overtly same phenotypic characteristics exhibited by nude mutant mice (Fig S1d and S1c; Mecklenburg et al., 2005) that are homozygous for the Foxn1nu mutated allele of Foxn1, a member of the forkhead domain family of transcription factors (Nehls et al., 1994; Meier et al., 1999; Mecklenburg et al., 2001). Foxn1nu/Foxn1nu mice have primarily been studied for their immunodeficiency based on athymic aplasia (Mecklenburg et al., 2005).
The overt phenotypic parallels between nude and Hoxc13 null mice compelled us to hypothesize that the two genes function in common pathways of keratinocyte differentiation in both hair and nails. The striking similarities in the histopathological changes of hair and nail between these mutants presented here strongly support this idea. The down-regulation of Foxn1 expression in postnatal skin of Hoxc13 null mice combined with results from HOXC13/Foxn1 transient co-transfection and chromatin immunoprecipitation (ChIP) assays suggest that Foxn1 is a direct regulatory target for HOXC13. Foxn1 is thus the second member of the forkhead transcription factor gene family regulated by HOXC13, as we have previously demonstrated HOXC13-dependent transcriptional control of Foxq1 in the HF medulla (Potter et al., 2006). Together, these results suggest that Hoxc13 is at the core of a regulatory network essential for both HF and nail differentiation.
RESULTS
Hair and nail defects of Hoxc13 null and Foxn1nu mice are strikingly similar
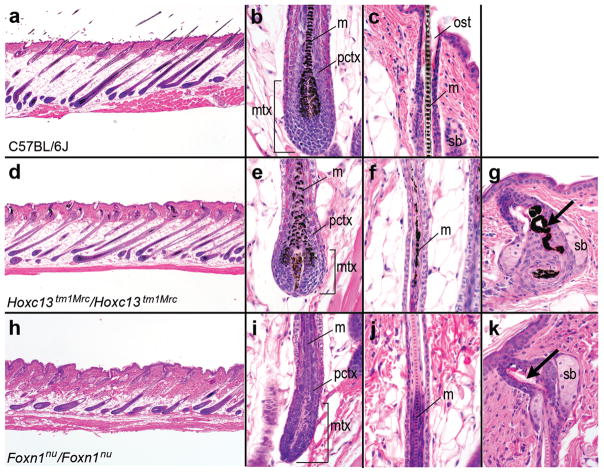
Histopathological analysis of skin from Hoxc13 null (Hoxc13tm1Mrc/Hoxc13tm1Mrc) and nude (Hsd-Foxn1nu/Foxn1nu) mice, as well as C57BL/6J mice (controls) was performed at 5d post natum (p.n.) and at ≥8 weeks of age. At 5d p.n. all HFs in dorsal skin of both mutants and the controls were near their final stage of morphogenesis (Fig 1a, d, h); at this stage HFs are histologically identical to follicles in late anagen of cycling hair (Stenn and Paus, 2001). Bulb regions of HFs from Hoxc13 null mice appeared superficially normal compared to controls, although the usually distinct columns of follicular keratinocytes forming the different components of the hair shaft appeared grossly disorganized (Fig 1b, e). This loss of organization in the upper matrix was reflected in particular by the disruption of the normal septate pattern of the HF medulla in more distal regions (Fig 1f). At the level of the sebaceous gland, the hair shaft became twisted and distorted and contained amorphous eosinophilic material (Fig 1g). These abnormal hair shafts apparently had insufficient structural rigidity to exit through the osteum like normal hair fibers (compare Fig 1c), a likely primary cause for the overall alopecic appearance of Hoxc13 null mice. The follicular matrix of nude mice exhibited a similar loss of organization (Fig 1i), although medulla formation appeared to be normal (Fig 1j). However, at the level of the sebaceous gland, the hair twisted within the infundibulum and broke off at the surface (Fig 1k), thus producing a phenocopy of the Hoxc13 null hair defect in this region. Anagen HFs of adult Hoxc13 null and nude mutant mice examined at ≥8 weeks showed essentially the same changes as those seen at 5d p.n. (data not shown).
Figure 1. Hoxc13 null and nude mice exhibit similar hair defects.
Histological comparison of HFs in H&E –stained sections (10 μm) of dorsal skin from 5d p.n. mice of the following strains: (a–c) C57/BL6L-Tyrc-2J (controls); (d–g) Hoxc13 null (homozygous B6.129-Hoxc13tm1Mrc/Hoxc13tm1Mrc); (h–k) nude (Hsd-Foxn1nu/Foxn1nu). In all strains, HFs were uniformly in late anagen phase of the hair cycle. Note that in both Hoxc13 null and nude mice the hair shaft became twisted and distorted at the level of the sebaceous gland (sb) and failed to penetrate the epidermis (arrows in panels g and k). For further explanations, see text; m: medulla; mtx: matrix; ost: osteum; pctx: precortex; scale bars: 500 μm in panels a, d, h, and 10 μm in the remaining panels.
Compared to C57BL/6J-Tyrc-2J controls (Fig 2a–d), the Hoxc13 null (homozygous B6.Cg-Tyrc-2J Hoxc13tm1Mrc) mice had a normal appearing proximal nail fold; however, unlike the control mice where the stratum granulosum (SG) ended half way into the invagination, the (SG) continued well into the matrix (Fig 2e–h). Instead of a regular translucent nail plate, these mice formed a structure that resembled a layer of cornified, stratified, squamous epithelium. Only near the proximal end of the nail bed a structure reminiscent of a normal nail plate was formed.
Figure 2. Nails of Hoxc13 null and nude mutant mice exhibit similar structural defects.
Histological comparison of nails in H&E –stained sections (10 μm) of rear foot digits derived from the following mouse strains at 5d p.n.: (a–d) C57/BL6J-Tyrc-2J mice (controls); (e–h) Hoxc13 null (homozygous B6.Cg-Tyrc-2JHoxc13tm1Mrc); nude (Hsd-Foxn1nu/Foxn1nu). Compared to control mice Hoxc13 null and nude mice had similar defects including reduced nail matrix (mtx), a defective nail plate (np), and an extended stratum granulosum (SG) with its distal limits (marked by arrows) reaching into the matrix as explained in the text; cu: cuticle; nb: nail bed; pnf: proximal nail fold; SG: stratum granulosum scale bars: 200 μm, panels a, e, i, and 5 μm in the remaining panels; distal points to the right.
The nude mice exhibited similar, but not identical, changes (Fig 2i–l). The SG extended from the proximal nail fold to the matrix (Fig 2j) as was seen for the Hoxc13 null mice. In contrast to the former, most nude mice had no evidence of a nail plate but rather a hyperplastic, cornified, epidermis-like layer, although this was not consistent between individuals (some mice had abortive nail plate-like structures). This finding differed from previous analyses of nude mice at 8–12 weeks of age in which a thinned nail plate was reported to form (Mecklenburg et al., 2004). This phenotypic variation may reflect age differences at time of analysis (5 days vs. 8–12 weeks) and differences in strain backgrounds (outbred Hsd-Foxn1nu/Foxn1nu used here vs. inbred NMRI-Foxn1nu/Foxn1nu mice in the Mecklenburg study). Likewise, the thin nail plate-like structure observed with Hoxc13 null mice at 5d p.n. was replaced with a hyperplastic, cornified epidermis at ≥8 weeks of age, which was similar to what was seen in the young nude mice (data not shown). Depending upon environmental conditions and genetic background the nail matrix either produces a cornified, hyperplastic epidermis or an abortive nail plate.
Down-regulation of Foxn1 in hair and nail of Hoxc13 null mice
DNA microarray analysis of 5d p.n. skin from Hoxc13tm1Mrc/Hoxc13tm1Mrc mice versus wild type littermates revealed differential expression of 180 genes (q <0.05), including 31 up- and 149 down-regulated genes (Table S1; GEO series: GSE23759). The most strongly dysregulated genes belonged to the gene ontology (GO) group Intermediate Filament/Cytoskeleton that included numerous hair keratin and keratin-associated protein (KAP) genes. Table 1 lists differentially expressed genes known to be active in skin and hair (n=47), all of which were down-regulated. The majority of these genes were keratin and KAP genes (n=37), and many were previously found to be differentially expressed in 5d p.n. skin of _Hoxc13-_overexpressing mice (Tkatchenko et al., 2001; Potter et al., 2006), as well as in dorsal skin of Dsg4lah/Dsg4lah mutant mice (Bazzi et al., 2009). Notably, Foxn1 was among the 13 down-regulated “non-keratin/KAP” genes belonging to diverse GO groups.
Table 1. Subset of genes down-regulated in skin of Hoxc13 null mice.
Subset of differentially expressed genes in dorsal skin of 5d p.n. Hoxc13 null mice that are known to be active in skin and hair as determined by querying the MGI database (http://www.informatics.jax.org/); genes are listed under current gene nomenclature symbols; since the nomenclature, particularly of keratin genes, was substantially revised recently (Schweizer et al., 2006), common previously used gene symbols are listed in the last column; for genes shown in bold type-face, evidence for direct regulation by FOXN1 has previously been demonstrated as discussed in the text; shading indicates genes down-regulated in skin of Dsg4 null mice (Bazzi et al., 2009); underlining denotes HOXC13 target genes validated by DNA binding studies (see text).
| Entrez Clone ID | Fold Change | Current Symbol | Gene Name | Previous/Alternative Gene Symbol |
|---|---|---|---|---|
| 71369 | −241.29 | Krtap16-10 | keratin associated protein 16-10 | |
| 170651 | −235.46 | Krtap16-1 | keratin associated protein 16-1 | |
| 71363 | −231.19 | Krtap7-1 | keratin associated protein 7-1 | |
| 170657 | −228.74 | Krtap16-9 | keratin associated protein 16-9 | |
| 71888 | −200.85 | Krt33a | keratin 33A | |
| 68484 | −193.37 | Krtap16-8 | keratin associated protein 16-8 | |
| 77918 | −188.00 | Krtap16-5 | keratin associated protein 16-5 | |
| 16700 | −185.72 | Krtap6-1 | keratin associated protein 6-1 | |
| 16704 | −180.84 | Krtap8-2 | keratin associated protein 8-2 | |
| 23927 | −177.32 | Krtap14 | keratin associated protein 14 | mKAP13, Pmg1 |
| 16672 | −169.26 | Krt34 | keratin 34 | Krt1-4, mHa4 |
| 76444 | −142.77 | Krtap4-7 | keratin associated protein 4-7 | KAP4.7 |
| 26560 | −135.74 | Krtap15 | keratin associated protein 15 | Pmg2 |
| 268905 | −133.33 | Krtap13-1 | keratin associated protein 13-1 | KAP13.1 |
| 16679 | −132.74 | Krt86 | keratin 86 | Krt2-10, Krt2-11, mHb6 |
| 53617 | −120.25 | Krt35 | keratin 35 | Krt1-24, mHa5 |
| 16701 | −98.67 | Krtap6-2 | keratin associated protein 6-2 | |
| 11571 | −94.56 | Crisp1 | cysteine-rich secretory protein 1 | Aeg1 |
| 170654 | −86.56 | Krtap16-4 | keratin associated protein 16-4 | |
| 16660 | −86.05 | Krt31 | keratin 31 | Krt1-1, Ha1 |
| 16671 | −69.95 | Krt33b | keratin 33B | Krt1-3, mHa3 |
| 57390 | −62.45 | Psors1c2 | psoriasis susceptibility 1 candidate 2 (human) | |
| 77914 | −39.87 | Krtap17-1 | keratin associated protein 17-1 | |
| 114566 | −39.41 | Krt82 | keratin 82 | Krt2-20 |
| 69473 | −33.65 | Krtap3-1 | keratin associated protein 3-1 | KAP3.1 |
| 71623 | −33.43 | Krtap5-2 | keratin associated protein 5-2 | |
| 69533 | −32.53 | Krtap26-1 | keratin associated protein 26-1 | |
| 16670 | −21.89 | Krt32 | keratin 32 | Krt1-2, mHa2 |
| 66708 | −17.04 | Krtap3-2 | keratin associated protein 3-2 | KAP3.2 |
| 114666 | −15.78 | Krtap5-5 | keratin associated protein 5-5 | |
| 16673 | −12.70 | Krt36 | keratin 36 | Krt1-22, Krt1-5 |
| 17752 | −11.40 | Mt4 | metallothionein 4 | MT-IV |
| 16694 | −8.24 | Krtap12-1 | keratin associated protein 12-1 | D10Jhu14e |
| 50775 | −7.89 | Krtap5-4 | keratin associated protein 5-4 | |
| 20216 | −7.36 | Acsm3 | acyl-CoA synthetase medium-chain family member 3 | |
| 60594 | −7.36 | Capn12 | calpain 12 | |
| 13506 | −5.72 | Dsc2 | desmocollin 2 | Dsc2a, Dsc2b |
| 56370 | −4.89 | Tagln3 | transgelin 3 | Np25 |
| 105866 | −4.61 | Krt72 | keratin 72 | |
| 16680 | −4.30 | Krt84 | keratin 84 | Krt2-16, Krt2-3, mHb4 |
| 237987 | −4.18 | Otop2 | otopetrin 2 | |
| 320864 | −3.73 | Krt26 | keratin 26 | |
| 72685 | −3.37 | Dnajc6 | DnaJ (Hsp40) homolog, subfamily C, member 6 | |
| 16846 | −2.71 | Lep | leptin | |
| 15218 | −2.32 | Foxn1 | forkhead box N1 | Whn, Hfh11 |
| 12163 | −2.19 | Bmp8a | bone morphogenetic protein 8a | Bmp7r1, OP2 |
| 20652 | −2.02 | Soat1 | sterol O-acyltransferase 1 | ACAT-1 |
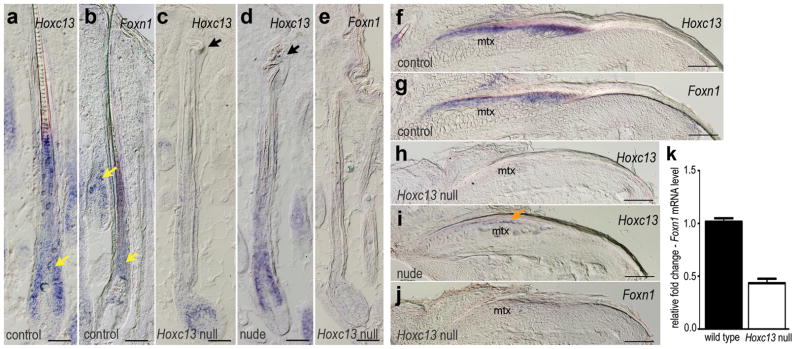
The Foxn1 expression differential in skin (−2.3 –fold; Table 1) was validated by Q-PCR indicating 2.4 –fold down-regulation (Fig 3k), and potential changes in the expression patterns of Hoxc13 and Foxn1 in HFs and nails were cross-examined in Hoxc13 null (homozygous B6.Cg-Tyrc-2J Hoxc13tm1Mrc) and nude mice at 5d p.n. by ISH. The normal Hoxc13 and Foxn1 patterns in HFs of C57BL/6J-Tyrc-2J control mice showed extensive overlap in several compartments including cuticle and cortex of the differentiating hair shaft, and the matrix and inner root sheath (Fig 3a, b). In hair of Hoxc13 null mice, expression of the truncated Hoxc13 transcript was detected exclusively in the upper matrix (Fig 3c). In HFs of nude mice, Hoxc13 was expressed in a pattern resembling its pattern in the controls, including the typical proximal boundary in the matrix at the mid-level of the dermal papilla (Fig 3d). In contrast, expression of Foxn1 was no longer detectable in Hoxc13 null HFs (Fig 3e), thus suggesting that Foxn1 expression requires Hoxc13 activity.
Figure 3. Disruption of Foxn1 expression pattern in HF and nail of Hoxc13 null mice.
ISH analysis of Hoxc13 and Foxn1 expression patterns in hair and nail from C57BL/6J-Tyrc-2J (control), Hoxc13 null (homozygous B6.Cg-Tyrc-2JHoxc13tm1Mrc), and nude mice. (a, b) Hoxc13 and Foxn1 expression (bluish stain) overlapped in cortical/precortical regions of control HFs (yellow arrowheads). (c) Reduced Hoxc13 expression in HF matrix of Hoxc13 null mice. HFs of nude mice showed characteristic aspects of the Hoxc13 pattern (d), whereas Foxn1 expression was undetectable in HFs of Hoxc13 null mice (e); note twisted hair shafts in Hoxc13 null and nude mice (arrowheads in c, d). Hoxc13 expression in nail matrix (mtx) of control mice (f) mirrored the Foxn1 pattern (g). Hoxc13 expression was undetectable in nail matrix of Hoxc13 null mice (h) but weakly present (orange arrow) in nails of nude mice (i), and Foxn1 expression was undetectable in Hoxc13 null nail matrix (j). (k) Bar graph showing the relative 2.4-fold change in Foxn1 expression in Hoxc13 null versus control (wild type) mice as measured by RT-PCR. Scale bars: 50 μm, a–e, and 100 μm in f–j.
In the differentiating nail epithelium of C57BL/6J-Tyrc-2J control mice, Hoxc13 and Foxn1 expression patterns in the upper matrix were essentially identical (Fig 3f, g), and the Foxn1 pattern agreed with previously published data (Lee et al., 1999). In Hoxc13 null mice, expression of the truncated Hoxc13 transcript was no longer detectable, probably due to the largely ablated and transformed matrix (Fig 3h). However, in the nail matrix of nude mice, residual Hoxc13 could still be observed (Fig 3i), whereas Foxn1 expression was not detectable in the truncated nail matrix of Hoxc13 null mice (Fig 3j).
HOXC13 regulates Foxn1 expression
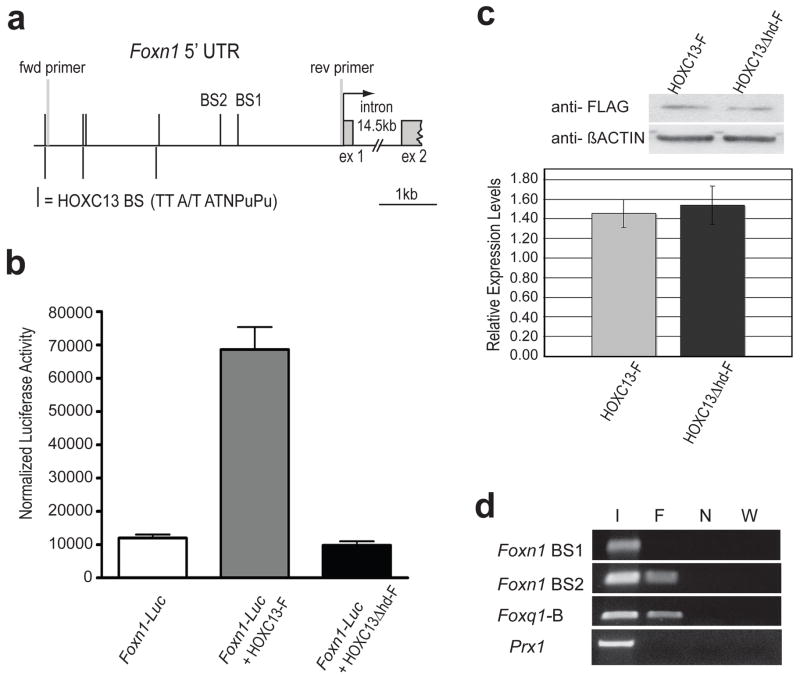
In-silico analysis of the Foxn1 promoter region revealed multiple matches to the HOXC13 consensus binding sequence (5′-TTA/TATNPuPu-3′) (Fig 4a) known to interact with both mouse and human HOXC13 (Jave-Suarez et al., 2002; Pruett et al., 2004; Potter et al., 2006). Transient co-transfection assays using C2C12 myofibroblasts were performed with FLAG (F)-tagged HOXC13 expression plasmids and a Foxn1-luciferase reporter gene construct (Foxn1-luc) that included proximal Foxn1 promoter sequences extending 5kb upstream of the Foxn1 transcriptional start site (Waterston et al., 2002; Fig 4a). As demonstrated in Figure 4b, significant differences were seen in luciferase activity between transfection groups [F(2,33)= 68.42, p < 0.001)]. Specifically, co-transfection with Foxn1-luc and full-length HOXC13 expression vector (HOXC13-F) indicated 6-fold up-regulation of luciferase activity relative to control assays without HOXC13-F (p<0.001) (Fig 4b). Notably, co-transfection with a HOXC13 expression vector in which the homeodomain had been deleted (HOXC13Δhd-F) failed to demonstrate significant luciferase activation compared to the control (Foxn1-luc alone; p<0.05), thus suggesting homeodomain-dependent activation of Foxn1. Western blot analysis of C2C12 cells transfected with either HOXC13-F or HOXC13Δhd-F using FLAG-specific antibodies showed roughly equal levels of protein expression for both the full-length and the truncated versions of the protein (Fig 4c).
Figure 4. HOXC13 directly regulates Foxn1 expression.
(a) HOXC13 consensus binding sequences (TTA/TATNPuPu, black bars) upstream of Foxn1 transcription start (angled arrow); putative binding sites (BS) 1 and 2 examined by ChIP and locations of PCR primers used for isolating the 5 kb region included in Foxn1-luc are indicated; boxed region: transcribed sequences; (b) Normalized luciferase activities resulting from co-transfection of C2C12 cells with Foxn1-luc and HOXC13 expression vectors as indicated; co-transfection with HOXC13Δhd-F, in which the homeodomain was deleted, resulted in expression levels similar to baseline levels. (c) Western blot analysis (top) using anti-FLAG antibodies confirmed expression of HOXC13-F and HOXC13Δhd-F in transfected C2C12 cells at approximately equal levels (n=3) as determined by densitometric quantification of chemiluminescent signals (see bar diagram below). (d) ChIP assays of C2C12 cells transfected with HOXC13-FLAG; PCR analysis of immunoprecipitated chromatin (F) indicated amplification of HOXC13-bound sequences specific for BS2 but not BS1 after 42 cycles; unprecipitated input DNA was used as positive control (I), while ChIP DNA from untransfected cells (N) and distilled water (W) were used as negative controls; additionally, control reactions using primers specific for Prx1 sequences containing no HOXC13 consensus binding sequences failed to yield PCR products with the same batch of immunoprecipitated DNA.
In vivo occupancy of the Foxn1 promoter region by HOXC13 was determined by chromatin immunoprecipitation (ChIP) of C2C12 cells transfected with HOXC13-F. Testing of precipitated chromatin by PCR amplification using two primer sets specific for putative HOXC13 binding sites (BS) 1 and 2, respectively, in the proximal Foxn1 promoter region (Fig 4a), demonstrated amplification of a _Foxn1_-specific DNA fragment encompassing BS2 but not BS1 (Fig 4d). Interestingly, unlike BS1, BS2 was found to reside in a sub-region of the Foxn1 promoter that is phylogenetically conserved between mouse, chimpanzee and Rhesus monkey (Fig S2). Control reactions were performed with precipitated chromatin and primers specific for a previously tested region of the Foxq1 promoter (_Foxq1_-B) containing a functional HOXC13 binding site (Potter et al., 2006) and a Prx1 promoter region not containing a HOXC13 consensus binding sequence; while a PCR amplification product was observed for _Foxq1_-B, none was detected for Prx1 (Fig 4d). Combined with the results from the co-transfection assays and the altered Foxn1 expression in vivo, these data demonstrate that HOXC13 mediates transcriptional regulation of Foxn1; the specific in vivo contribution of the particular binding site (BS2), however, will need to be determined through a systematic dissection of the Foxn1 promoter region with further in vivo validation.
DISCUSSION
The 2.3-fold downregulation of Foxn1 in 5d p.n. skin of Hoxc13 null mice was accompanied by a drastic down-regulation of hair structural genes, primarily keratin and KAP genes (Table 1). Earlier reports of _Foxn1_-dependent dysregulation either of some of the same genes, including Krt31, Krt32, Krt33b, Krt34, Krt35, Krt84, Krt86, and Krtap5-4 (Schorpp et al., 2000; Schlake et al., 2000; Schlake and Boehm_,_ 2001; Schlake, 2001) combined with the similar histopathological changes in hair and nail of Hoxc13 null and nude mice observed here, suggest that Hoxc13 and Foxn1 act in some of the same differentiation pathways. The data from HOXC13/Foxn1 co-transfection and ChIP analysis (Fig. 5) suggest that HOXC13 acts as transcriptional regulator of Foxn1.
Figure 5. Models of HOXC13-controlled network motifs involved in regulating HF differentiation.
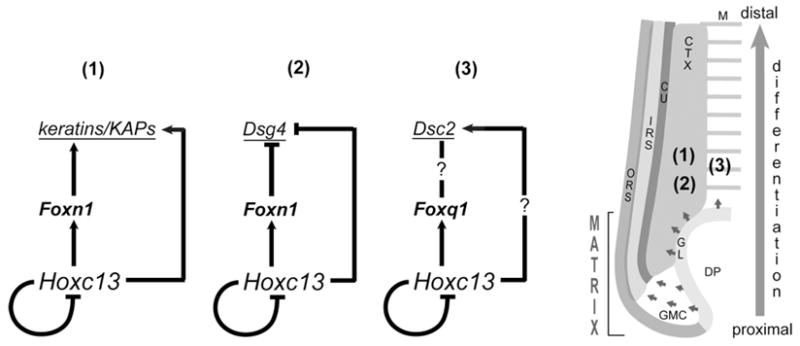
Left: schematics of three HOXC13-controlled feed-forward loop (FFL)-type network motifs (1), (2), and (3) as explained in the text; a HOXC13 negative feedback loop (Tkatchenko et al., 2001) is included in all three circuits but requires validation. Right: Schematic of bisected lower portion of anagen HF indicating the medulla (M), cortex (CTX) and cuticle (CU) of the hair shaft, and the internal root sheath (IRS) and outer root sheath (ORS) that transitions into the germinal layer (GL) surrounding the dermal papilla (DP); the germinal compartment in the lower matrix (GMC) contains proliferating, transit-amplifying cells (Langbein and Schweizer, 2005); the topography of regulatory network motifs shown on the left is indicated by the numbers.
The complexity of the Hoxc13 expression pattern in different HF compartments suggests that Hoxc13 is involved in controlling the differentiation of various distinct trichocyte lineages, each expressing a specific set of terminal differentiation markers. Studies involving electrophoretic mobility shift assays (EMSAs) and co-transfection assays suggested that HOXC13 controls the expression of human keratin genes KRT32 (previously KRTHA2), specifically expressed in the hair shaft cuticle, and KRT35 (KRTHA5), expressed in both cuticle and cortex (Jave-Suarez et al., 2002). Consistent with these data, the mouse versions of both genes (Krt32 and Krt35) are among the hair keratin genes strongly down-regulated in skin of Hoxc13 null mice (Table 1). Furthermore, we previously showed sequence-specific interaction of HOXC13 with cognate binding sites abundantly present in the Krtap16 KAP gene complex (Pruett et al., 2004). Most members of this gene complex (Krtap16-1, -4, -5, -8, -9, and -10) are expressed in the differentiating cortex (ibid), and they are among the most strongly down-regulated genes (up to 240-fold) in skin of Hoxc13 null mice (Table 1). It is tempting to speculate that this massive reduction in Krtap16 expression does not exclusively result from the loss of HOXC13 as a putative direct regulator but may reflect the collapse of a regulatory cascade involving Foxn1 (and possibly Hoxc12; see Shang et al., 2002) as a potential amplifier of HOXC13 activity in Krtap16 regulation (Fig 5).
This proposed co-regulation of Krtap16 and other KAP and keratin genes by HOXC13 and FOXN1 is consistent with findings demonstrating strong activation of Krt33b (previously Krt1-3, mHa3) and Krt85 (previously Krt2-18, mHb5) upon tetracycline-induced FOXN1 expression in HeLa cells that was dependent on the presence of the FOXN1 N-terminal DNA binding domain (Schlake et al., 2000). Accordingly, one may conceptualize a regulatory network of hair shaft differentiation that involves in addition to a hierarchical HOXC13-controlled FOXN1 pathway, parallel pathways in which keratin genes are regulated directly by HOXC13 and FOXN1, as well as other transcription factors known to be involved in hair shaft differentiation, such as LEF1/CTNNB1, MSX2, and SMAD4 (Millar, 2002; Qiao et al., 2006; Fuchs and Raghavan, 2002; Duverger and Morasso, 2008; Owens et al., 2008; Cai et al., 2009).
A shortcoming of this rather simple network model is that it fails to take likely HOXC13 co-factor requirements into account because pertinent information is presently limited. As a member of the AbdB-type HOX proteins, HOXC13 was predicted to interact most likely with MEIS- and PREP-type members of the TALE (three amino acid loop extension) superclass of homeodomain proteins (Jave-Suarez and Schweizer, 2006). However, failure to demonstrate nuclear co-localization of candidate MEIS and PREP proteins with HOXC13 in KRT32- and _KRT35_-expressing cells (ibid), seems to argue against this idea, thus providing a rationale for considering alternative co-factors. Both FOXN1 expressed in the cortex, cuticle and IRS, and FOXQ1 in the medulla might be potential candidates, particularly in view of data indicating that other members of the FOX family act as HOX co-factors in different systems. Specifically, FOXP1 was shown to determine spinal motor neuron (MN) columnar fate in cooperation with various HOX proteins (Dasen et al., 2008); this FOXP1 expression requires HOX activity, an apparent regulatory parallel to Drosophila, where the Hox protein Scr directly activates expression of forkhead (Ryoo and Mann, 1999). Furthermore, HOXA11 interfaces with FOXO1A in regulating prolactin (prl) gene expression in endometrial stromal cells (Lynch et al., 2009).
Accordingly, our data are consistent with the idea that HOXC13 and FOXN1 operate in a regulatory network motif known as coherent feed-forward loop (FFL) (Mangan and Alon, 2003). This type of FFL is one of the most abundantly occurring three-gene regulatory circuits found in biological systems in which transcription factor X regulates transcription factor Y and both regulate gene Z in such a way that the signs (activation or repression) of the direct and indirect paths of gene Z regulation are the same (ibid). Although requiring further validation, the proposed HOXC13-FOXN1-keratin/KAP gene regulatory circuit in the HF cortex (Fig 5) fits the model of a coherent FFL and, in the case of the Krtap16 gene cluster (Pruett et al., 2004), is supported by the presence of more than 30 copies of the core motif of the Forkhead domain binding sequence (TAAACA; Furuyama et al., 2000; Yan et al., 2006) within 23 kb of DNA, more than 6 times its random statistical frequency (data not shown). Common functions of coherent FFLs are modulation of the amplitude and timing (delay) of effecter gene activation or repression (Mangan and Alon, 2003), which are both critical for orchestrating differentiation processes such as hair shaft formation.
The regulation of Foxq1 by HOXC13 in the medulla (Potter et al. 2006) might be part of an FFL that potentially involves Desmocollin2 (Dsc2) as a common target; this is supported by in silico analysis of the Dsc2 promoter region that identified clusters of bona fide binding sites for both HOXC13 and Forkhead domain transcription factors (data not shown). Recent data suggesting transcriptional regulation of another desmosomal cadherin gene, Desmoglein4 (Dsg4), by HOXC13 and FOXN1 in the differentiating follicular cortex (Bazzi et al., 2009) are consistent with the concept of a HOXC13-controlled FFL-type network motif as well (Fig. 5).
Multifactorial regulation of Hox target genes has been studied extensively in Drosophila. A good example is the activation of the cell death gene reaper (rpr) by the Hox gene Deformed (Dfd) in a distinct subregion of the Dfd expression domain during Drosophila head development that requires interaction with at least eight other transcription factors on a minimal enhancer (Stöbe et al., 2009). Based on this paradigm, target gene specificity in distinct lineages of hair shaft differentiation within the HOXC13 expression domain may be expected to require different combinations of accessory transcription factors and some of these are likely to be induced by HOXC13.
In summary, the data demonstrating HOXC13-dependent activation of Foxn1 reported here and of Foxq1 shown previously (Potter et al., 2006) combined with the apparent control of downstream effecter genes (Dsg4 – Bazzi et al., 2009; Crisp1 – Peterson et al., 2005; hair keratin and KAP genes - Jave-Suarez et al., 2002; Pruett et al., 2004) collectively provide a framework for outlining a HOXC13-controlled regulatory network of hair shaft differentiation. Furthermore, mutated alleles of the HOXC13 target genes FOXN1 and DSG4 in humans have been linked to an inherited immunodeficiency with baldness (Frank et al., 1999) and localized autosomal recessive hypotrichosis (Kljuic et al., 2003), respectively. The emerging _Hoxc13_-regulated pathways and network motifs presented and discussed here provide critical entry points for further studies promising more detailed insight into the molecular control mechanisms of HF differentiation. This information will be a prerequisite for understanding pertinent disease processes and designing effective therapeutic approaches.
MATERIALS AND METHODS
Mice and histological analysis of tissues
Mouse strains used: B6.129-Hoxc13tm1Mrc (Godwin and Capecchi, 1998), C57BL/6J+/+,C57BL/6J-Tyrc-2J, and Hsd-Foxn1nu/Foxn1nu; B6.129-Hoxc13tm1Mrc were crossed with C57BL/6J-Tyrc-2J mice to generate albino B6.Cg-Tyrc-2JHoxc13tm1Mrc. Mice were maintained in a temperature and light/dark (12/12 hrs) cycle-controlled vivarium. All studies were done using Institutional Animal Care and Use Committee -approved protocols. Dorsal skin and digits (front and rear feet) were collected from euthanized (CO2 asphyxiation) mice at 5d and ≥8 weeks p.n., fixed in Fekete’s acid alcohol/formalin solution, and transferred to 70% ethanol (Seymour et al., 2004) prior to paraffin-embedding. Sections (6–10 μm) were stained with hematoxylin/eosin (H&E).
In Situ hybridization
Dissected tissues (scapular skin and rear feet) from homozygous Hsd-Foxn1nu, B6.Cg-Tyrc-2J, Hoxc13tm1Mrc, and C57BL/6J-Tyrc-2 mice at 5d p.n. were fixed and processed for cryo-sectioning (10 μm) as previously described (Potter et al., 2006). _Hoxc13_-specific probe was prepared by using pC13rev (Tkatchenko et al., 2001). Foxn1 probe template was generated by PCR using cDNA from skin of 5d p.n. FVB/NTac mice and primers 5′-TCCCAGCCTCTGCACCCAAT and 5′-TGCATGTCTCCCAGAGCACC; PCR products were cloned into pCRII-TOPO vector (Invitrogen) for the generation of digoxigenin (Roche Applied Science) -labeled antisense and sense (control) RNA probes (Pruett et al., 2004). Hybridization and colorimetric signal detection was performed as described (ibid).
Transient co-transfection assays
Transfection assays using C2C12 myofibroblasts were done as described (Potter et al., 2006). The reporter plasmid included 5 kb of the Foxn1 promoter region isolated from mouse genomic DNA by PCR using primers 5′–TCAGTCCGTCAGCCTGATTG and 5′-ACTTATGGCAATGCTCCTGC (Fig. 4a) and inserted into Photinus pyralis luciferase-containing pGL2-Basic (Promega). Homeodomain-deleted HOXC13 expression vector HOXC13Δhd-F was generated by removal of an EcoN1-XhoI fragment of about 550 bp containing the Hoxc13 homeobox and 3′UTR sequences from HOXC13-F (aka Hoxc13-FLAG; Potter et al., 2006) containing a full-length Hoxc13 cDNA tagged with an N-terminal FLAG epitope in pcDNA3.1 (Invitrogen). Renilla luciferase vector pRL-CMV (Promega) was used for normalization. HOXC13-F and HOXC13Δhd-F expression in transfected C2C12 cells was confirmed by Western blot analysis using anti-FLAG antibody (see supplemental material). Each transfection was performed in triplicate in at least three independent experiments. Normalized data was averaged (+/− standard error) and analyzed by analysis of variance (ANOVA) with Bonferroni post hoc analyses performed as applicable. Significance was set at _p_>0.05.
Chromatin immunoprecipitation (ChIP) and Western blot analysis
ChIP assays (Ren and Dynlacht, 2004) with C2C12 cells transfected with FLAG-tagged HOXC13 expression vector were performed as described (Potter et al., 2006). Precipitated Foxn1 DNA was detected by PCR using _Foxn1_-specific primer sets for binding sites (BS) 1 and 2 (Fig. 4); _Foxn1_BS1: 5′-AAGGCAGAATCCGGGCTGGA and 5′-CTCACATGCCTGCGFTTGTCC; _Foxn1_BS2: 5′-CCAGCATTGTTGGAAGGGTT and 5′-CTCCACAGGTCAGGAGCTGAG. A primer set (5′-CCTGAGTTACCTGCACTCTG and 5′-AGGACTGAGGAGGATTCTTG) specific for a Prx1 genomic region not containing HOXC13 binding sequences was used for control reaction. Annealing for all primers was at 60°C using standard conditions.
DNA microarray and quantitative (Q) –PCR analysis
Scapular skin from three 5d p.n. male Hoxc13tm1Mrc/Hoxc13tm1Mrc mice and three age- and sex-matched wild type littermates was used for RNA extraction, cDNA synthesis, and probe preparation for DNA microarray analysis using MOE430v2.0 GeneChip arrays (Affymetrix) and for Q-PCR analysis as described in detail (supplemental material).
Supplementary Material
Supp data
Acknowledgments
The authors gratefully acknowledge the technical assistance of Margaret Romano with processing tissue samples and Donna Jacob’s help with managing mouse strains. We thank Tim Stearns and Kathleen Silva from The Jackson Laboratory for help with DNA microarray data analysis and for providing tissue samples, respectively. This work was supported by NIH/NIAMS grant 2R01AR47204 and a National Alopecia Areata Foundation grant to AA.
References
- Akin ZN, Nazarali AJ. Hox genes and their candidate downstream targets in the developing central nervous system. Cell Mol Neurobiol. 2005;25:697–741. doi: 10.1007/s10571-005-3971-9. [DOI] [PubMed] [Google Scholar]
- Awgulewitsch A. Hox in hair growth and development. Naturwissenschaften. 2003;90:193–211. doi: 10.1007/s00114-003-0417-4. [DOI] [PubMed] [Google Scholar]
- Bazzi H, Demehri S, Potter CS, Barber AG, Awgulewitsch A, Kopan R, et al. Desmoglein 4 is regulated by transcription factors implicated in hair shaft differentiation. Differentiation; research in biological diversity. 2009;78:292–300. doi: 10.1016/j.diff.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich CJ, Ruddle FH, Stenn KS. Differential expression of the Hox 3.1 gene in adult mouse skin. Ann N Y Acad Sci. 1991;642:346–53. doi: 10.1111/j.1749-6632.1991.tb24400.x. discussion 53–54. [DOI] [PubMed] [Google Scholar]
- Cai J, Lee J, Kopan R, Ma L. Genetic interplays between Msx2 and Foxn1 are required for Notch1 expression and hair shaft differentiation. Dev Biol. 2009;326:420–30. doi: 10.1016/j.ydbio.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi MR. Hox genes and mammalian development. Cold Spring Harb Symp Quant Biol. 1997;62:273–81. [PubMed] [Google Scholar]
- Chuong CM, Noveen A. Phenotypic determination of epithelial appendages: genes, developmental pathways, and evolution. J Investig Dermatol Symp Proc. 1999;4:307–11. doi: 10.1038/sj.jidsp.5640235. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Oliver G, Ting SA, Jegalian BG, Chen HM, De Robertis EM. Gradients of homeoproteins in developing feather buds. Development (Cambridge, England) 1990;110:1021–30. doi: 10.1242/dev.110.4.1021. [DOI] [PubMed] [Google Scholar]
- Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–16. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Duboule D. The vertebrate limb: a model system to study the Hox/HOM gene network during development and evolution. Bioessays. 1992;14:375–84. doi: 10.1002/bies.950140606. [DOI] [PubMed] [Google Scholar]
- Duverger O, Morasso MI. Role of homeobox genes in the patterning, specification, and differentiation of ectodermal appendages in mammals. J Cell Physiol. 2008;216:337–46. doi: 10.1002/jcp.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Pignata C, Panteleyev AA, Prowse DM, Baden H, Weiner L, et al. Exposing the human nude phenotype. Nature. 1999;398:473–4. doi: 10.1038/18997. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–34. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin AR, Capecchi MR. Hoxc13 mutant mice lack external hair. Genes Dev. 1998;12:11–20. doi: 10.1101/gad.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jave-Suarez LF, Schweizer J. The HOXC13-controlled expression of early hair keratin genes in the human hair follicle does not involve TALE proteins MEIS and PREP as cofactors. Arch Dermatol Res. 2006;297:372–6. doi: 10.1007/s00403-005-0623-3. [DOI] [PubMed] [Google Scholar]
- Jave-Suarez LF, Winter H, Langbein L, Rogers MA, Schweizer J. HOXC13 is involved in the regulation of human hair keratin gene expression. J Biol Chem. 2002;277:3718–26. doi: 10.1074/jbc.M101616200. [DOI] [PubMed] [Google Scholar]
- Kanzler B, Viallet JP, Le Mouellic H, Boncinelli E, Duboule D, Dhouailly D. Differential expression of two different homeobox gene families during mouse tegument morphogenesis. Int J Dev Biol. 1994;38:633–40. [PubMed] [Google Scholar]
- Kljuic A, Bazzi H, Sundberg JP, Martinez-Mir A, O’Shaughnessy R, Mahoney MG, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113:249–60. doi: 10.1016/s0092-8674(03)00273-3. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Langbein L, Schweizer J. Keratins of the human hair follicle. Int Rev Cytol. 2005;243:1–78. doi: 10.1016/S0074-7696(05)43001-6. [DOI] [PubMed] [Google Scholar]
- Lee D, Prowse DM, Brissette JL. Association between mouse nude gene expression and the initiation of epithelial terminal differentiation. Dev Biol. 1999;208:362–74. doi: 10.1006/dbio.1999.9221. [DOI] [PubMed] [Google Scholar]
- Lynch VJ, Brayer K, Gellersen B, Wagner GP. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One. 2009;4:e6845. doi: 10.1371/journal.pone.0006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci U S A. 2003;100:11980–5. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Mecklenburg L, Nakamura M, Sundberg JP, Paus R. The nude mouse skin phenotype: the role of Foxn1 in hair follicle development and cycling. Exp Mol Pathol. 2001;71:171–8. doi: 10.1006/exmp.2001.2386. [DOI] [PubMed] [Google Scholar]
- Mecklenburg L, Paus R, Halata Z, Bechtold LS, Fleckman P, Sundberg JP. FOXN1 is critical for onycholemmal terminal differentiation in nude (Foxn1) mice. The Journal of investigative dermatology. 2004;123:1001–11. doi: 10.1111/j.0022-202X.2004.23442.x. [DOI] [PubMed] [Google Scholar]
- Mecklenburg L, Tychsen B, Paus R. Learning from nudity: lessons from the nude phenotype. Experimental dermatology. 2005;14:797–810. doi: 10.1111/j.1600-0625.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- Meier N, Dear TN, Boehm T. Whn and mHa3 are components of the genetic hierarchy controlling hair follicle differentiation. Mechanisms of development. 1999;89:215–21. doi: 10.1016/s0925-4773(99)00218-x. [DOI] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. The Journal of investigative dermatology. 2002;118:216–25. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–7. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- Owens P, Bazzi H, Engelking E, Han G, Christiano AM, Wang XJ. Smad4-dependent desmoglein-4 expression contributes to hair follicle integrity. Dev Biol. 2008;322:156–66. doi: 10.1016/j.ydbio.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Tkatchenko TV, Pruett ND, Potter CS, Jacobs DF, Awgulewitsch A. Epididymal Cysteine-Rich Secretory Protein 1 (CRISP-1) Encoding Gene is Expressed in Murine Hair Follicles and Downregulated in Mice Overexpressing Hoxc13. J Invest Dermatol. 2005;10:238–42. doi: 10.1111/j.1087-0024.2005.10114.x. [DOI] [PubMed] [Google Scholar]
- Potter CS, Peterson RL, Barth Jl, Pruett ND, Jacobs DF, Kern MJ, et al. Evidence that satin hair mutant gene Foxq1 is among multiple and functionally diverse presumptive regulatory targets for Hoxc13 during hair follicle differentiation. J of Biol Chem. 2006;281:29245–55. doi: 10.1074/jbc.M603646200. [DOI] [PubMed] [Google Scholar]
- Pruett ND, Tkatchenko TV, Jave-Suarez L, Jacobs DF, Potter CS, Tkatchenko AV, et al. Krtap16, characterization of a new hair keratin-associated protein (KAP) gene complex on mouse chromosome 16 and evidence for regulation by Hoxc13. J Biol Chem. 2004;279:51524–33. doi: 10.1074/jbc.M404331200. [DOI] [PubMed] [Google Scholar]
- Qiao W, Li AG, Owens P, Xu X, Wang XJ, Deng CX. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene. 2006;25:207–17. doi: 10.1038/sj.onc.1209029. [DOI] [PubMed] [Google Scholar]
- Reid AI, Gaunt SJ. Colinearity and non-colinearity in the expression of Hox genes in developing chick skin. Int J Dev Biol. 2002;46:209–15. doi: 10.1387/ijdb.011495. [DOI] [PubMed] [Google Scholar]
- Ren B, Dynlacht BD. Use of chromatin immunoprecipitation assays in genome-wide location analysis of mammalian transcription factors. Methods Enzymol. 2004;376:304–15. doi: 10.1016/S0076-6879(03)76020-0. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Mann RS. The control of trunk Hox specificity and activity by Extradenticle. Genes Dev. 1999;13:1704–16. doi: 10.1101/gad.13.13.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlake T. The nude gene and the skin. Exp Dermatol. 2001;10:293–304. doi: 10.1034/j.1600-0625.2001.100501.x. [DOI] [PubMed] [Google Scholar]
- Schlake T, Boehm T. Expression domains in the skin of genes affected by the nude mutation and identified by gene expression profiling. Mech Dev. 2001;109:419–22. doi: 10.1016/s0925-4773(01)00538-x. [DOI] [PubMed] [Google Scholar]
- Schlake T, Schorpp M, Maul-Pavicic A, Malashenko AM, Boehm T. Forkhead/winged-helix transcription factor Whn regulates hair keratin gene expression: molecular analysis of the nude skin phenotype. Dev Dyn. 2000;217:368–76. doi: 10.1002/(SICI)1097-0177(200004)217:4<368::AID-DVDY4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Schorpp M, Schlake T, Kreamalmeyer D, Allen PM, Boehm T. Genetically separable determinants of hair keratin gene expression. Dev Dyn. 2000;218:537–43. doi: 10.1002/1097-0177(200007)218:3<537::AID-DVDY1007>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, et al. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–74. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour R, Ichiki T, Mikaelian I, Boggess D, Silva KA, Sundberg JP. Necropsy methods. In: Hedrich HJ, editor. Laboratory mouse. London: Academic Press; 2004. pp. 495–516. [Google Scholar]
- Shang L, Pruett ND, Awgulewitsch A. Hoxc12 expression pattern in developing and cycling murine hair follicles. Mechanisms of development. 2002;113:207–10. doi: 10.1016/s0925-4773(02)00022-9. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Stöbe P, Stein MA, Habring-Muller A, Bezdan D, Fuchs AL, Hueber SD, et al. Multifactorial regulation of a hox target gene. PLoS Genet. 2009;5:e1000412. doi: 10.1371/journal.pgen.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatchenko AV, Visconti RP, Shang L, Papenbrock T, Pruett ND, Ito T, et al. Overexpression of Hoxc13 in differentiating keratinocytes results in downregulation of a novel hair keratin gene cluster and alopecia. Development (Cambridge, England) 2001;128:1547–58. doi: 10.1242/dev.128.9.1547. [DOI] [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–62. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Yan J, Xu L, Crawford G, Wang Z, Burgess SM. The forkhead transcription factor FoxI1 remains bound to condensed mitotic chromosomes and stably remodels chromatin structure. Mol Cell Biol. 2006;26:155–68. doi: 10.1128/MCB.26.1.155-168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp data