Functional CD47/signal regulatory protein alpha (SIRPα) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo (original) (raw)
Abstract
The homeostatic control mechanisms regulating human leukocyte numbers are poorly understood. Here, we assessed the role of phagocytes in this process using human immune system (HIS) BALB/c Rag2−/−IL-2Rγc−/− mice in which human leukocytes are generated from transplanted hematopoietic progenitor cells. Interactions between signal regulatory protein alpha (SIRPα; expressed on phagocytes) and CD47 (expressed on hematopoietic cells) negatively regulate phagocyte activity of macrophages and other phagocytic cells. We previously showed that B cells develop and survive robustly in HIS mice, whereas T and natural killer (NK) cells survive poorly. Because human CD47 does not interact with BALB/c mouse SIRPα, we introduced functional CD47/SIRPα interactions in HIS mice by transducing mouse CD47 into human progenitor cells. Here, we show that this procedure resulted in a dramatic and selective improvement of progenitor cell engraftment and human T- and NK-cell homeostasis in HIS mouse peripheral lymphoid organs. The amount of engrafted human B cells also increased but much less than that of T and NK cells, and total plasma IgM and IgG concentrations increased 68- and 35-fold, respectively. Whereas T cells exhibit an activated/memory phenotype in the absence of functional CD47/SIRPα interactions, human T cells accumulated as CD4+ or CD8+ single-positive, naive, resting T cells in the presence of functional CD47/SIRPα interactions. Thus, in addition to signals mediated by T cell receptor (TCR)/MHC and/or IL/IL receptor interactions, sensing of cell surface CD47 expression by phagocyte SIRPα is a critical determinant of T- and NK-cell homeostasis under steady-state conditions in vivo.
Keywords: hematopoiesis, humanized mice, leukocyte homeostasis
Homeostasis of hematopoietic cells depends on molecular signals provided by extracellular factors and cell–cell interactions with both hematopoietic cells and nonhematopoietic components. The dependency of lymphocytes on such signals has been intensely studied in mice, and several factors regulating lymphocyte numbers in steady-state conditions have been identified (1–3). Because of ethical and practical reasons, experimental exploration of human leukocyte homeostatic mechanisms in vivo is difficult. To address this topic, mouse models with components of the human immune system (HIS) have been generated by injecting human hematopoietic progenitor cells (hHPC) into newborn immunodeficient mice (4–6).
HIS mice generated in BALB/c Rag2−/−IL-2Rγc−/− newborns support multilineage human hematopoietic development, but human T- and natural killer- (NK) cell homeostasis remains suboptimal in this model (7, 8). Signs of disturbed human T-cell homeostasis include premature thymus aging, low T-cell frequency in peripheral lymphoid organs, high peripheral T-cell turnover, and high variability in naive T-cell proportion (7–9). HIS mice complemented with human IL-7 (10–12) or human MHC molecules (13) (i.e., two major factors controlling naive T-cell homeostasis) exhibit enhanced human T-cell numbers but only to a limited extent. Similarly, inoculation of human IL-15/IL-15Rα into HIS mice leads to improved but still suboptimal NK-cell accumulation (14). HIS mice generated in adult BALB/c Rag2−/−IL-2Rγc−/− mice, which contain more phagocytes than newborn animals, only show limited accumulation of human B cells (15). In contrast, HIS mice generated in immunodeficient mice based on the nonobese diabetic (NOD) genetic background, known for its defective phagocyte activity (4, 16), exhibit a large proportion of human T and NK cells in their lymphoid organs (10, 17, 18). Overall, these observations strongly suggest that phagocyte activity plays a role in T- and NK-cell number regulation.
Phagocyte activity is inhibited by interactions between CD47 and signal regulatory protein alpha (SIRPα; CD172a) (19). CD47, a ubiquitously expressed Ig-like membrane protein, can interact with several other cell surface receptors (e.g., thrombospondin, integrins, and other members of the SIRP family) (20). These receptors can interact with CD47 in cis or in trans, and these interactions are described as a two-way exchange of information (21). The resulting complexity of CD47 biology may explain its broad—but not fully elucidated—role in hematopoietic and immune processes. The expression pattern of SIRPα is restricted to neuronal and hematopoietic lineages, with a remarkable bias to immune cells exhibiting a phagocytic activity (e.g., macrophages, granulocytes, or dendritic cells) (19). CD47null mice are viable and apparently healthy; however, phenotypic differences with their CD47+ littermates are revealed in a pathogenic setting, because CD47−/− mice are unable to control Escherichia coli bacterial infection (22). CD47-deficient erythrocytes injected into CD47+ mice are rapidly cleared from the blood circulation by phagocytes of the recipient, suggesting that CD47 ligation is a critical discriminator of self (23). Similarly, experimental settings making use of bone marrow chimeras have shown that phagocyte tolerance to CD47−/− leukocytes is only observed in absence of CD47 expression on nonhematopoietic cells (24). These observations highlight that CD47/SIRPα interactions are a major determinant of escape from phagocyte-mediated cell clearance.
Based on this set of observations, we investigated in HIS mice whether CD47/SIRPα interactions would function as a mechanism of immune surveillance regulating human leukocyte numbers in lymphoid organs. It was previously shown that human hematopoietic reconstitution of HIS mice is improved when proper CD47/SIRPα interactions take place (25), but the relative in vivo impact on the diverse human leukocyte subsets was not analyzed. Here, we show that in vivo homeostasis of human T and NK cells is particularly sensitive to sensing of correct CD47 expression on cell surface (i.e., phagocytes selectively participate to their homeostatic control in steady-state conditions).
Results
Enforced Expression of Mouse CD47 by Human Cells Improves Human Xeno-Engraftment in Vivo.
To assess the role of CD47 in human hematopoietic cell maintenance in vivo, we made use of a humanized mouse model of the hemato-lymphoid cellular components (4–6). We and others have already shown that transplantation of hHPC into BALB/c Rag2−/−IL-2Rγc−/− newborn mice leads to multilineage human hematopoietic reconstitution (7, 8, 14). The human cells found in BALB-HIS mice cohabit with components of the mouse immune system (e.g., macrophages and dendritic cells). Most laboratory mouse strains, including BALB/c, express an allele of the SIRPα molecule that does not properly bind to human CD47 (25, 26). We, therefore, tested whether human hematopoietic cells with enforced mouse CD47 (mCD47) expression would exhibit improved survival in the BALB-HIS mice. Before transplantation, we transduced the hHPC either with the control or the mCD47-expressing pHEF lentiviral vector (Fig. S1_A_). In mCD47/BALB-HIS mice, mCD47 expression on human cells was restricted to the transduced cells (Fig. S1_B_).
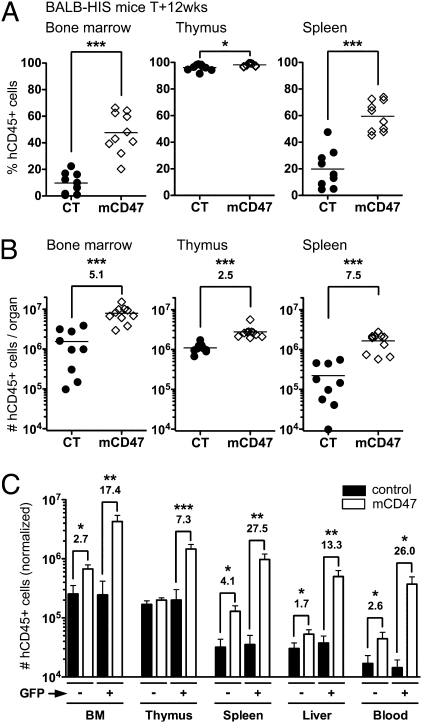
Twelve weeks after mCD47-hHPC transplantation, the frequency (Fig. 1_A_ and Fig. S2_A_) and number (Fig. 1_B_ and Fig. S2_B_) of human cells harvested from mCD47/BALB-HIS mice were enhanced in all lymphoid organs (2.5- to 7.5-fold more). We compared the proportion of GFP+ cells obtained in vitro on hHPC after transduction and in the reconstituted HIS mice (Fig. S2_C_). The improved human graft maintenance correlated with a selective advantage for mCD47-expressing human cells (Fig. S2_D_). No GFP+ cell accumulation was observed in the control group (27). We next analyzed the relative contribution of GFP− and GFP+ cells to human hematopoiesis in mCD47/BALB-HIS mice (Fig. 1_C_). In control BALB-HIS mice, both GFP− and GFP+ hHPC generated a similar amount of human cells, indicating that the lentiviral transduction did not induce any bias (27). In contrast, GFP+ hHPC expressing mCD47 systematically generated higher numbers of human cells (7.3- to 27.5-fold more) (Fig. 1_C_).
Fig. 1.
Human cell engraftment in BALB-HIS mice using mCD47-expressing hHPC. Human cell engraftment in 12-wk-old control (n = 9) and mCD47/BALB-HIS mice (n = 10). (A) Frequency and (B) total number of human cells harvested from BALB-HIS mice generated either with control- or mCD47-transduced hHPC (horizontal bar is the mean value). (C) Number of hCD45+ cells generated in vivo from a normalized amount (10,000) of GFP− or GFP+ hHPC (mean + SEM). When statistically significant, the increase in cell numbers observed in mCD47/BALB-HIS mice is indicated (fold increase over control). BM, bone marrow.
Human hematopoietic reconstitution of C57BL/6 Rag2−/−IL-2Rγc−/− animals has never been efficiently achieved (4–6). This lack of achievement is potentially because of the high activity of the murine myeloid cells in this genetic background (4). To test whether mCD47-enforced expression can bypass this apparent roadblock, we generated mCD47/B6-HIS mice with newborn C57BL/6 Rag2−/−IL-2Rγc−/− animals, and we observed a dramatic enhancement of human cell repopulation in all lymphoid organs (Fig. S3). The level of human cell accumulation in the mCD47/B6-HIS mice was comparable with what we obtained in mCD47/BALB-HIS mice (Fig. 1_C_ and Fig. S3_B_), showing that proper CD47 ligation enables optimal human cell accumulation, even in mouse recipients previously described as nonpermissive to hHPC engraftment.
Enforced mCD47 Expression Results in Improved Engraftment of hHPC in Vivo.
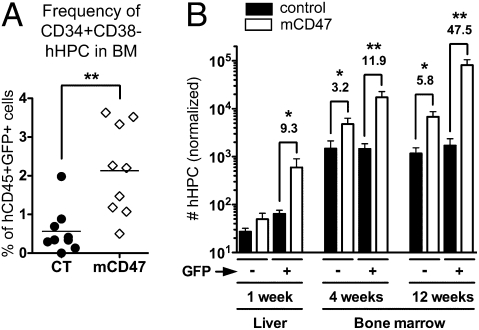
The improvement in human engraftment in mCD47/BALB-HIS mice could be caused by two nonmutually exclusive mechanisms: (i) the number of multipotent hHPC might be increased in these animals, therefore giving rise to enhanced numbers of human cells, and (ii) individual hematopoietic lineages may be conferred a specific survival advantage. To test the first possibility, we assessed whether CD34+CD38− (mostly CD117+CD133/2+) (Fig. S4_A_) hHPC engraftment capacity and long-term maintenance was enhanced by mCD47 expression. The frequency of hHPC was increased approximately fourfold in GFP+ human cells harvested from mCD47/BALB-HIS mice (Fig. 2_A_). The number of hHPC recovered from control animals was similar in both GFP− and GFP+ human cell fractions, with an ∼30-fold increase between weeks 1 and 4 after transplantation and no more accumulation after week 4 (Fig. 2_B_). A positive effect of mCD47-enforced expression on hHPC numbers was already detectable as early as 1 wk posttransplantation, with a 9.3-fold increase in GFP+ hHPC recovery (Fig. 2_B_). The number of hHPC recovered from the mCD47-expressing GFP+ cells was accumulating over time (∼30-fold between weeks 1 and 4 posttransplantation and ∼5-fold between weeks 4 and 12).
Fig. 2.
Kinetic of hHPC niche reconstitution in mCD47/BALB-HIS mice. (A) Frequency of CD34+CD38− hHPC among human GFP+ cells harvested in the bone marrow of 12-wk-old control (n = 9) and mCD47/BALB-HIS (n = 10) mice. (B) Normalized number of CD34+CD38− hHPC recovered at week 1 (liver; n = 5 control vs. n = 5 mCD47), week 4 (bone marrow; n = 8 control vs. n = 7 mCD47), and week 12 (bone marrow; n = 9 control vs. n = 10 mCD47) posttransplantation.
Overall, enforced expression of mCD47 by human cells improves short- and long-term human hHPC engraftment in humanized mice.
Enforced mCD47 Expression in hHPC Leads to Improved Human Thymopoiesis.
We next investigated whether specific hematopoietic lineages were exhibiting a selective survival advantage when expressing mCD47. We focused on T and NK cells, which are described to poorly accumulate in BALB-HIS mice (5, 6, 9). In the thymus, the frequencies of pro-T cells (CD1a+CD3−) and CD4+CD8+ double-positive (DP) immature thymocytes subsets were increased in mCD47/BALB-HIS mice, whereas the frequencies of CD4+ (SP4), CD8+ (SP8) single-positive thymocytes, and mature thymocytes (CD1a−CD3+) were reduced (Fig. S4 B–D). Enhanced thymopoiesis in mCD47/BALB-HIS mice was mostly caused by the specific accumulation of mCD47-expressing cells, because mCD47-expressing hHPC generated more pro-T cells (10.4-fold), immature DP thymocytes (6.9-fold), and mature T cells (10.6-fold) than their control GFP+ counterparts (Fig. S4_E_). The proportions of CD16−CD56+ and CD16hiCD56low thymic NK-cell subsets were similar between the two groups of animals (Fig. S4_F_). Because mCD47-transduced hHPC generated more human thymocytes (7.3-fold increase) (Fig. 1_C_), the total number of thymic NKp46+ NK cells generated from mCD47-hHPC was enhanced in a similar proportion (5.3-fold increase) (Fig. S4_F_).
Altogether, these results show that proper CD47 ligation on human cells permits to sustain active thymopoiesis over time, with global increase of thymus size, more physiological proportions of major thymocyte subsets, and enhanced numbers of immature thymocytes.
mCD47-Expressing T and NK Cells Selectively Accumulate in BALB-HIS Mice.
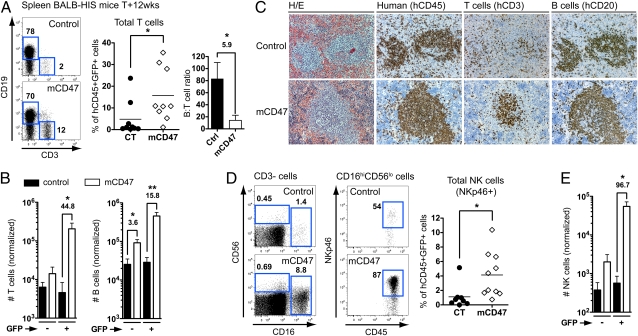
We next analyzed the accumulation of human T and NK cells in peripheral lymphoid organs. We consistently observed a preferential increase of T-cell frequency within mCD47/BALB-HIS GFP+ cells (∼16% of hCD45+GFP+ splenocytes vs. ∼5% in control animals), whereas B-cell frequency was not significantly different between the two groups, resulting in a 5.9-fold reduction in the B- to T-cell ratio (Fig. 3_A_ and Fig. S5_A_). The GFP+ mCD47-expressing hHPC fraction generated ∼45-fold more mature T cells in the spleen compared with control or nontransduced hHPC (Fig. 3_B_). Similar selective accumulation of T cells was observed in all analyzed lymphoid organs (bone marrow, liver, and blood). The number of B and myeloid cells generated by GFP+ hHPC was also increased in mCD47/BALB-HIS mouse spleens but to a lower extent (B cells: 15.8-fold increase; monocytes: 14.3-fold increase) compared with T cells (Fig. 3_B_ and Fig. S5_B_). Histological analysis of the spleen of the mCD47/BALB-HIS mice revealed an improved organization of human T cells, which formed clusters together with B-cell areas, whereas T cells were more randomly scattered inside the spleen of control animals (Fig. 3_C_). The frequency of total NKp46+ NK cells was enhanced in mCD47/BALB-HIS mice (∼4% of hCD45+GFP+ splenocytes vs. ∼1.2% in control animals), mostly because of the accumulation of CD16hiCD56loNKp46+ NK cells (∼6.6% of hCD45+CD3−GFP+ splenocytes vs. ∼1.1% in control animals, P = 0.0149) (Fig. 3_D_). Overall, mCD47-expressing hHPC generated ∼97-fold more NK cells in the spleen (Fig. 3_E_).
Fig. 3.
Accumulation of mCD47-expressing T, B, and NK cells in the spleen. Human T-, B-, and NK-cell subsets in the spleen of control (n = 8) and mCD47/BALB-HIS mice (n = 10). (A) Representative CD3 and CD19 expression on hCD45+ GFP+ splenocytes harvested from 12-wk-old mice. Graphs show frequency of T cells (CD3+) and B- to T-cell ratio among GFP+ human splenocytes. (B) Normalized number of human T and B cells (from 10,000 hHPC). (C) Histological analysis of the spleen for the presence of human hematopoietic cells (hCD45), T (hCD3), and B cells (hCD20). H/E, H&E. Pictures were obtained in successive sections from one representative animal from each group. (D) Representative CD16 and CD56 expression among hCD45+GFP+CD3− splenocytes and NKp46 expression among GFP+CD16hiCD56lo NK cells. The graph shows the frequency of total NK cells (pooled CD16−CD56+NKp46+ and CD16hiCD56loNKp46+) among hCD45+GFP+ cells. (E) Normalized number of human total NK cells (from 10,000 hHPC).
Altogether, these data show that, whereas mCD47 expression induced an accumulation of all leukocyte subsets in BALB-HIS mice, there was a selective increase of peripheral T- and NK-cell numbers, resulting in the reversal of the human B- to T-cell ratio.
mCD47-Expressing T Cells Accumulate in BALB-HIS Mice as Naïve, Resting Cells and Exhibit Improved Survival Capacity.
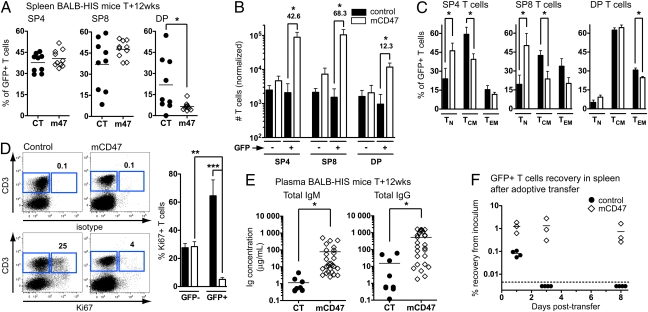
We next monitored the relative proportion of several T-cell subsets. The frequency of CD4+ and CD8+ T cells in the spleen did not significantly differ between groups, but mCD47 expression was accompanied by a marked decrease in the frequency of peripheral DP T cells (Fig. 4_A_), which may correspond to activated T cells normally found at low frequency in healthy individuals but that accumulate in inflammatory conditions (28). The mCD47-expressing hHPC generated ∼43-fold more CD4+ T cells, ∼68-fold more CD8+ T cells, and only ∼12-fold more DP T cells than their control counterparts (Fig. 4_B_). Using CCR7 and CD45RA cell surface markers (29), we observed that mCD47-expressing mature CD4+ and CD8+ T lymphocytes accumulated in lymphoid organs mostly as naive T cells (TN; CD45RA+CCR7+), whereas T cells mostly belonged to the central memory T-cell (TCM; CD45RA−CCR7+) subset in control mice (Fig. 4_C_). The frequency of effector memory T cells (TEM; CD45RA−CCR7−) did not significantly differ between the two groups (Fig. 4_C_). Furthermore, cycling (Ki67+) T cells represented only ∼4% of the mCD47-expressing GFP+ T cells in the spleen of mCD47/BALB-HIS mice, whereas a significant fraction of control GFP+ (∼65%) or nontransduced T cells (∼30%) were actively dividing (Fig. 4_D_) (9).
Fig. 4.
Phenotype and survival capacity of mCD47-expressing T cells. (A) Proportion of CD4+, CD8+, and DP cells among GFP+ spleen T cells in 12-wk-old control (n = 9) or mCD47/BALB-HIS (n = 10) mice. (B) Normalized numbers of CD4+, CD8+, and DP T cells (from 10,000 hHPC). (C) Proportion of TN, TCM, and TEM cells in the CD4+, CD8+, and DP GFP+ T cells. (D) Isotype control and Ki67 staining on splenocytes from one representative mouse from each group. The percentage of Ki67+ T cells is given. The numerical analysis is shown in the graph (n = 5 per group). (E) Plasma concentration of total human IgM and IgG in 12-wk-old control (n = 9) vs. mCD47/BALB-HIS (n = 27) mice (IgM: 1.1 ± 0.4 vs. 77.8 ± 25.6 μg/mL; IgG: 15.3 ± 7.9 vs. 529.1 ± 112.6 μg/mL). (F) Adoptive transfer of control or mCD47/BALB-HIS spleen T cells into adult, nonmanipulated BALB/c Rag2−/−IL-2Rγc−/− mice. The number of human GFP+ T cells recovered from the spleen of the recipient mice is plotted as a percentage of recovery from the initial inoculum (n = 4 control vs. n = 3 mCD47 per time point).
We assessed the functionality of human leukocyte subsets generated in mCD47/BALB-HIS mice. Improved T-cell homeostasis was accompanied by a marked accumulation of total plasma IgM and IgG in mCD47/BALB-HIS mice, with 68- and 35-fold increases, respectively, compared with control mice (Fig. 4_E_). We next tested the capacity of mCD47-expressing T cells to survive in lymphopenic-recipient mice after adoptive transfer. We inoculated human cells isolated from mCD47/BALB-HIS mice into nonmanipulated BALB/c Rag2−/−IL-2Rγc−/− mice (i.e., the same host environment). One day after mouse T-cell adoptive transfer, the recovery in the spleen typically represents 1–10% of the original inoculum (30). When injecting control BALB-HIS T cells, the D+1 recovery in the spleen was very limited (∼0.08% of the inoculum), and the cells became undetectable at D+3 (Fig. 4_F_). The initial recovery of mCD47-expressing T cells was 15-fold better (∼1.2% of the inoculum), and these T cells were maintained at a similar level for at least 8 d (Fig. 4_F_). The maintenance of mCD47-expressing T cells was accompanied by moderate T-cell division (Fig. S6_A_) and no overt phenotypic change over time (Fig. S6_B_). Culture of mCD47/BALB-HIS spleen NK cells in the presence of K562 human leukemia cells induced the surface expression of CD107a, a degranulation marker, at a level that was higher (Fig. S6_C_) or similar (14) to what was observed with control cells. Last, mCD47/BALB-HIS spleen T cells cultured with allogeneic human peripheral blood mononuclear cells extensively proliferated to a similar extent as control cells (Fig. S6_D_).
From these results, we conclude that proper CD47 ligation supports optimal T- and NK-cell homeostasis and their respective functional activities.
Engraftment of hHPC in BALB/c NOD.sirpa Rag2−/−IL-2Rγc−/− Mice.
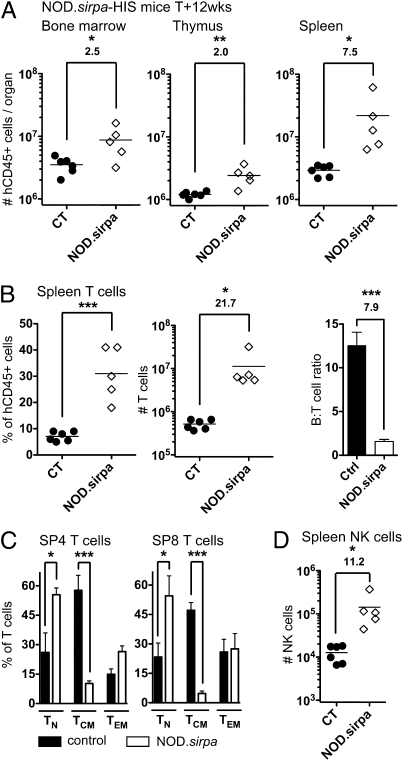
To confirm that CD47/SIRPα interactions are responsible for the aforementioned observations, we generated BALB/c Rag2−/−IL-2Rγc−/− mice congenic for the NOD.sirpa gene, which interacts with human CD47 (25). Similar to what we observed in mCD47/BALB-HIS mice, human cells harvested from NOD._sirpa_-HIS mice were increased 2.0- to 7.5-fold (Fig. 5_A_). The hHPC pool represented ∼3% of bone marrow human cells compared with ∼1% in control animals, and the number of hHPC was approximately eightfold higher in NOD-sirpa congenic mice (Fig. S7_A_). Splenic T cells accumulated ∼22-fold more in NOD._sirpa_-HIS mice than in control animals, resulting in an 7.9-fold reduction in the B- to T-cell ratio (Fig. 5_B_). As a comparison, B-cell number increased only 2.7-fold (control: 6.1 ± 0.2 × 106 vs. NOD.sirpa: 16.6 ± 6.9 × 106, P = 0.1251). NOD._sirpa_-HIS peripheral T cells mostly belonged to the TN cell subset (Fig. 5_C_). Furthermore, 11.2-fold more human NK cells were harvested in the spleen of NOD._sirpa_-HIS mice (Fig. 5_D_ and Fig. S7_B_). Although only rare mesenteric lymph nodes could be harvested from control BALB-HIS mice, various enlarged lymph nodes (e.g., mesenteric, brachial, axillary, or inguinal) could be isolated from NOD._sirpa_-HIS mice, resulting in approximately fivefold higher human cell numbers (Fig. S7_C_). T-cell proportion was also increased in the lymph nodes of NOD._sirpa_-HIS mice, resulting in a 7.3-fold reduction in the B- to T-cell ratio (Fig. S7_C_). Overall, NOD._sirpa_-HIS mice recapitulated the observations obtained in BALB-HIS mice, including a selective enhancement of human T- and NK-cell reconstitution.
Fig. 5.
Human cell reconstitution in BALB/c NOD.sirpa Rag2−/−IL-2Rγc−/− mice. Human cell repopulation in control (n = 6) and NOD._sirpa_-HIS mice (n = 5) 12 wk after hHPC injection. (A) Total number of human cells in the bone marrow, thymus, and spleen. (B) T-cell frequency, total T-cell number, and B- to T-cell ratio in the spleen. (C) Relative proportion of TN, TCM, and TEM cells within spleen CD4+ and CD8+ T cells. (D) Number of human NK cells (NKp46+) in the spleen.
Discussion
In this study, we have used mice humanized for components of the hemato-lymphoid system to identify the CD47/SIRPα signaling axis as a major mechanism controlling human T- and NK-cell homeostasis. By using two complementary experimental approaches, we show that CD47 is the major—if not the only—ligand of SIRPα in this setting. By ensuring proper ligation of SIRPα (mouse phagocytes) by CD47 (human cells), human hematopoietic cell maintenance was globally improved, in conjunction with selective accumulation of human T and NK cells (i.e., two subsets showing poor accumulation in the BALB-HIS mouse model).
There are several explanations for the improved maintenance of human cells in the presence of functional CD47/SIRPα interactions. First, sustained increase in hHPC numbers likely leads to increased numbers of their hematopoietic offspring. Second, human hematopoietic cells expressing mCD47 exhibited a marked competitive advantage during hematopoietic reconstitution. Third, in most lymphoid organs analyzed, we also observed a benefit of mCD47-enforced expression on the maintenance of nontransduced hematopoietic cells of the same hosts. This observation suggests that mCD47+ human cells induced partial mouse phagocyte tolerance to mCD47− cells. In mice, inoculation of CD47null hematopoietic cells into CD47+ mice results in their rapid clearance from the lymphoid system, including in T/B cell-deficient mice or NK cell-depleted animals (23, 31), whereas splenectomy or clodronate-mediated phagocyte depletion significantly reduces their elimination (23). In (WT→CD47KO) bone marrow chimeras generated with WT mouse progenitor cells and lethally irradiated CD47−/− animals, infused CD47−/− splenocytes were shown to survive for at least 3 d (24). In contrast, CD47−/− splenocytes injected either in (WT→WT) or (CD47KO→WT) bone marrow chimeras were cleared with a half-life of ∼5 h (24). It was, therefore, concluded that the lack of CD47 expression on nonhematopoietic cells induces phagocyte tolerance to CD47null leukocytes, which would normally be eliminated in the spleen. In BALB-HIS mice, in which nonhematopoietic cells are mCD47+hCD47null, mouse phagocytes may function as mediators of immunosurveillance (32) and actively limit the engraftment of mCD47null hHPC. Introduction of optimal CD47/SIRPα interactions enables the delivery of proper do not eat me signals to mouse phagocytes by human hematopoietic cells, even in limiting conditions such as in newborn C57BL/6 (Fig. S3) or macrophage-sufficient BALB/c adult mice (Fig. S8) or after injection of low hHPC numbers (Fig. S9). Of note, T- and NK-cell numbers generated in mCD47/BALB-HIS (from GFP+ hHPC) (Fig. 3), NOD._sirpa_-HIS (Fig. 5), and NSG-HIS mice (Fig. S9) were of the same order of magnitude.
Our data strongly suggest that human T-cell homeostasis is particularly sensitive to the CD47/SIRPα signaling axis. In the thymus, mCD47− human cells did not benefit from induction of mouse phagocyte tolerance by mCD47+ cells. In peripheral lymphoid organs, effective CD47/SIRPα interactions lead to enhanced B-cell and monocyte generation (∼15-fold increase in spleen) but to a limited extent compared with T cells (∼45-fold). As a consequence, mCD47+ T cells selectively accumulate in peripheral lymphoid organs, mostly as naive, resting cells, with improved function, which was evidenced by increased total plasma IgM and IgG concentration. Until the present report, CD47 role in T-cell development and peripheral survival remained particularly elusive, although evidences suggested that T cells were sensitive to CD47-mediated clearance mechanisms in vivo. In CD47−/− mice, a limited, nonsignificant reduction in peripheral T-cell frequency was reported (22). It was later shown that thymocyte numbers are reduced approximately twofold in CD47−/− mice (33). Phagocytes in CD47−/− mice are tolerant to other CD47null hematopoietic cells, and therefore, experiments have been designed to test the behavior of CD47−/− bone marrow cells injected in sublethally irradiated CD47+/+ hosts (i.e., during competitive reconstitution). In this experimental setup, a complete lack of de novo CD47−/− T-cell generation was observed over a 1-y period, whereas CD47−/− B and myeloid cells were detected for up to 25 and 35 wk after reconstitution, respectively (24). It, therefore, seems that T-cell lineage is particularly sensitive to phagocyte-mediated removal.
Our data highlight two other noticeable consequences of functional CD47/SIRPα interactions, namely improved NK-cell homeostasis and lymph node organogenesis. Similarly to T cells, selective accumulation of NK cells was obtained in presence of proper CD47/SIRPα interactions. Of note, this specific effect was observed at the periphery but not in the thymus of humanized mice, suggesting that homeostasis of thymic and peripheral NK-cell subsets might be differentially regulated (2, 34). Improved lymph node organogenesis was also systematically observed both in mCD47/BALB-HIS and NOD._sirpa_-HIS mice. In contrast, control BALB-HIS mice only exhibited rare mesenteric lymph nodes (7, 8). It is known that lymphoid tissue inducer cells are strongly reduced in IL-2Rγc−/− mice because of lack of IL-7 signaling (35). Adult Rag2−/−IL-2Rγc−/− mice lack Peyer's patches and exhibit rudimental anlagen of peripheral lymph nodes (4–6). Still, these anlagen are normally generated during embryonic life and can be maintain during neonatal life in an IL-7/IL-7R–dependent fashion by colonizing hematopoietic cells (e.g., through the adoptive transfer of T or NK cells but not B cells within 1 wk of birth) (36). In HIS mice, proper CD47/SIRPα interactions may allow for the rapid development of human cells, supporting maturation of Rag2−/−IL-2Rγc−/− mouse lymph node anlages. Indeed, we observed, in mCD47/BALB-HIS mice, low but significant numbers of Lin−CD34−CD117+CD127+ cells, which might represent human lymphoid tissue inducer cells (37).
Phagocytes exert well-described functions in the clearance of apoptotic cells and aged erythrocytes (38, 39) as well as immunosurveillance mediators of tumor cells and virus-infected cells (20, 32). Our results provide a role for CD47-dependent phagocyte-mediated mechanisms for the control of hematopoietic cell numbers. Next to MHC/T cell receptor (TCR) (13) and IL-7/IL-7R interactions (10–12) for T cells and IL-15/IL-15R interactions for NK cells (14), we, therefore, propose CD47/SIRPα interaction as another critical mechanism regulating human T- and NK-cell homeostasis under steady-state conditions. In humans, such a mechanism could be driven by differential expression of CD47 between various hematopoietic cell subsets, which was proposed in the case of senescent erythrocytes (38). The question of whether CD47 expression during an infection or in certain pathogenic settings could have a direct influence on the homeostasis of specific human hematopoietic cell populations still remains open.
Materials and Methods
Details are in SI Materials and Methods.
mCD47/BALB-HIS Mouse Generation and Analysis.
HIS mice were generated as previously described (7, 8, 27). Fetal liver CD34+CD38− lineage-negative (CD3, CD11c, CD19, CD56, and BDCA2) hHPC were sorted using a FACS-Aria (BD Biosciences). Newborn (<5 d old) sublethally irradiated (3.5 Gy) BALB/c Rag2−/−IL-2Rγc−/− mice were injected intrahepatic with ∼105 hHPC, transduced either with a control or codon-optimized mCD47-expressing pHEF lentiviral vector (11, 27). BALB/c Rag2−/−IL-2Rγc−/− NOD.sirpa congenic animals were generated, bred, and maintained at the Institut Pasteur (by N.D.H and J.P.D.S.) after backcrossing to BALB/c background for six generations. Cell suspensions were stained with fluorescent anti-human mAbs targeting the indicated cell markers and analyzed with an LSR-II cytometer (BD Biosciences). Dead cells were excluded based on DAPI incorporation.
Histology and ELISA.
Histological analysis was performed on formaldehyde-fixed, paraffin-embedded tissue samples. Stainings were performed on successive sections either with H&E or monoclonal antibodies to hCD45 (LCA 2B11+PD7/26; Dako), hCD20 (L26; Dako), or hCD3 (SP7; Neomarkers). The plasma samples were screened by ELISA for the presence of total human IgM [AffiniPure F(ab’)2 goat anti-hIgM and IgG AffiniPure goat anti-hIgG; Jackson ImmunoResearch). Plasma samples were tested in serial dilutions starting at a 1:2 dilution.
Human Cell Adoptive Transfers.
HIS mouse spleen cell suspensions were injected i.v. into adult (>10 wk old) nonirradiated BALB/c Rag2−/−IL-2Rγc−/− mice. Each recipient mouse received 1.5–3.5 × 106 hCD45+ cells. To correct for the variable frequency of GFP+ T cells in the original inoculum, the number of human T cells recovered from the recipient mice is plotted as a percentage of recovery from the inoculum. In some experiments, the human cells were labeled with the cell division tracking dye CellTrace-Violet (15 μM; Invitrogen).
Supplementary Material
Supporting Information
Acknowledgments
We thank Dr. Timo van den Berg and Dr. Mireille Centlivre for their valuable suggestions, Berend Hooibrink for expert maintenance of the flow cytometry platform, the Bloemenhove Clinic (Heemstede, The Netherlands) for providing fetal tissues, and the staff of the Animal Research Institute Amsterdam for animal care. This work was supported by grants from the Bill and Melinda Gates Foundation (Grand Challenges in Global Health Program GC4), Wijnand M. Pon Foundation, Collège de France, Fondation pour la Recherche Médicale (FRM), Institut Pasteur, and Institut National de la Santé et de la Recherche Médicale (INSERM). N.D.H. was supported by the Human Frontiers Science Program, and R.S. was supported by Dutch Cancer Society Grant NKI 2006-3530.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Almeida AR, Rocha B, Freitas AA, Tanchot C. Homeostasis of T cell numbers: From thymus production to peripheral compartmentalization and the indexation of regulatory T cells. Semin Immunol. 2005;17:239–249. doi: 10.1016/j.smim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 3.Takada K, Jameson SC. Naive T cell homeostasis: From awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 4.Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol. 2006;176:2053–2058. doi: 10.4049/jimmunol.176.4.2053. [DOI] [PubMed] [Google Scholar]
- 5.Manz MG. Human-hemato-lymphoid-system mice: Opportunities and challenges. Immunity. 2007;26:537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 7.Gimeno R, et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2-/- gammac-/- mice: Functional inactivation of p53 in developing T cells. Blood. 2004;104:3886–3893. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- 8.Traggiai E, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 9.Legrand N, et al. Transient accumulation of human mature thymocytes and regulatory T cells with CD28 superagonist in “human immune system” Rag2(-/-)gammac(-/-) mice. Blood. 2006;108:238–245. doi: 10.1182/blood-2006-01-0190. [DOI] [PubMed] [Google Scholar]
- 10.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 11.van Lent AU, et al. IL-7 enhances thymic human T cell development in “human immune system” Rag2-/-IL-2Rgammac-/- mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183:7645–7655. doi: 10.4049/jimmunol.0902019. [DOI] [PubMed] [Google Scholar]
- 12.O'Connell RM, et al. Lentiviral vector delivery of human interleukin-7 (hIL-7) to human immune system (HIS) mice expands T lymphocyte populations. PLoS One. 2010;5:e12009. doi: 10.1371/journal.pone.0012009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manz MG, Di Santo JP. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat Immunol. 2009;10:1039–1042. doi: 10.1038/ni1009-1039. [DOI] [PubMed] [Google Scholar]
- 14.Huntington ND, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weijer K, et al. Intrathymic and extrathymic development of human plasmacytoid dendritic cell precursors in vivo. Blood. 2002;99:2752–2759. doi: 10.1182/blood.v99.8.2752. [DOI] [PubMed] [Google Scholar]
- 16.Shultz LD, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 17.Ito M, et al. NOD/SCID/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa F, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barclay AN. Signal regulatory protein alpha (SIRPα)/CD47 interaction and function. Curr Opin Immunol. 2009;21:47–52. doi: 10.1016/j.coi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005;175:7781–7787. doi: 10.4049/jimmunol.175.12.7781. [DOI] [PubMed] [Google Scholar]
- 21.Sarfati M, Fortin G, Raymond M, Susin S. CD47 in the immune response: Role of thrombospondin and SIRP-alpha reverse signaling. Curr Drug Targets. 2008;9:842–850. doi: 10.2174/138945008785909310. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg FP, et al. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 23.Oldenborg PA, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, et al. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci USA. 2007;104:13744–13749. doi: 10.1073/pnas.0702881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takenaka K, et al. Polymorphism in Sirpα modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. Species- and cell type-specific interactions between CD47 and human SIRPα. Blood. 2006;107:2548–2556. doi: 10.1182/blood-2005-04-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Lent AU, et al. In vivo modulation of gene expression by lentiviral transduction in “human immune system” Rag2-/- gamma c -/- mice. Methods Mol Biol. 2010;595:87–115. doi: 10.1007/978-1-60761-421-0_6. [DOI] [PubMed] [Google Scholar]
- 28.Parel Y, Chizzolini C. CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmun Rev. 2004;3:215–220. doi: 10.1016/j.autrev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 30.Hao Y, Legrand N, Freitas AA. The clone size of peripheral CD8 T cells is regulated by TCR promiscuity. J Exp Med. 2006;203:1643–1649. doi: 10.1084/jem.20052174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blazar BR, et al. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med. 2001;194:541–549. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guimont-Desrochers F, et al. Absence of CD47 in vivo influences thymic dendritic cell subset proportions but not negative selection of thymocytes. Int Immunol. 2009;21:167–177. doi: 10.1093/intimm/dxn135. [DOI] [PubMed] [Google Scholar]
- 34.Vosshenrich CA, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 35.Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol. 2005;174:21–25. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 36.Coles MC, et al. Role of T and NK cells and IL7/IL7r interactions during neonatal maturation of lymph nodes. Proc Natl Acad Sci USA. 2006;103:13457–13462. doi: 10.1073/pnas.0604183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cupedo T, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 38.Oldenborg PA. Role of CD47 in erythroid cells and in autoimmunity. Leuk Lymphoma. 2004;45:1319–1327. doi: 10.1080/1042819042000201989. [DOI] [PubMed] [Google Scholar]
- 39.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information