Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats (original) (raw)
Abstract
The dystrophin–glycoprotein complex (DGC) provides an essential link from the muscle fibre cytoskeleton to the extracellular matrix. In dystrophic humans and mdx mice, mutations in the dystrophin gene disrupt the structure of the DGC causing severe damage to muscle fibres. In frog muscles, transmission of force laterally from an activated fibre to the muscle surface occurs without attenuation, but lateral transmission of force has not been demonstrated in mammalian muscles. A unique ‘yoke’ apparatus was developed that attached to the epimysium of muscles midway between the tendons and enabled the measurement of lateral force. We now report that in muscles of young wild-type (WT) mice and rats, compared over a wide range of longitudinal forces, forces transmitted laterally showed little or no decrement. In contrast, for muscles of mdx mice and very old rats, forces transmitted laterally were impaired severely. Muscles of both mdx mice and very old rats showed major reductions in the expression of dystrophin. We conclude that during contractions, forces developed by skeletal muscles of young WT mice and rats are transmitted laterally from fibre to fibre through the DGC without decrement. In contrast, in muscles of dystrophic or very old animals, disruptions in DGC structure and function impair lateral transmission of force causing instability and increased susceptibility of fibres to contraction-induced injury.
Non-technical summary
The force developed by a single fibre in frog muscles is transmitted laterally to the muscle surface with little or no loss. To demonstrate this phenomenon in mammals, a ‘yoke’ apparatus was developed that attached to the surface of whole, parallel-fibred muscles and permitted measurements of the lateral transmission of forces. We then demonstrated that for wild-type mice and rats longitudinal and lateral transmission of forces in muscles were not different. In contrast, for skeletal muscles of dystrophic mice and very old rats, in which the dystrophin-associated glycoprotein complex (DGC) of fibres was disrupted, the forces transmitted laterally were impaired severely. We conclude that during contractions of skeletal muscles, an intact DGC is essential for the lateral transmission of force and disruptions of the DGC lead to sarcomere instability and contraction-induced injury.
Introduction
A major breakthrough occurred in the understanding of muscle mechanics when Street (1983) provided the first physiological evidence that confirmed, in experiments on in vitro semitendinosus muscles of frogs, that two pathways of force transmission existed, one longitudinal and the other lateral. These experiments demonstrated for the first time that most, if not all of the force developed by a single muscle fibre longitudinally was transmitted laterally through the adjacent extracellular matrix (ECM) and muscle fibres to the epimysium of the skeletal muscle. Despite this early demonstration of the existence of the lateral transmission of force in skeletal muscles of frogs, the inability to perform similar experiments on any mammalian skeletal muscle resulted in a complete lack of direct evidence that this phenomenon also occurred in the skeletal muscles of mammalian species. Although no measurements have been made that demonstrate lateral transmission of force in mammalian muscles, many investigators (Pardo et al. 1983; Ervasti & Campbell, 1993; Worton, 1995; Rybakova et al. 2000; Paul et al. 2002; Bloch & Gonzalez-Serratos, 2003; Campbell & Stull, 2003; Ervasti, 2003; Huijing, 2003; Bloch et al. 2004; Abmayr & Chamberlain, 2006; Anastasi et al. 2008; Claflin & Brooks, 2008) have assumed that the lateral transmission of force must function as effectively in mammalian muscles, as evidenced in the muscles of frogs (Street, 1983), despite the absence of any direct evidence that such is the case.
The pathway proposed by these investigators for the lateral transmission of force has focused primarily on the potential of costameres, first described by Pardo and colleagues (1983), to provide the necessary linkage for the transfer of force laterally from the z-discs of skeletal muscle fibres to the ECM (Ervasti, 2003; Bloch et al. 2004). Within striated skeletal muscle fibres, the dystrophin-associated glycoprotein complex (DGC), situated primarily within costameres, appears to provide the necessary connection between the force-generating structures, the sarcomeres, laterally through the sarcolemma and basement membrane into the ECM that is shared with the surrounding muscle fibres (Ervasti & Campbell, 1991; Worton, 1995; Henry & Campbell, 1996; Bloch & Gonzalez-Serratos, 2003; Ervasti, 2003; Michele & Campbell, 2003; Bloch et al. 2004; Lapidos et al. 2004; Anastasi et al. 2008). During contractions, these potential pathways for the lateral transmission of forces provide the possibility of stabilizing the lengths of sarcomeres that vary in their capability of developing force (Macpherson et al. 1997; Panchangam et al. 2008). The DGC appears to be essential in mammalian skeletal muscles, since a loss of its components causes muscular dystrophy in both humans (Hoffman et al. 1987; Worton, 1995; Bloch et al. 2004) and mice (Rybakova et al. 2000; Li et al. 2006). In dystrophic (mdx) mice, whose skeletal muscles lack the DGC, even repeated isometric contractions cause a severe contraction-induced injury (Claflin & Brooks, 2008) and lengthening contractions cause an injury that is even more severe (DelloRusso et al. 2001; Li et al. 2006). The conclusion that dystrophin and the DGC protect skeletal muscle fibres from contraction-induced injury is supported by the protection from contraction-induced injury provided the skeletal muscles of dystrophic mice through the expression of a mini-dystrophin fusion gene that restored dystrophin expression and DGC function in skeletal muscles (Li et al. 2006).
Unlike muscular dystrophy, with a single underlying cause arising from the loss of dystrophin, the age-related changes in skeletal muscles arise from multiple underlying causes that are largely unknown. The age-related structural and functional deficits that have been demonstrated in skeletal muscles of mice (Brooks & Faulkner, 1988), rats (Larsson et al. 1991) and humans (Dedrick & Clarkson, 1990; Frontera et al. 1991; Ploutz-Snyder et al. 2001) include a loss of motor units (Doherty et al. 1993); muscle atrophy; fatigability and weakness (Young et al. 1984, 1985; Brooks & Faulkner, 1988; Frontera et al. 1991); a decrease in maximum and sustained power (Faulkner et al. 2008); and an increased susceptibility to contraction-induced injury (Dedrick & Clarkson, 1990; DelloRusso et al. 2001; Ploutz-Snyder et al. 2001; Li et al. 2006). The possibility that during the ageing of mammalian skeletal muscles, the loss of dystrophin expression leads to an impaired lateral transmission of force has not been investigated previously. Here we demonstrate for the first time that force is transferred laterally without decrement in skeletal muscles of young wild-type (WT) mice and rats. In contrast, in skeletal muscles of both mdx mice and very old rats, the lateral transmission of force is impaired severely. Throughout the manuscript the terms WT, mdx and very old muscles will be used when appropriate, rather than designating the species. The observations on WT, mdx and very old muscles suggest that disruptions associated with muscular dystrophy, or acquired disruptions of the DGC associated with ageing, may interfere with the mechanical connections between skeletal muscle fibres and the ECM and that such disruptions may lead to impairments in the lateral transmission of force and the subsequent muscle dysfunctions that are associated with both dystrophy and ageing.
Methods
To investigate the effect of the lack of dystrophin on the lateral transmission of force, adult (12–15 months of age) WT (_n_= 6) and mdx (_n_= 6) male mice from the C57BL/10ScSn-mdx/J strain were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). In addition, to determine the age-related influences, F344 × Brown Norway F1 young rats of 3 months of age (_n_= 6), old rats of 30–33 months of age (_n_= 6), and very old rats of 36–38 months of age were obtained from the National Institutes of Aging. The inclusion of the two ‘old age’ groups was based on the linear decline in the skeletal muscles of humans of the number of motor units (Doherty et al. 1993) and the number of fibres in skeletal muscles (Lexell et al. 1988) that result in a linear decline in strength, power, maximum oxygen uptake and consequently in the records for running and weight lifting (Faulkner et al. 2008). Similar declines in the structure and function of skeletal muscles with ageing have been reported for mice (Brooks & Faulkner, 1988), rats (Kanda et al. 1986) and humans (Lexell et al. 1988). Prior to the present experiments, animals were housed in a pathogen-free barrier-protected animal room in the Unit for Laboratory Animal Medicine, University of Michigan. The procedures used in the present study were approved by the University of Michigan Committee on the Use and Care of Animals and were conducted in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals. The experiments and all treatments of the mice and rats comply with The Journal of Physiology's policy on animal experimentation (Drummond, 2009).
Experimental procedures
In preparation for the measurement of the forces developed by skeletal muscles, each mouse or rat was anaesthetized with an initial intraperitoneal injection of pentobarbital sodium (40 to 60 mg kg−1). Throughout an experiment, supplemental dosages maintained a depth of anaesthesia that prevented responses to tactile stimuli and a heated platform maintained core temperature at 37°C. For each mouse or rat, an incision was made on the left leg longitudinally from the knee to the ankle and a blunt dissection technique freed the muscle from the surrounding connective tissue. For the adult WT and mdx mice, the distal tendon of the anterior tibialis (ATB) muscle was exposed and cut just proximal to the superior transverse ligament and silk 4–0 sutures were tied to the distal tendon as close as possible to the muscle without damageing muscle fibres. For the rats, a similar dissection was used to prepare the extensor digitorum longus (EDL) muscle. For each of the two muscles, the distal tendon of the muscle was folded back to form a short loop and tied again. The mouse or rat was placed in a prone position on the platform of the apparatus with the left leg fixed at both the ankle and knee. An ‘S’ hook was attached to the force transducer (BG-1000; Kulite Semiconductor products, Leonia, NJ, USA) and then hooked through the loop of the distal tendon of the muscle. The exposed muscle and tendon were kept moist by periodic applications of isotonic saline. For a given preparation of either muscle, single twitches of the whole muscle were initiated by square wave pulses of 0.2 ms duration administered to the appropriate motor nerve. The stimulation voltage and muscle length (_L_o) were adjusted to produce the maximum isometric twitch force (_P_t). With the muscle at _L_o, tetanic contractions were produced by the 0.2 ms square wave pulses applied for 300 ms at a frequency of 40 Hz. Subsequent tetanic contractions were produced with increments of 30 Hz in the stimulus frequency until the force reached a plateau. For each of the two muscles, the maximum isometric tetanic force (_P_o) was produced typically at between 150 and 200 Hz. Two minutes were allowed between contractions to enable the muscle to recover. A trace of a maximum isometric contraction of an anterior tibialis (ATB) muscle of a WT mouse is presented in Fig. 2_A_. Note that in the absence of the ‘yoke-apparatus’, there is a more rapid rise in tension, a well-defined plateau in maximum force, and the attainment of a slightly higher maximum force (Fig. 2_Aa_ compared with Fig. 2_Ab_).
Figure 2. Comparison of the longitudinal and lateral transmission of forces in WT and mdx mice.
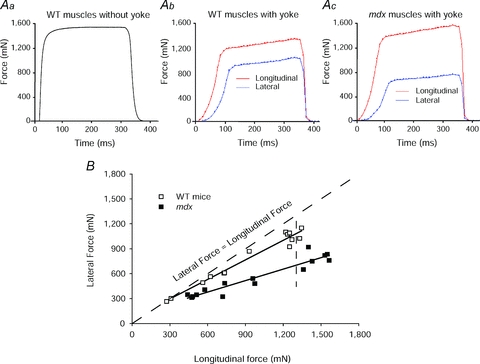
Aa, a record of the maximum isometric tetanic force (_P_o) of an ATB muscle of a WT mouse. Note ATB and EDL muscles of WT and mdx mice and young and old WT rats display a very similar type of isometric force trace. Ab and c, records obtained following the attachment of the ‘yoke’ apparatus. The forces were measured during maximum stimulation of the motor nerve with the muscle at optimum length for the production of force (_L_o). Following the attachment of the ‘yoke’ apparatus, the longitudinal and lateral force traces of maximally activated WT (b) and mdx (c) muscles of mice displayed a slower initial rise in force and then a secondary even slower rise. After the attachment of the ‘yoke’, the _P_o values of the WT and mdx muscles were ∼25% lower than those of the muscles before the attachment of the ‘yoke’. Note the much closer relationship for the values for the lateral and longitudinal forces for the WT muscle than for the mdx muscle. B, relationship of longitudinal to lateral force transmission in anterior tibialis (ATB) muscles of mdx and WT mice.
‘Yoke apparatus’
For the measurement of the lateral transmission of force from activated fibres to the surface of the muscles, a small plastic ‘yoke-apparatus’ was designed and fabricated (Fig. 1_A_ and B). A number of ‘yokes’ of different internal circumferences were cut on a laser-cutting apparatus, with the smallest ‘yoke’ with internal diameters from 2 mm to 4 mm and the largest ‘yoke’ from 4 mm to 8 mm, The availability of ‘yokes’ of different sizes enabled, for a given experiment, the selection of a ‘yoke’ of the appropriate size. Four holes for the attachments of sutures were spaced equally around the ‘yoke’. After the measurement of maximum isometric force was made, the hook on the distal tendon of the muscle that attached to the force transducer was removed and a ‘yoke’ of the appropriate size was slipped onto the muscle such that the ‘yoke’ fit snugly over the belly of the muscle at a point equidistant from the two ends. Once positioned, silk 6–0 sutures attached the ‘yoke’ firmly to the epimysium of the muscle at each of four points around the belly of the muscle. A separate set of four sutures attached to the ‘yoke’ were brought together with a single attachment to the force transducer (Fig. 1_A_ and B). The four anchor points on the ‘yoke’ attached to a single hook on the force transducer provided the measurement of the force transmitted laterally (Fig. 1_B_). Although the ‘yoke’ attachment withstood forces up to ∼1800 mN for ATB muscles of mice and ∼2000 mN for EDL muscles of rats, tearing of the epimysium occurred at higher forces and a maximum isometric force could not be measured laterally. For the ATB muscle of the mouse and the EDL muscle of the rat, there is essentially no variation in the lengths of the fibres throughout either muscle. For EDL muscles of the mouse, the fibre length (_L_f)/muscle length (_L_m) ratio is 0.51 ± 0.18 and for the ATB muscle 0.61 ± 0.04 (Burkholder et al. 1994). Our data on EDL muscles of young (_L_f/_L_m= 0.45), adult (_L_f/_L_m= 0.44) and old (_L_f/_L_m= 0.45) mice (Brooks & Faulkner, 1988) and unpublished data on rats are in good agreement with these values. The homogeneity in the lengths of the individual fibres and the lengths of these fibres equalling almost half the length of the muscle are the major reasons for the extensive use of these muscles in studies of ageing, fatigability and contractility (Brooks & Faulkner, 1996; Renganathan et al. 1997; DelloRusso et al. 2001; Li et al. 2006). For the ATB muscles of mice and the EDL muscles of rats, there was a loss in maximum isometric force measured longitudinally after the attachment of the ‘yoke’ apparatus to the muscle, but the deficit was not significantly different between the WT and mdx mice, or among the three age groups of rats. Following the attachment of the ‘yoke’ apparatus, the maximum isometric force decreased on the average by 30% for the muscles of WT and mdx mice and 20% for the muscles of the young, old and very old rats. Fortunately, the losses in force due to the attachment of the ‘yoke’ were linearly related to the forces developed in response to the stimulation of different numbers of motor units. Consequently, the relationship between lateral and longitudinal force was not affected by the ‘yoke’ (Figs 1, 2, and 3). We conclude that the decrease in the maximum force following the attachment of the ‘yoke’ apparatus was attributable to the suturing of the ‘yoke’ apparatus to the epimysium of the muscle that led to accidental damage to single fibres close to the periphery of the muscles. The values for maximum specific longitudinal forces of the ATB muscles of WT (250 ± 15 kN m−2, _n_= 6) and mdx (200 ± 10 kN m−2, _n_= 6) mice and for EDL muscles of young rats (265 ± 15 kN m−2, _n_= 12) with an age-related decrease of 20% for the muscles old rats (205 ± 10 kN m−2, _n_= 5) and 30% for the muscles of very old rats (180 ± 5 kN m−2, _n_= 6) are consistent with published data on the effect of age on these muscles in mice (Brooks & Faulkner 1988) and of age on these muscles in rats (Carlson et al. 2002).
Figure 1. The apparatus used to measure the longitudinal and lateral transmission of force.
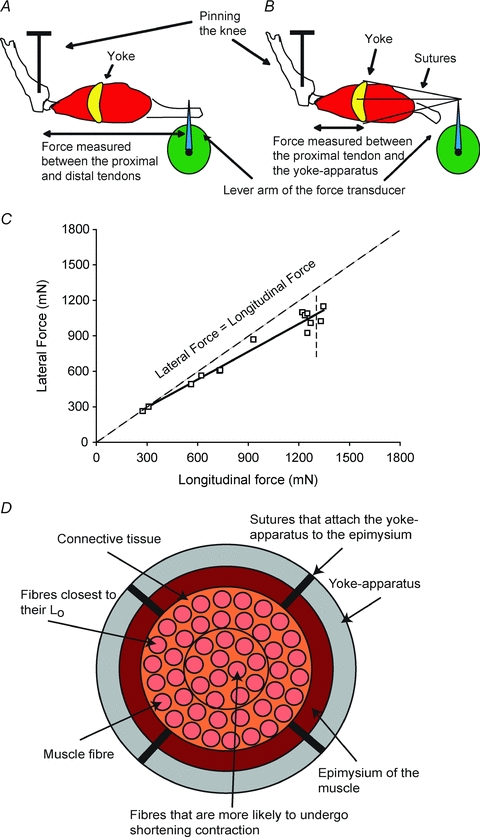
A, transmission of the force was measured from the proximal to the distal tendon to assess the longitudinal transmission of force. B, transmission of the force was measured from the proximal tendon laterally from the isometrically contracting fibres to the yoke. C, longitudinal and lateral force transmission in WT anterior tibialis anterior muscle. Note the closeness of the data to the line of identity between lateral and longitudinal force. D, diagram showing a cross-section of a skeletal muscle during the measurement of lateral transmission of forces. Note that throughout the contraction the fibres close to the surface of the muscle remain isometric between the proximal tendon and the yoke at an optimum length for force development. In contrast, the fibres toward the center of the muscle cross-section tend to shorten slightly and contribute less force than the fibres at optimum length.
Figure 3. Lateral and longitudinal transmission of force for EDL muscles of young, old and very old rats.
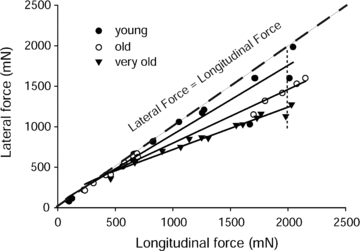
Values are given as the maximum isometric tetanic forces measured from proximal to distal tendon (longitudinal force) and from the yoke to the proximal tendon (lateral force) in cases of whole muscles as well as those from decreased sets of motor units.
Paired measurements of forces transmitted longitudinally and laterally
For a given experiment, the appropriate branch of the deep peroneal nerve was identified that innervated either the ATB muscle of mice, or the EDL muscle of rats. The branch of the peroneal nerve was severed to provide a sufficient length for the stimulation of the whole nerve to obtain the maximum longitudinal force generated by the whole muscle from the proximal to the distal tendon (see Table 1 for values and Figs 2_Aa–c_ for the force traces of the contractions). Note that prior to the attachment of the ‘yoke’, the force trace for both the WT and mdx muscles displayed an almost vertical rise in force with a sharp transition to the plateau at maximum force (Fig. 2_Aa_). In contrast, after the attachment of the ‘yoke’, the force trace for both WT and mdx muscles (Fig. 2_Ab_ and c) displayed a much slower initial rise in tension followed by a secondary slower rise to maximum force identified as when the force traces began to show a decline (Fig. 2_Ab_ and c). Despite considerable impact of the ‘yoke’ on the rate of development of force, the maximum longitudinal forces of the EDL muscles of young, old and very old rats measured after the attachment of the ‘yoke’ was decreased by 20% and that of the ATB muscles of the WT and mdx mice was decreased by 30% (Table 1). Following the measurement of the maximum force transmitted longitudinally, the distal tendon was detached from the force transducer and the ‘yoke’ was attached for the measurement of the forces transmitted laterally from the proximal tendon to the ‘yoke’ (Figs 1_C_, 2_B_ and 3). Progressive dissections of the motor nerve produced a wide range of paired longitudinal and lateral forces eventually down to one or two motor units for ATB muscles of both WT and mdx mice (Figs 1_C_ and 2_B_) and for EDL muscles of young, old and very old rats (Fig. 3). When measurements of the paired forces were completed, the proximal tendon was severed to separate the muscle from the leg, the ‘yoke’ was removed from the muscle and the muscle was blotted and weighed. The animal was then given an overdose of anaesthesia and a bilateral pneumothorax performed to ensure killing.
Table 1.
Summary of the measurements made on EDL muscles of rats and ATB muscles of mice
| Groups | Muscle mass (mg) | Muscle length (mm) | Specific force (kN m−2) | _P_o before yoke attachment (mN) | _P_o after yoke attachment (mN) | Lateral transmission of force (mN) |
|---|---|---|---|---|---|---|
| EDL muscles of rats | ||||||
| Young (_n_= 12) | 147 ± 4 | 31 ± 2 | 265 ± 15 | 2995 ± 365 | 2300 ± 340 | 1830 ± 230 |
| Old (_n_= 5) | 135 ± 9 | 27 ± 3 | 205 ± 10 | 2230 ± 190 | 1790 ± 150 | 1350 ± 160 |
| Very old (_n_= 6) | 143 ± 3 | 30 ± 1 | 180 ± 5 | 1885 ± 160 | 1570 ± 190 | 1030 ± 120 |
| ATB muscles of mice | ||||||
| Control (_n_= 6) | 53 ± 1 | 14.2 ± 0.1 | 250 ± 15 | 1695 ± 130 | 1220 ± 150 | 1050 ± 100 |
| mdx (_n_= 6) | 83 ± 1 | 14.2 ± 0.7 | 200 ± 10 | 2140 ± 70 | 1470 ± 85 | 815 ± 70 |
Histological analysis
Skeletal muscles were removed from anaesthetized rats and embedded fresh in a TFM™ tissue freezing medium (Triangle Biomedical Science, Durham, NC, USA). They were then snap frozen in liquid nitrogen-cooled isopentane. Transverse cryosections (8 μm) were cut through the middle of the muscle and were sectioned in a Leica CM3050 cryostat. For histological staining, frozen sections were fixed in 10% neutral buffered formalin and then stained with 0.1% picrosirus red/0.1% fast green for 30 min for the identification of the interstitial connective tissue. For immunohistological staining, sections were blocked with 5% BSA in phosphate buffered saline (PBS) for 1 h and stained with primary antibodies to collagen IV (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and pan-laminin (Sigma-Aldrich Corp., St Louis, MO, USA) followed by Cy3 conjugated secondary antibodies. All antibodies were diluted in blocking solution, and slides were washed in three washes of PBS between incubation. Following the staining procedure, the coverslips were mounted on the slides with Permafluor (Thermo Scientific). Slides were viewed and captured on an Olympus BX51 fluorescence microscope with a DP-71 digital camera. Images were analysed by ImageJ software to measure cross sectional areas (available at http://rsbweb.nih.gov/ij/).
Protein expression
KCl washed microsomes were prepared from homogenates of hind-limb muscles from young and very old rats. Briefly, muscles were homogenized using a dounce homogenizer in buffer containing (in mm): 20 sodium pyrophosphate, 20 sodium phosphate, 1 MgCl2, 300 sucrose, 5 EDTA, pH 7.1. Homogenates were centrifuged for 25 min at 14,000 g. The supernatant was collected and centrifuged for 35 min at 30,100 g. The pellet was then resuspended and incubated in buffer containing 50 mm Tris, pH 7.4, 0.3 m sucrose, 0.6 m KCl for 30 min at 4°C with gentle stirring. The resultant KCl washed microsomes were then collected by centrifugation at 140,000 g for 30 min and resuspended in 50 mm Tris, pH 7.4, 0.3 m sucrose. All buffers contained a combination protease inhibitor cocktail and were used ice cold. Membrane protein concentration was determined by the Lowry assay and equivalent amounts of protein were loaded on 3–15% gradient SDS-PAGE and Western blotting on PVDF membranes. Equivalent loading was confirmed by Ponceau S staining of the transferred membrane. Blots were blocked with 5% non-fat dry milk in TBS-T (50 mm Tris, pH 7.5, 150 mm NaCl + 0.01% Tween 20). Protein expression was determined using antibodies against dystrophin (Mandra, Sigma), glycosylated α-dystroglcyan (IIH6, Upstate Biotechnology/Millipore), β-dystroglycan (Santa Cruz Biotechnology), B1 integrin (Chemicon/Millipore) and dysferlin (Hamlet, Vector Laboratories, Inc., Burlingame, CA, USA) followed by horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) both diluted in blocking buffer and incubated for 1 h at room temperature. Blots were washed three times with TBS-T after each incubation. Blots were developed with ECL reagents (Pierce/Thermo Scientific) according to manufacturer directions and visualized and quantified using a Fluorchem imageing sytem and AlphaView software for densitometry (Alpha Innotech/Cell Biosciences, Santa Clara, CA, USA). To normalize the changes in protein expression, the data obtained on densitometry of the skeletal muscles of old and very old rats were divided by the mean of the expression levels in the young rats to demonstrate the relative magnitude of the change in protein expression with age.
Results
Lateral transmission of force in skeletal muscles of young, wild-type mice and rats
The measurements of paired values for lateral and longitudinal forces developed by the muscles of young WT mice (shown in Fig. 1_C_), young WT and mdx mice (shown in Fig. 2_C_) and young, old and very old rats (shown in Fig. 3) allowed comparisons from maximum whole muscle force to that of just a few motor units. The low forces, elicited by activating a small number of motor units in WT muscles of mice and rats, were transmitted equally well laterally and longitudinally and, even with high levels of motor unit activation, the forces at the ‘yoke’ averaged only slightly less than those measured at the distal tendon. For the WT muscles of both species, the small loss in the lateral, compared with the longitudinal, transmission of force observed at the highest forces generated was attributed to: (i) the compliance of the attachments of the ‘yoke’ to the surface of the muscle and (ii) a gradient in the amount of shortening that occurred in the lengths of fibres between the fibres at the periphery and those at the core of the muscles with the fibres in the core of the muscle shortening slightly more than those nearer to the ‘yoke’ (Fig. 1_D_). Both of these factors resulted in some fibres shortening to lengths that were less than optimum for the development of maximum force (Gordon et al. 1966). Despite these factors and the substantially higher forces developed in the experiments reported here, the results are in excellent agreement with the single fibre data obtained on the muscles of frogs (Street, 1983). We conclude that, as demonstrated previously in the muscles of frogs, the forces developed by WT muscles of young mice and rats are transmitted equally well laterally and longitudinally.
Lateral transmission of force is impaired in dystrophin-deficient mdx mice
Given that null mutations in the dystrophin gene lead to a loss of dystrophin expression in the sarcolemma of mdx muscles, we tested the hypothesis that the lateral transmission of force is impaired significantly in mdx compared with WT muscles. In contrast to the close approximation to the line of identity for the forces generated by the WT muscles and transmitted laterally to the ‘yoke’, those generated by the mdx muscles and transmitted laterally to the ‘yoke’ diverged at even the lowest forces measured. For the data plotted, the difference between the slopes of the best-fit lines for each data set (0.46 for the mdx and 0.78 for the WT muscles) indicate clearly a much closer relationship between the force measured at the ‘yoke’ and the force measured at the distal tendon for the WT than for the mdx muscles. For the WT muscles, the forces transmitted laterally and longitudinally did not diverge significantly from the line of identity until force levels exceeded 900 mN, a value ∼50% of the maximum isometric force developed by the WT muscles. The magnitude of the differences between the lateral compared with the longitudinal transmission of force generated by the mdx compared with the WT muscles was greatest at the highest forces measured, with a deficit of ∼50% for the mdx compared with ∼20% for the WT muscles. The impaired capability of the mdx muscles to transmit force laterally provides a direct demonstration that the DGC is a major factor in the lateral transmission of force and provides strong support for the models proposed for the lateral transmission of force in mammalian muscle that were until now hypothetical (Pardo et al. 1983; Hoffman et al. 1987; Ervasti & Campbell, 1993; Worton, 1995; Rybakova et al. 2000; Paul et al. 2002; Bloch & Gonzalez-Serratos, 2003; Ervasti, 2003; Huijing, 2003; Michele & Campbell, 2003; Bloch et al. 2004; Lapidos et al. 2004; Anastasi et al. 2008; Claflin & Brooks, 2008).
Lateral transmission of force is impaired with ageing
As with muscular dystrophy, advanced age leads to major decrements in muscle function that include a loss in the ability of mammals to generate force (Young et al. 1984, 1985; Brooks & Faulkner, 1988; Faulkner et al. 2008) and power (Faulkner et al. 2008). Due to the inability of old muscles and very old muscles to produce the same level of force as young muscles, the comparison of the lateral transmission of forces between age groups was achieved by taking the ratio of lateral to longitudinal transmissions. Figure 3 shows an age-related difference in the lateral transmission of force within the EDL muscles among the three age groups, young, old and very old. The difference in the lateral transmission of force between the old and the very old (_P_≤ 0.001) muscles is much greater than the difference between young and old muscles (P > 0.05), even though the difference in age between the young and old muscles is greater than that between the old and very old muscles. The deficit in the lateral transmission of forces for both old and very old muscles was particularly noticeable at very high forces, greater than 1000 mN. For forces less than 1000 mN, the young and old muscles expressed similar levels for the lateral transmission of force, whereas very old muscles expressed much lower values for the lateral transmission of force at all levels of force production.
Alterations in the interactions of muscle fibres with ECM within muscles of very old rats
Many of the molecular components responsible for the lateral transmission of force in skeletal muscles are unknown particularly those at the interface between the costameres and the ECM (Rybakova et al. 2000; Bloch & Gonzalez-Serratos, 2003; Ervasti, 2003; Lapidos et al. 2004). Despite the lack of knowledge regarding the specific components, lateral transmission of force involves proteins that link the costameres functionally to the ECM as well as the actual composition of the ECM (Kjaer, 2004; Gao et al. 2008_a_,b;). To test whether these components were altered in old and very old muscles, expression levels of components of the DGC and the integrin complex, key costameric ECM receptor complexes in skeletal muscle, were compared in membranes isolated from young (6 months old) and very old (35–36 months old) muscles. The expression of dystrophin was reduced by more than 60% in the membrane fractions of very old compared with young muscles (Fig. 4_A_ and B). This large change in dystrophin protein expression was quite unique, as expression levels of other components of the DGC, including β-dystroglycan and α-dystroglycan, were not changed significantly (Fig. 4_A_ and B). Conversely, the expression levels of β1 integrin were increased significantly in very old muscles (Fig. 4_A_ and B).
Figure 4. Expression of dystrophin–glycoprotein complex and integrin complex proteins in aged muscle membranes.
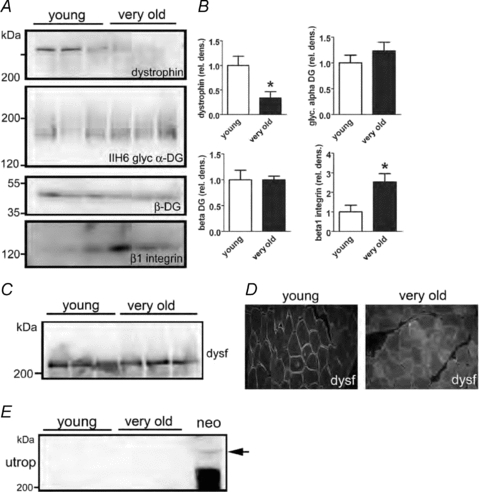
One hundred micrograms of total hindlimb muscle membrane protein preparations from three young (12 month) and three very old rats (34–35 months) was analysed by SDS-PAGE and Western blotting (A) and quantified by densitometry (B) *P < 0.05 by Student's t test, data are means ±s.e.m.C and D, mislocalization of dysferlin and no upregulation of utrophin in aged muscle. While expression levels of dysferlin in membrane fractions is normal by Western blotting (C), immunolocalization of dysferlin (D) shows redistribution of dysferlin expression into cytoplasmic membrane compartments. E, no upregulation of utrophin was observed in aged muscle, although full length utrophin protein was detected in a positive control sample from neonatal muscle.
To determine the specificity of the decrease in dystrophin expression in very old muscles, the expression levels of dysferlin, a membrane protein important in muscle vesicle trafficking and repair of membrane damage (Bansal et al. 2003), was examined. Similar to the null mutations in dystrophin, null mutations in dysferlin can cause muscular dystrophy, even though dysferlin is not an integral component of the DGC (Bansal et al. 2003). Although in isolated membranes from very old muscles, the expression levels of dysferlin were normal (Fig. 4_C_), with immunolocalization, the dysferlin expressed was redistributed to membranes within the cytoplasm (Fig. 4_D_). In several forms of muscular dystrophy that are associated with null mutations in components of the DGC, dysferlin is redistributed secondarily from the sarcolemma to membrane compartments within the cytoplasm. In very old muscles that showed reduced dystrophin expression, no up-regulation of the dystrophin homologue utrophin was observed (Fig. 4_E_). Consequently, for very old muscles, the loss of dystrophin expression was not compensated for by an increased expression of utrophin. This placed the very old rats in a similar situation to that of dystrophic mice, with skeletal muscles that are seriously impaired in their ability to transmit force laterally.
Very old muscles also show significant remodelling of the ECM (Kjaer, 2004; Gao et al. 2008_a_,b;). Electron micrographs of very old muscles showed a significant increase in the thickness of the basal lamina surrounding the muscle fibres (Fig. 5_A_). The thickening of the basal lamina was especially noticeable in the area of contact amongst three neighbouring muscle fibres (Fig. 5_A_). In very old muscles, capillaries located in this area were completely surrounded by a thick basal lamina. To quantify collagen deposition in very old muscles, muscle sections were stained with picrosirius red and fibrillar collagen content was quantified by ImageJ analysis. (Fig. 5_B_ and C). The very old muscles showed a more than 5-fold increase in fibrillar collagen expression. Immunolocalization techniques were used to measure the expression of key molecular components of the basal lamina. The components included laminin and type IV collagen indicating that some key components were preserved in the very old muscles (Fig. 5_B_). The apparent thickness of the staining for each of these components was increased, which was consistent with our observations made with electron microscopy. Furthermore, in very old muscles, QRT-PCR showed no detectable increase in collagen IV, procollagen I and laminin mRNA expression (data not shown). These observations suggest that during the ageing of skeletal muscles, the accumulation of the components of the basal lamina and the connective tissue was likely associated with a decreased degradation of the ECM proteins. In summary, while alterations were observed in the deposition of the ECM in very old muscles, the molecular composition of the basal lamina and matrix appeared to be preserved. Furthermore, the integrin mediated linkages appeared to be preserved, or even increased. These observations, combined with our results that the loss of dystrophin was sufficient to cause a decrease in the transmission of the lateral force, shift the emphasis for the age-related decline in the lateral transmission of force squarely onto an acquired loss of dystrophin expression.
Figure 5. Increase in accumulation of interstitial connective tissue and basal lamina thickness in skeletal muscles of old rats.
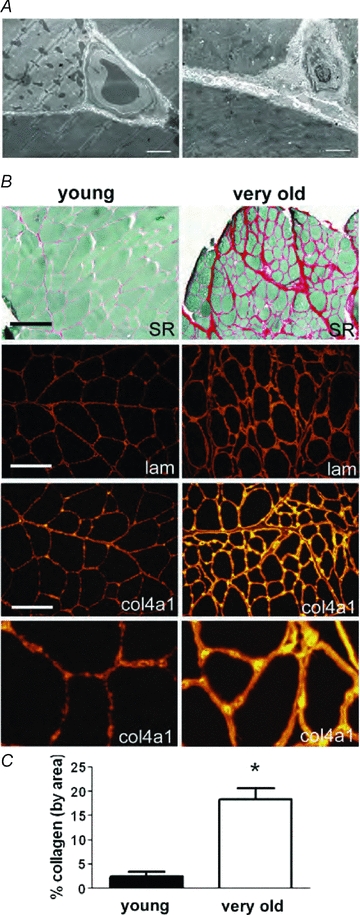
A, electron micrographs of three adjacent fibres of an EDL muscle showing thickening of the basal lamina in skeletal muscles of old compared to young rats. Three adjacent fibres from skeletal muscles of young (left) and old (right) rats are shown. Bar = 2 μm. B, increased abundance of fibrillar collagen and thickness of collagen IV and laminin in the basal lamina of muscle from very old rats. Picrosirius red staining followed by area analysis was used to quantify the fibrillar collagen expression in aged muscle. Immunolocalization of type IV collagen and laminin show increased thickness and staining intensity of the basal lamina. Bar = 200 μm, top two panels. Bar = 100 μm, immunofluorescence panels. Magnification of the bottom panels was increased identically for detailed comparative purposes. C, collagen composition. Two entire midbelly sections of picrosirius red stained sections from each muscle were quantified using a montage of 10× objective images, and averaged for each animal. *P < 0.005 by Student's t test, data are means ±s.e.m., _n_= 3 animals in each.
Discussion
The experiment performed by Street (1983) demonstrated for the first time that when a single fibre partially dissected from the semitendinosis muscle of a frog was activated maximally, the force was transmitted laterally across the adjacent fibres to the epimysium with little or no decrement. The skeletal muscles of frogs have a much simpler architecture and design than mammalian skeletal muscles (Patel & Lieber, 1997; Huijing, 2003), but the transverse lattice elements (‘costameres’) were already under investigation in mammals (Pardo et al. 1983) and potential roles for the dystrophin–glycoprotein complex (Ervasti & Campbell, 1993; Paul et al. 2002; Michele & Campbell, 2003; Lapidos et al. 2004) in the lateral transmission of force were quite evident. We now demonstrate in skeletal muscles of WT mice and rats that forces generated during activation of either the whole muscle or of varying numbers of motor units are transmitted laterally to the epimysium with little or no decrement. In contrast, in the skeletal muscles of mdx mice and very old rats the lateral transmission of force was impaired severely. In each case, the magnitude of the impairment in the lateral transmission of force was correlated closely with a loss of dystrophin expression. Our data indicate that either the loss of dystrophin in mdx mice, or the acquired disruption of the DGC, as a consequence of the ageing of WT rats, leads to significant losses in the capability of skeletal muscle fibres to transmit forces laterally.
Within single fibres, the stability of the sarcomeres is highly dependent on shear linkages of sarcomeres to the ECM (Huijing, 2003). The DGC, first described by Ervasti et al. (1990), resides within complex intracellular structures termed costameres (Pardo et al. 1983). The costameres are positioned in register with the z-discs of the myofibrils located on the periphery of each fibre (Pardo et al. 1983; Ervasti & Campbell, 1991; Worton, 1995; Henry & Campbell, 1996; Bloch & Gonzalez-Serratos, 2003; Ervasti, 2003; Michele & Campbell, 2003; Bloch et al. 2004; Lapidos et al. 2004; Anastasi et al. 2008). Consequently, costameres are ideally positioned to transmit the forces developed by activated fibres laterally throughout a muscle. During each of the three types of contractions, shortening, isometric and lengthening (Faulkner, 2003; Claflin & Brooks, 2008), the lateral transmission of force stabilizes sarcomere lengths and forces from fibre to fibre eventually out to the epimysium, as demonstrated by Street (1983) in muscles of frogs, and now by us in muscles of mice and rats. These lateral linkages are critical during each type of contraction, but most critical during lengthening contractions, when forces may be as much as twofold greater than those developed during isometric contractions (DelloRusso et al. 2001; Faulkner, 2003; Li et al. 2006). Our results on the very old muscles of rats show for the first time that an age-related loss of dystrophin is sufficient to cause a marked loss in the lateral transmission of force, which provides important experimental evidence that the DGC and costameres play critical roles in lateral transmission of force in skeletal muscles.
The possibility that dystrophin plays an important role in the stability of skeletal muscle fibres and its absence in dystrophic muscles contributes to the fragility of dystrophic muscle has been proposed by a number of investigators (Ervasti et al. 1990; Worton, 1995; Henry & Campbell, 1996; Ervasti, 2003; Michele & Campbell, 2003; Lapidos et al. 2004). Following protocols of lengthening contractions, the skeletal muscles of mdx mice (DelloRusso et al. 2001; Li et al. 2006) and old rats (Brooks & Faulkner, 1996) and humans (Dedrick & Clarkson, 1990; Frontera et al. 1991; Ploutz-Snyder et al. 2001) exhibit high levels of contraction induced injury. Compared with the force deficits elicited by a lengthening contraction protocol administered to skeletal muscles of young WT mice, force deficits for mdx mice were sevenfold greater (DelloRusso et al. 2001; Li et al. 2006) and for those of old compared with young rats twofold greater (Brooks & Faulkner, 1996). The large force deficits sustained by dystrophin-deficient muscles of mdx mice, even during isometric contraction protocols (Claflin & Brooks, 2008), reflect the compromised lateral transfer of forces between activated fibres, as each fibre acts as an independent longitudinal force generator. These observations are strongly supportive of the critical role that has been proposed for the costameres in the maintenance of sarcomere stability (Rybakova et al. 2000; Bloch & Gonzalez-Serratos, 2003; Ervasti, 2003; Huijing, 2003; Bloch et al. 2004; Kjaer, 2004). During lengthening contractions in which high forces are developed within fibres, the weaker groups of sarcomeres are at great risk of being stretched beyond overlap of thick and thin filaments and of sustaining a contraction-induced injury (Macpherson et al. 1997; Panchangam et al. 2008). When no mechanism is present for the lateral support from adjacent fibres, as in dystrophic muscles, any variability in development of forces along the lengths of fibres leads to heterogeneities in sarcomere lengths and to additional sarcomere injury (Macpherson et al. 1997; Claflin & Brooks, 2008; Panchangam et al. 2008). Consequently, the lateral transmission of force appears to be critical both in the protection of fibres from sustaining the initial contraction-induced damage and for the healing of the damage after a contraction-induced injury (Dedrick & Clarkson, 1990; DelloRusso et al. 2001; Ploutz-Snyder et al. 2001). Consequently, lateral pathways of support provide the only mechanism that enables the longitudinal strains to be shunted around the injured segment of a fibre while it is healing (Rader et al. 2006). The parallels between the loss of dystrophin, the disruption of the DGC, the decrease in the lateral transmission of force and the greatly increased susceptibility to contraction-induced injury in the skeletal muscles of both dystrophic mice and humans and in very old mammals provide strong support for the interdependence of these variables and their vital importance in the normal maintenance of the viability of skeletal muscle fibres.
Descriptive changes of the losses in the structure and function of ageing skeletal muscles are well-documented in humans (Grimby & Saltin, 1983; Young et al. 1984, 1985; Faulkner et al. 2008), rats (Larsson et al. 1991) and mice (Brooks & Faulkner, 1988). These losses are explained, at least in part, by the linear decrease in the number of fibres in skeletal muscles of humans that begins in the mid-fifties (Lexell et al. 1988) and is coupled with the timing and linear losses in the number of motor neurons (Lexell et al. 1988) and consequently in the number of motor units (Campbell et al. 1973; Doherty et al. 1993). Studies of alterations in excitation–contraction coupling have clarified, at least in part, the impaired production of muscle force by the skeletal muscles of old animals (Renganathan et al. 1997), but many issues that contribute to the decreased force production and the susceptibility of the skeletal muscles of old and very old animals to atrophy and to injury remain unresolved. Consequently, although the gross whole skeletal muscle changes that occur with ageing are well-described (Faulkner et al. 2008), the underlying molecular bases for many of these structural and functional changes that occur in skeletal muscles and single fibres with ageing are complex and largely unknown.
Our observation that the lateral transmission of force is disrupted in the skeletal muscles of mdx mice and old and very old rats focuses attention on molecules involved in the interactions between the DGC in muscle fibres and the ECM (Kjaer, 2004; Gao et al. 2008_a_,b;) as potential causes of some of the disease and age-related dysfunctions. Costameric proteins, including β1-containing integrins and the DGC, play critical roles in linking sarcomeres to the basal lamina and subsequently to the ECM (Worton, 1995; Rybakova et al. 2000; Paul et al. 2002; Ervasti, 2003; Lapidos et al. 2004). In the muscles of very old rats, the loss of lateral transmission of force was associated with a significant loss of dystrophin expression, while integrin and ECM protein expression was increased. Interestingly, when extrapolated to maximum isometric force, the magnitude of the loss in the lateral transmission of force observed for the muscles of very old rats (30%) was only slightly more than half the magnitude of the loss associated with the total loss of dystrophin in the young mdx mice of (50%). These values correlate reasonably well with the 60% loss in dystrophin expression in very old rats compared with the 100% loss of dystrophin expression in the mdx mice. Consequently, the acquired loss of dystrophin expression in the skeletal muscles of very old rats has the potential of not only playing a critical role in the impairment in the lateral transmission of force, but also being involved in the broader, whole-body characteristics of ageing, such as frailty, fatigability and susceptibility to injury (Pirozzolo & Maletta, 1982; Grimby & Saltin, 1983; Faulkner et al. 2008).
We propose that both dystrophin and the DGC play critical roles in the transmission of the lateral force in skeletal muscles, although the possibility exists that other secondary morphological changes in mdx muscles in mice and/or very old muscles in rats might contribute to the observed deficits in lateral force. While even very old muscles do not show cycles of degeneration/regeneration, both older dystrophic muscles in mice (Pastoret & Sebille, 1995; Head, 2010) and very old muscles in rats show changes in the ECM that include fibrosis, increased variation in fibre size (Fig. 5), fibre atrophy and the occasional splitting of muscle fibres (see online Supplemental Material, Supplementary Fig. 1). The possibility exists that for the skeletal muscles of both very old WT rats and mdx mice, the ECM, including the epimysium, becomes stiffer due to fibrosis (Kjaer, 2004; Gao et al. 2008_a_,b;). This possibility is supported by the similarities in the rates and the eventual magnitude in the loss in the development of lateral force – 30% for the very old muscles of rats and 50% for the mdx muscles of mice. This observation suggests that the overall compliance of the skeletal muscles in each of these two mammalian species, is similar. Many of the morphological changes observed for the dystrophic and very old muscles contribute to defects in the longitudinal as well as the lateral transmission of force. In that respect, an extremely interesting observation is that for each of these two models, compared with the longitudinal transmission of force, the lateral transmission of force is affected disproportionately. The hypertrophy of the EDL and soleus muscles of mdx compared with WT mice has been reported previously (Lynch et al. 2001). The hypertrophy of the mdx muscles results in the mdx muscles developing maximum forces longitudinally that are not different from those developed longitudinally by the WT muscles (Lynch et al. 2001). Despite the lack of any difference in the longitudinal forces developed by mdx and WT muscles, the lateral forces developed by the mdx muscles are compromised severely. The approaches developed in this study caused deficits in the lateral transmission of force in animal model systems that produced both morphological and physiological changes, primarily through the disruption of the DGC. In future studies, such approaches offer opportunities to identify the intracellular and extracellular molecular components of the longitudinal and lateral transmission of force in dystrophic and very old skeletal muscles, as well as their contributions to other forms of muscle dysfunction.
Acknowledgments
We thank Robert G. Dennis for assistance in the design and fabrication of the yoke apparatus, Dennis R. Claflin for his helpful comments on the manuscript, Cheryl Hassett of the Faulkner Laboratory for methodological assistance to K.R. with the operations and collection of data. Carol S. Davis assisted in the preparation of the manuscript for publication. This work was supported by funds to J.A.F. from the Contractility Core of the Nathan Shock Centre P30 AG13283 and the Functionality Core of the Program Project PO1 AG15434 and to D.E.M. from NIH R01-HL080388.
Glossary
Abbreviations
ATB
anterior tibialis
DGC
dystrophin-associated glycoprotein complex
ECM
extracellular matrix
EDL
extensor digitorum longus
Author contributions
The work was performed in the research laboratories of D.E.M. in the Department of Molecular & Integrative Physiology and J.A.F. in the Biogerontology Laboratories in the Biomedical Sciences Research Building at the University of Michigan Medical School. K.S.R, D.E.M. and J.A.F. contributed to the conception and design of the experiments. K.S.R., M.L.P., J.M.VM., A.R., T.Y.K., D.E.M. and J.A.F. contributed to the overall collection, analysis and interpretation of the data. K.S.R, D.E.M. and J.A.F. drafted the article and revised it critically for intellectual content. All of the authors approved of the final version of the manuscript.
Supplementary material
Supplementary Figure 1.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Abmayr S, Chamberlain JS. The structure and function of dystrophin. In: Winder S, editor. The Molecular Mechanisms of Muscular Dystrophy. Georgetown: Landes Bioscience; 2006. [Google Scholar]
- Anastasi G, Cutroneo G, Santoro G, Arco A, Rizzo G, Bramanti P, Rinaldi C, Sidoti A, Amato A, Favaloro A. Costameric proteins in human skeletal muscle during muscular inactivity. J Anat. 2008;213:284–295. doi: 10.1111/j.1469-7580.2008.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31:73–78. doi: 10.1097/00003677-200304000-00004. [DOI] [PubMed] [Google Scholar]
- Bloch RJ, Reed P, O’Neill A, Strong J, Williams M, Porter N, Gonzalez-Serratos H. Costameres mediate force transduction in healthy skeletal muscle and are altered in muscular dystrophies. J Muscle Res Cell Motil. 2004;25:590–592. [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol. 1996;497:573–580. doi: 10.1113/jphysiol.1996.sp021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, McComas AJ, Petito F. Physiological changes in aging muscles. J Neurol Neurosurg Psych. 1973;36:174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KP, Stull JT. Skeletal muscle basement membrane-sarcolemma-cytoskeleton interaction minireview series. J Biol Chem. 2003;278:12599–12600. doi: 10.1074/jbc.R300005200. [DOI] [PubMed] [Google Scholar]
- Carlson BM, Borisov AB, Dedkov EI, Khalyfa A, Kostrominova TY, Macpherson PC, Wang E, Faulkner JA. Effects of long-term denervation on skeletal muscle in old rats. J Gerontol A Biol Sci Med Sci. 2002;57:B366–B374. doi: 10.1093/gerona/57.10.b366. [DOI] [PubMed] [Google Scholar]
- Claflin DR, Brooks SV. Direct observation of failing fibers in muscles of dystrophic mice provides mechanistic insight into muscular dystrophy. Am J Physiol Cell Physiol. 2008;294:C651–C658. doi: 10.1152/ajpcell.00244.2007. [DOI] [PubMed] [Google Scholar]
- Dedrick ME, Clarkson PM. The effects of eccentric exercise on motor performance in young and older women. Eur J Appl Physiol Occup Physiol. 1990;60:183–186. doi: 10.1007/BF00839156. [DOI] [PubMed] [Google Scholar]
- DelloRusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil. 2001;22:467–475. doi: 10.1023/a:1014587918367. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–874. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM. Costameres: the Achilles’ heel of Herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Faulkner JA. Terminology for contractions of muscles during shortening, while isometric, and during lengthening. J Appl Physiol. 2003;95:455–459. doi: 10.1152/japplphysiol.00280.2003. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Davis CS, Mendias CL, Brooks SV. The aging of elite male athletes: age-related changes in performance and skeletal muscle structure and function. Clin J Sport Med. 2008;18:501–507. doi: 10.1097/JSM.0b013e3181845f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech. 2008a;41:465–469. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Waas AM, Faulkner JA, Kostrominova TY, Wineman AS. Micromechanical modeling of the epimysium of the skeletal muscles. J Biomech. 2008b;41:1–10. doi: 10.1016/j.jbiomech.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby G, Saltin B. The ageing muscle. Clin Physiol. 1983;3:209–218. doi: 10.1111/j.1475-097x.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Head SI. Branched fibres in old dystrophic mdx muscle are associated with mechanical weakening of the sarcolemma, abnormal Ca2+ transients and a breakdown of Ca2+ homeostasis during fatigue. Exp Physiol. 2010;95:641–656. doi: 10.1113/expphysiol.2009.052019. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol. 1996;8:625–631. doi: 10.1016/s0955-0674(96)80103-7. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Huijing PA. Muscular force transmission necessitates a multilevel integrative approach to the analysis of function of skeletal muscle. Exerc Sport Sci Rev. 2003;31:167–175. doi: 10.1097/00003677-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Kanda K, Hashizume K, Nomoto E, Asaki S. The effects of aging on physiological properties of fast and slow twitch motor units in the rat gastrocnemius. Neurosci Res. 1986;3:242–246. doi: 10.1016/0168-0102(86)90006-4. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94:1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- Larsson L, Ansved T, Edstrom L, Gorza L, Schiaffino S. Effects of age on physiological, immunohistochemical and biochemical properties of fast-twitch single motor units in the rat. J Physiol. 1991;443:257–275. doi: 10.1113/jphysiol.1991.sp018833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Li S, Kimura E, Ng R, Fall BM, Meuse L, Reyes M, Faulkner JA, Chamberlain JS. A highly functional mini-dystrophin/GFP fusion gene for cell and gene therapy studies of Duchenne muscular dystrophy. Hum Mol Genet. 2006;15:1610–1622. doi: 10.1093/hmg/ddl082. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol. 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson PC, Dennis RG, Faulkner JA. Sarcomere dynamics and contraction-induced injury to maximally activated single muscle fibres from soleus muscles of rats. J Physiol. 1997;500:523–533. doi: 10.1113/jphysiol.1997.sp022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Campbell KP. Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem. 2003;278:15457–15460. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- Panchangam A, Claflin DR, Palmer ML, Faulkner JA. Magnitude of sarcomere extension correlates with initial sarcomere length during lengthening of activated single fibers from soleus muscle of rats. Biophys J. 2008;95:1890–1901. doi: 10.1529/biophysj.107.118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci U S A. 1983;80:1008–1012. doi: 10.1073/pnas.80.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoret C, Sebille A. Mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci. 1995;129:97–105. doi: 10.1016/0022-510x(94)00276-t. [DOI] [PubMed] [Google Scholar]
- Patel TJ, Lieber RL. Force transmission in skeletal muscle: from actomyosin to external tendons. Exerc Sport Sci Rev. 1997;25:321–363. [PubMed] [Google Scholar]
- Paul AC, Sheard PW, Kaufman SJ, Duxson MJ. Localization of α7 integrins and dystrophin suggests potential for both lateral and longitudinal transmission of tension in large mammalian muscles. Cell Tissue Res. 2002;308:255–265. doi: 10.1007/s00441-002-0526-y. [DOI] [PubMed] [Google Scholar]
- Pirozzolo FJ, Maletta GJ. The Aging Motor System. New York: Praeger Publishers; 1982. [Google Scholar]
- Ploutz-Snyder LL, Giamis EL, Formikell M, Rosenbaum AE. Resistance training reduces susceptibility to eccentric exercise-induced muscle dysfunction in older women. J Gerontol A Biol Sci Med Sci. 2001;56:B384–B390. doi: 10.1093/gerona/56.9.b384. [DOI] [PubMed] [Google Scholar]
- Rader EP, Song W, Van Remmen H, Richardson A, Faulkner JA. Raising the antioxidant levels within mouse muscle fibres does not affect contraction-induced injury. Exp Physiol. 2006;91:781–789. doi: 10.1113/expphysiol.2005.033043. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J Membr Biol. 1997;157:247–253. doi: 10.1007/s002329900233. [DOI] [PubMed] [Google Scholar]
- Rybakova IN, Patel JR, Ervasti JM. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J Cell Biol. 2000;150:1209–1214. doi: 10.1083/jcb.150.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983;114:346–364. doi: 10.1002/jcp.1041140314. [DOI] [PubMed] [Google Scholar]
- Worton R. Muscular dystrophies: diseases of the dystrophin-glycoprotein complex. Science. 1995;270:755–756. doi: 10.1126/science.270.5237.755. [DOI] [PubMed] [Google Scholar]
- Young A, Stokes M, Crowe M. Size and strength of the quadriceps muscles of old and young women. Eur J Clin Invest. 1984;14:282–287. doi: 10.1111/j.1365-2362.1984.tb01182.x. [DOI] [PubMed] [Google Scholar]
- Young A, Stokes M, Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol. 1985;5:145–154. doi: 10.1111/j.1475-097x.1985.tb00590.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.