Comparison of Human Induced Pluripotent and Embryonic Stem Cells: Fraternal or Identical Twins? (original) (raw)
Human induced pluripotent stem cells (hiPSCs) have been hailed as an effective replacement for human embryonic stem cells (hESCs) and a prime candidate cell source for regenerative medicine aims. Both hESCs and hiPSCs share the important properties of self-renewal and pluripotency; that is, they are theoretically capable of generating unlimited amounts of any differentiated cell in the human body. However, accumulating reports of gene expression differences between hESCs and hiPSCs have led many to question the equivalence of these two promising cell types. Seemingly random variation in the differentiation propensity of hiPSCs to neural,1 cardiovascular,2 and hemangioblastic lineages3 has frustrated investigators hoping to better exploit their potential for disease modeling and cell replacement therapies. In light of these somewhat dispiriting results, the recent publication of genome-wide reference “scorecards” for monitoring the quality and utility of 32 human pluripotent stem cell lines is a welcome advance.4 Such advances are crucial to aiding our ability to predict a cell line's differentiation propensity in a high-throughput fashion.
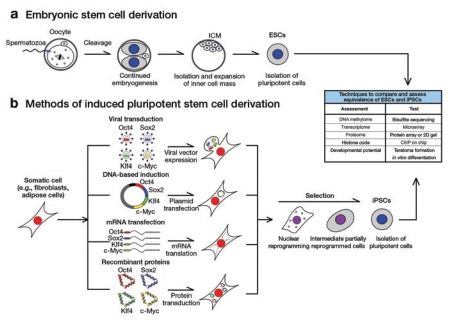
hESCs are derived from the inner cell mass of fresh or frozen embryos at the blastocyst stage of development5 (Figure 1a). Most importantly, hESCs self-renew to allow for indefinite maintenance of the undifferentiated state in vitro and thereby retain the ability to differentiate into derivatives of the three embryonic germ layers that subsequently form all the tissues of a developing fetus. Consequently, hESCs are a promising candidate cell source for the generation of differentiated cells for use in cell replacement therapies, as well as a valuable tool for disease modeling and drug screening applications. Unfortunately, however, hESC derivation remains ethically controversial in the United States and somewhat challenging logistically because of a limited supply of donor human embryos. Therefore, the landmark discovery that hiPSCs with remarkable similarity to hESCs could be derived relatively easily from somatic tissues was hailed as a significant advance.6,7 In contrast to hESCs, hiPSCs are derived by “reprogramming” of somatic cells to a pluripotent state through the overexpression of a key set of transcription factors (Figure 1b). This process does not require the destruction of human embryos ex utero, thereby circumventing much of the ethical debate surrounding hESC derivation. In addition, because the techniques for hiPSC derivation are easily applicable to adult somatic cell types, cell lines can be easily derived from a variety of genetic backgrounds. This allows not only for the creation of patient-specific hiPSCs that are theoretically secure against immune rejection but also for novel studies of heritable genetic disorders in their human cell types.8
Figure 1.
Schematic of human embryonic stem cell (hESC) and human induced pluripotent stem cell (hiPSC) derivation protocols. (a) ESCs are derived from the inner cell mass (ICM) of the blastocyst, whereas (b) iPSCs can be derived from a variety of somatic cell types using a variety of reprogramming techniques. Commonly used assays to determine the equivalence of hESCs and hiPSCs at the molecular and functional levels are listed in the table on the right.
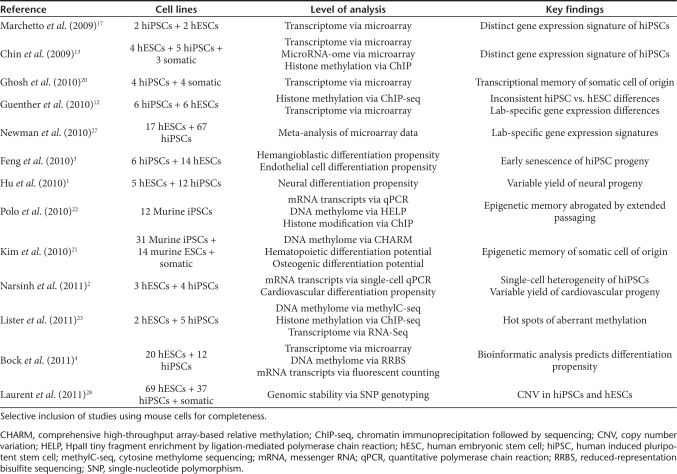
hiPSCs are similar to hESCs in terms of their morphology, feeder dependence, surface marker expression, and in vivo teratoma formation capacity.6,7 Despite these similarities, reports of variability in the in vitro differentiation potential of hiPSCs with respect to hESCs have called into question the functional and molecular equivalence of the two cell types (Table 1). For instance, a reduced and more variable yield of neural1 and cardiovascular progeny2 has been observed in hiPSCs, irrespective of the presence of reprogramming transgenes in the hiPSC genome. In addition, hiPSC-derived early blood progenitor and endothelial cells appear to undergo premature senescence.2,3 What underlies these differences in yield of useful differentiated cell types? Such results are perhaps unsurprising in light of the fact that murine iPSC lines differ from one another in terms of their developmental potency when tested in the definitive tetraploid blastocyst complementation assay.9,10,11 Namely, only certain murine iPSC lines are capable of generating “all-iPSC” mice upon injection into tetraploid blastocysts. Yet because embryo-based assays of pluripotency are not feasible using human cell types, the need for a better understanding of the molecular surrogates of pluripotency has become critical to our comprehension of these seemingly random variations in developmental potential.
Table 1. Comparisons of iPSCs with ESCs and their somatic cells of origin.
Microarray-based analysis of global gene expression profiles has been an invaluable tool in the characterization of the transcriptional state of cells and the identification of key differences between cell types. Although global gene expression profiles of hESCs and hiPSCs are largely similar,12 subtle differences in the expression of messenger RNAs (mRNAs)13 and micro RNAs14 have been reported. Importantly, residual transgene expression15 and genetic background16 have both been found to perturb the global gene expression profile of human pluripotent stem cells (PSCs), and such effects cannot be excluded in the above-mentioned -omic studies.13,14 Fortunately, the confounding effect of residual transgene expression can be overcome through the use of newly developed transgene-free hiPSCs. However, it bears mentioning that even transgene-free hiPSCs have displayed transcriptional differences from their hESC counterparts in one report.17 On the other hand, controlling for genetic background by comparing genetically matched hESCs and hiPSCs has not yet been undertaken. However, genetically matched comparison of murine iPSCs and ESCs recently revealed consistent differences in the expression of the Dlk1-Dio3 imprinted gene cluster.18,19 Specifically, expression of the Dlk1-Dio3 locus served as a marker of “fully pluripotent” murine iPSC lines that were capable of forming viable “all-iPSC” offspring in the tetraploid blastocyst complementation assay. Validation of this imprinted region as a potential marker for “full pluripotency” across hiPSC lines is ongoing.
Some of the differences between hiPSCs and hESCs appear to be related to the hiPSC's somatic cell of origin in the form of an “epigenetic memory,” a term that refers to persisting epigenetic marks from the cell type of origin in the resulting hiPSC that continue to affect gene expression. Gene expression differences indicative of an epigenetic memory have been demonstrated in hiPSCs derived from fibroblasts, adipose tissue, and keratinocytes20 as well as in murine iPSCs.21,22 Notably, continuous passaging22 or treatment with chromatin-modifying drugs21 seems to abrogate transcriptional differences attributable to epigenetic memory in murine iPSCs, indicating that this phenomenon may affect differentiation propensity only transiently.
A recent comparison of the DNA methylome in hiPSCs versus hESCs at single-base resolution revealed further insight into the epigenomics of reprogramming.23 Predominantly, DNA methylation patterns between hiPSCs and hESCs were similar, but differentially methylated regions were identified. Approximately 45% of these differentially methylated regions were attributed to a failure to reprogram the somatic cell epigenome (epigenetic memory), whereas ~55% were found to be specific to hiPSCs (not found in the somatic cell of origin or in hESCs). Their results suggest that aberrant methylation patterns dissimilar to the start and end points of reprogramming are frequently generated in susceptible “hotspot” regions of the genome. However, the reference standard consisted of only two to four hESC lines, which may limit the ability to generalize these results in light of the known variability among hESC lines.24 Continued study of a wider variety of hESC and hiPSC lines will be required to fully understand the appropriate range of variability and better define the “gold standard.”
To this end, the recent comprehensive characterization of a large number of hESC and hiPSC lines warrants special mention.4 Bock et al. established reference maps of variation in the transcriptome and DNA methylome of 20 representative hESC lines. By comparing 12 well-characterized hiPSC lines to this reference standard, they were able to make “deviation scorecards” and arrive at several interesting conclusions. First, although most genes exhibit similar degrees of variation in hiPSC and hESC lines, a small number of genes exhibited substantially increased deviation from the hESC reference standard in hiPSCs. Interestingly, only a very small fraction of this gene expression variation was attributable to epigenetic memory of the somatic cell of origin (fibroblasts). Second, as suggested by previous studies, an hiPSC-specific gene expression and DNA methylation signature was able to distinguish most, but not all, hiPSC lines from hESC lines. Thus, hESCs and hiPSCs can be thought of as two overlapping clouds in which some, but not all, hiPSCs can be distinguished from hESCs. However, no unique epigenetic or transcriptional deviation was found to be shared by all tested hiPSC lines. Interestingly, expression of MEG3, which has been proposed as a surrogate marker of developmental potency and is located in the aforementioned Dlk1-Dio3 imprinted region, was not found to correlate with the quality or utility of hESC or hiPSC lines for biomedical research applications such as in vitro differentiation.
In light of such variability, it seems likely that different cell lines will ultimately be best suited for different applications. Undoubtedly, the teratoma formation assay does not provide the necessary speed or detail to comprehensively predict differentiation propensity in a high-throughput fashion. To aid in the ability to prospectively identify cell lines with enhanced differentiation potential toward a particular lineage, Bock et al. created a “lineage scorecard” based on quantitative expression profiling of 500 lineage-related genes in differentiating embryoid bodies.4 Remarkably, the scorecard prediction of neural lineage differentiation propensity was highly correlated with the observed efficiency of differentiation to motor neurons (Pearson's r = 0.87). With continued validation, these assays could serve as more efficient and informative measures of a newly derived hiPSC line's pluripotent quality and differentiation potential. Streamlining the process of selecting, monitoring, and predicting the quality and utility of newly derived PSC lines is a welcome advance to the field.
Despite the excellent performance of the lineage scorecard in predicting neural differentiation propensity, several potential confounding factors warrant mention. Practically speaking, the lineage scorecard assay was demonstrated using fluorescent mRNA counting technology that is not yet widely available.25 Next, heterogeneity among single cells in hESC populations in vitro has previously been shown to underlie important cell fate decisions, with initial evidence suggesting an increased degree of heterogeneity among single hiPSCs than among single hESCs.2 To what degree could heterogeneity in the cell population contribute to observed variations in yield of differentiated progeny? Also, correlation of lineage scorecard predictions between biological replicates performed by two different researchers in different labs was modest (Pearson's r = 0.59). In light of these results, to what extent are the observed differences between cell lines attributable to experimental variables such as physical handling, media renewal, or passage number? Finally, the 12 hiPSC lines used in the study did not vary in terms of the originating somatic cell type (fibroblasts) or the reprogramming technique (retroviral transgenesis).26 Because the influence of such parameters on differentiation propensity has been demonstrated in alternative settings,15,20 future studies may warrant inclusion of transgene-free hiPSCs derived from a greater variety of somatic cell types.
In light of the aforementioned studies, it seems that a complex cell state such as pluripotency may not be adequately characterized by the assessment of a half-dozen molecular markers. Although initial comparisons on a global scale revealed considerable similarity between hESCs and hiPSCs, closer inspection at finer resolution reveals differences, for instance, at the single-base-pair23 or single-cell2 level. If related cell types are thought of as analogous to siblings, hESCs and hiPSCs can perhaps be thought of as twins—but are they fraternal or identical? Fraternal twins often look considerably alike. Identical twins are much more difficult to distinguish from each other, but there are appreciable differences upon closer inspection. For hESCs and hiPSCs, whether these differences are functionally consequential or simply related to the scale of analysis remains largely unknown. Perhaps the epigenetic marks that set hiPSCs apart from hESCs carry an unfairly negative connotation, because epigenetic memory can be used judiciously to bias hiPSCs toward a cell fate of interest. An investigator desiring large quantities of blood cells, for example, may opt to use blood-derived hiPSCs so as to enhance yield for this application.21 The ability to prime PSCs selectively toward the desired cell lineage—using epigenetic memory, cytokines, genetic modification, small molecules, or otherwise—may therefore occupy increasing interest in the future. Undoubtedly, monitoring the effect of any perturbation on the global hiPSC transcriptome and epigenome in a high-throughput fashion will aid in our ability to derive PSC lines that faithfully serve their intended purpose.
Acknowledgments
We acknowledge funding support from the National Institutes of Health (NIH DP2OD004437, RC1AG036142, and R01AI085575), the Edward Mallinckrodt Jr Foundation (J.C.W.), and HHMI (K.H.N.). Because of space limitations, we were unable to cite all the important papers relevant to induced pluripotent stem cell biology; we apologize to investigators not mentioned here who have made significant contributions to this field.
REFERENCES
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsinh KH, Sun N, Sanchez-Freire V, Lee AS, Almeida P, Hu S, et al. J Clin Invest. e-pub ahead of print 7 February 2011; 2011. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Lu SJ, Klimanskaya I, Gomes I, Kim D, Chung Y, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, et al. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- Kang L, Wang J, Zhang Y, Kou Z., and, Gao S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 2009;5:135–138. doi: 10.1016/j.stem.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Frampton GM, Soldner F, Hockemeyer D, Mitalipova M, Jaenisch R, et al. Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell. 2010;7:249–257. doi: 10.1016/j.stem.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KD, Venkatasubrahmanyam S, Jia F, Sun N, Butte AJ., and, Wu JC. MicroRNA profiling of human-induced pluripotent stem cells. Stem Cells Dev. 2009;18:749–758. doi: 10.1089/scd.2008.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink T, Hochedlinger K, Bell G., and, Jaenisch R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc Natl Acad Sci USA. 2006;103:933–938. doi: 10.1073/pnas.0510485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MCN, Yeo GW, Kainohana O, Marsala M, Gage FH., and, Muotri AR. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One. 2009;4:e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Luo GZ, Yang W, Zhao X, Zheng Q, Lv Z, et al. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J Biol Chem. 2010;285:19483–19490. doi: 10.1074/jbc.M110.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh Z, Wilson KD, Wu Y, Hu S, Quertermous T., and, Wu JC. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One. 2010;5:e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, et al. Nature. e-pub ahead of print 2 February 2011; 2011. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegrucci C., and, Young LE. Differences between human embryonic stem cell lines. Hum Reprod Update. 2007;13:103–120. doi: 10.1093/humupd/dml041. [DOI] [PubMed] [Google Scholar]
- Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, et al. Nat Biotechnol. e-pub ahead of print 3 February 2011; 2011. A functionally characterized test set of human induced pluripotent stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AM., and, Cooper JB. Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell. 2010;7:258–262. doi: 10.1016/j.stem.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]