Phosphatidylserine is a critical modulator for Akt activation (original) (raw)
Association of Akt with phosphatidylserine enhances binding to PIP3, inducing conformational changes in Akt that promote its phosphorylation-mediated activation.
Abstract
Akt activation relies on the binding of Akt to phosphatidylinositol-3,4,5-trisphosphate (PIP3) in the membrane. Here, we demonstrate that Akt activation requires not only PIP3 but also membrane phosphatidylserine (PS). The extent of insulin-like growth factor–induced Akt activation and downstream signaling as well as cell survival under serum starvation conditions positively correlates with plasma membrane PS levels in living cells. PS promotes Akt-PIP3 binding, participates in PIP3-induced Akt interdomain conformational changes for T308 phosphorylation, and causes an open conformation that allows for S473 phosphorylation by mTORC2. PS interacts with specific residues in the pleckstrin homology (PH) and regulatory (RD) domains of Akt. Disruption of PS–Akt interaction by mutation impairs Akt signaling and increases susceptibility to cell death. These data identify a critical function of PS for Akt activation and cell survival, particularly in conditions with limited PIP3 availability. The novel molecular interaction mechanism for Akt activation suggests potential new targets for controlling Akt-dependent cell survival and proliferation.
Introduction
The serine/threonine protein kinase Akt (protein kinase B) regulates various cellular processes including cell survival, growth, proliferation, and metabolism (Brazil and Hemmings, 2001). Akt contains three distinctive domains including N-terminal pleckstrin homology (PH) domain, C-terminal regulatory domain (RD), and central kinase domain (KD; Bellacosa et al., 1991; Thomas et al., 2002). It has been well established that Akt is activated through two essential steps involving membrane translocation and phosphorylation (Alessi and Cohen, 1998; Downward, 1998). Cytosolic Akt is recruited to the plasma membrane via the interaction between the PH domain and membrane phosphatidylinositol 3,4,5-trisphosphate (PIP3) produced by phosphoinositide-3 (PI3) kinase upon growth factor receptor stimulation. The membrane–Akt interaction results in conformational changes of Akt, enabling its activation through phosphorylation at T308 of the KD and at S473 of the RD by phosphoinositide-dependent protein kinases (PDKs; Alessi et al., 1996; Andjelković et al., 1997; Stokoe et al., 1997; Bellacosa et al., 1998; Watton and Downward, 1999). Besides the well-known interaction between the PH domain and PIP3, involvement of other plasma membrane components or Akt individual domains in Akt activation has not been explored.
Phosphatidylserine (PS) is the major anionic phospholipid class in eukaryotic biomembranes and is highly enriched in the inner leaflet of the plasma membrane (Op den Kamp, 1979; Vance and Steenbergen, 2005; Calderon and Kim, 2008). It has been demonstrated that PS participates in various signaling events such as membrane translocation and activation of protein kinase C (PKC; Newton and Keranen, 1994; Verdaguer et al., 1999) or Raf-1 kinase (Improta-Brears et al., 1999; Kim et al., 2000). Moreover, docosahexaenoic acid (DHA), which can increase PS in neuronal membranes (Kim et al., 2000; Kim, 2007), has been shown to promote neuronal survival and facilitate the membrane translocation of the Akt-PH domain (Akbar et al., 2005), prompting us to investigate the role of plasma membrane PS in Akt activation. In this study, we demonstrate a novel molecular mechanism by which membrane PS critically participates in dynamic PIP3-dependent Akt signaling through the interaction with individual domains of Akt. Mass spectrometric probing of Akt conformation along with molecular interaction analyses revealed that PS and PIP3 jointly influence the Akt–membrane interaction required for interdomain conformational changes of Akt for phosphorylation. We found that both PH and RD domains interacted with the membrane in a PIP3- and PS-dependent manner, exposing T308 and S473 for phosphorylation, respectively. Remarkably, significant interaction occurred between RD and membrane PS in vitro, enabling Akt phosphorylation at S473 by mammalian target of rapamycin (mTOR) complex 2 (mTORC2; mTOR–rictor complex) even without PIP3. In living cells, insulin-like growth factor (IGF)-induced Akt translocation and phosphorylation and subsequent downstream signaling were also modulated in a PS-dependent manner. Susceptibility to cell death and Akt signaling tested under a serum-starved condition also exhibited PS dependency, which demonstrates that PS–Akt interaction is an important physiological mechanism in Akt activation.
Results
Modulation of PIP3-dependent Akt activation by membrane PS
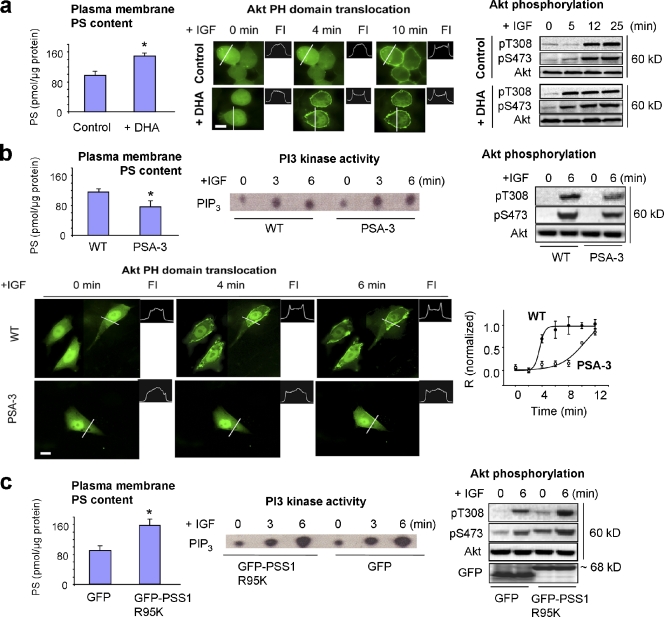
To test the involvement of the plasma membrane PS in Akt signaling, Akt translocation and phosphorylation at T308 and S473 were examined after altering the plasma membrane PS contents in living cells using three independent approaches: increasing PS by DHA supplementation, using CHO mutant cells lacking PS synthase-1 (PSA-3) where the PS content can be lowered (Kuge et al., 1986; Saito et al., 1998), and overexpressing PS synthase 1 (PSS1) mutant R95K, which is known to be resistant to the product inhibition, thus increasing the PS content (Kuge et al., 1998). Supplementation of Neuro 2A cells with DHA significantly increased the PS content in the isolated plasma membrane (Fig. S1 a), resulting in facilitated membrane translocation of GFP-PH domain (Fig. 1 a; quantitative data is shown in Fig. S1 b) and phosphorylation of Akt upon IGF stimulation (Akbar et al., 2005). In contrast, the PSA-3 mutant cells contained a significantly lower level of plasma membrane PS, and showed noticeably inhibited IGF-triggered membrane translocation and phosphorylation of Akt in comparison to the wild-type (WT) CHO cells (Fig. 1 b). The observed impairment of Akt activation was not caused by inhibited phosphoinositide-3 kinase (PI3 kinase), as the PI3 kinase activity was similar between the PSA-3 and WT cells (Fig. 1 b). In another model where the plasma membrane PS content was raised by expressing the R95K mutant of PSS1 compared with the case with GFP-PSS1 WT or GFP alone (Fig. S2 a; Kuge et al., 1998), Akt translocation (Fig. S2 b) and phosphorylation was promoted, whereas the PI3 kinase activity remained unchanged (Fig. 1 c). A key function of Akt is to promote cell survival by phosphorylating and inhibiting numerous downstream pro-apoptotic factors such as Bad and Forkhead box O1 (FOXO1)/O3 (Datta et al., 1997; Brunet et al., 1999). The activated Akt also phosphorylates and inhibits glycogen synthase kinase 3β (GSK-3β) that not only is involved in glucose metabolism but also induces apoptosis (Parcellier et al., 2008). These Akt downstream events also correlated with plasma membrane PS levels. As shown in Fig. S2 c, IGF-induced phosphorylation of Akt, GSK-3β, and FOXO1 was promoted in the cells where the PS level was higher. The results from these three independent models consistently indicated that endogenous Akt activation and downstream signaling is significantly influenced by the plasma membrane PS content.
Figure 1.
Effects of plasma membrane PS on membrane translocation of Akt-PH domain and Akt activation. (a) Increasing plasma membrane PS in Neuro 2A cells by DHA supplementation facilitated IGF-induced Akt membrane translocation and phosphorylation. The membrane translocation is depicted by a representative fluorescence intensity profile across a transverse section in indicated cells (FI). (b) Decreasing plasma membrane PS using PSA-3 mutant CHO cells impaired Akt-PH domain translocation and Akt phosphorylation without affecting PI3 kinase activity. Shown are representative micrographs, time course of translocation by using averaged relative R values, and PI3 kinase activity. (c) Increasing plasma membrane PS by expressing the PSS1(R95K) facilitated IGF-induced Akt activation without altering PI3 kinase activity in Neuro 2A cells. GFP-PSS1 was observed at the apparent molecular mass of ~68 kD. The dimeric form of GFP (∼52 kD) was detected in samples expressing GFP alone. The plasma membrane PS contents are expressed as mean ± SD (error bars; n = 3), representing two independent experiments. *, P < 0.05. Bars, 10 µm.
Effects of PS on interdomain conformational changes of Akt
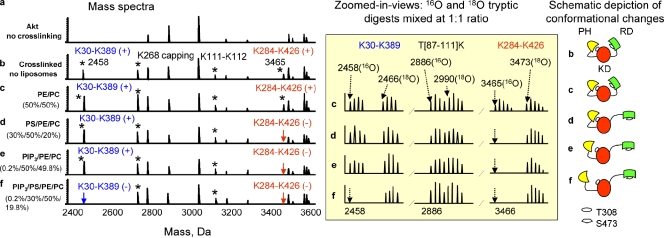
To gain insight into the molecular mechanism for the involvement of membrane PS in Akt activation, we examined the conformational status of Akt altered upon membrane interaction, which is the accepted basis of membrane translocation and subsequent activation. The 3D structure of full-length Akt was probed by chemical cross-linking and quantitative mass spectrometry (MS) as we and others have previously described (Young et al., 2000; Huang and Kim, 2006). Inactive full-length Akt 1 (Akt hereafter) was incubated with or without unilamellar vesicles containing varying compositions of phospholipids, subsequently modified with a lysine-specific bifunctional cross-linker, disuccinimidyl suberate (DSS; maximum span length, 24 Å), and analyzed by MS after tryptic digestion (Fig. 2 and Fig. S3), as described previously (Huang and Kim, 2006). The two interdomain cross-linking pairs, K30 of the PH domain to K389 of the KD (K30-K389) and K284 of KD to K426 of RD (K284-K426), identified from inactive Akt that had not been interacted with (Huang and Kim, 2006), allowed us to examine the alteration of proximity (within 24 Å) between the domains involved upon membrane interaction, as schematically depicted in Fig. 2. Interaction of inactive Akt with liposomes containing 50:50 phosphatidylethanolamine (PE)/ phosphatidylcholine (PC) did not alter the cross-linking results (Fig. 2 c). When they interacted with membranes containing both PS and PIP3 at a typical concentration for the inner leaflet of the neuronal plasma membrane under a stimulated condition (0.2:30:50:19.8 PIP3/PS/PE/PC), both interdomain cross-linked pairs were no longer observed within the 24-Å spatial distance constraint (Fig. 2 f), which suggests that the PH and RD moved away from KD, presumably exposing T308 and S473 for phosphorylation and activation. Individual modification of these lysine residues was observed, indicating that they were still accessible. The intensity changes of the cross-linked peaks were confirmed by quantitative 18O tryptic labeling (Yao et al., 2001; Huang et al., 2005; Huang and Kim, 2006) as illustrated in zoomed-in-views in Fig. 2. Note that the quantitative analysis showed that the intensity of the K30-K389 cross-linking (2458 D) decreased slightly after interaction with liposomes containing PIP3, but not PS, compared with that of the control without membrane interaction (2466 D; Fig. 2 e). When liposomes contained either PS (30:50:20 PS/PE/PC) or PIP3 (0.2:50:49.8 PIP3/PE/PC), the cross-linking between the RD and KD (K284-K426) disappeared; however, the cross-linking between the PH and KD (K30-K389) was still observed (Fig. 2, d and e). These data indicated that either PS or PIP3 can interact with Akt to induce an open conformation of the RD to expose S473 for subsequent phosphorylation. However, the presence of both PIP3 and PS in the membrane was required to properly unfold the PH domain for exposure of T308.
Figure 2.
Effects of membrane phospholipids on interdomain conformational changes of Akt due to membrane interaction probed by mass spectrometry. The appearance of two interdomain cross-linked peptides (K30-K389 and K284-K426) depended on the phospholipid composition of interacting membranes. Cross-linked peptides are marked with asterisks (left). The zoomed-in-views (middle) show quantitative comparison of these cross-linked peptides using 16O/18O labeling as described in Materials and methods. Although the16O/18O ratio from non–cross-linked peptide pairs such as T[87–111]K (2886 D vs. 2990 D) remained the same, the 16O/18O ratio from the interdomain cross-linked peptide pairs separated by 8 D changed according to the membrane phospholipid composition. Schematic presentations of the interdomain conformational changes of Akt deduced by the cross-linking data are shown (right).
Effects of PS on Akt–membrane interaction and phosphorylation in vitro
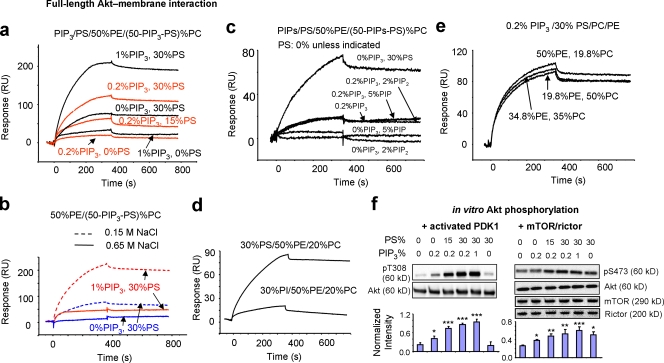
To further examine the molecular basis for the PS-dependent Akt–membrane interaction involved in Akt translocation and activation (Fig. 1), the binding of Akt to unilamellar vesicles of various phospholipid compositions in a physiological salt concentration (0.15 M NaCl) was monitored in vitro using biomolecular interaction analysis based on surface plasmon resonance (SPR). At a given PS composition (30%), binding of Akt to liposomes increased with increasing proportion of PIP3 (Fig. 3 a), which is consistent with the established notion that Akt specifically binds to PIP3 (Alessi et al., 1997; Brazil and Hemmings, 2001). Likewise, the extent of binding was significantly increased with increasing PS proportion at a given PIP3 composition (0.2%; Fig. 3 a). Without PS in the liposome, the extent of binding decreased dramatically, which suggests that the presence of PS is also critical for Akt–membrane interaction. Remarkably, considerable binding of Akt to the vesicle containing PS/PE/PC (30:50:20) was observed even in the absence of PIP3 (Fig. 3 a). PS has recently been shown to play an important role in regulating cellular localization of proteins via electrostatic interaction (Yeung et al., 2008). The observed Akt–PS interaction was apparently driven by electrostatic force, as the Akt binding to liposomes significantly decreased by raising the salt concentration (0.65 M NaCl; Fig. 3 b; Smith and Storch, 1999). Nevertheless, this electrostatic interaction was specific to PS, as other acidic phospholipids including phosphatidylinositol (PI), PI 4-phosphate (PIP), and PI 4,5-bisphosphate (PIP2), were not effective even at concentrations considerably higher than those physiologically expected. As shown in Fig. 3 (c and d), no significant binding was observed between Akt and the membrane containing 30% PI (Fig. 3 d), or 5% or 2% PIP2 (Fig. 3 c), which is consistent with the previous finding that the Akt binding to PIP2 is insignificant compared with PIP3 (James et al., 1996). In addition, the binding of Akt to membranes containing 0.2% PIP3 were identical regardless of the presence of either 5% PIP (0.2:5:50:44.8 PIP3/PIP/PE/PC) or 2% PIP2 (0.2:2:50:47.8 PIP3/PIP2/PE/PC; Fig. 3 c), which indicates that unlike PS (Fig. 3 a), neither PIP nor PIP2 promoted Akt–PIP3 interaction. Furthermore, changing proportions of PE or PC at a given PS and PIP3 composition did not affect the binding of Akt to membranes significantly (Fig. 3 e). These results indicated that PIP3 and PS are important membrane components for Akt–membrane interaction.
Figure 3.
Effects of phospholipids on the membrane interaction and phosphorylation of Akt. (a) Representative SPR sensorgrams of the binding of full-length Akt to liposomes with various phospholipid compositions (x:y:50:[50 − x − y] PIP3/PS/PE/PC). 200 nM Akt was injected onto the L1 chip coated with liposomes. The response of the control liposomes containing PE/PC (50:50), which was insignificant, was subtracted from each sensorgram to correct for background drift. The sensorgram was obtained using PBS as the running buffer. (b) The effect of salt concentration on the binding of Akt to liposomes containing PIP3 and/or PS. (c–e) Representative SPR sensorgrams showing insignificant effects of PIP2, PIP (c), PI (d), and PE/PC on Akt-membrane interaction. (f) Western blot data showing PS- or PIP3-dependent Akt phosphorylation of T308 and S473 by PDK1 and mTORC2, respectively. The band intensity of pT308 or pS473 were normalized to the Akt level (data are mean ± SD [error bars], n ≥ 3). Statistical significance was tested against liposomes containing 0% PS and 0% PIP3. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The immediate consequence of the Akt–membrane interaction is the conformational change of Akt exposing the phosphorylation sites to be phosphorylated by upstream enzymes. In agreement with the SPR binding results, in vitro phosphorylation of both T308 and S473 by active PDK1 and mTORC2, respectively, increased with increasing proportion of PIP3 or PS (Fig. 3 f). Akt phosphorylation at T308, presumably exposed after the PH domain interacts with membrane, was insignificant when Akt was incubated with liposomes containing no PIP3. The extent of T308 phosphorylation was reduced significantly when vesicles contained PIP3 (0.2%) but no PS in comparison to the case where vesicles contained both PS (15 or 30%) and PIP3 (0.2%; Fig. 3 f). This observation is consistent with the incomplete unfolding of the PH domain from the KD after the interaction of Akt with liposomes containing PIP3 but no PS (Fig. 2 e), which supports the notion that both PIP3 and PS are required for the optimal exposure of T308 for phosphorylation. In contrast, a significant phosphorylation of S473 by mTORC2, a known physiological PDK2, was observed when Akt was incubated with liposomes containing 30% PS even without PIP3 (Fig. 3 f). Liposomes containing PIP3 but no PS also resulted in considerable S473 phosphorylation by mTORC2 (Fig. 3 f). Similar results were obtained using MAPK-activated protein kinase II (MAPKAPK2), an enzyme known to phosphorylate Akt at S473 in vitro (Fig. S4; Alessi et al., 1996); this consistently supports the role of PS in conformational changes to expose S473 for phosphorylation. These data also agreed well with the cross-linking results (Fig. 2) where either PS or PIP3 caused unfolding of the RD from KD, presumably exposing S473. These data strongly suggested an important involvement of PS in PIP3-triggered Akt activation. Moreover, open RD conformation induced by Akt–membrane PS interaction allowing significant in vitro phosphorylation of S473 located in the RD might suggest a direct interaction of the RD with PS.
Molecular interaction between membrane PS and individual domains of Akt
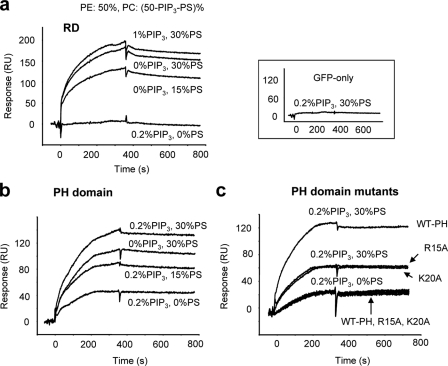
To assess the involvement of Akt individual domains in the interaction with membrane PS, the GFP-tagged RD or PH domain was expressed in Neuro 2A cells, captured by anti-GFP antibody immobilized on the sensor chip, and made to interact with liposomes with varying lipid compositions. We observed a significant interaction of the RD with PS in the absence of PIP3 (Fig. 4 a). The binding of the RD to liposomes was dependent largely on the PS concentration in the membrane, as the binding was insignificant without PS and increased only slightly by including 1% PIP3 in the liposomes containing 30% PS. The binding of the PH domain to the membrane was also PS-dependent at a given PIP3 content in the liposomes (Fig. 4 b), which supports the facilitated translocation of Akt-PH domain to the plasma membrane containing the higher PS content observed in Fig. 1. There was no binding between GFP and the membrane containing both PIP3 and PS (Fig. 4, inset).
Figure 4.
Membrane interaction of the individual domains of Akt affected by PIP3 and PS. SPR sensorgrams representing the membrane binding of Akt RD (a), PH domain (b), and PH domain mutants (c). The GFP-RD or GFP-PH domain of Akt was captured by anti-GFP antibody immobilized on a CM5 chip followed by the injection of liposomes containing PIP3/PS/PE/PC (x:y:50:[50 − x − y]). Both RD and PH domains showed PS-dependent binding. PH domain mutation (R15A or K20A) significantly impairs the Akt binding to membrane without affecting Akt-PIP3 interaction (c). Inset, SPR sensorgram showing negligible binding of liposomes to GFP alone.
The biological significance of the interaction of PH or RD with membrane PS can be revealed when the interaction is disrupted either by depletion of PS from the membrane or eliminating the PS-interacting residues in these domains. As PS cannot be completely depleted from the biomembranes, the disruption of PS–Akt interaction was attempted by mutating possible interaction sites in the PH domain for which the 3D structure is well established. The crystal structure of the PH domain indicates that R15, K20, R67, and R69 are located outside the PIP3-binding pocket but form a positively charged flat plane, which has been suggested to interact with negatively charged membrane surface (Thomas et al., 2002) where PS is a major component (Yeung et al., 2008). We mutated these potential PS-binding residues and examined the membrane binding using the SPR-based approach described in the Materials and methods. Among these basic residues, R15 and K20 were found to be important for PS binding. Mutation of the residues to alanine did not alter the Akt binding to PIP3 when evaluated using liposomes containing 0.2:50:49.8 PIP3/PE/PC (Fig. 4 c), which suggests that the local conformation of the PIP3-binding pocket and therefore affinity for PIP3 was not altered by the mutation. However, we found that the binding of R15A or K20A to the membrane containing both PS and PIP3 (0.2:30:50:19.8 PIP3/PS/PE/PC) decreased >50% compared with the WT control, which indicates that these basic residues participate in the Akt–PS binding, which may be relevant to Akt–membrane interaction in vivo.
Significance of Akt interaction with membrane PS in living cells
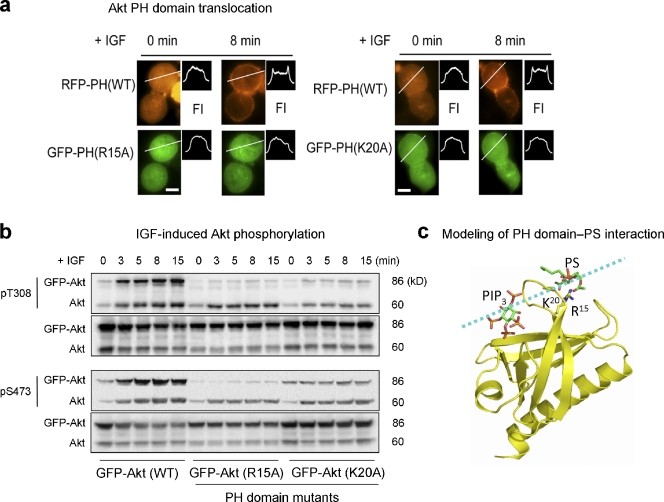
To demonstrate the biological significance of the Akt–PS interaction, IGF-induced Akt activation was examined after overexpressing the R15A and K20A mutants where the binding between the PH domain and membrane PS is disrupted. We found that the membrane translocation of the mutant PH domain (R15A or K20A) was insignificant in comparison to the coexpressed WT RFP-PH, which readily translocated to the plasma membrane upon IGF stimulation (Fig. 5 a). Western blot analysis showed that T308 or S473 phosphorylation of the GFP-Akt mutants (R15 or K20) was nearly abolished in comparison to WT control (Fig. 5 b), which indicates that interaction of R15 or K20 with negatively charged membrane PS is indeed critical for Akt activation. Meanwhile, the phosphorylation of endogenous Akt at both T308 and S473 did not change significantly, regardless of the type of mutants expressed (Fig. 5 b), which indicates that the PIP3 production or PI3K activity was not affected by the expression of the mutants. The interaction of these residues with membrane PS was further supported by computer modeling (Morris et al., 1998) based on the crystal structure (PDB accession no. 1H10; Thomas et al., 2002) and our mutation data (Fig. 5 b), as shown in Fig. 5 c. The lowest energy conformation generated by Lamarckian genetic algorithm depicted the putative binding of PS to R15 and K20 via hydrogen bonding.
Figure 5.
Akt membrane translocation and phosphorylation impaired by disrupting Akt–PS interaction via mutation of the PS-binding residues in the PH domain. (a) Representative micrographs showing IGF-induced membrane translocation of Neuro 2A cells coexpressing RFP-PH (WT) and GFP-PH (R15A or K20A). The membrane translocation is depicted by a representative fluorescence intensity profile across a transverse section in indicated cells (FI). Bars, 10 µm. (b) Time course of IGF-induced Akt phosphorylation at T308 and S473 in Neuro 2A cells expressing GFP-Akt WT or GFP-Akt mutants. (c) Computer modeling depicting binding of PS to R15 and K20 located outside the PIP3-binding pocket in the PH domain. The broken line indicates a putative membrane surface. The lowest energy conformations generated by using a Lamarckian genetic algorithm (Morris et al., 1998) showed the putative binding of PS to R15 and K20 via hydrogen bonding.
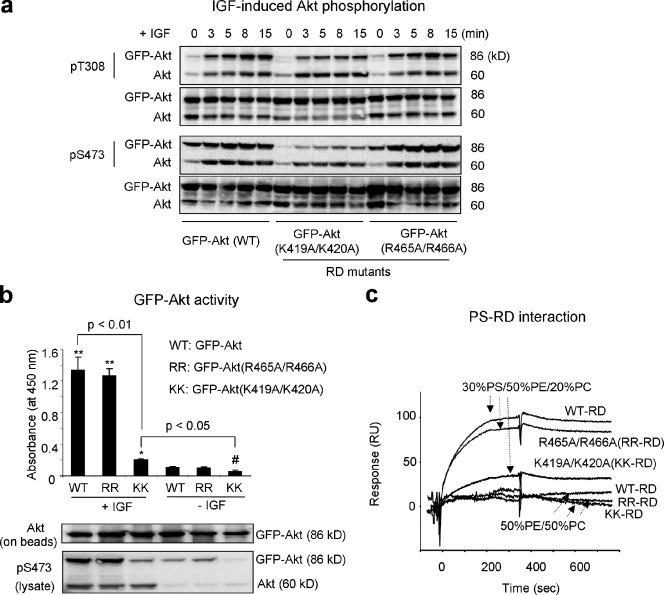
The significance of the PS–RD interaction was similarly tested using mutation. Because the crystal structure of RD is not available, we tested Akt mutants where each of all six basic residues in the RD was mutated to alanine. Among these mutants, we found that K419A/K420A double mutation significantly impaired the phosphorylation of the expressed Akt, particularly at S473 (Fig. 6 a). In contrast, other mutants such as R465A/R466A showed insignificant effects on Akt phosphorylation (Fig. 6 a and Fig. S5). Consistently, an in vitro kinase activity assay using overexpressed GFP-Akt WT or mutants after IGF stimulation and immunopurification indicated a significant loss of Akt activity by K419A/K420A mutation in comparison to the WT or R465A/466A mutant (Fig. 6 b). When the corresponding GFP-RD mutants were prepared and evaluated for their binding to membrane PS using SPR, the K419A/K420A showed significantly less binding than WT, whereas the binding of R465A/R466A mutant was similar to that of WT (Fig. 6 c), which indicates that K419 and K420 were the PS-binding residues. It is worthwhile to note that T308 phosphorylation was also partially reduced in the K419A/K420A mutant, possibly because of impaired S473 phosphorylation, which has been shown to promote T308 phosphorylation (Sarbassov et al., 2005). However, the possibility of the RD–PS interaction contributing directly to T308 phosphorylation cannot be excluded. The fact that IGF-induced translocation of the PH domain and T308 and S473 phosphorylation are greatly impaired by eliminating PS-binding residues in both PH domain (R15, K20) and RD (K419/K420) without affecting endogenous Akt activation indicated that interaction of membrane PS with RD and PH domains is also crucial for proper Akt activation in vivo_._
Figure 6.
Akt activation impaired by disrupting Akt–PS interaction via mutation of the PS-binding residues in the RD. (a) Time course of IGF-induced Akt phosphorylation at T308 and S473 in Neuro 2A cells expressing GFP-Akt WT or full-length GFP-Akt mutants. Mutation of the K419A/K420A impaired Akt phosphorylation, particularly at S473 located in the RD region. (b) Kinase activity of GFP-Akt WT or mutants immunoprecipitated from Neuro 2A cell lysates. Representative Western blotting data are shown to indicate the IGF-induced phosphorylation status of GFP-Akt or mutants in the lysate and the amount of GFP-Akt or mutants immunoprecipitated on the beads. Error bars indicate ± SD. (c) The effect of the mutation on the binding of Akt-RD to membrane PS. Statistical significance was tested against GFP-Akt WT without IGF-stimulation unless indicated. * or **, significant increase (*, P < 0.05; **, P < 0.01); #, significant decrease (P < 0.05).
Significance of Akt–PS interaction in cell survival
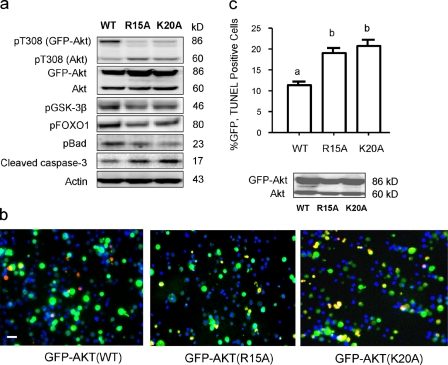
To further demonstrate the cellular significance of Akt–PS interaction, we examined cell survival and Akt downstream signaling events under a serum-starved condition where growth factors are depleted, hence PIP3 production is minimized. The R15A or K20A Akt mutant overexpressed in Neuro 2A cells showed only trivial phosphorylation, whereas WT-Akt showed significant phosphorylation (Fig. 7 a). Phosphorylation of GSK-3β, FOXO1, and Bad, the downstream signaling of the Akt activation, was also reduced in cells expressing these mutants. There appears to be competition between the expressed WT-Akt and endogenous Akt under this PIP3-limited condition, as the endogenous Akt phosphorylation in WT-expressing cells was slightly reduced in comparison to that in mutant-expressing cells. This seems to be the reason that the downstream signaling was not affected as dramatically as one would expect from the extent of differences between WT and mutant Akt phosphorylation. Nevertheless, the combined level of phosphorylated Akt (expressed and endogenous) was significantly lower in the mutant-expressing cells, which was reflected by the reduced phosphorylation of GSK-3β, FOXO1, and Bad, and increased active caspase-3 in comparison to the WT-expressing cells. The down-regulated survival signals and elevated caspase-3 activation in mutant Akt-expressing cells corroborate with the TUNEL assay data (Fig. 7, b and c), which indicates that cells expressing the mutant Akt where the Akt–PS interaction is disturbed are more susceptible to apoptosis than cells expressing WT-Akt.
Figure 7.
Effects of mutation of PS-binding residues on Akt downstream signaling and apoptotic cell death induced by serum starvation. Neuro 2A cells were transfected with GFP-Akt WT or mutants for 36 h and subjected to serum starvation for 48 h. (a) GFP-Akt (R15A) or GFP-Akt (K20A) expressed in Neuro 2A cells showed significantly impaired phosphorylation. Phosphorylation of GSK-3β, FOXO1, and Bad, the downstream signaling of the Akt activation, was also reduced in cells expressing these mutants, with a concomitant increase in active caspase-3. (b) Cells expressing the mutants showed significantly more TUNEL-positive cells (green + red = orange–∼yellow) in comparison to cells expressing GFP-Akt WT. TUNEL-positive cells were stained red using Click-iT TUNEL assay kit with Alexa Fluor 594 Azide. Nuclei were stained with Hoechst 33342. Bar, 30 µm (c) The percentages of GFP- and TUNEL-positive cells were determined by counting the total of 500–1,000 GFP-Akt WT or GFP-Akt mutant expressing cells from six randomly selected fields. Inset, Western blot analysis indicating comparable expression of WT and mutant GFP-Akt. Results represent two independent experiments performed in duplicates. Serum-sufficient cells transfected with WT or mutants showed negligible cell death. Statistical analysis was performed by post-hoc Tukey honestly significant difference test at the significance level of P < 0.05. Different letters indicate statistically significant differences.
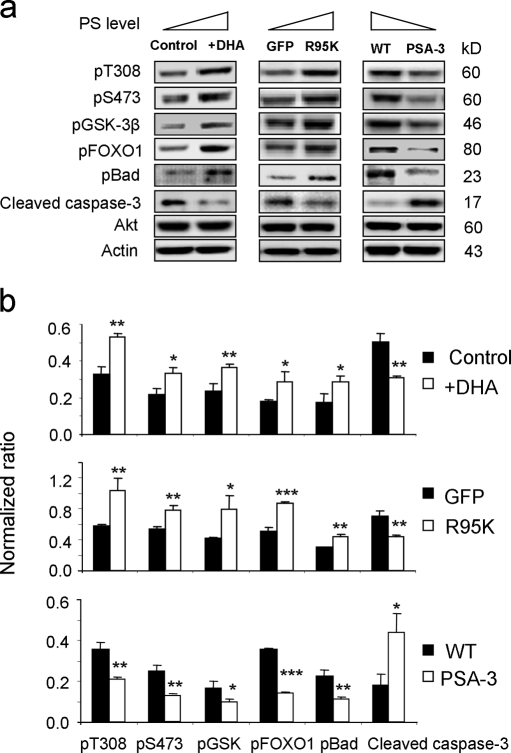
To demonstrate the importance of PS in endogenous Akt signaling in the context of cell survival, we examined the same signaling events using three cellular models we used in this study to manipulate the PS levels. We found that raising PS by DHA supplementation or by expressing PSS1(R95K) mutant resulted in more phosphorylation of Akt, GSK-3β, FOXO1, and Bad, and less induction of active caspase-3 under the serum-starved condition in comparison to the control cells. In contrast, PSA-3 cells, which contained reduced levels of PS, showed elevated active caspase-3 and reduced phosphorylation of Akt, GSK-3β, FOXO1, and Bad (Fig. 8). These data strongly support the significance of PS in endogenous Akt activation and its downstream signaling leading to cell survival under the condition where PIP3 production is limited.
Figure 8.
Effects of plasma membrane PS on Akt activation, downstream signaling, and apoptotic cell death induced by serum starvation. (a) Increasing plasma membrane PS in Neuro 2A cells by DHA supplementation or expressing the PSS1 (R95K) mutant enhanced phosphorylation of Akt and its downstream targets, resulting in less caspase-3 activation. In contrast, the PSA-3 mutant, where the PS level is lower in comparison to the WT, showed increased caspase-3 activation with decreased Akt signaling. (b) Quantitative analysis of the Western blot data. The band intensity was normalized to either Akt (pT308 and pS473) or actin level (pGSK-3β, pFOXO1, pBad, and active caspase-3). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
The interaction of Akt with the plasma membrane is a critical step in Akt activation. The consensus view is that the dynamics of membrane PIP3 regulate the Akt activation through the interaction with the PH domain. In the present study, we demonstrate a novel Akt activation mechanism by which membrane PS participates in the PIP3-dependent membrane translocation and interdomain conformational changes of Akt through the interaction with not only the PH domain but also the RD of Akt.
Under no circumstances is plasma membrane devoid of PS. As a result, the role of PS has not been properly considered in Akt activation. Our data for the first time demonstrate that membrane PS significantly affects Akt signaling in living cells. We demonstrated that IGF-triggered Akt translocation/phosphorylation and downstream signaling were facilitated after increasing PS by DHA supplementation or R95K mutation, and inhibited in PSA-3 cells that contain less PS (Fig. 1, Fig. S1, and Fig. S2). Disrupting Akt–PS interaction by mutating the PS-interacting residues, R15 and K20 in the PH domain, greatly impaired Akt translocation, phosphorylation (Fig. 5, a and b), and Akt downstream signaling such as phosphorylation of GSK-3β and pro-apoptotic factors (FOXO1 and Bad), and consequently enhanced cell death in a serum-starved condition (Fig. 7). Endogenous Akt activation as well as its downstream signaling events also responded to the PS content under both serum-starved (Fig. 8) and IGF-stimulated conditions (Fig. S2 c), which strongly supports the cellular significance of PS in Akt activation. The in vitro data indicated PS-dependent Akt membrane binding (Fig. 3 a) and phosphorylation (Fig. 3 f) but no significant Akt binding to other negatively charged phospholipids (Fig. 3, c–e), which suggests specific interaction between Akt and PS despite its electrostatic nature (Fig. 3 b). The computer modeling based on the mutation data indeed supports specific PS–PH domain interaction (Fig. 5 c). These in vitro and in vivo data indicated that Akt–PS interaction is an important and physiologically relevant membrane-binding module in Akt activation besides the well-established PIP3–PH interaction.
Our in vitro data indicated that the full-length Akt binds to PS even in the absence of PIP3 (Fig. 3 a). In cells, negatively charged lipids in the plasma membrane are known to be clustered with basic residues of cationic proteins including K-Ras, Rac1, MARCKS, GAP-43, and GRK5 (McLaughlin et al., 2005; Yeung et al., 2008). This may explain negligible Akt localization at the plasma membrane under the resting state, despite the ability of both PH and RD domains to interact with membrane PS in vitro. It has been also demonstrated that these strongly cationic proteins normally associated with the plasma membrane can be released when the plasma membrane surface charge decreases under stimulated or stressed conditions (Yeung et al., 2008). The plasma membrane PS thus exposed may become available for the interaction with Akt through the PS-binding residues, which was found to be important for establishing stable Akt binding to PIP3 (Figs. 3 a and 4), subsequent conformational changes (Fig. 2), and phosphorylation (Figs. 3 f and 5 b).
In this study, we used large unilamellar vesicles containing 0.2% PIP3 and 0–30% PS to represent the inner plasma membrane surface where Akt interacts with PIP3 and PS. The PIP3 concentration in resting cells has been found to be 50 nM and can increase up to 2 µM under a stimulated condition (Stephens et al., 1991). The local concentrations of PIP3 at the membrane can go up to 200 µM (Insall and Weiner, 2001), which is estimated to be ∼0.02%. In this regard, the percentage of PIP3 used in the present study was 10-fold higher than the estimated value, but was still significantly lower than those customarily used in in vitro assays (Alessi et al., 1997). The PS content in mammalian cells ranges from 2 to 15% depending on tissue, cell, or organelle types, with a higher PS content in the brain in comparison to the peripheral tissues (Kim, 2008). Among subcellular organelles, the plasma membrane is particularly enriched with PS (Vance and Steenbergen, 2005). Considering exclusive localization of PS in the inner leaflet, the PS content in the cytosolic face of the plasma membrane can reach up to 30%. The phospholipid composition of the vesicles shown in this study was intended to mimic the physiologically relevant range of PS composition in the plasma membrane. The vesicles containing 0% PS, which does not represent physiological membranes, were used only as a control.
The significant interaction observed between Akt and PS even in the absence of PIP3 appeared to conflict with early in vitro studies showing a negligible Akt binding to vesicles containing 10 µM PS/10 µM PC measured by SPR (James et al., 1996), or to sucrose-loaded vesicles containing 100–150 µM PS/20 µM PE/100–200 µM PC determined by the percentage of Akt remaining on the pellet after centrifugation (Stephens et al., 1998). The reasons for this discrepancy are not clear. One plausible explanation could be that the Akt–PS interaction depends on PS molecular species in a similar manner to Akt-PIP3 binding that is specifically dependent on PIP3 species (Alessi et al., 1997; Stephens et al., 1998). Our results were obtained using liposomes with a high proportion of polyunsaturated phospholipids (18:0, 22:6 PS/18:0, 22:6 PE/16:0,18:1 PC), which is typical for the inner leaflet of the plasma membranes. Alternatively, the concentration of PS used in the previous studies might have been too low to observe the effect of PS (James et al., 1996; Stephens et al., 1998), especially with the high proportion of PIP3 used in the study (∼5%; James, et al., 1996). Higher concentrations of large unilamellar vesicles of phospholipids (∼400 µM PS/∼700 µM PE/∼300 µM PC) used in our study may more appropriately represent the cellular membrane phospholipids, the concentration of which is estimated to be in the ∼2 mM range if the cell diameter is assumed to be ∼20 µm. We can also speculate that the inclusion of BSA may have contributed to the lack of Akt association with PS-containing liposomes in the previous study (Stephens et al., 1998), as BSA has been indicated to structurally perturb anionic lipid membranes (Shoemaker and Vanderlick, 2002) and to cause aggregation of neutral PC vesicles (Schenkman et al., 1981; Sato et al., 1999).
Like most members of the AGC superfamily, Akt is activated by phosphorylation mechanisms (Stokoe et al., 1997; Alessi and Cohen, 1998; Downward, 1998), specifically phosphorylation at T308 and S473 by upstream PDK enzymes. It is now well understood that the Akt phosphorylation is affected by the conformational status of Akt upon membrane interaction (Downward, 1998; Milburn et al., 2003; Huang and Kim, 2006; Calleja et al., 2007, 2009). Our in vitro cross-linking data revealed that PIP3 and PS jointly influence the interdomain conformation of Akt for exposing T308 and S473 for phosphorylation and activation (Fig. 2), providing a mechanism for the observed PIP3- and PS-dependent Akt phosphorylation (Fig. 3 f). In a recent in vivo study using fluorescent lifetime imaging microscopy (Calleja et al., 2007), it was found that the PH domain interacts with the KD, causing a folded (PH-in) conformer that blocks the access of T308 by PDK1 in inactive Akt molecules. When Akt translocates to the plasma membrane, presumably because of interaction with phosphoinositides, the interdomain interaction was altered, resulting in an open (PH-out) conformer to expose T308 for phosphorylation (Calleja et al., 2007). In agreement with these findings, we also found the open conformation to expose T308 at the membrane interaction states. Nevertheless, our data indicated that PIP3 without PS has only limited ability to cause a spatial interdomain arrangement of Akt to expose T308.
Our data also revealed a close interaction between the regulatory and kinase domains in the inactive state of Akt that maintains a folded RD-KD structure. Interaction of RD primarily with membrane PS altered its association with the KD, resulting in an open conformer that exposes S473 (Fig. 2) for phosphorylation by mTORC2 (Fig. 3 f). PIP3 also induced an open RD conformation, although the direct interaction between PIP3 and RD seemed to be minimal (Fig. 4 a). It is possible that interdomain (e.g., PH-kinase) conformational changes upon binding of PIP3 to the PH domain subsequently alter proximity between RD and KDs. Compared with the phosphorylation of T308 on the KD, which is known to be essential for Akt activation (Alessi et al., 1996), the mechanism and regulation of the phosphorylation of S473 on the RD is less well characterized. Nevertheless, the S473 phosphorylation has been shown to facilitate T308 phosphorylation, augment Akt activity (Alessi et al., 1996; Sarbassov et al., 2005), and assist in maintaining Akt stability (Lawlor and Alessi, 2001) to ensure its downstream function (Datta et al., 1999), and therefore is required for full activation of Akt. Our results suggest an important complementary mechanism in survival signaling in which PS may substitute for PIP3 in S473 phosphorylation. Although the membrane phospholipid composition is relatively well-maintained, the loss of PS by up to 30% has been observed particularly in neural tissues after long-term n-3 fatty acid depletion (Hamilton et al., 2000) or ethanol exposure (Wen and Kim, 2004). In contrast, DHA supplementation has been shown to increase the PS content in neuronal membranes (Kim et al., 2000; Akbar et al., 2005), which suggests a specific role of DHA in neuronal membrane modification (Glomset, 2006). Akt activation in individual cell types under normal conditions is likely determined by the PIP3 generation capacity and cell-specific factors such as the PS content, copy numbers of Akt, PDK-1, and mTORC2, etc. Under suboptimal conditions where PIP3 production is limited, cell survival may significantly rely on the PS-dependent modulation of Akt activation. This PS-dependent molecular mechanism may provide an explanation for the increased neuronal susceptibility to cell death under n-3 fatty acid deficiency (Akbar et al., 2005) or long-term ethanol exposure (Akbar et al., 2006).
Although the detailed mechanism is unclear, it has been demonstrated that Akt interacts with its upstream kinase, PDK1, in cytoplasm, whereas Akt maintains its inactive form in living cells (Calleja et al., 2007). Upon growth factor stimulation, both Akt and PDK1 are concomitantly recruited to the plasma membrane through the interaction of their PH domains with membrane PIP3. It has been reported that vesicles containing PIP3 alone are at least 15-fold less effective in comparison to those containing PIP3, PC, and PS in activating PDK1 (Alessi et al., 1997). This significant reduction of the PDK1 activity suggests the possibility that the membrane interaction of PDK1 may also be dependent on not only PIP3 but also PS, as shown in this study for the Akt–membrane interaction. In such a case, the interaction of the PH domain with PS may be a part of PH domain’s general selectivity for the plasma membrane targeting, in addition to the well-established PH-PIP3 binding.
It has been demonstrated that the initial interaction of general receptor for phosphoinositides isoform 1 (GRP-1) with anionic lipids is too weak for stable membrane docking of the PH domain (Corbin et al., 2004). Similarly, the electrostatic interactions of Akt only with PS in the membrane may not be strong enough for membrane translocation without tight binding between PIP3 and the PH domain. However, the presence of anionic phospholipids in the membrane has been shown to facilitate the finding of rarer target lipids such as PIP3 or diacylglycerol for the translocation of the PH domain of GRP-1 (Corbin et al., 2004) or PKC (Nalefski and Newton, 2001), respectively. Once the target lipids are found, high affinity binding is established, resulting in stable membrane docking. Our study also strongly suggests that membrane translocation and activation of Akt may require Akt–PS interactions in addition to specific binding of Akt to PIP3 (Fig. 5, a and b; and Fig. 6 a).
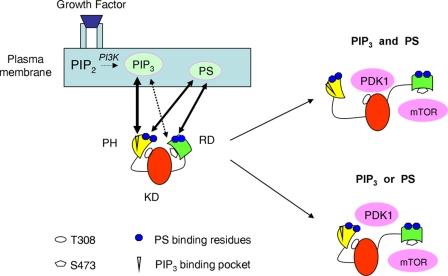
Fig. 9 is a schematic presentation of the membrane PS involvement in Akt activation according to our data. Upon growth factor receptor stimulation, Akt is recruited to the plasma membrane through the specific binding of PIP3 to the PH domain. Concurrent interactions of PS with the basic residues in the PH domain outside the PIP3-binding pocket and in the RD (K419/K420) secure and facilitate the Akt membrane binding and conformational changes for subsequent phosphorylation. Although both PS and PIP3 are required for an optimal exposure of T308 for phosphorylation by PDK1, either PS or PIP3 can expose S473 for phosphorylation by mTORC2.
Figure 9.
Schematic presentation of the PS involvement in Akt activation. PS and PIP3 jointly regulate the membrane binding and interdomain conformational changes of Akt for unfolding the PH and RD to expose T308 and S473 for phosphorylation and activation. When the growth factor receptor is stimulated, PIP3 is generated in the membrane, which in turn triggers translocation of cytosolic Akt to the plasma membrane through the specific binding of PIP3 to the PH domain. Although necessary, the PIP3–PH interaction alone is not sufficient for securing Akt binding to the plasma membrane. Interaction of PS with the PS-binding residues in the PH domain outside the PIP3-binding pocket is also required for the membrane binding and conformational changes to expose T308 for phosphorylation by PDK1. Near the plasma membrane, presumably after or concurrent with PIP3–PH binding, the PS-binding residues in the RD also interact with PS, resulting in an open conformation, allowing S473 phosphorylation by mTORC2. Although direct binding of PIP3 with the RD is minimal, PIP3 can also induce an open RD conformation to expose S473 for phosphorylation. The involvement of KD has not been evaluated separately.
In conclusion, we demonstrate that the well-established binding of Akt to PIP3 through the PH domain is necessary but not sufficient for Akt activation. We found that the PIP3-dependent Akt activation also requires membrane PS, which directly interacts with the PH and RD domains of Akt through specific binding sites. Furthermore, we showed that membrane PS plays an important role in salvaging Akt activation and downstream signaling, leading to cell survival under adverse conditions where PIP3 signal is limited. The novel molecular interaction mechanism in Akt signaling involving membrane PS and individual domains of Akt, particularly the less-known RD, revealed in this study may provide insight for possible new targets for controlling physiological and pathophysiological processes of cell survival.
Materials and methods
Cell culture, transfection, fatty acid supplementation, and IGF stimulation
Neuro 2A (mouse neuroblastoma) cells were maintained in DME with 5% FBS in a humidified atmosphere of 95% air and 5% CO2 at 37°C. CHO-K1 (WT) and PSA-3 mutant cells (a gift from O. Kuge, Kyushu University, Fukuoka, Japan) were initially raised in F12 medium with 5% FBS and 20 µM PS from bovine brain for 1 wk. Cells were further cultured in the same medium without PS for 7–8 d before being used for further experiments. Cells were transfected with GFP-or RFP-tagged mutants (or WT) using Fugene-6 transfection reagent (Roche) for 36 h. For fatty acid supplementation, Neuro 2A cells were transfected with or without GFP-Akt PH domain overnight, and supplemented with or without 20 µM DHA for 36 h in the presence of 40 µM vitamin E and 2% FBS. After overnight serum starvation, cells were harvested for phospholipid analyses by high-performance liquid chromatography (HPLC)/MS or stimulated with IGF-1 (10 or 100 ng/ml for Neuro 2A or CHO cells, respectively) for Western blotting or microscopic analysis.
Preparation of unilamellar vesicles
Unilamellar vesicles (1 mg/ml of total phospholipids) containing various components of PE (18:0, 22:6), PC (16:0, 18:1), PS (18:0, 22:6), and PIP3 (18:1, 18:1 or 18:0, 20:4; Avanti Polar Lipids, Inc.) were prepared by extruding the multilamellar vesicles through a 0.1-µm polycarbonate membrane as described previously (Kim et al., 2004). Samples were analyzed by HPLC/MS (Wen and Kim, 2004) to verify the final concentrations of lipid components.
In vitro phosphorylation of Akt
Recombinant inactive Akt (Millipore) reconstituted in Hepes buffer (0.1 µg/µL, 2 µl) was incubated with 3 µl PDK1 at 0.005 µg/µL (Millipore) or 15 µl mTOR complex solution (EMD), which also contains rictor or 0.5 µl MAPKAPK2 at 0.1 µg/µL (EMD) at 30°C for 45 min in the presence of various liposome preparations (15 µl) and ATP/Mg2+ cocktail (in the case of mTORC2, ATP/Mn2+ was used in the presence of DTT and glycerol phosphate). The mixtures were subjected to Western blot analysis using anti–phospho-T308 Akt, anti–phospho-S473 Akt and anti-Akt antibodies (Cell Signaling Technology) with an enhanced chemiluminescent substrate (Thermo Fisher Scientific). Bands were visualized by a Gel Logic 440 imaging system and quantitated using 1D imaging analysis software (both from Kodak).
Microscopic study of GFP- or RFP-Akt membrane translocation
Akt membrane translocation was monitored by microscopy using an inverted microscope (IX81; Olympus) with a GFP or RFP filter and a 60× oil objective lens. The serum-starved cells were maintained at 35°C using a Delta T4 culture dish controller (Bioptechs). After recording the initial image, medium was exchanged with IGF-containing buffer (10 or 100 ng/ml IGF for Neuro 2A or CHO cells, respectively). The time series of images were recorded using a 12-bit 20-MHz camera (coolSnap FX; Roper Scientific) and analyzed using Microsuite (Soft Imaging Systems) as described previously (Akbar et al., 2005). The R value ([plasma membrane fluorescence − cytosolic fluorescence]/cytosolic fluorescence) representing the relative increase of fluorescence intensity in the plasma membrane over the cytosol was calculated in three to five cells per condition and normalized to the plateau value.
In vitro PI3 kinase assay
PI3 kinase activity assay was performed using a modified protocol described previously (Varticovski et al., 1994). In brief, cells were stimulated with IGF and harvested. Cell lysate containing 1 mg of protein was incubated with 10 µg of anti-PI3 kinase p85 antibody (Millipore) for 1 h at 4°C followed by an additional 1-h incubation with 30 µl of protein A agarose beads at 4°C. After washing, the immunoprecipitated enzyme was collected and incubated with phospholipids substrate (4 µg each of PIP2, PC, and PS) in the presence of 20 µM ATP/MgAc cocktail containing10 µCi [32P]ATP (PerkinElmer) for 1 h at 37°C. The reaction was stopped by adding HCl/MeOH (1:1). Phospholipids were extracted and separated on thin layer chromatography (TLC) plates. Radioactive spots containing [32P]PIP3 were detected by exposing the TLC plates to x-ray film.
In vitro Akt kinase assay
Neuro 2A cells expressing GFP-Akt WT (or mutants) with or without IGF stimulation were lysed with cell lysis buffer (Cell Signaling Technology) containing additional Halt phosphatase inhibitors (Pierce). Cell lysates containing 1.2 mg of protein were precleared with 20 µl protein A/protein G plus agarose beads. The precleared lysates were incubated with 10 µl of anti-GFP antibody (rabbit IgG fraction; Invitrogen) for 30 min at RT, followed by incubation with 50 µl of protein A/protein G plus agarose beads for 70 min at RT on an orbital shaker. The kinase activity of the immunoprecipitated GFP-Akt was measured with a K-LISA Akt activity kit (EMD) according to the manufacturer’s instructions. In brief, the beads containing immunoprecipitated GFP-Akt were incubated with a biotinylated peptide substrate in the presence of ATP/MgCl2 mixture. The phosphorylated substrate was separated from the beads and captured on streptavidin-coated wells and detected with a phosphoserine detection antibody, followed by HRP–antibody conjugate and color development with TMB substrate. The relative kinase activity was determined by measuring the absorbance at 450 nm. The immunoprecipitated GFP-Akt detached from beads by boiling in Laemmli buffer for 5 min was subjected to Western blot analysis to estimate the amount of Akt used in the assay.
Cross-linking reaction, tryptic digestion, 18O labeling of tryptic digests, and nano-electrospray ionization mass spectrometric analysis
Recombinant inactive Akt were dialyzed against 50 mM Hepes, pH 7.4, containing 50 mM NaCl at 4°C to remove the primary amine-containing Tris-HCl buffer. 10 µL of Akt at 2.5 µM was incubated with 30 µl liposomes with varying compositions at 30°C for 45 min. The mixtures were incubated with a 50-M excess of freshly prepared DSS (Pierce) in DMSO (1% final concentration of DMSO) at room temperature for 10 min. At this cross-linking condition, intermolecular cross-linked dimers or multimers were not observed according to the SDS-PAGE analysis. The cross-linking reaction was quenched with 1 M Tris-HCl. For quantitative analyses, each of the liposome-interacted cross-linked samples digested in H216O was mixed with the control (cross-linked Akt without liposome interaction), digested in H218O at a ratio of 1:1, and subjected to mass spectrometric analysis using a high-resolution QSTAR pulsar quadruple-time-of-flight (Qq-TOF) mass spectrometer (Applied Biosystems) equipped with a nano-electrospray ionization source, according to the method described previously (Huang and Kim, 2006). When digested with trypsin in H218O, non–cross-linked peptides incorporated two 18O atoms into the C terminus, resulting in a mass increase by 4 D (2,990 D). The cross-linking between two tryptic peptides such as the interdomain cross-linked pairs incorporated a total of four 18O atoms into two C termini, increasing the peptide mass by 8 D. Quantitative comparison is achieved using 16O/18O intensity ratios.
Cloning of PSS1 from mouse brain, GFP tagging, and point mutation of PSS1 and Akt
Total RNA from mouse brain was isolated using TriZOL reagent (Invitrogen). 1 µg of isolated RNA was used for first strand cDNA synthesis by using 200 units of Superscript III RT (M-MLV Reverse transcription; Invitrogen), according to manufacturer’s instructions. cDNA of PSS1 and human Akt (GenBank/EMBL/DDBJ accession no. NM_005163) was cloned into pEGFP-C1 vector (Takara Bio Inc.) to prepare EGFP-PSS1 and EGFP-Akt, respectively, at Veritas Laboratory Inc. For point mutations, QuickChange II Site-Directed Mutagenesis kit (Agilent Technologies) was used according to manufacturer’s instructions. These mutations included EGFP-PSS1 (R95K; CGC to AAG), EGFP-Akt (R15A, R436, or R465/ R466; AGG to GCG), and EGFP-Akt (K20A, K419 or K420A, or K419/K420; AAG to GCG). After transformation, five colonies were picked and amplified in LB medium, and plasmid DNA was purified by using a Qiaprep Spin Miniprep kit (QIAGEN). Purified plasmids were sequenced to confirm the mutations by using an ABI BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems).
Biomolecular interaction analysis by SPR
SPR measurements were performed on a Biacore X instrument (Uppsala). A CM5 sensor chip was used for the interaction between liposomes and GFP-RD or GFP-PH domains. Anti-GFP antibody (Invitrogen) was immobilized onto the chip by amine coupling according to the manufacturer’s instructions. In brief, after a 7-min injection (flow rate of 10 µl/min) of 1:1 mixture of _N_-ethyl-_N_′-(3-dimethylaminopropyl) carbodiimide hydrochloride and _N_-hydroxysuccinimide, anti-GFP antibody (25 µg/ml in NaOAc buffer, pH 5.0) was injected using a 7-min injection at 10 µl/min. Remaining activated groups were blocked by injecting 1 M ethanolamine/HCl, pH 8.5. The cell lysate containing GFP-tagged Akt domains expressed in Neuro 2A cells was manually injected into the experimental cell until 15–20 RU of GFP-tagged protein was captured onto the antibody surface (Kim et al., 2000). No lysate was injected into the control cell. Liposomes (30 µl) were injected at 5 µl/min into both flow cells, and all sensorgrams were recorded against the control. Regeneration of the anti-GFP surface was achieved by injecting 10 mM sodium acetate, pH 4.0, followed by radioimmune precipitation assay (RIPA) buffer. Alternatively, an L1 sensor chip was selected for the interaction of recombinant full-length Akt and liposomes using a modified protocol described previously (Ananthanarayanan et al., 2003). In brief, 35 µl liposome preparations containing various phospholipid compositions (1 mg/ml) were injected into the experimental cell at 5 µl/min. The control cell was similarly coated with the control vesicles containing PE/PC (50:50). The lipid layer in both cells was stabilized by injecting 12 µl of 50 mM NaOH at 50 µl/min several times until a steady signal was obtained. Akt (200 nM, 90 µl) was injected at 15 µl/min into both cells. The sensor surface was regenerated by completely removing the protein and liposomes with a 30-s injection of 40% isopropanol in 50 mM NaOH at 50 µl/min. The response obtained from the liposomes containing PE/PC (50:50) was used as an additional reference and subtracted from all sensorgrams to compensate for background drift. PBS (pH 7.4, 795 mg/L Na2HPO4, 144 mg/L KH2PO4, and 9,000 mg/ml NaCl) without calcium and magnesium) or Hepes were used as the running buffer.
Analysis of plasma membrane PS
Isolation of the plasma membrane was performed using differential centrifugation as described previously (McKeel and Jarett, 1970). The isolated fractions including the plasma membrane, microsomes, and mitochondria were verified by Western blot analyses using distinct marker proteins, cadherin, calnexin, and VDAC (antibodies were obtained from Cell Signaling Technology), respectively. Analyses of the phospholipids in the plasma membrane preparations with or without DHA supplementation were performed on an Agilent 1100 MSD coupled with an Agilent 1100 HPLC system using a Prodigy C18 column (150 × 2.0 mm, 5 mm; Phenomenex) as described previously (Wen and Kim, 2004).
Induction of cell death and TUNEL assay
Neuro 2A cells were either transfected with Akt WT, Akt mutants, GFP, GFP-PSS1, or GFP-PSS1(R95K), or supplemented with DHA for 36 h, subsequently serum-starved for 48 h, and subjected to Western blot analysis or TUNEL assay. Similarly, CHO-K1 (WT) and PSA-3 cells raised as described in the Cell culture section were similarly serum starved for 48 h and subjected to Western blot analysis. Antibodies for phospho-Bad(S136), phospho-FOXO1(T24)/FOXO3(T32), phospho-GSK-3β(S9), cleaved caspase-3(D175) (Cell Signaling Technology), and actin (Santa Cruz Biotechnology, Inc.) were used with an enhanced chemiluminescent substrate cocktail containing 5–10% SuperSignal West Femto maximum sensitivity substrate (Pierce). For the TUNEL assay, the apoptotic nuclei containing free 3′ OH termini were detected by using Click-iT TUNEL Alexa Fluor Imaging assay kit (Invitrogen) according to manufacturer’s instructions with slight modifications. Cells were fixed with freshly prepared paraformaldehyde (4% in PBS, pH 7.4), permeabilized using 0.25% Triton X-100 in PBS. After incubating with TUNEL reaction mixture and nuclear staining with Hoechst 33342, coverslips containing cells were mounted on slides and used for fluorescence microscopy. The percentage of GFP- and TUNEL-positive cells in relation to the total number of cells expressing GFP was determined by counting 500–1,000 GFP-positive cells from six randomly selected fields using an inverted semimotorized microscope (IX81; Olympus).
Computer modeling of the PS–PH domain interaction
The PS head group was docked to the proposed interaction site using AutoDock version 4.0, and the energy optimization was performed using Lamarckian genetic algorithm as described previously (Morris et al., 1998). The lowest mean energy was selected as a plausible model for the binding of the PS head group to the PH domain of Akt (PDB accession no. 1H10) when considering the feasibility of embedding the tail in the membrane.
Statistical analysis
Statistical analyses were performed using Student’s t tests unless specified otherwise.
Online supplemental material
Fig. S1 shows the effect of the increase of plasma membrane PS by DHA supplementation on Akt membrane translocation. Fig. S2 shows the effect of PS on Akt activation and downstream signaling. Fig. S3 shows identification of cross-linking sites by tandem MS (MS/MS). Fig. S4 shows the effects of PS and PIP3 on the in vitro phosphorylation of S473 by MAPKAPK2. Fig. S5 shows Akt phosphorylation in Neuro 2A cells affected by the mutation of the basic residues in the RD. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201005100/DC1.
Acknowledgments
The authors thank Hui Lu and Morten Kallberg of University of Illinois at Chicago Bioengineering for assistance in computer modeling.
This research was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Footnotes
Abbreviations used in this paper:
DHA
docosahexaenoic acid
DSS
disuccinimidyl suberate
FOXO1
Forkhead box O1
GSK-3β
glycogen synthase kinse-3β
HPLC
high-performance liquid chromatography
IGF
insulin-like growth factor
KD
kinase domain
MAPKAPK2
MAPK-activated protein kinase II
MS
mass spectrometry
MS/MS
tandem MS
mTOR
mammalian target of rapamycin
mTORC2
mTOR complex 2
PC
phosphatidylcholine
PDK
phosphoinositide-dependent protein kinase
PE
phosphatidylethanolamine
PH
pleckstrin homology
PI
phosphatidylinositol
PI3 kinase
phosphoinositide-3 kinase
PIP
PI-4-phosphate
PIP2
PI 4,5-bisphosphate
PIP3
PI 3,4,5-trisphosphate
PS
phosphatidylserine
PSS1
PS synthase 1
RD
regulatory domain
SPR
surface plasmon resonance
WT
wild type
References
- Akbar M., Calderon F., Wen Z., Kim H.-Y. 2005. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA. 102:10858–10863 10.1073/pnas.0502903102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar M., Baick J., Calderon F., Wen Z., Kim H.-Y. 2006. Ethanol promotes neuronal apoptosis by inhibiting phosphatidylserine accumulation. J. Neurosci. Res. 83:432–440 10.1002/jnr.20744 [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Cohen P. 1998. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 8:55–62 10.1016/S0959-437X(98)80062-2 [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B.A. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541–6551 [PMC free article] [PubMed] [Google Scholar]
- Alessi D.R., James S.R., Downes C.P., Holmes A.B., Gaffney P.R.J., Reese C.B., Cohen P. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7:261–269 10.1016/S0960-9822(06)00122-9 [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan B., Stahelin R.V., Digman M.A., Cho W. 2003. Activation mechanisms of conventional protein kinase C isoforms are determined by the ligand affinity and conformational flexibility of their C1 domains. J. Biol. Chem. 278:46886–46894 10.1074/jbc.M307853200 [DOI] [PubMed] [Google Scholar]
- Andjelković M., Alessi D.R., Meier R., Fernandez A., Lamb N.J.C., Frech M., Cron P., Cohen P., Lucocq J.M., Hemmings B.A. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515–31524 10.1074/jbc.272.50.31515 [DOI] [PubMed] [Google Scholar]
- Bellacosa A., Testa J.R., Staal S.P., Tsichlis P.N. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 254:274–277 10.1126/science.1833819 [DOI] [PubMed] [Google Scholar]
- Bellacosa A., Chan T.O., Ahmed N.N., Datta K., Malstrom S., Stokoe D., McCormick F., Feng J., Tsichlis P. 1998. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 17:313–325 10.1038/sj.onc.1201947 [DOI] [PubMed] [Google Scholar]
- Brazil D.P., Hemmings B.A. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657–664 10.1016/S0968-0004(01)01958-2 [DOI] [PubMed] [Google Scholar]
- Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 96:857–868 10.1016/S0092-8674(00)80595-4 [DOI] [PubMed] [Google Scholar]
- Calderon F., Kim H.-Y. 2008. Detection of intracellular phosphatidylserine in living cells. J. Neurochem. 104:1271–1279 10.1111/j.1471-4159.2007.05079.x [DOI] [PubMed] [Google Scholar]
- Calleja V., Alcor D., Laguerre M., Park J., Vojnovic B., Hemmings B.A., Downward J., Parker P.J., Larijani B. 2007. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 5:e95 10.1371/journal.pbio.0050095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja V., Laguerre M., Larijani B. 2009. 3-D structure and dynamics of protein kinase B-new mechanism for the allosteric regulation of an AGC kinase. J Chem Biol. 2:11–25 10.1007/s12154-009-0016-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J.A., Dirkx R.A., Falke J.J. 2004. GRP1 pleckstrin homology domain: activation parameters and novel search mechanism for rare target lipid. Biochemistry. 43:16161–16173 10.1021/bi049017a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S.R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M.E. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 91:231–241 10.1016/S0092-8674(00)80405-5 [DOI] [PubMed] [Google Scholar]
- Datta S.R., Brunet A., Greenberg M.E. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927 10.1101/gad.13.22.2905 [DOI] [PubMed] [Google Scholar]
- Downward J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262–267 10.1016/S0955-0674(98)80149-X [DOI] [PubMed] [Google Scholar]
- Glomset J.A. 2006. Role of docosahexaenoic acid in neuronal plasma membranes. Sci. STKE. 2006:pe6 10.1126/stke.3212006pe6 [DOI] [PubMed] [Google Scholar]
- Hamilton L., Greiner R., Salem N., Jr, Kim H.-Y. 2000. n-3 fatty acid deficiency decreases phosphatidylserine accumulation selectively in neuronal tissues. Lipids. 35:863–869 10.1007/S11745-000-0595-x [DOI] [PubMed] [Google Scholar]
- Huang B.X., Kim H.-Y. 2006. Interdomain conformational changes in Akt activation revealed by chemical cross-linking and tandem mass spectrometry. Mol. Cell. Proteomics. 5:1045–1053 10.1074/mcp.M600026-MCP200 [DOI] [PubMed] [Google Scholar]
- Huang B.X., Dass C., Kim H.-Y. 2005. Probing conformational changes of human serum albumin due to unsaturated fatty acid binding by chemical cross-linking and mass spectrometry. Biochem. J. 387:695–702 10.1042/BJ20041624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Improta-Brears T., Ghosh S., Bell R.M. 1999. Mutational analysis of Raf-1 cysteine rich domain: requirement for a cluster of basic aminoacids for interaction with phosphatidylserine. Mol. Cell. Biochem. 198:171–178 10.1023/A:1006981411691 [DOI] [PubMed] [Google Scholar]
- Insall R.H., Weiner O.D. 2001. PIP3, PIP2, and cell movement—similar messages, different meanings? Dev. Cell. 1:743–747 10.1016/S1534-5807(01)00086-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S.R., Downes C.P., Gigg R., Grove S.J.A., Holmes A.B., Alessi D.R. 1996. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem. J. 315:709–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-Y. 2007. Novel metabolism of docosahexaenoic acid in neural cells. J. Biol. Chem. 282:18661–18665 10.1074/jbc.R700015200 [DOI] [PubMed] [Google Scholar]
- Kim H.-Y. 2008. Biochemical and biological functions of docosahexaenoic acid in the nervous system: modulation by ethanol. Chem. Phys. Lipids. 153:34–46 10.1016/j.chemphyslip.2008.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-Y., Akbar M., Lau A., Edsall L. 2000. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J. Biol. Chem. 275:35215–35223 10.1074/jbc.M004446200 [DOI] [PubMed] [Google Scholar]
- Kim H.-Y., Bigelow J., Kevala J.H. 2004. Substrate preference in phosphatidylserine biosynthesis for docosahexaenoic acid containing species. Biochemistry. 43:1030–1036 10.1021/bi035197x [DOI] [PubMed] [Google Scholar]
- Kuge O., Nishijima M., Akamatsu Y. 1986. Phosphatidylserine biosynthesis in cultured Chinese hamster ovary cells. II. Isolation and characterization of phosphatidylserine auxotrophs. J. Biol. Chem. 261:5790–5794 [PubMed] [Google Scholar]
- Kuge O., Hasegawa K., Saito K., Nishijima M. 1998. Control of phosphatidylserine biosynthesis through phosphatidylserine-mediated inhibition of phosphatidylserine synthase I in Chinese hamster ovary cells. Proc. Natl. Acad. Sci. USA. 95:4199–4203 10.1073/pnas.95.8.4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor M.A., Alessi D.R. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903–2910 [DOI] [PubMed] [Google Scholar]
- McKeel D.W., Jarett L. 1970. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J. Cell Biol. 44:417–432 10.1083/jcb.44.2.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Hangyás-Mihályné G., Zaitseva I., Golebiewska U. 2005. Reversible - through calmodulin - electrostatic interactions between basic residues on proteins and acidic lipids in the plasma membrane. Biochem. Soc. Symp. 72:189–198 [DOI] [PubMed] [Google Scholar]
- Milburn C.C., Deak M., Kelly S.M., Price N.C., Alessi D.R., Van Aalten D.M.F. 2003. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem. J. 375:531–538 10.1042/BJ20031229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. 1998. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 19:1639–1662 [DOI] [Google Scholar]
- Nalefski E.A., Newton A.C. 2001. Membrane binding kinetics of protein kinase C betaII mediated by the C2 domain. Biochemistry. 40:13216–13229 10.1021/bi010761u [DOI] [PubMed] [Google Scholar]
- Newton A.C., Keranen L.M. 1994. Phosphatidyl-L-serine is necessary for protein kinase C’s high-affinity interaction with diacylglycerol-containing membranes. Biochemistry. 33:6651–6658 10.1021/bi00187a035 [DOI] [PubMed] [Google Scholar]
- Op den Kamp J.A. 1979. Lipid asymmetry in membranes. Annu. Rev. Biochem. 48:47–71 10.1146/annurev.bi.48.070179.000403 [DOI] [PubMed] [Google Scholar]
- Parcellier A., Tintignac L.A., Zhuravleva E., Hemmings B.A. 2008. PKB and the mitochondria: AKTing on apoptosis. Cell. Signal. 20:21–30 10.1016/j.cellsig.2007.07.010 [DOI] [PubMed] [Google Scholar]
- Saito K., Nishijima M., Kuge O. 1998. Genetic evidence that phosphatidylserine synthase II catalyzes the conversion of phosphatidylethanolamine to phosphatidylserine in Chinese hamster ovary cells. J. Biol. Chem. 273:17199–17205 10.1074/jbc.273.27.17199 [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 307:1098–1101 10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- Sato Y., Kaneko K., Mikami K., Mizugaki M., Suzuki Y. 1999. Isolation of bovine serum albumin fragment P-9 and P-9-mediated fusion of small unilamellar vesicles. Biol. Pharm. Bull. 22:1360–1365 [DOI] [PubMed] [Google Scholar]
- Schenkman S., Araujo P.S., Dijkman R., Quina F.H., Chaimovich H. 1981. Effects of temperature and lipid composition on the serum albumin-induced aggregation and fusion of small unilamellar vesicles. Biochim. Biophys. Acta. 649:633–647 10.1016/0005-2736(81)90168-1 [DOI] [PubMed] [Google Scholar]
- Shoemaker S.D., Vanderlick T.K. 2002. Intramembrane electrostatic interactions destabilize lipid vesicles. Biophys. J. 83:2007–2014 10.1016/S0006-3495(02)73962-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.R., Storch J. 1999. The adipocyte fatty acid-binding protein binds to membranes by electrostatic interactions. J. Biol. Chem. 274:35325–35330 10.1074/jbc.274.50.35325 [DOI] [PubMed] [Google Scholar]
- Stephens L.R., Hughes K.T., Irvine R.F. 1991. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 351:33–39 10.1038/351033a0 [DOI] [PubMed] [Google Scholar]
- Stephens L., Anderson K., Stokoe D., Erdjument-Bromage H., Painter G.F., Holmes A.B., Gaffney P.R., Reese C.B., McCormick F., Tempst P., et al. 1998. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 279:710–714 10.1126/science.279.5351.710 [DOI] [PubMed] [Google Scholar]
- Stokoe D., Stephens L.R., Copeland T., Gaffney P.R.J., Reese C.B., Painter G.F., Holmes A.B., McCormick F., Hawkins P.T. 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 277:567–570 10.1126/science.277.5325.567 [DOI] [PubMed] [Google Scholar]
- Thomas C.C., Deak M., Alessi D.R., van Aalten D.M.F. 2002. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr. Biol. 12:1256–1262 10.1016/S0960-9822(02)00972-7 [DOI] [PubMed] [Google Scholar]
- Vance J.E., Steenbergen R. 2005. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 44:207–234 10.1016/j.plipres.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Varticovski L., Harrison-Findik D., Keeler M.L., Susa M. 1994. Role of PI 3-kinase in mitogenesis. Biochim. Biophys. Acta. 1226:1–11 [DOI] [PubMed] [Google Scholar]
- Verdaguer N., Corbalan-Garcia S., Ochoa W.F., Fita I., Gómez-Fernández J.C. 1999. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. EMBO J. 18:6329–6338 10.1093/emboj/18.22.6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watton S.J., Downward J. 1999. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr. Biol. 9:433–436 10.1016/S0960-9822(99)80192-4 [DOI] [PubMed] [Google Scholar]
- Wen Z., Kim H.-Y. 2004. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J. Neurochem. 89:1368–1377 10.1111/j.1471-4159.2004.02433.x [DOI] [PubMed] [Google Scholar]
- Yao X., Freas A., Ramirez J., Demirev P.A., Fenselau C. 2001. Proteolytic 18O labeling for comparative proteomics: model studies with two serotypes of adenovirus. Anal. Chem. 73:2836–2842 10.1021/ac001404c [DOI] [PubMed] [Google Scholar]
- Yeung T., Gilbert G.E., Shi J., Silvius J., Kapus A., Grinstein S. 2008. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 319:210–213 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- Young M.M., Tang N., Hempel J.C., Oshiro C.M., Taylor E.W., Kuntz I.D., Gibson B.W., Dollinger G. 2000. High throughput protein fold identification by using experimental constraints derived from intramolecular cross-links and mass spectrometry. Proc. Natl. Acad. Sci. USA. 97:5802–5806 10.1073/pnas.090099097 [DOI] [PMC free article] [PubMed] [Google Scholar]