Impact of the U.S. Food and Drug Administration Cardiovascular Assessment Requirements on the Development of Novel Antidiabetes Drugs (original) (raw)
Lingering questions related to cardiovascular (CV) safety of type 2 diabetes treatments resulted in new U.S. Food and Drug Administration (FDA) regulations requiring careful assessment of CV risk. These new requirements will provide the medical community with robust data to estimate CV risk associated with new therapeutic agents. To meet these requirements, phase 2 and 3 development programs will need to be larger and more comprehensive and will include high-risk patients. In addition, it is likely that most (if not all) newly approved drugs will be required to conduct post-approval CV safety outcome studies. The purpose of this article is to review the drivers for the new FDA requirements and how these new requirements will affect the development of novel antidiabetes drugs.
The goals of antidiabetes treatment are to forestall the metabolic effects of high glucose levels and to prevent microvascular and macrovascular complications. Compelling data in type 2 diabetic patients support the conclusion that improved long-term glycemic control reduces the risk of microvascular complications (1,2). Based on several large outcome studies (e.g., the Diabetes Control Complications Trial and the UK Prospective Diabetes Study [UKPDS]), glycosylated hemoglobin (HbA1c) was established as a surrogate biomarker of glycemic control and therapeutic goals were set accordingly (3).
Cardiovascular disease is the leading cause of death in patients with type 2 diabetes; more than 60% die of CV disease, and an even greater proportion have serious CV-related complications. Diabetes is associated with a two- to fourfold increase in the risk of coronary heart disease and death (4). Patients with type 2 diabetes who have not had a myocardial infarction (MI) have a risk of infarction similar to that of nondiabetic patients who have had a prior MI (5–7). Pooled data from patients with acute coronary syndrome (ACS) in 11 independent Thrombolysis in Myocardial Infarction (TIMI) study group clinical trials from 1997 to 2006 suggest that, despite modern therapies for ACS, diabetes confers a significant adverse prognosis, with mortality rates of 7.2–8% during the first year after an event (8). Thus, while microvascular complications can lead to significant morbidity and premature mortality, the greatest cause of death in people with diabetes is by far CV disease (9).
The ability of glucose lowering to alter CV outcome is not as clear as its ability to reduce microvascular complications. The UKPDS (2) demonstrated a nonsignificant 16% reduction in CV complications (combined fatal or nonfatal MI and sudden death) with intensive glycemic treatment. In an analysis of the study cohort, a continuous association was noted such that, for every percentage point of median HbA1c lowering, there was a statistically significant 18% reduction in CV disease events, with no glycemic threshold (9). Long-term follow-up demonstrated a significant 15% reduction in CV disease among patients in the intensive glycemic treatment group (10). The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study, which included 1,441 patients with type 1 diabetes, demonstrated a significant reduction in CV events (57%) in the intensively treated group after >17 years of follow-up (11).
Based on the proven correlation between HbA1c and microvascular complications and lack of clear CV benefits, most clinical development programs for novel antidiabetes drugs have focused on glucose lowering. As a consequence, most patients recruited to confirmatory studies required for regulatory approval have had limited duration of diabetes and few complications. Although these types of confirmatory studies established the glucose-lowering properties of novel drugs, CV safety assessment in the context of the clinical development of glucose-lowering agents has been limited.
Cardiovascular safety concerns have been raised with respect to several antidiabetes compounds approved or under development for the treatment of type 2 diabetes. In July 2008, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee met to discuss the role of CV assessment in the premarketing and postmarketing settings. The FDA determined that concerns about CV risk should be more thoroughly addressed during drug development; their newly issued guidelines will result in profound changes in the ways new antidiabetes drugs are evaluated and brought to market in the future (12).
GLUCOSE LOWERING AND CV DISEASE RISK REDUCTION
The University Group Diabetes Program, launched in 1960, was an early placebo-controlled multicenter clinical trial devised to determine which, if any, of the treatments for type 2 diabetes was efficacious in reducing CV risk (13). Patients treated with tolbutamide, a first-generation sulfonylurea drug, had a significantly higher rate of CV death than patients given placebo or insulin. Resulting concerns over potential sulfonylurea-related cardiotoxicity led to additional studies that both supported and conflicted with this finding.
One study to examine this question was the UKPDS (2,14,15). Although glucose lowering, with sulfonylureas or insulin, was associated with a reduction in the development and/or progression of retinopathy, nephropathy, and possibly neuropathy, the improvements in CV complications were not statistically significant. In a substudy of 342 overweight patients, treatment with metformin was associated with risk reductions for several CV end points compared with the conventional treatment group as follows: MI (−39%), any diabetes-related end point (−32%), diabetes-related death (−42%), and all-cause mortality (−36%). However, early addition of metformin therapy for patients not achieving glycemic targets with sulfonylurea treatment was associated with an increased risk of diabetes-related death compared with continued sulfonylurea treatment alone (2). Meta-analysis raised the suspicion that metformin/sulfonylurea combination therapy may be deleterious to the heart (16).
Several large outcome studies demonstrated no reduction in CV events with intensive glycemic control (9,17,18). In addition, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study terminated its glycemic control arm when increased mortality was observed among participants randomized to the intensive glycemic control treatment arm (target HbA1c <6%) (9,19). In the ACCORD study, 80% of patients were intensively treated with insulin. Recently, a retrospective study that included tens of thousands of patients who were followed after starting insulin treatment (as an add-on to oral monotherapy) showed a U-shaped correlation between HbA1c values and mortality and CV events. Compared with oral combination agents, there was a 50% increase in mortality in insulin-treated patients. This result suggested the possibility that insulin treatment in certain circumstances may be deleterious to the heart (20). However, the interpretation of the study results is limited, since this was a retrospective study; therefore, large prospective studies examining the correlation between insulin therapy and CV events are needed.
Although the primary end point (reduction in a composite of all-cause mortality, nonfatal MI [including silent MI], stroke, ACS, endovascular or surgical intervention in the coronary or leg arteries, and amputation above the ankle) was not met in the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) study, pioglitazone treatment did result in significant risk reduction in major adverse CV event composite secondary end points (21). Consistent with the reported side effect profile for pioglitazone, there was an increased rate of edema and heart failure observed in that trial (22).
In 2009, the Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) study found that there was no increase in CV hospitalization or death with rosiglitazone added to metformin or a sulfonylurea, compared with the combination of metformin and a sulfonylurea, but the rate of heart failure leading to hospital admission or death was significantly increased (23). In addition, a meta-analysis of rosiglitazone treatment suggested an increased CV risk with this therapy (24).
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial was designed to test treatment strategies for patients with coronary artery disease and diabetes (25). Overall, there was no significant difference in the rates of death or major CV events between patients undergoing prompt revascularization (either coronary artery bypass grafting or percutaneous coronary intervention) and patients undergoing medical therapy or between strategies of insulin sensitization and insulin provision.
The aim of the Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus (HEART2D) study was to demonstrate a difference between two insulin strategies—one targeting postprandial hyperglycemia and the other targeting fasting and premeal hyperglycemia—on time to first CV event in survivors of acute MI (26). There was no difference in CV event rates between the two strategies.
Can CV safety biomarkers predict CV outcome?
The uncertainly regarding CV safety raises questions of the reliability of CV biomarkers as predictors of clinical events. Several examples suggest that even treatments with positive effects on CV safety biomarkers may not ultimately lead to reduced CV risk:
- A significant decrease in common carotid intima-media thickness progression in nondiabetic coronary artery disease patients was observed after treatment for 48 weeks with rosiglitazone (27). However, as discussed, meta-analysis of the CV events observed in the clinical studies raised questions as to the safety of this agent (24).
- Muraglitazar, a dual (α/γ) peroxisome proliferator–activated receptor (PPAR) activator in the glitazar class that activates PPAR-α and -γ, was shown to improve hyperglycemia and lipid abnormalities (i.e., reduce triglycerides and increase HDL cholesterol levels) simultaneously (28). However, meta-analysis of the CV events observed in the clinical studies (29) suggested an increased risk of CV disease, leading to nonapproval of this investigational agent.
- Torcetrapib, an inhibitor of cholesterylester transfer protein (CETP), has been shown to increase HDL cholesterol by 60–100% and to lower LDL cholesterol by up to 20% (30). However, a large outcome study demonstrated that torcetrapib therapy resulted in an increased risk of mortality and morbidity (30).
- Sibutramine treatment is associated with a decrease in body weight and waist circumference as well as reduction in fasting blood glucose and HbA1c. Treatment benefits were seen in plasma triglycerides and HDL, without significant variations in serum total or LDL cholesterol (31). However, results from the Sibutramine Cardiovascular Outcomes Trial (SCOUT), which was a randomized double-blind comparison of sibutramine versus placebo, in addition to standard care for weight management in overweight/obese subjects who are at increased risk of a CV event, suggested risk associated with this agent. The preliminary results led to taking sibutramine off the European market.
New regulatory FDA requirements
CV safety concerns have been raised with several antidiabetes compounds (most notably with agonists of the PPAR class) that were approved or under development for the treatment of type 2 diabetes (24,29).
In July 2008, the Endocrinologic and Metabolic Drugs Advisory Committee of the FDA met to discuss CV risk with oral antidiabetes agents and the role of risk assessment in the premarketing and postmarketing setting. After considering the discussion at this meeting, as well as other available data and information, the FDA determined that effects on CV risk should be more thoroughly addressed during antidiabetes agent development (12).
The resulting FDA guidance document identifies several key areas that will need to be addressed by study sponsors:
- An upper bound of the 95% CI for the risk ratio of important CV events of <1.3 should be used as a key criterion for excluding unacceptable CV risk for new treatments of type 2 diabetes.
- Study patients must include individuals with relatively advanced disease, elderly patients, and patients with some degree of renal impairment.
- A minimum of 2 years’ CV safety data must be provided.
- All phase 2 and 3 studies should include a prospective independent adjudication of CV events. Adjudicated events should include CV mortality, MI, and stroke and can include hospitalization for ACS, urgent revascularization procedures, and possibly other end points.
- To satisfy the new statistical guidelines, the analysis of CV events may include a meta-analysis of all placebo-controlled trials, add-on trials (i.e., drug vs. placebo, each added to standard therapy), and active-controlled trials, and/or an additional single, large safety trial may be conducted that alone, or added to other trials, would be able to satisfy this upper bound before a new drug application/biologics license application (NDA/BLA) is approved.
Consequences of the new guidelines
The new guidelines will result in profound changes to the way that novel antidiabetes drugs are developed:
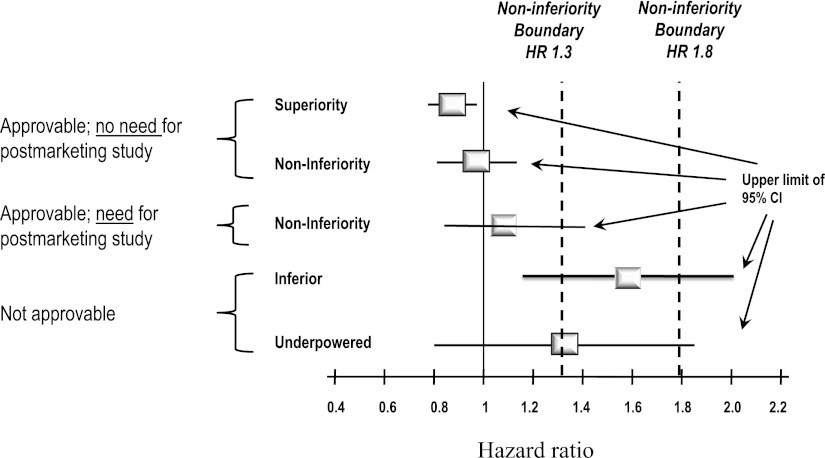
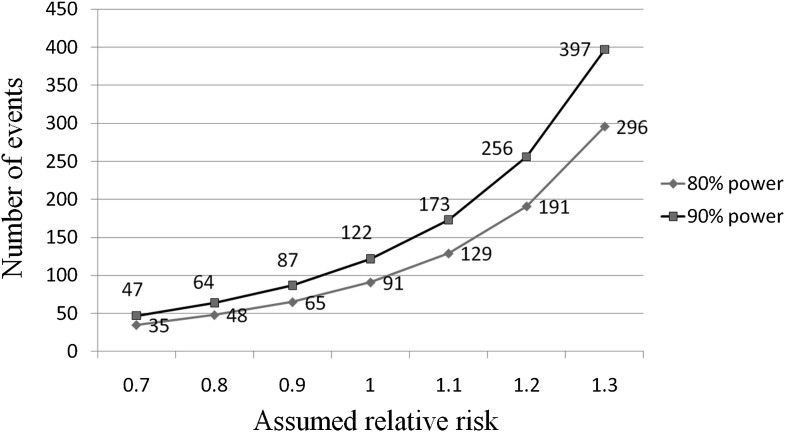
- The most important change is the need to establish lack of CV toxicity. The FDA guidelines provide statistical hurdles for approval. If the premarketing application contains clinical data showing that the upper bound of the two-sided 95% CI for the estimated increased risk (i.e., risk ratio) is between 1.3 and 1.8, and the overall risk-benefit analysis supports approval, a postmarketing trial generally will be necessary to definitively demonstrate that the upper bound of the two-sided 95% CI for the estimated risk ratio is <1.3. If the premarketing application contains clinical data showing that the upper bound of the two-sided 95% CI for the estimated increased risk (i.e., risk ratio) is <1.3 and the overall risk-benefit analysis supports approval, a postmarketing CV trial may not be necessary (Fig. 1). The implications of this new stipulation are significant; to reach the initial approvability bar of 1.8, a clinical development program will need to prospectively target 100–140 CV events (Fig. 2). To achieve the more stringent test of <1.3, a development program will need ∼600–700 events. Recently approved antidiabetes drugs (i.e., saxagliptin, liraglutide), in contrast, had ∼40 major CV events each, even though >5,000 subjects participated in the development program (saxagliptin). Thus, programs will need to enroll greater numbers of patients and will need to shift focus to include more high-risk CV patients, a fact that will result in far more complex and expensive development plans.
- Patient selection will also change. Until recently, many clinical development programs concentrated on demonstrating an effect on the surrogate marker HbA1c. To register and obtain a product label that includes monotherapy and add-on therapy, most recruited subjects need have only limited diabetes duration (a few years), and most were treated with a single background antidiabetes medication. These programs limited or excluded obviously high-risk and complicated patients. Such programs were criticized for the limited information they provided, and their applicability to larger patient populations was questioned (32). The new guidelines mandate a shift in patient selection. To demonstrate that the drug is safe in patients with higher CV risk and to allow for meaningful estimates of CV risk, phase 2 and 3 programs will need to include patients at higher CV risk, i.e., patients with relatively advanced disease, elderly patients, and patients with some degree of renal impairment.
- Whereas prior development programs collected data on cardiac adverse events, these events were not independently adjudicated. Adjudication is a process by which an independent CV research group reviews each presumed CV event and makes a decision whether the event truly represents a cardiac event. This process arguably provides a higher level of confidence in the diagnosis. The new guidelines require sponsors to establish an independent CV end point committee to prospectively adjudicate, in a blinded fashion, CV events during all phase 2 and 3 clinical trials. Adjudicated events should include CV mortality, MI, and stroke and can include hospitalization for ACS, urgent revascularization procedures, and possibly other end points.
- The FDA’s prior guidelines at the time of submission of the marketing application for products intended for the treatment of type 2 diabetes required that phase 2 and 3 trial data be available for at least 2,500 subjects exposed to the investigational product, with at least 1,300–1,500 of these subjects exposed to the investigational product for ≥1 year and at least 300–500 subjects exposed to the investigational product for ≥18 months. The new guidelines require longer controlled trials (e.g., minimum of 2 years) to obtain enough events and to provide data on longer-term CV risk for these chronically used therapies. This requirement obviously will translate into much larger and longer phase 3 clinical development programs.
Figure 1.
FDA CV safety: CI bars. The FDA guidelines provide statistical hurdles for approval. Five hypothetical examples of possible hazard ratios and the upper limit of the 95% CI of a development plan are shown as well as the regulatory consequences of each outcome.
Figure 2.
Total CV events needed to fall below the FDA target cutoff of 1.8. Assuming a novel antidiabetes drug is neutral in terms of CV disease, a program should accrue ~120 CV events to provide adequate power to meet the FDA requirement.
Current antidiabetes CV outcome studies landscape
Preregistration trials.
To provide the required CV safety data, several pharmaceutical companies are now conducting CV outcome studies as part of their phase 3 development plans (Table 1). Examples of such studies are included below.
Table 1.
Examples of ongoing pre- and postapproval outcome studies
| Trial name | Drug | Primary endpoint | Number of subjects (years) |
|---|---|---|---|
| EXAMINE | alogliptin | MACE | 5,400 (5) |
| CANVAS | canagliflozin | MACE | 4,500 (4) |
| T-emerge 8 | taspoglutide | CV events | 2,000 (2.5) |
| ALECARDIO | aleglitazar | Superiority: MACE | 6,000 ACS (4.5) |
| TECOS | sitagliptin | Noninferiority: MACE + unstable angina | 14,000 (5) |
| SAVOR | saxagliptin | Superiority: MACE | 12,000 (5) |
| EXSCEL | exenatide LAR | Superiority: MACE | 12,000 (5.5) |
| LEADER | liraglutide | MACE | 9,000 (5) |
The Canagliflozin Cardiovascular Assessment Study (CANVAS) will assess canagliflozin in the treatment of patients with type 2 diabetes, with regard to CV risk for major adverse cardiac events. The study will evaluate canagliflozin compared with placebo on CV events, including CV death, heart attack, and stroke in patients with type 2 diabetes, whose diabetes is not well controlled at the beginning of the study and who have a history of CV events or are at high risk for CV events. Patients will receive capsules of canagliflozin (either 100 or 300 mg) or matching placebo; the study duration is estimated to be ~4 years (33).
The EXAMINE study (Examination of Cardiovascular Outcomes: Alogliptin vs. Standard of Care in Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndrome) was designed to evaluate the CV safety of alogliptin versus placebo in addition to standard care in subjects with type 2 diabetes and ACS. The study plans to enroll 5,400 subjects and last for ~4.75 years (34).
Postregistration trials.
Saxagliptin (Onglyza) was the first antidiabetes agent to receive FDA approval after issuance of the new CV guidelines and thus represents a good example of the impact of the new regulations. Saxagliptin was approved by the FDA in July 2009. One of the postmarketing requirements was to demonstrate lack of CV toxicity. The Saxagliptin Assessment of Vascular Outcomes Recorded (SAVOR-TIMI 53) study is thus the first example of a post-approval commitment under the new guidance (35). SAVOR is a multicenter randomized double-blind placebo-controlled phase 4 study that evaluates treatment with saxagliptin, a dipeptidyl peptidase-4 inhibitor, in adult type 2 diabetic patients with CV risk factors. This 5-year study will follow ~12,000 patients with type 2 diabetes, who have either a history of previous CV events or multiple risk factors for vascular disease, and will include patients with renal impairment.
Pooled analysis of all eight registrational trials comprising the saxagliptin clinical program with 4,607 patients (5,051 patient-years) and multiple comparators suggested no increased CV risk with saxagliptin treatment. In fact, although this systematic overview had inherent and important limitations, the data support a potential reduction in CV events with saxagliptin (36). Based on this hypothesis-generating data, the objectives of the SAVOR-TIMI 53 trial are to test the hypothesis of whether treatment with 2.5 mg or 5 mg saxagliptin compared with placebo when added to a patient’s current standard care will result in a reduction in the composite end point of CV death, nonfatal MI, or nonfatal ischemic stroke and exclude unacceptable CV toxicity.
A second example of the impact of the new guidelines is liraglutide. The clinical development program for liraglutide, as was the case for saxagliptin, was completed before issuance of the FDA guidance, but analyses of CV events in the phase 2 and 3 trials of liraglutide showed that this drug met the standard for ruling out unacceptably increased CV risk. The overall rates of CV events in the preapproval clinical trials were low, however, and the more stringent criteria outlined for postapproval evaluations were not met; the FDA is therefore requiring a post-approval study of CV safety (37).
CONCLUSIONS
Lingering questions related to CV safety of type 2 diabetes treatments resulted in new FDA regulations to carefully assess CV risk. The new requirements will provide the medical community with robust data to estimate CV risk associated with new therapeutic agents. To meet these requirements, phase 2 and 3 clinical trial programs will be larger and more comprehensive and will include high-risk patients. In addition, sponsors of most, if not all, newly approved drugs will be required to conduct post-approval CV safety outcome studies. Several questions remain:
- Will the time, money, and resources channeled to address a “theoretical CV risk” for a new drug limit the assessment of drug-specific issues and benefits?
- How will the medical community and health authorities assess the CV risk of generic drugs that do not have the CV safety data that will be generated by novel antidiabetes agents?
- Potential time, cost, and risk implications may limit incentives for companies to develop new antidiabetes therapies. The result may be development of fewer antidiabetes drugs and fewer companies capable of developing these drugs.
Acknowledgments
B.H. is an employee of AstraZeneca. I.R. is the co-principle investigator of SAVOR and has consulted for AstraZeneca and Bristol-Myers Squibb. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This publication is based on the presentations at the 3rd World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, F. Hoffmann-La Roche, Janssen-Cilag, Johnson & Johnson, Novo Nordisk, Medtronic, and Pfizer.
References
- 1.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 2007;147:149–155 [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 6.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care 2005;28:2901–2907 [DOI] [PubMed] [Google Scholar]
- 7.Schramm TK, Gislason GH, Køber L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation 2008;117:1945–1954 [DOI] [PubMed] [Google Scholar]
- 8.Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA 2007;298:765–775 [DOI] [PubMed] [Google Scholar]
- 9.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 11.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Center for Drug Evaluation and Research. Guidance for industry diabetes mellitus: evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes [Internet], 2008. Available from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf Accessed 23 November 2010
- 13.Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes 1970;19(Suppl.):789–830 [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 15.Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 16.Rao AD, Kuhadiya N, Reynolds K, Fonseca VA. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality? A meta-analysis of observational studies. Diabetes Care 2008;31:1672–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 18.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010;375:481–489 [DOI] [PubMed] [Google Scholar]
- 21.Wilcox R, Kupfer S, Erdmann E; PROactive Study investigators Effects of pioglitazone on major adverse cardiovascular events in high-risk patients with type 2 diabetes: results from PROspective pioglitAzone Clinical Trial In macro Vascular Events (PROactive 10). Am Heart J 2008;155:712–717 [DOI] [PubMed] [Google Scholar]
- 22.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 23.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125–2135 [DOI] [PubMed] [Google Scholar]
- 24.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471 [DOI] [PubMed] [Google Scholar]
- 25.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009;32:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidhu JS, Kaposzta Z, Markus HS, Kaski JC. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol 2004;24:930–934 [DOI] [PubMed] [Google Scholar]
- 28.Kendall DM, Rubin CJ, Mohideen P, et al. Improvement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (alpha/gamma) peroxisome proliferator-activated receptor activator, in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a double-blind, randomized, pioglitazone-comparative study. Diabetes Care 2006;29:1016–1023 [DOI] [PubMed] [Google Scholar]
- 29.Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005;294:2581–2586 [DOI] [PubMed] [Google Scholar]
- 30.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–2122 [DOI] [PubMed] [Google Scholar]
- 31.Vettor R, Serra R, Fabris R, Pagano C, Federspil G. Effect of sibutramine on weight management and metabolic control in type 2 diabetes: a meta-analysis of clinical studies. Diabetes Care 2005;28:942–949 [DOI] [PubMed] [Google Scholar]
- 32.Nathan DM. Finding new treatments for diabetes: how many, how fast...how good? N Engl J Med 2007;356:437–440 [DOI] [PubMed] [Google Scholar]
- 33.Johnson & Johnson Pharmaceutical Research & Development. CANVAS: CANagliflozin cardioVascular assessment study [Internet], 2010. Available from http://www.jnj.com/connect/news/all/Johnson-Johnson-R-D-Reports-Phase-2b-Clinical-Trial-Results-Evaluating-Canagliflozin Accessed 23 November 2010
- 34.Takeda Global Research and Development Center. Cardiovascular outcomes study of alogliptin in subjects with type 2 diabetes and acute coronary syndrome (EXAMINE) [Internet], 2010. Available from http://www.takeda.com/press/article_34996.html Accessed 23 November 2010
- 35.AstraZeneca. Does saxagliptin reduce the risk of cardiovascular events when used alone or added to other diabetes medications (SAVOR-TIMI 53) [Internet], 2010. Available from http://clinicaltrials.gov/ct2/show/NCT01107886 Accessed 23 November 2010
- 36.Frederich R, Alexander JH, Fiedorek FT, et al. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med 2010;122:16–27 [DOI] [PubMed] [Google Scholar]
- 37.Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide: the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010;362:774–777 [DOI] [PubMed] [Google Scholar]