Intracellular Transport of Plant Viruses: Finding the Door out of the Cell (original) (raw)
Abstract
Plant viruses are a class of plant pathogens that specialize in movement from cell to cell. As part of their arsenal for infection of plants, every virus encodes a movement protein (MP), a protein dedicated to enlarging the pore size of plasmodesmata (PD) and actively transporting the viral nucleic acid into the adjacent cell. As our knowledge of intercellular transport has increased, it has become apparent that viruses must also use an active mechanism to target the virus from their site of replication within the cell to the PD. Just as viruses are too large to fit through an unmodified plasmodesma, they are also too large to be freely diffused through the cytoplasm of the cell. Evidence has accumulated now for the involvement of other categories of viral proteins in intracellular movement in addition to the MP, including viral proteins originally associated with replication or gene expression. In this review, we will discuss the strategies that viruses use for intracellular movement from the replication site to the PD, in particular focusing on the role of host membranes for intracellular transport and the coordinated interactions between virus proteins within cells that are necessary for successful virus spread.
Keywords: Macromolecular trafficking, membrane biology, membrane proteins, movement proteins, plasmodesmata, plant–virus interactions
INTRODUCTION
Among the major plant pathogen groups (bacteria, fungi, nematodes, and viruses), viruses are unique because they live exclusively in the symplast of their host. This ‘lifestyle’ requires that plant viruses move between cells to re-initiate infections in order to accumulate in sufficient levels and tissues to guarantee their survival. A second feature that distinguishes plant viruses from other types of pathogens is their small genome size. A virus must accomplish successful infection of a plant utilizing a very limited amount of genetic material; some plant virus genomes may encode as few as three proteins, whereas 10–15 proteins would be the upper limit for most viruses. Consequently, any gene present in a viral genome after generations of selection must have an essential function(s). One class of protein encoded in every virus genome is the movement protein (MP)—a protein shown through genetic and cell biological studies to be required for virus intercellular movement and to modify plasmodesmata (PD): the symplasmic tunnels between cells that are the gateway for this movement.
The requirement for viruses to modify PD for their movement was recognized many years ago as it became obvious that, no matter the form of the virus capsid in a cell, it would be too large to fit through an unmodified PD. Importantly, even the naked genomic viral nucleic acids have too large an effective diameter to pass through PD. Thus, one of the key functions of the viral MP is to enlarge PD to allow passage of some form of the virus into the adjacent cell (Wolf et al., 1989). The MP also plays an active, if less defined, role in the physical movement of the virus across the PD. Plant virus MPs can be divided into two broad categories, based on the degree of structural changes they induce in the plasmodesma and the form of the viral nucleic acid–protein complex that travels through the PD (Scholthof, 2005; Benitez-Alfonso et al., 2010; Niehl and Heinlein, 2011). Some viral MPs, as exemplified by the MP of Tobacco mosaic virus (TMV), increase the size exclusion limit (SEL) of the PD to allow for movement of an MP–RNA aggregate, but do not cause obvious visual changes to the PD structure. Other MPs, such as those of Cowpea mosaic virus (CPMV) or Cauliflower mosaic virus (CaMV), are involved in dramatically restructuring the PD by apparently removing the desmotubule, the appressed endoplasmic reticulum (ER) within PD, leading to an expansion of the pore to allow for movement of virions with diameters up to 50 nm. These viruses are thought to convert the PD into a tubule. Several recent reviews have focused on the structure of PD and the contribution of viral MPs to intercellular movement (Benitez-Alfonso et al., 2010; Niehl and Heinlein, 2011; Tilsner et al., 2011; Ueki and Citovsky, 2011).
As our knowledge of the function of viral MPs and virus movement has increased, it has become apparent that viruses must also use an active mechanism to move from the site of replication within the cell to the PD. Most plant viruses replicate in cells in association with host membranes, which can be modified to form a reaction vessel for the viral replicase (Laliberté and Sanfaçon, 2010). Other viruses such as CaMV are considered to replicate in inclusion bodies of viral origin, but, even in this case, recent evidence suggests that the inclusions are associated with the host cytoskeleton and ER (Harries et al., 2009a). Consequently, there is a requirement for the viral nucleic acid to travel some distance in the cytoplasm from the replication site to the PD. It has been estimated that molecules up to 500 kDa can diffuse freely in the cytoplasm of eukaryotic cells (Seksek et al., 1997; Luby-Phelps, 2000) but plant virus nucleic acids and virions far exceed this size and are thought to need an active mechanism for intracellular movement (Harries et al., 2010; Niehl and Heinlein, 2011). In essence, if PD are the doorway out of the cell, then plant viruses must possess the tools to find the door as well as the keys to unlock the door.
In early models of cell-to-cell movement, the viral MP alone was thought to be responsible for intracellular movement as well as intercellular movement. However, evidence has accumulated now for the involvement of other categories of viral proteins in intracellular movement, including those originally associated with replication or gene expression. In this review, we have chosen to highlight two aspects involved in virus movement that appear critical for this activity: virus protein interactions with host membranes for intracellular transport and the coordinated interactions between virus proteins within cells that are necessary for successful virus spread. Multiple recent reviews discuss other aspects of virus movement in greater detail and the reader is guided to these for additional information in this area (Lucas et al., 2009; Harries et al., 2010; Verchot-Lubicz et al., 2010; Harries and Ding, 2011; Niehl and Heinlein, 2011; Tilsner et al., 2011). Also, the reader is directed to an earlier review by Epel (2009) that specifically discusses the influence of host membranes on virus movement for further historical perspective on this topic.
INTRACELLULAR MOVEMENT OF PLANT VIRUSES WITH MPS NOT ASSOCIATED WITH TUBULE FORMATION
Genus Tobamovirus
TMV, a monopartite RNA virus and a member of the family Virgaviridae, is one of the most intensively studied of the plant viruses utilized to understand virus intercellular movement. TMV encodes an MP that belongs to the 30K superfamily of viral MPs (Melcher, 2000) and the literature on its MP has helped form the classic definition of an MP (reviewed in Haywood et al., 2002; Harries and Nelson, 2008). Because our knowledge of the TMV MP has set the standard for MPs of this type, it is useful to discuss this protein in some detail here. There is a large body of work, particularly through genetic analyses using mutant viruses, indicating that the TMV MP is necessary for intercellular spread (reviewed in Niehl and Heinlein, 2011). Early studies identified TMV mutants with altered MP sequences that were defective in intercellular movement at restrictive temperature (Jockusch, 1968; Nishiguchi et al., 1978) but whose normal movement was restored in MP transgenic plants (Deom et al., 1987; Meshi et al., 1987). In more detailed molecular and cell biological studies, the TMV MP was determined to localize to PD and increase the SEL (Wolf et al., 1989; Atkins et al., 1991; Ding et al., 1992). TMV MP also bound ssRNA (Citovsky et al., 1990) and facilitated the transport of both itself and viral RNA (vRNA) between cells (Waigmann and Zambryski, 1995; Nguyen et al., 1996; Kotlizky et al., 2001).
Despite the large amount of evidence that TMV MP is necessary for intercellular virus movement through manipulation of the PD, we only have a few pieces of evidence at this point that give us some clue as to its function at the PD. For example, recent results indicate that the TMV MP has microfilament severing activity and that this may be related to the ability of the MP to increase the SEL (Su et al., 2010). These data support earlier findings that disruption of actin filaments can result in an increase in SEL (Ding et al., 1996). TMV MP's ability to increase PD SEL is also likely due to decreases in callose deposition at the PD. It was recently found that TMV MP interacts with a host receptor, an ankyrin repeat containing protein (ANK), at the PD and that this interaction results in a decrease in callose and an increase in virus intercellular movement (Ueki et al., 2010). It is also interesting to note that TMV MP interacts with calreticulin, a protein involved in Ca2+ sequestering and at times associated with the ER and its lumen, at the PD (Chen et al., 2005). Although the significance of this interaction remains unclear, overexpression of calreticulin redirects MP from the PD to microtubules and significantly slows virus spread. Phosphorylation may also play a role at the PD, since a cell wall-specific kinase, PAPK1, co-localizes with the TMV MP at PD in Arabidopsis and was shown to phosphorylate TMV MP in vitro (Lee et al., 2005). It is possible that this kinase is essential for completing the transport of a viral MP–vRNA complex to the next cell to initiate the next round of infection. Indeed, it was shown that a TMV MP–vRNA complex cannot establish infection in protoplasts, but can establish infection when introduced into plants (Karpova et al., 1997).
In fact, TMV MP can be phosphorylated in additional multiple locations (e.g. Watanabe et al., 1992; Citovsky et al., 1993; Haley et al., 1995) and these phosphorylation sites may be critical for its movement function (Waigmann et al., 2000). The MP of Tomato mosaic virus is also phosphorylated in vitro by CK2 kinase (Matsushita et al., 2003) and a CK2-like kinase from Nicotiana tabacum can phosphorylate the Potato virus X TBGp1 MP (Módena et al., 2008). The association of MP with ER membranes has been linked to TMV replication and movement (Heinlein et al., 1998; Reichel and Beachy, 1998; Más and Beachy, 1999; Guenoune-Gelbart et al., 2008) and it is possible that MP phosphorylation/dephosphorylation events at the ER might be key in controlling these processes in infected cells. Indeed, Karger et al. (2003) demonstrated that an MP-specific ER-associated protein kinase could phosphorylate a specific threonine residue (Thr104) in the TMV MP. Mutation of Thr104 to mimic phosphorylation resulted in a severe decrease in intercellular virus spread. Recent evidence suggests that other amino acids (C-terminal portion) of the MP are phosphorylated at the ER in the early stages of infection before the virus has traveled to the cell wall; however, the effects of this phosphorylation on replication or movement are not yet clear (Tyulkina et al., 2010). While the TMV MP is essential for virus intercellular movement, it is not the only viral or host component that acts to facilitate movement. TMV replicates in virus replication complexes (VRCs) that contain virus particles, virus-encoded 183 and 126-kDa proteins, MP, and vRNA as well as host components such as the ER, ribosomes, β-tubulin, and EF1-α (Shalla, 1964; Esau and Cronshaw, 1967; Beachy and Zaitlin, 1975; Heinlein et al., 1998; Más and Beachy, 1999; dos Reis Figueira et al., 2002; Ding, X.S. and Nelson, R.S., unpublished). The size of VRCs in infected cells is directly related to the severity of the infection (Liu et al., 2005) and, although the function of the VRCs is not entirely clear, as the name implies, they are believed to be sites of virus replication and translation (Heinlein et al., 1998; Más and Beachy, 1999).
If TMV likely replicates in VRCs associated with the ER, what mechanism governs the transport of its RNA to the PD? When considering this question, it is important to recognize that the ER itself is intimately involved in trafficking cellular macromolecules. The ER is a core component of the endomembrane system—a system that is fluid and yet composed of unique membrane components within individual organelles (Hanton et al., 2007; Moreau et al., 2007; Bassham et al., 2008; Sparkes et al., 2009). Protein and membrane trafficking is often discussed in the context of vesicles moving from the ER to the Golgi and then the plasma membrane (PM; Figure 1). ER-to-Golgi transport involves vesicles containing a host coat protein complex II (COP-II). Transport of membrane and proteins from the ER to the peroxisome and chloroplast have also been suggested. This transport from the ER is associated with biosynthetic activities and, as such, is a forward, or anterograde, activity. Anterograde transport is balanced by retrograde transport. Most often, retrograde transport is associated with endocytosis where cargo is returned from the PM through endosomes to the vacuole. Transport from the Golgi to the ER involves vesicles containing a host coat protein I (COP-I). These characteristics require a regulatory system composed of an extraordinary number of signaling and receptor molecules (Bassham et al., 2008). Proteins that regulate this system to maintain order include unique proteins involved in forming vesicles (e.g. coat GTPases, GTP-exchange factors (GEFs), vesicle coat proteins), labeling vesicles (e.g. v-SNARES), and receiving vesicles at the receptor membrane (e.g. Rab-GTPases, t-SNARES). Beyond classical signaling and receptor proteins, the influence of integral and peripheral membrane proteins on the specificity and fluidity of membrane themselves (e.g. lipid raft regions) for protein association and movement also must be considered (Tilsner et al., 2011). It seems very likely that TMV utilizes aspects of this host endomembrane system for its intracellular movement.
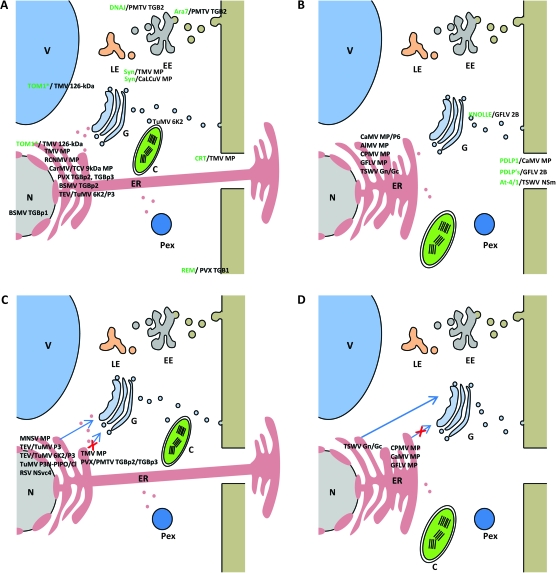
Figure 1.
Cellular Membranes and Virus Intra/Intercellular Movement.
(A, B) Schematics of the host cell endomembrane system illustrate the association of virus proteins with a known movement function (black text) from both non-tubule-forming viruses (A) and tubule-forming viruses (B) with host membranes. Only those proteins whose localization has been demonstrated in plants are shown. Host proteins that are known to mediate such interactions are shown where applicable (green text). PVX and BSMV are shown as representatives of the potex-like and hordei-like viruses, respectively. There are additional virus proteins in these categories that associate with membranes but that are not included due to space limitations. DNAJ, class of chaperone proteins; Ara7, Rab5 GTPase ortholog and a marker for early endosomes; Syn, synaptotagmin, a clathrin-associated, SNARE-interacting protein; CRT, calreticulin; REM, remorin, a plasma membrane protein; KNOLLE, T-SNARE; PDLPs, plasmodesmal localized proteins; At-4/1, Arabidopsis protein localized to plasmodesmata.
(C, D) The results of inhibiting secretory pathways with brefeldin A (BFA) treatments or dominant negative mutations (ex. Sar1, Arf1) on the movement of non-tubule-forming (C) and tubule-forming (D) viruses from the ER to the cell periphery. A blue arrow blocked with a red X denotes that these viruses do not move through the endomembrane secretory system, since BFA treatment and/or mutations did not inhibit the movement of these virus proteins to the cell periphery. An unblocked arrow indicates that these virus proteins do move through the secretory system, since BFA treatment and/or mutations did diminish movement. V, vacuole; G, Golgi apparatus; N, nucleus; ER, endoplasmic reticulum; C, chloroplast; Pex, peroxisome; EE, early endosome; LE, late endosome. * TOM1 associates with vacuolar and probably ER as well as other unidentified membranes. These membrane interactions may be mediated by the host membrane-associated protein TOM2A.
As noted, both the TMV MP and the replicase components, the 126 and 183-kDa proteins, associate with the ER. Early studies showed that TMV infections cause severe modifications to the ER: with conversion of tubular ER into large aggregates that revert to tubular ER late in the infection (Reichel and Beachy, 1998). This ER modification was visually phenocopied by expression of MP–GFP in the absence of virus. The MP associates with membranes and has two putative transmembrane domains (Reichel and Beachy, 1998; Más and Beachy, 1999; Brill et al., 2004; Fujiki et al., 2006), although the presence of transmembrane domains has been questioned (see within Tilsner et al., 2011). More recently, it has been shown through dual labeling experiments that ectopically expressed MP–mRFP sometimes flows with GFP-labeled ER (Sambade et al., 2008). The association of the TMV MP with membranes can be inhibited by eliminating MP phosphorylation at a specific amino acid that may destabilize the MP (Fujiki et al., 2006). However, even though association of the TMV MP with the ER was essential for virus intercellular movement, it was not sufficient for movement (Fujiki et al., 2006). In addition, as noted previously, an ER-associated kinase specific for TMV MP was identified and mutation of a specific amino acid to mimic phosphorylation inhibited virus intercellular transport (Karger et al., 2003). Thus, phosphorylation in this instance may turn off MP activity rather than act as a positive regulator.
In addition to the TMV MP, the 126-kDa protein also has characteristics that suggest it may have a role in intracellular movement. The TMV 126-kDa protein is known primarily for its involvement in symptom formation (Shintaku et al., 1996), its role in enhancing the replication of TMV (Ishikawa et al., 1986; Lewandowski and Dawson, 2000), and its ER association (Heinlein et al., 1998), but evidence also exists that it is required for virus movement. Hirashima and Watanabe (2001, 2003) demonstrated that a chimeric TMV with amino acid changes in the helicase domain of the 126-kDa protein was defective in intercellular movement, but replicated normally. The TMV 126-kDa protein, a major component of VRCs, is capable of forming inclusion bodies (126-bodies) when ectopically expressed (Ding et al., 2004). TMV VRCs and 126-bodies form an association with ER possibly through the membrane-bound host protein, TOM1 (Yamanaka et al., 2000; Hagiwara et al., 2003), and also are associated with actin microfilaments and microtubules (McLean et al., 1995; Heinlein et al., 1998; Liu et al., 2005). In addition, VRCs containing the 126-kDa protein have been shown to move on actin filaments (Kawakami et al., 2004; Liu et al., 2005). This movement correlates with findings showing that the VRCs are paired across cell walls of adjoining cells near or at the infection front, but just six cells back from that front, VRCs are no longer paired and often have moved from the wall (Szécsi et al., 1999). The involvement of microfilaments in sustained movement has been demonstrated in studies with latrunculin B (Lat B), a pharmacological agent that disrupts microfilament structure. TMV lesions that develop in Lat B-treated tissues are smaller than those in control tissues (Kawakami et al., 2004; Liu et al., 2005; Harries et al., 2009b). Furthermore, silencing of myosin XI-2, a protein responsible for transport of cargo on microfilaments, also results in a significant reduction in lesion size (Harries et al., 2009b). One possible explanation for the 126-kDa protein's involvement in virus movement could be through an interaction with MP and, as previously noted, both of these proteins are found in VRCs that form in TMV-infected cells at or near the infection front. The requirement by TMV for an additional protein for its movement highlights the fact that some proteins not classically defined as MPs still have crucial roles in this activity.
Since both the MP and the 126-kDa protein are known to associate with membranes and are involved in virus movement, it is reasonable to consider that the ER is a conduit through which vRNA is moved to the PD through interactions with the MP and 126-kDa protein. Indeed, as noted, the ER spans the cytoplasm and passes through the PD. Guenoune-Gelbart et al. (2008) suggested that diffusion of TMV vRNA and MP (and possibly other components) through the ER within the PD may be a mechanism to facilitate TMV cell-to-cell spread. These authors utilized ER lumen and membrane markers to show that both viral replicase components, the 126 and 183-kDa proteins, as well as the MP were required for maximum flow between cells. Further evidence for the involvement of the ER in intracellular spread can be seen in the finding that the intercellular movement of Turnip vein clearing virus (TVCV), a virus closely related to TMV, is insensitive to disruption of actin filaments or microtubules (Harries et al., 2009b). The absence of any cytoskeletal requirement by TVCV for movement suggests that this virus may utilize membrane transport alone for its motility. For TMV, it is possible that a membrane association is a basic requirement and the cytoskeleton an added requirement for its intercellular movement, the latter as noted in the previous paragraph. Indeed, it was shown that TMV does not require intact microfilaments early during intercellular spread (first 24 h), but does require them for sustained virus intercellular movement (2–6 d post infection; Hofmann et al., 2009; Harries et al., 2009b). Microfilaments may still modulate early spread of TMV, since normal virus spread requires full membrane fluidity, free of excessive microfilament-mediated membrane anchoring (Hofmann et al., 2009).
The finding that both the replicase and MP of TMV are needed for maximum transport of membrane and ER lumen markers through the PD raises the interesting possibility that TMV vRNA could be transported by multiple proteins and via multiple mechanisms at different times during a single infection. For example, the replicase proteins might act to shuttle TMV RNA along cytoskeletal and membrane elements to the PD, where the RNA is passed off to the MP for intercellular transport, possibly through membrane diffusion (Epel, 2009). Or it is possible that a membrane-assisted transport system involving the MP and replicase traffics TMV RNA to the PD and that the actinomyosin network in conjunction with the replicase is need to transport the VRC from the PD back to the interior of the cell for further modification (degradation or virus replication). The possibility for multi-protein shuttling of vRNA or virions is certainly not exclusive to TMV, as we discuss in some detail for other viruses.
Indeed, although membranes and phosphorylation have a role in TMV movement, the method by which TMV MP itself arrives at the PD remains a mystery. Treatment of cells with brefeldin A (at low concentrations, 10 μg ml−1), which generally inhibits COP-II and COP-I-mediated transport, did not inhibit MP transport to the PD whether expressed ectopically or during virus infection (Tagami and Watanabe, 2007; Wright et al., 2007; Genoves et al., 2010). In addition, intercellular transport of tobamoviruses was not inhibited by BFA treatment nor by overexpression of a defective coat GTPase, Sar1p, which inhibits the COP-II transport system specifically (Tagami and Watanabe, 2007; Genoves et al., 2010). Higher concentrations of BFA (50 μg ml−1 and above) did disturb both the ER network and movement of MP to the cell wall either during virus infection or when expressed ecotopically (Heinlein et al., 1998; Wright et al., 2007). This suggests that the MP utilizes the ER but not the COP-II vesicle trafficking system to reach the cell wall.
Genus Dianthovirus
The subcellular localization of the MP of Red clover necrotic mosaic virus (RCNMV) has provided further insights into how a viral RNA might be transported from the ER to the PD. RCNMV is a bipartite positive-stranded RNA virus and a member of the family Tombusviridae. It encodes an MP with sequence and functional characteristics similar to the TMV MP. For example, the RCNMV MP can bind single-stranded RNA, increase the SEL of the PD, and is localized to the cell wall (Osman and Buck, 1991; Fujiwara et al., 1993; Giesman-Cookmeyer and Lommel, 1993; Tremblay et al., 2005). Furthermore, the MPs of TMV and RCNMV can be exchanged between viruses for functional complementation (Giesman-Cookmeyer et al., 1995). Like the TMV MP, an RCNMV MP–GFP fusion expressed from a virus first appeared at the cell wall, but was subsequently found at the ER as punctate spots that co-localized with the RCNMV 27-kDa replicase component (Kaido et al., 2009). However, the RCNMV MP–GFP fusion expressed ectopically was localized exclusively to the cell wall. It was determined that the ER localization of the MP–GFP was associated with replication of RNA1 of this virus, but not with RNA2 or the replicase alone. This laboratory later determined that the C-terminal 70 amino acids of the RCNMVC MP were crucial for ER localization of the MP–GFP fusion and for intercellular movement of the recombinant virus that encoded the MP. Importantly, however, the C-terminal deletion did not alter the ability of the MP to increase the SEL of the PD, bind ssRNA, or bind ssRNA in vitro (Kaido et al., 2011). This very important observation suggests that ER localization of the MP–GFP is required for RCNMV to move between cells, even though other functions typically associated with MPs were intact.
Genus Carmovirus
In addition to the MPs that belong to the 30K superfamily, other types of MPs encoded by RNA viruses in the Tombusviridae have been shown to associate with the ER. For example, some members of the Carmovirus genus encode two small proteins of 7 and 9 kDa that are both necessary for virus intercellular movement and are unrelated to the MP of TMV. They have been studied for their association with membranes. For example, the 9-kDa MPs from Carnation mottle virus (CarMV) and Turnip crinkle virus (TCV) were shown through in vitro studies to insert into ER without the aid of additional viral or plant host components (Vilar et al., 2002; Martínez-Gil et al., 2010). This ability to insert into membranes in vitro was not observed for the TMV MP (referenced in Heinlein et al., 1998). Both the CarMV and TCV 9-kDa MPs contain two membrane-spanning domains, and it has been shown with the CarMV MP that both the N- and highly charged C-termini of its MP faced the cytoplasm, in theory to bind the soluble, RNA-bound viral 7-kDa protein through their β-sheet domains to complete virus intercellular movement. The p8 protein of TCV, which is a homolog of the CarMV 7-kDa protein, also associates with membrane structures (Li et al., 1998). Interestingly, the N and C-termini of the TCV 9-kDa MP faced the lumen (Martínez-Gil et al., 2010), which leads to speculation as to how it interacts with the TCV p8 protein, presumed to be in the cytoplasm. The authors did not rule out the possibility that the protein has other orientations in vivo. Integration of these MPs into the ER was determined to be through a co-translational and signal recognition particle-dependent fashion (Saurí et al., 2005; Martínez-Gil et al., 2010). Both Sec61α and the translocating chain-associated membrane protein bind the transmembrane domains of the 9-kDa proteins.
Interestingly, there are even differences in the membrane topologies of MPs within the Carmovirus genus. The 7-kDa MP of a second member of the genus Carmovirus, Melon necrotic spot virus (MNSV), was determined to have a single membrane-spanning domain, with its N-terminus in the cytoplasm and C-terminus in the lumen (Martínez-Gil et al., 2007). These studies, like those described for CarMV, were conducted utilizing in vitro translation or overexpression of fusion proteins in E. coli and not in planta. It will be worthwhile to determine whether these findings apply in the natural host of these viruses.
Genus Tombusvirus
In contrast to the carmoviruses, research with a different member of the family Tombusviridae has highlighted the intricate involvement of membranes in the process of viral replication. Tombusviruses are also monopartite sense-stranded RNA viruses, like TMV and the carmoviruses, but they encode two viral proteins that influence their movement that are different from those of the other viruses. The p22 protein of Tomato bushy stunt virus (TBSV) is its classical MP. This protein partitioned with crude membrane fractions (e.g. Desvoyes et al., 2002). The p19 protein, which influences spread of TBSV in some hosts and is a suppressor of silencing, is a soluble protein (Scholthof et al., 1995). TBSV is one of two plant viruses, the other being Brome mosaic virus (BMV), that has been modified to replicate in yeast (e.g. Panavas and Nagy, 2005). Utilizing the extensive yeast knockout library, genes encoding multiple membrane-associated proteins were determined to be necessary for virus accumulation. Although their function in movement versus virus replication and their association with particular virus components require further study, it is important to note that four groups of proteins involved in lipid metabolism, membrane-association, vesicle-mediated transport, or vacuolar targeting were obtained. Within the latter three groups, proteins were identified that influenced transport to the ER, Golgi, or vacuoles, as well as those necessary for membrane fusions or that act as transporters across membranes (reviewed in Nagy and Pogany, 2010). The Nagy laboratory has further studied the involvement of multiple ESCRT (endosomal sorting complexes required for transport) proteins in virus replication (Barajas et al., 2009). Dominant negative construction of plant-derived Vps23p, Vps24p, Snf7p, and Vps4p inhibited virus accumulation in the plant host. The Vps23p protein, which, in yeast, is recruited to targeted cargo proteins, interacted with the p33 viral replication protein. In the absence of Vps23 and Vps24 in yeast, the RNA was more susceptible to degradation, thus suggesting that the replication complex at the peroxisome did not form correctly and was susceptible to degradation. McCartney et al. (2005) determined that the p33 protein targets from the cytosol to peroxisomes and later to a peroxisomal ER subdomain. This sorting was disrupted by co-expression of a dominant-negative inhibitor of Arf1, which inhibits the formation of COP-I complexes and trafficking between ER and Golgi in general. Thus, p33 targeting appears to depend on maintaining an ability to form vesicles. Research with TBSV has thus contributed to a greater understanding of the role of host membranes at the site of replication, essentially the starting point of the virus's journey to the PD. It will be important to determine what direct role, if any, these and the other membrane trafficking proteins that are less studied have in virus intracellular movement.
Triple Gene Block Viruses
A large number of helical RNA viruses (nine genera representing three families: Alphaflexiviridae, Betaflexiviridae, and Virgaviridae) are classified together due to an unusual grouping of three open reading frames in their genome. This conserved genetic module of genes, called the triple gene block (TGB), encodes proteins that function together in a regulated manner to enable intercellular movement of these viruses. There are two major classes of TGB modules, referred to as potex-like (for Potato virus X (PVX) as type member) and hordei-like (for Barley stripe mosaic virus (BSMV) as type member). Although many similarities exist between these two groups in the activities of their TGB proteins, significant differences also exist. The TGB-containing viruses now are perhaps the most intensively studied group of viruses for plant intercellular movement activity. Because of the complexity of the findings with these viruses, the reader is advised to read reviews written specifically for these viruses (e.g Verchot-Lubicz et al., 2010). We present in the following paragraphs an overview of the membrane interactions displayed by these proteins and also discuss how they interact with each other to allow virus intracellular and intercellular movement. It is interesting to note that the single MPs encoded by viruses such as TMV can be interchanged with the TGB proteins and still support movement of the recombinant viruses (Solovyev et al. 1996b; Solovyev et al. 1997; Ajjikuttira et al., 2005). This indicates some independence between movement and replication functions for the TGB proteins from these viruses (Verchot-Lubicz et al., 2010); however, it is more often the case that moving individual open reading frames, even within the TGB-containing viruses, leads to greatly reduced or no intercellular movement by the recombinant (e.g. Lim et al., 2008). Thus, although moving MPs and movement-associated proteins between viruses within or between genera and even families can sometimes provide necessary functions for intracellular and intercellular movement of the complimentary virus, they are likely not optimized for function in the non-native system.
The TGB proteins are identified as TGB1, TGB2, and TGB3: the order in which they appear on the virus genome. Their open reading frames overlap with one another (1 with 2 and 2 with 3). TGB1s for all of these viruses bind RNA and have NTPase/helicase activity (e.g. Donald et al., 1997; Kalinina et al., 2002; Leshchiner et al., 2006; Makarov et al., 2009). Interestingly, the potexvirus TGB1 can move independently within the cell while the TGB1 of the hordei-like viruses requires TGB2 and TGB3 for its movement (reviewed in Verchot-Lubicz et al., 2010). Although it has been reported that a hordei-like GFP–TGB1 fusion does align with perinuclear membranes and form punctae at the plasma membrane (Lawrence and Jackson, 2001), most studies on both hordei-like and potex-like viruses indicate that TGB1s are located in the cytoplasm or nucleoplasm (e.g. Liou et al., 2000; Zamyatnin et al., 2004; Wright et al., 2010). For the TGB1 from Potato mop-top virus (PMTV), the N-terminal 84 amino acids are sufficient for localization to the nucleolus (Wright et al., 2010). However, recently it was determined that a small hydrophilic plasma membrane protein, remorin, inhibited the movement of PVX and that this protein interacts with TGB1 from this virus (Raffaele et al., 2009). Thus, at least for this potex-like virus, there is an indication of an association of its TGB1 with a membrane. For both groups of viruses, TGB2 and TGB3 are integral membrane proteins (reviewed in Verchot-Lubicz et al., 2010). TGB2 proteins are from 12 to 14 kDa, have significant sequence similarity, and two predicted _trans_-membrane domains with a conserved hydrophilic loop (Morozov and Solovyev, 2003). Results from multiple studies have predicted that TGB2 proteins form a U-shaped membrane domain with the central connecting loop in the ER lumen (e.g. Zamyatnin et al., 2006; Hsu et al., 2008). Consistent with these findings are the results from Zamyatnin et al. (2006), who used bimolecular fluorescence complementation (BiFC) to determine that the N- and C-termini of a hordei-like TGB2 protein were located in the cytoplasm. A potex-like TGB2 was shown to have a similar topology in yeast (Lee et al., 2010). The TGB2 proteins have been reported to bind RNA in a sequence non-specific manner and some TGB2s increased the PD SEL (e.g. Tamai and Meshi 2001; Haupt et al., 2005; Hsu et al., 2009). At least some associate with the microfilament network (e.g. Haupt et al., 2005; Ju et al., 2005). TGB3 sequences are poorly conserved across these viruses and range in size from 7 to 24 kDa (reviewed in Verchot-Lubicz et al., 2010). The larger TGB3s, associated with the hordei-like viruses, have two _trans_-membrane domains with a central hydrophilic region (Solovyev et al., 1996a). Early computer modeling placed the N and C-termini of these proteins in the ER lumen; however, results from a later study indicate that the N-terminus and likely the C-terminus are in the cytoplasm for PMTV TGB3 expressed in planta (Tilsner et al., 2010). The hordei-like 15-kDa TGB3 from a benyvirus also has two _trans_-membrane domains but lacks the conserved N-terminal cysteine-rich region observed on the larger TGB3s (e.g. Morozov and Solovyev 2003). The small TGB3s from potex-like viruses, of 7–8-kDa mass, have a single _trans_-membrane domain, with the C-terminal portion of the protein predicted to sit in the cytosol. Mutational analysis supports the presence of the N-terminal _trans_-membrane domain and the C-terminal cytosolic domain (e.g. Krishnamurthy et al., 2003). Additionally, a TGB3 from a second potex-like virus appeared to have its N-terminus in the ER lumen, although this study was conducted in yeast rather than in a plant (Lee et al., 2010). The TGBp3 of the hordei-like PMTV increases the SEL of PD (Haupt et al., 2005).
TGB proteins are known to interact with each other. For example, interactions between the BSMV TGB3 and TGB1 have been observed from yeast extracts and the residues necessary for BSMV or PMTV TGB2 and TGB3 interactions have been mapped to the central hydrophilic loops of both proteins (Cowan et al., 2002; Lim et al., 2008). Interactions between TGB2 and TGB3 have some superficial similarity to the interactions observed between the Carmovirus 9 and 8-kDa proteins, in that TGB2 has RNA binding ability like the 8-kDa protein, but they differ in that both of the TGB proteins have _trans_-membrane domains. No interaction was observed between TGB2 and TGB1 (Cowan et al., 2002; Lim et al., 2008). For the potex-like TGBs, no definitive interaction between the TGB2 and TGB3 proteins or with TGB1 has been reported; however, there is some evidence that TGB2 interacts with itself during in vitro assays (e.g. Hsu et al., 2009).
Although the membrane localization and RNA binding attributes of, and interaction capacity for, many TGBs are documented, it is not known how these proteins function together to transport viral RNA within or between cells (Verchot-Lubicz et al., 2010). The composition of the viral movement complex of the TGB viruses is not known and the possibility exists that TGB proteins may move independently and in advance to the PD area to prepare the site for virus transport. Certainly, for some of the TGB-containing viruses, TGB2 and 3 are needed to passage TGB1 to the PD, but TGB2 and 3 do not move between cells (e.g. Zamyatnin et al., 2004; Haupt et al., 2005; Schepetilnikov et al. 2005). TGB2 from a potex-like virus interacted with a β-1,3 glucanase responsible for callose degradation at the PD (Fridborg et al., 2003) and, thus, although it does not move between cells, it modifies the PD to perhaps aid viral RNA movement. TGB3 from PVX targeted GFP-fused TGB2 to peripheral bodies and GFP-TGB3 formed peripheral bodies in a COP-II independent manner when expressed alone (Solovyev et al. 2000; Schepetilnikov et al. 2005). The TGB3 from the potex-like Bamboo mosaic virus (BaMV) directed the BaMV TGB2 from ER-like structures also to peripheral bodies (Lee et al., 2010). Peripheral bodies containing TGB3 resided in tubules containing the ER-shaping protein, reticulon (Lee et al., 2010). A sorting signal in the TGB3 of BaMV was important for its oligomerization and association with peripheral ER in yeast and for virus intercellular movement in the plant, Chenopodium quinoa (Wu et al. 2011). Also, it is known that TGB2 and TGB3 from the hordei-like PMTV associated with ER and formed motile granules that used the ER-actin network for intracellular movement (Haupt et al., 2005). When co-expressed ectopically or during a virus infection, it appeared that TGB3 was recruited to TGB2 vesicles for a portion of the infection cycle. The TGB3 of PMTV accumulated at the PD in the absence of TGB2. For the BSMV and PMTV TGB proteins, it was clear that deviations in their expression ratios significantly influenced virus movement (Lim et al., 2008; Tilsner et al., 2010). Although not discussed in detail, it is important to note here that some observations regarding virus protein interactions may have more to do with retrieval of viral and host factors from the PM for reuse. For example, at least a subset of the TGB2 vesicles are thought to be derived from the PM through treatment of tissue with FM4-64, a fluorescent marker that initially labels PM (Ueda et al., 2001; Haupt et al., 2005). Also, TGB2 co-localized in vesicles with a Rab5 ortholog, Ara7, that is a marker for the early endosome and it interacted with a J-domain chaperone that is essential for endocytic trafficking in other organisms (Haupt et al., 2005). It will be important to further understand the characteristics of each TGB protein; for example, does the hordei-like TGB2 bind RNA and reside in the virus movement complex (Verchot-Lubicz et al., 2010)?
Genus Potyvirus
For members of the Potyviridae, a large and economically important family of monopartite RNA viruses, there have been several breakthrough experiments showing a relationship of virally encoded proteins with membranes and intracellular transport. Potyviruses encode two membrane-associated proteins, the 6-kDa protein (6K2) and the P3 protein (Schaad et al., 1997; Eiamtanasate et al., 2007). Vesicles containing the 6K protein are the site for potyvirus genome replication (Cotton et al., 2009). Both of these proteins, here encoded by either Tobacco etch virus or Turnip mosaic virus (TuMV), now have been shown to produce mobile granules when ectopically expressed in cells (Wei and Wang, 2008; Wei et al., 2010b; Cui et al., 2010). These researchers also determined that this mobility was associated with the actomyosin network. The 6K2 granules were determined to associate with the ER exit sites (ERES) through their co-localization with Sec23 and Sec24, known markers for this location (Wei and Wang, 2008). This implied an interaction with the COP-II transport system. Co-expression of dominant-negative mutants of Sar1 and Arf1, the coat GTPases for COP-II- and COP-I-mediated transport, or of RabD2a, a GTPase involved in vesicle docking during ER/Golgi transport, inhibited localization of 6K2 at the ERES further implicating vesicular trafficking as part of the transport system for this protein. Similar findings were obtained for the P3 protein and, additionally, it was determined that P3 inclusions co-localized with 6K2 vesicles (Cui et al., 2010). The 6K2 vesicles were predominantly targeted to chloroplasts and thus the vesicular trafficking pathway was postulated to involve movement of 6K2-containing vesicles from the ER to the chloroplasts (Wei et al., 2010b), an unusual direction for membrane transport. The P3 protein was not previously associated with potyvirus movement, but it was known to interact with the P1 protein, the MP for these viruses (Carrington et al., 1998; Merits et al., 1999). Earlier studies determined that potyviral replication complexes are mobile (Cotton et al., 2009) and thus it appears that intracellular movement of the potyvirus replication complex, containing 6K2 and P3 and associated at least at times with the MP, occurs for this virus. This again points out a link between virus replication complexes and intracellular movement. An interaction at the PD between the viral cylindrical inclusion (CI) body protein, which was known to be involved in potyvirus intercellular movement, and the viral P3N-PIPO protein appears essential for locating the CI protein of TuMV to the PD (Wei et al., 2010a). The early secretory pathway (COP-II) rather than the actinomyosin system was required for delivery of P3N-PIPO and P3N-PIPO with CI to the PD as brefeldin A and a mutant of Sar1 both inhibited transport (Wei et al., 2010a). The association of CI with the wall was transient and this transient association of a viral protein complex with the cell wall is similar to the transient association of the TMV VRC with the cell wall (Szécsi et al., 1999). During TuMV infection, the coat protein of the virus also associated with the CI and P3N-PIPO complex as had been seen for other CI complexes and as was expected based on the requirement of the CP for intercellular movement by this virus (Wei et al., 2010a).
Genus Closterovirus
Beet yellows virus (BYV) is a member of the Closterovirdae, a family mostly composed of monopartite RNA viruses with large genomes of 15–20 kb. For BYV, there are five proteins involved in intercellular movement (Peremyslov et al., 1999; Alzhanova et al., 2000). Of these five proteins, the HSP70h and p6 protein have been characterized as associated with membranes. The HSP70h has been located at modest levels to vesicles and the chloroplast (Medina et al., 1999). The p6 protein, which is considered this virus's conventional MP, was associated with the rough ER during either p6 overexpression or virus infection (Peremyslov et al., 2004). This protein was determined to have a single span _trans_-membrane N-terminal domain and a C-terminal domain that is present in the cytoplasmic face of the ER (Peremyslov et al., 2004; Zamyatnin et al., 2006). How the membrane interaction displayed by the p6 protein is related to virus movement is unknown. In addition, how this protein and the HSP70h protein function together with the other three movement-associated proteins from this virus remains to be determined.
Other Viruses not Known to Form Tubules
Recent work involving three other viruses, one a minus-sense monopartite RNA virus, the second a four-part minus-sense RNA virus, and the third a single-stranded DNA virus, has further shed light on virus movement and membrane. Sonchus yellow net virus, a member of the Rhabdoviridae family, encodes a putative MP, sc4, and a nucleocapsid protein, N, that interact with host proteins associated with membranes and microtubules (Min et al., 2010). These interactions were identified through yeast two-hybrid screens and their interaction was verified in plants using BiFC assays. The authors hypothesized that a movement complex involving these viral and host proteins and viral RNA transports within the cell from the nuclear export site to the PD. Rice stripe virus, a member of the genus Tenuivirus, encodes a putative MP, NSvc4, which moves between cells (Xiong et al. 2008). This protein, as a C-terminal fusion with YFP, utilizes COP-II and myosin VIII-mediated systems to reach the PD during its ectopic expression (Yuan et al. 2011). Cabbage leaf curl virus (CaLCuV), a member of the Geminviridae family, encodes an MP that, like other geminivirus orthologs of this protein, is necessary for intercellular movement of this DNA virus (Sanderfoot et al., 1996; Rojas et al., 2001). It was recently determined that a plant synaptotagmin, a member of a family of clathrin-associated, SNARE-interacting transport proteins (Bassham et al., 2008), interacts with the CaLCuV intercellular MP and knockdown of its activity delayed virus systemic infection and intercellular spread of the MP (Lewis and Lazarowitz, 2010). A dominant-negative synaptotagmin mutant depleted PM-derived endosomes and yielded large intracellular vesicles attached to the PM. The authors suggested that the plant synaptogamin regulates endocytosis and this recycling pathway is required for virus movement. Interestingly, this same synaptotagmin interacted with the TMV MP and down-regulation of its expression inhibited the intercellular spread of this virus. Thus, there may be commonality in the transport requirements between an RNA virus and a DNA virus.
INTRACELLULAR MOVEMENT OF PLANT VIRUSES WITH MPS ASSOCIATED WITH TUBULE FORMATION
Several genera of plant viruses move from cell to cell as virions or nucleocapsid complexes by inducing a drastic modification of the PD into tubules. The changes to PD structure induced by the tubule formers involves the elimination of the desmotubule (reviewed in Benitez-Alfonso et al., 2010; Niehl and Heinlein, 2011) and an increase in the effective PD SEL from 5 to up to 50 nm (Kitajima and Lauritis, 1969; Lucas et al., 1993). The removal of the desmotubule from the PD indicates that the endomembrane system is not involved during the act of intercellular transport by these viruses, although it may be necessary for intracellular movement from the site of virion assembly to the tubule. Virus genera with members utilizing tubules for intercellular movement include the Caulimovirus, Comovirus, Tospovirus, Bromovirus, and Nepovirus (Kitajima and Lauritis, 1969; van Lent et al., 1991; Perbal et al., 1993; Ritzenthaler et al., 1995; Storms et al., 1995; Kasteel et al., 1997; Melcher, 2000). With viruses such as the RNA-based comovirus, Cowpea mosaic virus (CPMV), and the DNA-based caulimovirus, Dahlia mosaic virus, electron micrographs revealed virions in single file within the tubules (Kitajima and Lauritis, 1969; van Lent et al., 1991). It has been demonstrated with a number of viruses including Cauliflower mosaic virus (CaMV), Tomato spotted wilt virus (TSWV), CPMV, and Grapevine fanleaf virus (GFLV) that the MPs are necessary and sufficient for the formation of tubule structures (van Lent et al., 1991; Perbal et al., 1993; Ritzenthaler et al., 1995; Storms et al., 1995). In fact, the expression of the MP gene of these viruses in protoplasts will result in the elaboration of tubules from the surface of the protoplast into the medium. However, it is largely unknown how the virions or nucleoprotein complexes make their way from their site of assembly and accumulation in the cytoplasm to the plasma membrane where tubules develop. With each of the viruses in this section, we discuss the likely viral protein candidates that could have a role in intracellular movement.
Genus Caulimovirus
It is well established that the CaMV MP accumulates in foci at the PM in protoplasts and is the only viral protein necessary for the formation of tubules through which CaMV virions move (Perbal et al., 1993; Kasteel et al., 1996; Huang et al., 2000). To date, the CaMV MP has been shown to physically interact with a host protein involved in vesicular transport (Huang et al., 2001b). The protein, MP17, was identified in a yeast two-hybrid screen of Arabidopsis thaliana proteins. It belongs to the PRA1 gene family in Arabidopsis, a family of proteins that regulate vesicle trafficking between different cellular compartments including the Golgi, ER, and endosomes (Kamei et al., 2008). MP17 co-localizes with the CaMV MP in punctate spots at the initiation sites of tubules, and mutations in the CaMV MP that abolish cell-to-cell movement also abolish its interaction with MP17 (Huang et al., 2001b). Treatment of protoplasts with cytoskeletal assembly inhibitors had no effect on tubule formation or on the trafficking of a CaMV GFP–MP to foci at the cell periphery, whereas brefeldin A treatment inhibited tubule formation but not the development of foci (Huang et al., 2000). These results suggested that the cytoskeleton was not involved in movement of the MP to the cell periphery, but that the endomembrane system may be necessary for tubule formation.
The CaMV MP also physically interacts within PD with a PD-located protein (PDLP1) (Amari et al., 2010; Fernandez-Calvino et al., 2011). PDLP1 is found at the PD-associated membrane, with its N-terminus in the apoplast and its C-terminus in the cytoplasm. PDLP1 belongs to a small gene family of Arabidopsis proteins that modulates cell-to-cell trafficking and it is targeted to the PD through the secretory pathway in a COP-II-dependent manner (Thomas et al., 2008). Cytological evidence suggests that PDLPs interact with the MP of CaMV and with the MP of the unrelated virus, GFLV, at the base of tubules (Amari et al., 2010). Inoculation of CaMV to Arabidopsis plants in which PDLP1, PDLP2, and PDLP3 genes were mutated resulted in significantly fewer plants that developed systemic symptoms by 21 dpi. The authors concluded that intercellular virus movement was impaired, because replication of CaMV in protoplasts of the triple mutant was comparable to replication in wild-type Arabidopsis protoplasts (Amari et al., 2010).
Although the CaMV MP is responsible for the formation of tubules through which CaMV virions move, the MP does not appear to physically interact with virions. Instead, the interaction between virions and MP is mediated by the CaMV P3 protein, a 15-kDa protein required both for intercellular movement and aphid transmission (Leh et al., 1999; Stavolone et al., 2005). The P3 protein forms a rod-like tretramer structure in which the C-terminus of the protein is anchored to virions (Leclerc et al., 1998, 2001; Leh et al., 2001). The N-terminus of P3 forms a coiled-coil domain that interacts with a trimer of the MP (Stavolone et al., 2005). Immunogold labeling and electron microscopy have shown that MP and P3 co-localize with virions only within PD. Indeed, Stavolone and coworkers (2005) suggest that the complex of P3 and virions travels to the PD independently from the MP.
All of these findings involve observations at or around the PD, so the question remains as to how the CaMV genome gets to this location from its replication site in the cell. It is generally accepted that CaMV virions in the cytoplasm are found within large, amorphous inclusion bodies that are composed of the CaMV P6 protein (Li and Leisner, 2002; Haas et al., 2005). The P6 protein is a multifunctional protein that has roles in the development of chlorotic symptoms (Daubert et al., 1984; Baughman et al., 1988) and host range (Daubert et al., 1984; Schoelz et al., 1986), as well as expression of genes on the 35S RNA (Ryabova et al., 2002) and suppression of gene silencing (Love et al., 2007). No subcellular structures had been shown to be associated with P6 inclusion bodies until Harries et al. (2009a) demonstrated that P6 inclusion bodies actually co-localize with the ER, microtubules, and actin microfilaments. Harries et al. (2009a) expressed GFP-tagged P6 protein through agroinfiltration in Nicotiana species and showed that the P6–GFP inclusions are capable of movement on microfilaments. In addition, treatment of N. edwardsonii leaves with LatB abolished CaMV local lesions, suggesting that actin microfilaments are necessary for lesion formation. Microtubules do not appear to have a role in movement (Harries et al., 2009a) and it is not yet known whether the ER is involved. Since the P6 inclusion bodies themselves are capable of movement in the cell, it is plausible that P6 inclusion bodies, or perhaps much smaller aggregates of the P6 protein and virions, could deliver the virions to the tubules for movement to adjacent cells. Significantly, the C-terminal portion of the P6 protein interacts with the coat protein (CP; Himmelbach et al., 1996; Ryabova et al., 2002) whereas the N-terminus of P6 interacts with the MP (Hapiak et al., 2008), so it is tempting to speculate that the P6 protein might facilitate transfer of virions to the MP localized at the tubules.
Genus Nepovirus
In contrast to CaMV MP, the nine-terminal amino acids of the MP of the nepovirus GFLV interact with its virus CP directly (Belin et al., 1999). In infected plants, the GFLV virions can be found in crystalline and paracrystalline arrays in the cytoplasm (Savino et al., 1985). GFLV induces the formation of a perinuclear compartment where it replicates on membranes derived from the ER (Ritzenthaler et al., 2002). Although the GFLV MP could potentially transport the GFLV virions to the cell periphery for transfer through the tubules, this has not been proven. Ritzenthaler and coworkers (2002) have suggested that MP may initially be synthesized within the perinuclear compartment, because it initially is produced as part of a polyprotein that includes the CP (Margis et al., 1993). However, the GFLV CP was detected in the perinuclear compartment, whereas the MP was almost exclusively found in the tubules (Ritzenthaler et al., 2002). These observations suggest, but do not prove, that GFLV CP and MP utilize independent mechanisms to travel to the sites of tubule formation.
Furthermore, it is not clear how the GFLV MP itself is trafficked to the PD to initiate the formation of tubules. Tubule development in tobacco BY-2 cells expressing GFLV MP is inhibited by brefeldin A, indicating an involvement of the secretory pathway (Laporte et al., 2003). In addition, oryzalin treatment results in the abnormal localization of tubules, indicating that microtubules are necessary for correct targeting of MP to cross walls. The GFLV MP co-immunoprecipates with KNOLLE, a member of a family of t-SNARE proteins for vesicle-mediated trafficking (Laporte et al., 2003). Although the association of KNOLLE with GFLV MP might explain how MP reaches the cell periphery, its exact role in viral movement has yet to be elucidated. The GFLV MP protein also interacts with PDLPs at the base of tubule, but the PDLPs are not thought to have a role in trafficking of MP to the PD (Amari et al., 2010). Since PDLPs also co-localize with the MP of CaMV, Amari and coworkers (2010) speculated that they might be universal targets for localization of tubule-forming MPs to the cell membrane, but this hypothesis will need to be verified with other tubule-forming viruses. These studies with GFLV have focused more on host proteins necessary for tubule formation and perhaps trafficking of the MP itself to the cell periphery rather than the intracellular movement of GFLV virions to the tubules.
Genus Comovirus
CPMV is a comovirus that is distinguished from most of the viruses in this review in that its genome encodes two coat proteins: the large (37-kDa) CP and the small (23-kDa) CP. Interestingly, the C-terminus of the 48-kDa MP of CPMV interacts with the large CP but does not bind to the small CP (Carvalho et al., 2003). Furthermore, a deletion of the C-terminal 48 amino acids of the CPMV MP abolished the interaction between MP and large CP, but did not affect tubule formation. The MP deletion mutant formed empty tubules and the virus carrying the deletion could not move between cells (Lekkerkerker et al., 1996). This study showed that the interaction between CPMV MP and large CP was necessary for virus intercellular movement, but it did not determine whether the MP–large CP interaction was required for intracellular movement or only interactions at the PD.
Regarding virus replication, CPMV induces the formation of amorphous inclusion bodies that contain small membraneous vesicles (de Zoeten et al., 1974; Carette et al., 2000). The inclusion bodies are frequently found next to nuclei and are thought to be constructed on ER membranes which proliferate in cowpea cells infected with CPMV (de Zoeten et al., 1974; Carette et al., 2000). Carette and coworkers (2002a) analyzed the distribution of CPMV RNA, MP, and CP in protoplasts at 36 h post inoculation (hpi). At this time point, the viral RNA accumulated in the large inclusion body, whereas an antibody that recognized both the 48-kDa MP and co-C-terminal 58-kDa protein localized those proteins to the nucleus. Both viral RNA and the 110-kDa polymerase co-localize with the vesicles in the inclusion body, so it is likely the site for replication of CPMV (Carette et al., 2002a). By contrast, the virions accumulated on the cell periphery rather than in the inclusion body. We interpret these results to mean that, after encapsidation, the virions are rapidly transported to the plasma membrane for movement to adjacent cells. Treatments of protoplasts with the cytoskeleton inhibitors oryzalin and LatB indicated that microfilaments, but not microtubules, were necessary for formation of the large inclusion body. Carette and coworkers (2002a) suggested that the CPMV replication complexes might use the actin network to traffic to and accumulate in the inclusion body.
It is not known exactly how the CPMV virions and MP are trafficked from the replication site in the inclusion body or from the nucleus to the modified PD. Interestingly, at the site of replication, the CPMV 60-kDa helicase interacts with two proteins in the VAMP33 family of SNARE-like proteins (most often VAMPs are v-SNAREs; Bassham et al., 2008) and they co-localize to the ER-derived vesicles in the inclusion body (Carette et al., 2002b). However, this may indicate a role for vesicular membranes in replication rather than movement. Pouwels et al. (2002) expressed a CPMV MP–GFP construct in protoplasts to show that development of punctate structures on the cell periphery was unaffected by LatB, oryzalin, and brefeldin A. This experiment indicated that neither the cytoskeleton nor the secretory system was involved in intracellular movement of the MP to the plasma membrane, so it is not known how MP is trafficked to the cell periphery. Of the three pharmacological agents, only brefeldin A inhibited tubule formation. The authors speculated that the brefeldin A might inhibit the PM-targeting of vesicles containing host elements required for tubule formation. Significantly, Huang and coworkers (2000) obtained the same results when they examined the effect of pharmacological agents on the capacity of CaMV MP to form tubules in protoplasts.
Genus Tospovirus
Of all the plant viruses that utilize tubules for cell-to-cell movement, TSWV is unique, because its virions are surrounded by a membrane of host origin (German et al., 1992). To understand how TSWV moves intra- and intercellularly, it is important to examine how virions are assembled in the cell. The TSWV 29-kD N-protein is the capsid protein; it is tightly bound to the three genomic RNAs to form large, amorphous nucleocapsid aggregates (NCA) as early as 18 h after inoculation (Kikkert et al., 1999). For development of the membrane bound virions, a fraction of the nucleocapsid structures become enveloped in Golgi cisternae. As the particle matures, the Golgi membranes are wrapped around the nucleocapsids to form a double-enveloped particle. The mature, single-enveloped particle subsequently forms through fusion of double-enveloped particles with each other or with the ER, and the two TSWV glyocoproteins Gn and Gc migrate from the ER to the Golgi complex, where they are incorporated into the virion membrane (Ribeiro et al., 2009). The membrane-bound nucleocapsids are the form that is taken up by the thrips vector for transmission to other plants. However, a large fraction of the nucleocapsids are not destined for formation into membrane-bound virions, but instead remain localized in the NCAs (Kikkert et al., 1999). The nucleocapsid particles in the NCA presumably are responsible for intercellular movement.
Storms et al. (1995) noted that the tubules formed in N. rustica mesophyll tissue by the TSWV NSm MP had a diameter of 40–45 nm, which would be too small to allow passage of the 110-nm-wide membrane-bound virions. They speculated that the nucleoprotein particle was the form that moves to adjacent cells through tubules. Kormelink et al. (1991) showed that the NSm protein was associated with the NCA, and Soellick et al. (2000) demonstrated that the NSm and the N capsid proteins physically interact in a yeast two-hybrid assay. Consequently, the NSm could be responsible for movement of the nucleoprotein particles from the NCA to the site of tubule assembly and through the tubules to the adjacent cell. The NSm protein has been shown to interact in yeast two-hybrid screens with two different host proteins: a DnaJ-like chaperone (Soellick et al., 2000; von Bargen et al., 2001) and At-4/1, an Arabidopsis protein that localizes to punctate spots on the cell periphery (von Bargen et al., 2001; Paape et al., 2006). Proteins with DnaJ domains have the capacity to bind to Hsp-70, which, in turn, has been implicated in movement of the Beet yellows closterovirus (Peremyslov et al., 1999). The interaction of the DnaJ protein with TSWV NSm has led to speculation that it might mediate trafficking of the NSm and nucleocapsid protein to tubules (Soellick et al., 2000; von Bargen et al., 2001). However, the interactions have not been confirmed in vivo and, more importantly, it has not been proven that the DnaJ protein has a functional role in movement of TSWV. The At-4/1 protein has been localized to the ER and to the cell wall in the vicinity of PD (Paape et al., 2006), but, as with DnaJ, it has not been proven yet to have a functional role in movement of TSWV. Interestingly, the NSm protein complemented cell-to-cell movement of TMV mutants incapable of this activity (Lewandowski and Adkins, 2005). This suggests that the TSWV NSm protein, possibly through interactions with the proteins described above, is able to mimic the function of the TMV MP and allow TMV to move between cells.
Other Tubule-Forming Viruses
Results from studies with other tubule-forming viruses closely related to TMV (members of the alphavirus supergroup) also provide tantalizing information on membrane involvement during virus movement. Cucumber mosaic virus (CMV) 1a protein, an apparent ortholog to the TMV 126-kDa protein, is required for CMV intercellular movement and associates with the tonoplast (Cillo et al., 2002). At this time, it is unknown whether its membrane association influences virus movement. The MP of CMV forms tubules extending from the surface of protoplasts (Canto and Palukaitis, 2005), but the membrane content (presumed to include at least the plasma membrane due to location) and interaction of the MP with these structures were not determined. However, unlike all of the other tubule-forming viruses, these tubules are not necessary for CMV intercellular movement. The MP of another member of the alphavirus supergroup that includes TMV and CMV, Alfalfa mosaic virus (AlMV), localizes to the ER (Huang and Zhang, 1999). Later studies determined that a mutant MP that did not fractionate with membrane fractions also did not go to the PD (Huang et al., 2001a). When transcripts of this mutant MP were inoculated to transgenic plants expressing AlMV replicase, there was no accumulation of this protein in systemic or local tissue around the inoculation site, indicating this protein lacked movement function. More recently, the MP of Prunus necrotic ringspot virus, an ilarvirus whose MP can complement the function of the related AlMV MP, was shown to contain a hydrophobic region that associated with a membrane, but did not span it (Martínez-Gil et al. 2009). Through a mutational analysis it was concluded that the hydrophobic region was required for cell-to-cell movement of AlMV RNA 3 (Martínez-Gil et al. 2009). These findings are a reminder that hydrophobic interactions between viral proteins (e.g. MPs) and plant membranes may not require a transmembrane domain for their function.
CONCLUSIONS
There has been an explosion of information in the last 10–15 years about the cellular and molecular mechanisms that facilitate intra- and intercellular virus movement in plants. For example, the finding by McLean et al. (1995) that the TMV MP interacts with cytoskeletal elements has spawned a large body of work examining virus–cytoskeletal interactions (reviewed in Harries et al., 2010; Niehl and Heinlein, 2011). There is now a rapidly growing list of viral proteins that have been shown to interact with the cytoskeleton (reviewed in Harries et al., 2010), although, in many instances, the significance of these interactions for movement remains to be clarified. Similarly, the discovery that numerous diverse viruses replicate in association with membranes (reviewed in Laliberté and Sanfaçon, 2010) raised the possibility that viruses might also utilize host membranes for their transport. As discussed above, there is a wealth of valuable information about the interactions between viruses and membranes and it is clear that many viruses traffic through the host cell's endomembrane system. We have included a schematic of a cell showing the association of various viral proteins involved in intercellular virus movement with membranes (Figure 1). An understanding of virus transport is of obvious interest to virologists and those who wish to ameliorate virus infections. However, it may also serve to unlock a greater understanding of general macromolecular trafficking in plants. Despite the important recent strides in dissecting virus movement, due to the great diversity of plant viruses, there is no unifying model for virus movement and there is clearly still much to learn.
Progress in the future will depend in large part upon identifying additional host factors that interact with virus proteins and on uncovering additional movement functions of virus proteins other than MPs. As we discussed above, there is now evidence from numerous viruses that multiple virus proteins are critical for movement during a single infection. An attempt to understand the interactions between these proteins and other host factors will be critical if progress is to be made in understanding virus movement. In particular, identifying the interaction of virus proteins with specific host factors can provide many insights into possible trafficking mechanisms. Numerous methods are available and have been successfully used to identify virus/host interactors, including yeast two-hybrid screening (Fridborg et al., 2003; Paape et al., 2006; Lewis and Lazarowitz, 2010), co-immunoprecipitation (Laporte et al., 2003), and far-Western analysis (Yoshioka et al., 2004), but validating results in vivo is critical, regardless of what method is used to identify interactors. For example, Min et al. (2010) recently screened Sonchus yellow net virus proteins against an N. benthamiana yeast two-hybrid library and validated their positive interactions using BiFC. This approach is particularly powerful because it gives valuable information regarding the subcellular localization of the interaction, which, in turn, may yield more information about the biological significance of these proteins. Beyond the demonstration of an interaction in vivo, it is important to determine its functional importance. It would certainly be worthwhile to employ a similar approach for other virus proteins in the future. Since virus genomes contain just a handful of proteins, the more we understand about the host cell ‘keys’ that allow viruses to travel to and through the PD ‘doors’, the closer we will come to gaining a clear picture of the varied mechanisms utilized by viruses for their movement.
Funding
This work was supported by an Agriculture and Food Research Initiative Competitive Grant (No. 2010-65108-20525) from the USDA National Institute of Food and Agriculture to J.E.S. and R.S.N., The National Center for Research Resources (NIH Award No. P20RR016475) to P.A.H., and the Samuel Roberts Noble Foundation, Inc. to R.S.N.
Acknowledgments
The authors wish to acknowledge Mustafa Morsy and Julia Dyachok for critical reading of the manuscript. No conflict of interest declared.
References
- Ajjikuttira P, Loh CS, Wong SM. Reciprocal function of movement proteins and complementation of long-distance movement of Cymbidium mosaic virus RNA by Odontoglossum ringspot virus coat protein. J. Gen. Virol. 2005;86:1543–1553. doi: 10.1099/vir.0.80772-0. [DOI] [PubMed] [Google Scholar]
- Alzhanova DV, Hagiwara Y, Peremyslov VV, Dolja VV. Genetic analysis of the cell-to-cell movement of beet yellows closterovirus. Virology. 2000;268:192–200. doi: 10.1006/viro.1999.0155. [DOI] [PubMed] [Google Scholar]
- Amari K, et al. A family of plasmodesmal proteins with receptor-like properties for plant viral movement proteins. PLoS Path. 2010;6 doi: 10.1371/journal.ppat.1001119. e1001119. doi 10.1371/journal.ppat.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins D, Hull R, Wells B, Roberts K, Moore P, Beachy RN. The Tobacco mosaic virus 30K movement protein in transgenic tobacco plants is localized to plasmodesmata. J. Gen. Virol. 1991;72:209–211. doi: 10.1099/0022-1317-72-1-209. [DOI] [PubMed] [Google Scholar]
- Barajas D, Jiang Y, Nagy PD. A unique role for the host ESCRT proteins in replication of tomato bushy stunt virus. PLoS Path. 2009;5 doi: 10.1371/journal.ppat.1000705. e1000705. doi:10.1371/journal.ppat.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Brandizzi F, Otegui MS, Sanderfoot AA. The Arabidopsis Book. 2008. The secretory system of Arabidopsis; p. e0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman GA, Jacobs JD, Howell SH. Cauliflower mosaic virus gene VI produces a symptomatic phenotype in transgenic tobacco plants. Proc. Natl Acad. Sci. U S A. 1988;85:733–737. doi: 10.1073/pnas.85.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy RN, Zaitlin M. Replication of tobacco mosaic virus.6: replicative intermediates and TMV-RNA-related RNAs associated with polyribosomes. Virology. 1975;63:84–97. doi: 10.1016/0042-6822(75)90373-6. [DOI] [PubMed] [Google Scholar]
- Belin C, Schmitt C, Gaire F, Walter B, Demangeat G, Pinck L. The nine C-terminal residues of the grapevine fanleaf nepovirus movement protein are critical for systemic virus spread. J. Gen. Virol. 1999;80:1347–1356. doi: 10.1099/0022-1317-80-6-1347. [DOI] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Faulkner C, Ritzenthaler C, Maule AJ. Plasmodesmata: gateways to local and systemic virus infection. Mol. Plant–Microbe Interact. 2010;23:1403–1412. doi: 10.1094/MPMI-05-10-0116. [DOI] [PubMed] [Google Scholar]
- Brill LM, Dechongkit S, DeLaBarre B, Stroebel J, Beachy RN, Yeager M. Dimerization of recombinant tobacco mosaic virus movement protein. J. Virol. 2004;78:3372–3377. doi: 10.1128/JVI.78.5.3372-3377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto T, Palukaitis P. Subcellular distribution of mutant movement proteins of cucumber mosaic virus fused to green fluorescent proteins. J. Gen. Virol. 2005;86:1223–1228. doi: 10.1099/vir.0.80351-0. [DOI] [PubMed] [Google Scholar]
- Carette JE, Guhl K, Wellink J, Van Kammen A. Coalescence of the sites of cowpea mosaic virus RNA replication into a cytopathic structure. J. Virol. 2002a;76:6235–6243. doi: 10.1128/JVI.76.12.6235-6243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette JE, Stuiver M, Van Lent J, Wellink J, Van Kammen AB. Cowpea mosaic virus infection induces a massive proliferation of endoplasmic reticulum but not Golgi membranes and is dependent on de novo membrane synthesis. J. Virol. 2000;74:6556–6563. doi: 10.1128/jvi.74.14.6556-6563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette JE, Verver J, Martens J, van Kampen T, Wellink J, van Kammen A. Characterization of plant proteins that interact with cowpea mosaic virus ‘6OK’ protein in the yeast two-hybrid system. J. Gen. Virol. 2002b;83:885–893. doi: 10.1099/0022-1317-83-4-885. [DOI] [PubMed] [Google Scholar]
- Carrington JC, Jensen PE, Schaad MC. Genetic evidence for an essential role for potyvirus CI protein in cell-to-cell movement. Plant J. 1998;14:393–400. doi: 10.1046/j.1365-313x.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- Carvalho CM, Wellink J, Ribeiro SG, Goldbach RW, van Lent JWM. The C-terminal region of the movement protein of Cowpea mosaic virus is involved in binding to the large but not to the small coat protein. J. Gen. Virol. 2003;84:2271–2277. doi: 10.1099/vir.0.19101-0. [DOI] [PubMed] [Google Scholar]
- Chen M-H, Tian G-W, Gafni Y, Citovsky V. Effects of calreticulin on viral cell-to-cell movement. Plant Physiol. 2005;138:1866–1876. doi: 10.1104/pp.105.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo F, Roberts IM, Palukaitis P. In situ localization and tissue distribution of the replication-associated proteins of Cucumber mosaic virus in tobacco and cucumber. J. Virol. 2002;76:10654–10664. doi: 10.1128/JVI.76.21.10654-10664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V, Knorr D, Schuster G, Zambryski P. The P-30 protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell. 1990;60:637–647. doi: 10.1016/0092-8674(90)90667-4. [DOI] [PubMed] [Google Scholar]
- Citovsky V, McLean BG, Zupan JR, Zambryski P. Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase. Genes Dev. 1993;7:904–910. doi: 10.1101/gad.7.5.904. [DOI] [PubMed] [Google Scholar]
- Cotton S, Grangeon R, Thivierge K, Mathieu I, Ide C, Wei T, Wang A, Laliberte J-F. Turnip mosaic virus RNA replication complex vesicles are mobile, align with microfilaments, and are each derived from a single viral genome. J. Virol. 2009;83:10460–10471. doi: 10.1128/JVI.00819-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan GH, Lioliopoulou F, Ziegler A, Torrance L. Subcellular localisation, protein interactions, and RNA binding of potato mop-top virus triple gene block proteins. Virology. 2002;298:106–115. doi: 10.1006/viro.2002.1435. [DOI] [PubMed] [Google Scholar]
- Cui X, Wei T, Chowda-Reddy RV, Sun G, Wang A. The Tobacco etch virus P3 protein forms mobile inclusions via the early secretory pathway and traffics along actin microfilaments. Virology. 2010;397:56–63. doi: 10.1016/j.virol.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Daubert SD, Schoelz J, Debao L, Shepherd RJ. Expression of disease symptoms in cauliflower mosaic virus genomic hybrids. J. Mol. Appl. Genet. 1984;2:537–548. [PubMed] [Google Scholar]
- De Zoeten GA, Assink AM, Van Kammen A. Association of cowpea mosaic virus induced double stranded RNA with a cytopathological structure in infected cells. Virology. 1974;59:341–355. doi: 10.1016/0042-6822(74)90449-8. [DOI] [PubMed] [Google Scholar]
- Deom CM, Oliver MJ, Beachy RN. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science. 1987;237:389–394. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- Desvoyes B, Faure-Rabasse S, Chen MH, Park JW, Scholthof HB. A novel plant homeodomain protein interacts in a functionally relevant manner with a virus movement protein. Plant Physiol. 2002;129:1521–1532. doi: 10.1104/pp.004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Haudenshield JS, Hull RJ, Wolf S, Beachy RN, Lucas WJ. Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell. 1992;4:915–928. doi: 10.1105/tpc.4.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Kwon MO, Warnberg L. Evidence that actin filaments are involved in controlling the permeability of plasmodesmata in tobacco mesophyll. Plant J. 1996;10:157–164. [Google Scholar]
- Ding XS, Liu JZ, Cheng NH, Folimonov A, Hou YM, Bao YM, Katagi C, Carter SA, Nelson RS. The Tobacco mosaic virus 126-kDa protein associated with virus replication and movement suppresses RNA silencing. Mol. Plant–Microbe Interact. 2004;17:583–592. doi: 10.1094/MPMI.2004.17.6.583. [DOI] [PubMed] [Google Scholar]
- Donald RG, Lawrence DM, Jackson AO. The barley stripe mosaic virus 58-kilodalton beta (b) protein is a multifunctional RNA binding protein. J. Virol. 1997;71:1538–1546. doi: 10.1128/jvi.71.2.1538-1546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis Figueira A, Golem S, Goregaoker SP, Culver JN. A nuclear localization signal and a membrane association domain contribute to the cellular localization of the Tobacco mosaic virus 126-kDa replicase protein. Virology. 2002;301:81–89. doi: 10.1006/viro.2002.1560. [DOI] [PubMed] [Google Scholar]
- Eiamtanasate S, Juricek M, Yap YK. C-terminal hydrophobic region leads PRSV P3 protein to endoplasmic reticulum. Virus Genes. 2007;35:611–617. doi: 10.1007/s11262-007-0114-z. [DOI] [PubMed] [Google Scholar]
- Epel BL. Plant viruses spread by diffusion on ER-associated movement-protein-rafts through plasmodesmata gated by viral induced host beta-1,3-glucanases. Semin. Cell Dev. Biol. 2009;20:1074–1081. doi: 10.1016/j.semcdb.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Esau K, Cronshaw J. Relation of tobacco mosaic virus to host cells. J. Cell Biol. 1967;33:665–678. doi: 10.1083/jcb.33.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Calvino L, et al. Arabidopsis plasmodesmal proteome. Plos One. 2011;6 doi: 10.1371/journal.pone.0018880. e18880. doi:10.1371/journal.pone.0018880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridborg I, Grainger J, Page A, Coleman M, Findlay K, Angell S. TIP, a novel host factor linking callose degradation with the cell-to-cell movement of Potato virus X. Mol. Plant–Microbe Interact. 2003;16:132–140. doi: 10.1094/MPMI.2003.16.2.132. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Kawakami S, Kim RW, Beachy RN. Domains of tobacco mosaic virus movement protein essential for its membrane association. J. Gen. Virol. 2006;87:2699–2707. doi: 10.1099/vir.0.81936-0. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Giesman-Cookmeyer D, Ding B, Lommel SA, Lucas WJ. Cell-to-cell trafficking of macromolecules through plasmodesmata potentiated by the red clover necrotic mosaic virus movement protein. Plant Cell. 1993;5:1783–1794. doi: 10.1105/tpc.5.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoves A, Navarro JA, Pallas V. The intra- and intercellular movement of melon necrotic spot virus (MNSV) depends on an active secretory pathway. Mol. Plant–Microbe Interact. 2010;23:263–272. doi: 10.1094/MPMI-23-3-0263. [DOI] [PubMed] [Google Scholar]
- German TL, Ullman DE, Moyer JW. Tospoviruses: diagnosis, molecular biology, phylogeny, and vector relationships. Annu. Rev. Phytopathol. 1992;30:315–348. doi: 10.1146/annurev.py.30.090192.001531. [DOI] [PubMed] [Google Scholar]
- Giesman-Cookmeyer D, Lommel SA. Alanine scanning mutagenesis of a plant virus movement protein identifies three functional domains. Plant Cell. 1993;5:973–982. doi: 10.1105/tpc.5.8.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesman-Cookmeyer D, Silver S, Vaewhongs AA, Lommel SA, Deom CM. Tobamovirus and dianthovirus movement proteins are functionally homologous. Virology. 1995;213:38–45. doi: 10.1006/viro.1995.1544. [DOI] [PubMed] [Google Scholar]
- Guenoune-Gelbart D, Elbaum M, Sagi G, Levy A, Epel BL. Tobacco mosaic virus (TMV) replicase and movement protein function synergistically in facilitating TMV spread by lateral diffusion in the plasmodesmal desmotubule of Nicotiana benthamiana. Mol. Plant–Microbe Interact. 2008;21:335–345. doi: 10.1094/MPMI-21-3-0335. [DOI] [PubMed] [Google Scholar]
- Haas M, et al. The open reading frame VI product of Cauliflower mosaic virus is a nucleocytoplasmic protein: its N terminus mediates its nuclear export and formation of electron-dense viroplasms. Plant Cell. 2005;17:927–943. doi: 10.1105/tpc.104.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara Y, Komoda K, Yamanaka T, Tamai A, Meshi T, Funada R, Tsuchiya T, Naito S, Ishikawa M. Subcellular localization of host and viral proteins associated with tobamovirus RNA replication. EMBO J. 2003;22:344–353. doi: 10.1093/emboj/cdg033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley A, Hunter T, Kiberstis P, Zimmern D. Multiple serine phosphorylation sites on the 30 kDa TMV cell-to-cell movement protein synthesized in tobacco protoplasts. Plant J. 1995;8:715–724. doi: 10.1046/j.1365-313x.1995.08050715.x. [DOI] [PubMed] [Google Scholar]
- Hanton S, Matheson L, Chatre L, Rossi M, Brandizzi F. Post-Golgi protein traffic in the plant secretory pathway. Plant Cell Rep. 2007;26:1431–1438. doi: 10.1007/s00299-007-0390-z. [DOI] [PubMed] [Google Scholar]
- Hapiak M, et al. Cauliflower mosaic virus gene VI product N-terminus contains regions involved in resistance-breakage, self-association and interactions with movement protein. Virus Res. 2008;138:119–129. doi: 10.1016/j.virusres.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries P, Ding B. Cellular factors in plant virus movement: at the leading edge of macromolecular trafficking in plants. Virology. 2011;411:237–243. doi: 10.1016/j.virol.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Harries PA, Nelson RS. Movement of viruses in plants. In: Mahy BWJ, van Regenmortel MHV, editors. Encyclopedia of Virology. Oxford: Elsevier Ltd; 2008. pp. 348–355. [Google Scholar]
- Harries PA, Palanichelvam K, Yu WC, Schoelz JE, Nelson RS. The cauliflower mosaic virus protein P6 forms motile inclusions that traffic along actin microfilaments and stabilize microtubules. Plant Physiol. 2009a;149:1005–1016. doi: 10.1104/pp.108.131755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries PA, Park J-W, Sasaki N, Ballard KD, Maule AJ, Nelson RS. Differing requirements for actin and myosin by plant viruses for sustained intercellular movement. Proc. Natl Acad. Sci. U S A. 2009b;106:17594–17599. doi: 10.1073/pnas.0909239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries PA, Schoelz JE, Nelson RS. Intracellular transport of viruses and their components: utilizing the cytoskeleton and membrane highways. Mol. Plant–Microbe Interact. 2010;23:1381–1393. doi: 10.1094/MPMI-05-10-0121. [DOI] [PubMed] [Google Scholar]
- Haupt S, Cowan GH, Ziegler A, Roberts AG, Oparka KJ, Torrance L. Two plant-viral movement proteins traffic in the endocytic recycling pathway. Plant Cell. 2005;17:164–181. doi: 10.1105/tpc.104.027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood V, Kragler F, Lucas WJ. Plasmodesmata: pathways for protein and ribonucleoprotein signaling. Plant Cell. 2002;14:S303–S325. doi: 10.1105/tpc.000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein M, Padgett HS, Gens JS, Pickard BG, Casper SJ, Epel BL, Beachy RN. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell. 1998;10:1107–1120. doi: 10.1105/tpc.10.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach A, Chapdelaine Y, Hohn T. Interaction between cauliflower mosaic virus inclusion body protein and capsid protein: implications for viral assembly. Virology. 1996;217:147–157. doi: 10.1006/viro.1996.0102. [DOI] [PubMed] [Google Scholar]
- Hirashima K, Watanabe Y. Tobamovirus replicase coding region is involved in cell-to-cell movement. J. Virol. 2001;75:8831–8836. doi: 10.1128/JVI.75.18.8831-8836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima K, Watanabe Y. RNA helicase domain of tobamovirus replicase executes cell-to-cell movement possibly through collaboration with its nonconserved region. J. Virol. 2003;77:12357–12362. doi: 10.1128/JVI.77.22.12357-12362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C, Niehl A, Sambade A, Steinmetz A, Heinlein M. Inhibition of Tobacco mosaic virus movement by expression of an actin-binding protein. Plant Physiol. 2009;149:1810–1823. doi: 10.1104/pp.108.133827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H, Chou YL, Tseng YH, Lin YH, Lin TM, Lin NS, Hsu YH, Chang BY. Topological properties of the triple gene block protein 2 of Bamboo mosaic virus. Virology. 2008;379:1–9. doi: 10.1016/j.virol.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Hsu H, Tseng Y, Chou Y, Su S, Hsu Y, Chang B. Characterization of the RNA-binding properties of the triple-gene-block protein 2 of Bamboo mosaic virus. Virology J. 2009 doi: 10.1186/1743-422X-6-50. 6, 50. doi:10.1186/1743-422X-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Zhang L. Association of the movement protein of alfalfa mosaic virus with the endoplasmic reticulum and its trafficking in epidermal cells of onion bulb scales. Mol. Plant–Microbe Interact. 1999;12:680–690. [Google Scholar]
- Huang M, Jongejan L, Zheng HQ, Zhang L, Bol JF. Intracellular localization and movement phenotypes of Alfalfa mosaic virus movement protein mutants. Mol. Plant–Microbe Interact. 2001a;14:1063–1074. doi: 10.1094/MPMI.2001.14.9.1063. [DOI] [PubMed] [Google Scholar]
- Huang Z, Andrianov VM, Han Y, Howell SH. Identification of Arabidopsis proteins that interact with the cauliflower mosaic virus (CaMV) movement protein. Plant Mol. Biol. 2001b;47:663–675. doi: 10.1023/a:1012491913431. [DOI] [PubMed] [Google Scholar]
- Huang Z, Han Y, Howell SH. Formation of surface tubules and fluorescent foci in Arabidopsis thaliana protoplasts expressing a fusion between the green fluorescent protein and the cauliflower mosaic virus movement protein. Virology. 2000;271:58–64. doi: 10.1006/viro.2000.0292. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Meshi T, Motoyoshi F, Takamatsu N, Okada Y. In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 1986;14:8291–8305. doi: 10.1093/nar/14.21.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch H. 2 Mutants of tabacco mosaic virus temperature-sensitive in 2 different functions. Virology. 1968;35:94–101. doi: 10.1016/0042-6822(68)90308-5. [DOI] [PubMed] [Google Scholar]
- Ju H-J, Samuels TD, Wang Y-S, Blancaflor E, Payton M, Mitra R, Krishnamurthy K, Nelson RS, Verchot-Lubicz J. The Potato virus X TGBp2 movement protein associates with endoplasmic reticulum-derived vesicles during virus infection. Plant Physiol. 2005;138:1877–1895. doi: 10.1104/pp.105.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaido M, Funatsu N, Tsuno Y, Mise K, Okuno T. Viral cell-to-cell movement requires formation of cortical punctate structures containing Red clover necrotic mosaic virus movement protein. Virology. 2011;413:205–215. doi: 10.1016/j.virol.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Kaido M, Tsuno Y, Mise K, Okuno T. Endoplasmic reticulum targeting of the Red clover necrotic mosaic virus movement protein is associated with the replication of viral RNA1 but not that of RNA2. Virology. 2009;395:232–242. doi: 10.1016/j.virol.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Kalinina NO, Rakitina DV, Solovyev AG, Schiemann J, Morozov SY. RNA helicase activity of the plant virus movement proteins encoded by the first gene of the triple gene block. Virology. 2002;296:321–329. doi: 10.1006/viro.2001.1328. [DOI] [PubMed] [Google Scholar]
- Kamei CLA, Boruc J, Vandepoele K, Van den Daele H, Maes S, Russinova E, Inzé D, De Veylder L. The PRA1 gene family in Arabidopsis. Plant Phys. 2008;147:1735–1749. doi: 10.1104/pp.108.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karger EM, et al. Dysfunctionality of a tobacco mosaic virus movement protein mutant mimicking threonine 104 phosphorylation. J. Gen. Virol. 2003;84:727–732. doi: 10.1099/vir.0.18972-0. [DOI] [PubMed] [Google Scholar]
- Karpova OV, Ivanov KI, Rodionova NP, Dorokhov YL, Atabekov JG. Nontranslatability and dissimilar behavior in plants and protoplasts of viral RNA and movement protein complexes formed in vitro. Virology. 1997;230:11–21. doi: 10.1006/viro.1997.8472. [DOI] [PubMed] [Google Scholar]
- Kasteel DT, van der Wel NN, Jansen KA, Goldbach RW, van Lent JW. Tubule-forming capacity of the movement proteins of alfalfa mosaic virus and brome mosaic virus. J. Gen. Virol. 1997;78:2089–2093. doi: 10.1099/0022-1317-78-8-2089. [DOI] [PubMed] [Google Scholar]
- Kasteel DTJ, Perbal MC, Boyer JC, Wellink J, Goldbach RW, Maule AJ, van Lent JWM. The movement proteins of cowpea mosaic virus and cauliflower mosaic virus induce tubular structures in plant and insect cells. J. Gen. Virol. 1996;77:2857–2864. doi: 10.1099/0022-1317-77-11-2857. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Watanabe Y, Beachy RN. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc. Natl Acad. Sci. U S A. 2004;101:6291–6296. doi: 10.1073/pnas.0401221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkert M, Van Lent J, Storms M, Bodegom P, Kormelink R, Goldbach R. Tomato spotted wilt virus particle morphogenesis in plant cells. J. Virol. 1999;73:2288–2297. doi: 10.1128/jvi.73.3.2288-2297.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima EW, Lauritis JA. Plant virions in plasmodesmata. Virology. 1969;37:681–685. doi: 10.1016/0042-6822(69)90288-8. [DOI] [PubMed] [Google Scholar]
- Kormelink R, Kitajima EW, Dehaan P, Zuidema D, Peters D, Goldbach R. The nonstructural protein (NSS) encoded by the ambisense-S RNA segment of tomato spotted wilt virus is associated with fibrous structures in infected plant cells. Virology. 1991;181:459–468. doi: 10.1016/0042-6822(91)90878-f. [DOI] [PubMed] [Google Scholar]
- Kotlizky G, Katz A, van der Laak J, Boyko V, Lapidot M, Beachy RN, Heinlein M, Epel BL. A dysfunctional movement protein of Tobacco mosaic virus interferes with targeting of wild-type movement protein to microtubules. Mol. Plant–Microbe Interact. 2001;14:895–904. doi: 10.1094/MPMI.2001.14.7.895. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy K, Heppler M, Mitra R, Blancaflor E, Payton M, Nelson RS, Verchot-Lubicz J. The Potato virus X TGBp3 protein associates with the ER network for virus cell-to-cell movement. Virology. 2003;309:135–151. doi: 10.1016/s0042-6822(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Laliberté JF, Sanfaçon H. Cellular remodeling during plant virus infection. Annu. Rev. Phytopathol. 2010;48:69–91. doi: 10.1146/annurev-phyto-073009-114239. [DOI] [PubMed] [Google Scholar]
- Laporte C, Vetter G, Loudes AM, Robinson DG, Hillmer S, Stussi-Garaud C, Ritzenthaler C. Involvement of the secretory pathway and the cytoskeleton in intracellular targeting and tubule assembly of Grapevine fanleaf virus movement protein in tobacco BY-2 cells. Plant Cell. 2003;15:2058–2075. doi: 10.1105/tpc.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DM, Jackson AO. Interactions of the TGB1 protein during cell-to-cell movement of Barley stripe mosaic virus. J. Virol. 2001;75:8712–8723. doi: 10.1128/JVI.75.18.8712-8723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc D, Burri L, Kajava AV, Mougeot JL, Hess D, Lustig A, Kleemann G, Hohn T. The open reading frame III product of cauliflower mosaic virus forms a tetramer through a N-terminal coiled-coil. J. Biol. Chem. 1998;273:29015–29021. doi: 10.1074/jbc.273.44.29015. [DOI] [PubMed] [Google Scholar]
- Leclerc D, Stavolone L, Meier E, Guerra-Peraza O, Herzog E, Hohn T. The product of ORF III in cauliflower mosaic virus interacts with the viral coat protein through its C-terminal proline rich domain. Virus Genes. 2001;22:159–165. doi: 10.1023/a:1008121228637. [DOI] [PubMed] [Google Scholar]
- Lee J-Y, Taoka K-i, Yoo B-C, Ben-Nissan G, Kim D-J, Lucas D-J. Plasmodesmal-associated protein kinase in tobacco and Arabidopsis recognizes a subset of non-cell-autonomous proteins. Plant Cell. 2005;17:2817–2831. doi: 10.1105/tpc.105.034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-C, Wu C-H, Wang C-W. Traffic of a viral movement protein complex to the highly curved tubules of the cortical endoplasmic reticulum. Traffic. 2010;11:912–930. doi: 10.1111/j.1600-0854.2010.01064.x. [DOI] [PubMed] [Google Scholar]
- Leh V, Jacquot E, Geldreich A, Hermann T, Leclerc D, Cerutti M, Yot P, Keller M, Blanc S. Aphid transmission of cauliflower mosaic virus requires the viral PIII protein. EMBO J. 1999;18:7077–7085. doi: 10.1093/emboj/18.24.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh V, Jacquot E, Geldreich A, Kaas M, Blanc S, Keller M, Yot P. Interaction between the open reading frame III product and the coat protein is required for transmission of cauliflower mosaic virus by aphids. J. Virol. 2001;75:100–106. doi: 10.1128/JVI.75.1.100-106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekkerkerker A, Wellink J, Yuan P, van Lent J, Goldbach R, van Kammen AV. Distinct functional domains in the cowpea mosaic virus movement protein. J. Virol. 1996;70:5658–5661. doi: 10.1128/jvi.70.8.5658-5661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshchiner AD, Solovyev AG, Morozov SY, Kalinina NO. A minimal region in the NTPase/helicase domain of the TGBp1 plant virus movement protein is responsible for ATPase activity and cooperative RNA binding. J. Gen. Virol. 2006;87:3087–3095. doi: 10.1099/vir.0.81971-0. [DOI] [PubMed] [Google Scholar]
- Lewandowski DJ, Adkins S. The tubule-forming NSm protein from Tomato spotted wilt virus complements cell-to-cell and long-distance movement of Tobacco mosaic virus hybrids. Virology. 2005;342:26–37. doi: 10.1016/j.virol.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Lewandowski DJ, Dawson WO. Functions of the 126-and 183-kDa proteins of tobacco mosaic virus. Virology. 2000;271:90–98. doi: 10.1006/viro.2000.0313. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Lazarowitz SG. Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc. Natl Acad. Sci. U S A. 2010;107:2491–2496. doi: 10.1073/pnas.0909080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-Z, Qu F, Morris TJ. Cell-to-cell movement of turnip crinkle virus is controlled by two small open reading frames that function in trans. Virology. 1998;244:405–416. doi: 10.1006/viro.1998.9125. [DOI] [PubMed] [Google Scholar]
- Li YZ, Leisner SM. Multiple domains within the Cauliflower mosaic virus gene VI product interact with the full-length protein. Mol. Plant–Microbe Interact. 2002;15:1050–1057. doi: 10.1094/MPMI.2002.15.10.1050. [DOI] [PubMed] [Google Scholar]
- Lim HS, Bragg JN, Ganesan U, Lawrence DM, Yu JL, Isogai M, Hammond J, Jackson AO. Triple gene block protein interactions involved in movement of Barley stripe mosaic virus. J. Virol. 2008;82:4991–5006. doi: 10.1128/JVI.02586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou DY, Hsu YH, Wung CH, Wang WH, Lin NS, Chang BY. Functional analyses and identification of two arginine residues essential to the ATP-utilizing activity of the triple gene block protein 1 of bamboo mosaic potexvirus. Virology. 2000;277:336–344. doi: 10.1006/viro.2000.0610. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Blancaflor EB, Nelson RS. The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 2005;138:1853–1865. doi: 10.1104/pp.105.065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love AJ, Laird J, Holt J, Hamilton AJ, Sadanandom A, Milner JJ. Cauliflower mosaic virus protein P6 is a suppressor of RNA silencing. J. Gen. Virol. 2007;88:3439–3444. doi: 10.1099/vir.0.83090-0. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity. diffusion, intracellular surface area. International Review of Cytology: A Survey of Cell Biology. 2000;192:189–221. doi: 10.1016/s0074-7696(08)60527-6. [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Ding B, Vanderschoot C. Plasmodesmata and the supracelllular nature of plants. New Phytol. 1993;125:435–476. doi: 10.1111/j.1469-8137.1993.tb03897.x. [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Ham LK, Kim JY. Plasmodesmata: bridging the gap between neighboring plant cells. Trends Cell Biol. 2009;19:495–503. doi: 10.1016/j.tcb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Makarov VV, Rybakova EN, Efimov AV, Dobrov EN, Serebryakova MV, Solovyev AG, Yaminsky IV, Taliansky ME, Morozov SY, Kalinina NO. Domain organization of the N-terminal portion of hordeivirus movement protein TGBp1. J. Gen. Virol. 2009;90:3022–3032. doi: 10.1099/vir.0.013862-0. [DOI] [PubMed] [Google Scholar]
- Margis R, Ritzenthaler C, Reinbolt J, Pinck M, Pinck L. Genome organization of grapevine fanleaf nepovirus RNA2 deduced from the 122K polyprotein P2 in vitro cleavage products. J. Gen. Virol. 1993;74:1919–1926. doi: 10.1099/0022-1317-74-9-1919. [DOI] [PubMed] [Google Scholar]
- Martínez-Gil L, Johnson AE, Mingarro I. Membrane insertion and biogenesis of the turnip crinkle virus p9 movement protein. J. Virol. 2010;84:5520–5527. doi: 10.1128/JVI.00125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Gil L, Sanchez-Navarro JA, Cruz A, Pallas V, Perez-Gil J, Mingarro I. Plant virus cell-to-cell movement is not dependent on the transmembrane disposition of its movement protein. J. Virol. 2009;83:5535–5543. doi: 10.1128/JVI.00393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Gil L, Sauri A, Vilar M, Pallas V, Mingarro I. Membrane insertion and topology of the p7B movement protein of Melon necrotic spot virus (MNSV) Virology. 2007;367:348–357. doi: 10.1016/j.virol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Más P, Beachy RN. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA. J. Cell Biol. 1999;147:945–958. doi: 10.1083/jcb.147.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y, Ohshima M, Yoshioka K, Nishiguchi M, Nyunoya H. The catalytic subunit of protein kinase CK2 phosphorylates in vitro the movement protein of Tomato mosaic virus. J. Gen. Virol. 2003;84:497–505. doi: 10.1099/vir.0.18839-0. [DOI] [PubMed] [Google Scholar]
- McCartney AW, Greenwood JS, Fabian MR, White KA, Mullen RT. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell. 2005;17:3513–3531. doi: 10.1105/tpc.105.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean BG, Zupan J, Zambryski PC. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell. 1995;7:2101–2114. doi: 10.1105/tpc.7.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina V, Peremyslov VV, Hagiwara Y, Dolja VV. Subcellular localization of the HSP70-homolog encoded by beet yellows closterovirus. Virology. 1999;260:173–181. doi: 10.1006/viro.1999.9807. [DOI] [PubMed] [Google Scholar]
- Melcher U. The ‘30K’ superfamily of viral movement proteins. J. Gen. Virol. 2000;81:257–266. doi: 10.1099/0022-1317-81-1-257. [DOI] [PubMed] [Google Scholar]
- Merits A, Guo DY, Jarvekulg L, Saarma M. Biochemical and genetic evidence for interactions between potato A potyvirus-encoded proteins P1 and P3 and proteins of the putative replication complex. Virology. 1999;263:15–22. doi: 10.1006/viro.1999.9926. [DOI] [PubMed] [Google Scholar]
- Meshi T, Watanabe Y, Saito T, Sugimoto A, Maeda T, Okada Y. Function of the 30-kd protein of tobacco mosaic virus: involvement in cell-to-cell movement and dispensability for replication. EMBO J. 1987;6:2557–2563. doi: 10.1002/j.1460-2075.1987.tb02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min BE, Martin K, Wang RY, Tafelmeyer P, Bridges M, Goodin M. A host-factor interaction and localization map for a plant-adapted rhabdovirus implicates cytoplasm-tethered transcription activators in cell-to-cell movement. Mol. Plant–Microbe Interact. 2010;23:1420–1432. doi: 10.1094/MPMI-04-10-0097. [DOI] [PubMed] [Google Scholar]
- Módena NA, Zelada AM, Conte F, Mentaberry A. Phosphorylation of the TGBp1 movement protein of Potato virus X by a Nicotiana tabacum CK2-like activity. Virus Res. 2008;137:16–23. doi: 10.1016/j.virusres.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Moreau P, Brandizzi F, Hanton S, Chatre L, Melser S, Hawes C, Satiat-Jeunemaitre B. The plant ER–Golgi interface: a highly structured and dynamic membrane complex. J. Exp. Bot. 2007;58:49–64. doi: 10.1093/jxb/erl135. [DOI] [PubMed] [Google Scholar]
- Morozov SY, Solovyev AG. Triple gene block: modular design of a multifunctional machine for plant virus movement. J. Gen. Virol. 2003;84:1351–1366. doi: 10.1099/vir.0.18922-0. [DOI] [PubMed] [Google Scholar]
- Nagy PD, Pogany J. Global genomics and proteomics approaches to identify host factors as targets to induce resistance against Tomato bushy stunt virus. Natural and Engineered Resistance to Plant Viruses. Pt Ii. 2010;76:123–177. doi: 10.1016/S0065-3527(10)76004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Lucas WJ, Ding B, Zaitlin M. Viral RNA trafficking is inhibited in replicase-mediated resistant transgenic tobacco plants. Proc. Natl Acad. Sci. U S A. 1996;93:12643–12647. doi: 10.1073/pnas.93.22.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehl A, Heinlein M. Cellular pathways for viral transport through plasmodesmata. Protoplasma. 2011;248:75–99. doi: 10.1007/s00709-010-0246-1. [DOI] [PubMed] [Google Scholar]
- Nishiguchi M, Motoyoshi F, Oshima N. Behaviour of a temperature sensitive strain of tobacco mosaic virus in tomato leaves and protoplasts. J. Gen. Virol. 1978;39:53–61. [Google Scholar]
- Osman TAM, Buck KW. Detection of the movement protein of red clover necrotic mosaic virus in a cell wall fraction from infected Nicotiana clevelandii plants. J. Gen. Virol. 1991;72:2853–2856. doi: 10.1099/0022-1317-72-11-2853. [DOI] [PubMed] [Google Scholar]
- Paape M, Solovyev AG, Erokhina TN, Minina EA, Schepetilnikov MV, Lesemann DE, Schiemann J, Morozov SY, Kellmann JW. At-4/1, an interactor of the Tomato spotted wilt virus movement protein, belongs to a new family of plant proteins capable of directed intra- and intercellular trafficking. Mol. Plant–Microbe Interact. 2006;19:874–883. doi: 10.1094/MPMI-19-0874. [DOI] [PubMed] [Google Scholar]
- Panavas T, Nagy PD. Mechanism of stimulation of plus-strand synthesis by an RNA replication enhancer in a tombusvirus. J. Virol. 2005;79:9777–9785. doi: 10.1128/JVI.79.15.9777-9785.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal MC, Thomas CL, Maule AJ. Cauliflower mosaic virus gene-I product (P1) forms tubular structures which extend from the surface of infected protoplasts. Virology. 1993;195:281–285. doi: 10.1006/viro.1993.1375. [DOI] [PubMed] [Google Scholar]
- Peremyslov VV, Hagiwara Y, Dolja VV. HSP70 homolog functions in cell-to-cell movement of a plant virus. Proc. Natl Acad. Sci. U S A. 1999;96:14771–14776. doi: 10.1073/pnas.96.26.14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Pan Y-W, Dolja VV. Movement protein of a closterovirus is a type III integral transmembrane protein localized to the endoplasmic reticulum. J. Virol. 2004;78:3704–3709. doi: 10.1128/JVI.78.7.3704-3709.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels J, Van der Krogt GNM, Van Lent J, Bisseling T, Wellink J. The cytoskeleton and the secretory pathway are not involved in targeting the cowpea mosaic virus movement protein to the cell periphery. Virology. 2002;297:48–56. doi: 10.1006/viro.2002.1424. [DOI] [PubMed] [Google Scholar]
- Raffaele S, et al. Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement. Plant Cell. 2009;21:1541–1555. doi: 10.1105/tpc.108.064279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel C, Beachy RN. Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum. Proc. Natl Acad. Sci. U S A. 1998;95:11169–11174. doi: 10.1073/pnas.95.19.11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro D, Goldbach R, Kormelink R. Requirements for ER-arrest and sequential exit to the Golgi of tomato spotted wilt virus glycoproteins. Traffic. 2009;10:664–672. doi: 10.1111/j.1600-0854.2009.00900.x. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler C, et al. Grapevine fanleaf virus replication occurs on endoplasmic reticulum-derived membranes. J. Virol. 2002;76:8808–8819. doi: 10.1128/JVI.76.17.8808-8819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler C, Schmit AC, Michler P, Stussigaraud C, Pinck L. Grapevine fanleaf nepovirus P38 putative movement protein is located on tubules in vivo. Mol. Plant–Microbe Interact. 1995;8:379–387. [Google Scholar]
- Rojas MR, Jiang H, Salati R, Xoconostle-Cazares B, Sudarshana MR, Lucas WJ, Gilbertson RL. Functional analysis of proteins involved in movement of the monopartite begomovirus, tomato yellow leaf curl virus. Virology. 2001;291:110–125. doi: 10.1006/viro.2001.1194. [DOI] [PubMed] [Google Scholar]
- Ryabova LA, Pooggin MM, Hohn T. Viral strategies of translation initiation: Ribosomal shunt and reinitiation. Progress in Nucleic Acid Research and Molecular Biology. 2002;72:1–39. doi: 10.1016/S0079-6603(02)72066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambade A, Brandner K, Hofmann C, Seemanpillai M, Mutterer J, Heinlein M. Transport of TMV movement protein particles associated with the targeting of RNA to plasmodesmata. Traffic. 2008;9:2073–2088. doi: 10.1111/j.1600-0854.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- Sanderfoot AA, Ingham DJ, Lazarowitz SG. A viral movement protein as a nuclear shuttle: the geminivirus BR1 movement protein contains domains essential for interaction with BL1 and nuclear localization. Plant Physiol. 1996;110:23–33. doi: 10.1104/pp.110.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurí A, Saksena S, Salgado J, Johnson AE, Mingarro I. Double-spanning plant viral movement protein integration into the endoplasmic reticulum membrane is signal recognition particle-dependent, translocon-mediated, and concerted. J. Biol. Chem. 2005;280:25907–25912. doi: 10.1074/jbc.M412476200. [DOI] [PubMed] [Google Scholar]
- Savino V, Cherif C, Martelli GP. A natural serological variant of grapevine fanleaf virus. Phytopathologia Mediterranea. 1985;24:29–34. [Google Scholar]
- Schaad MC, Jensen PE, Carrington JC. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 1997;16:4049–4059. doi: 10.1093/emboj/16.13.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetilnikov, M.V., Manske, U., Solovyev, A.G., Zamyatnin, A.A., Schiemann, J., and Morozov S. Yu. (2005). The hydrophobic segment of Potato virus X TGBp3 is a major determinant of the protein intracellular trafficking. J. Gen. Virol. 86, 2379–2391. [DOI] [PubMed]
- Schoelz J, Shepherd RJ, Daubert S. Region VI of cauliflower mosaic virus encodes a host range determinant. Mol. Cell. Biol. 1986;6:2632–2637. doi: 10.1128/mcb.6.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof HB. Plant virus transport: motions of functional equivalence. Trends Plant Sci. 2005;10:376–382. doi: 10.1016/j.tplants.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Scholthof HB, Scholthof KBG, Kikkert M, Jackson AO. Tomato bushy stunt virus spread is regulated by 2 nested genes that function in cell-to-cell movement and host-dependent systemic invasion. Virology. 1995;213:425–438. doi: 10.1006/viro.1995.0015. [DOI] [PubMed] [Google Scholar]
- Seksek O, Biwersi J, Verkman AS. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Biol. 1997;138:131–142. doi: 10.1083/jcb.138.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalla TA. Assembly and aggregation of tobacco mosaic virus in tomato leaflets. J. Cell Biol. 1964;21:253ff. doi: 10.1083/jcb.21.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintaku MH, Carter SA, Bao YM, Nelson RS. Mapping nucleotides in the 126-kDa protein gene that control the differential symptoms induced by two strains of tobacco mosaic virus. Virology. 1996;221:218–225. doi: 10.1006/viro.1996.0368. [DOI] [PubMed] [Google Scholar]
- Soellick TR, Uhrig JF, Bucher GL, Kellmann JW, Schreier PH. The movement protein NSm of tomato spotted wilt tospovirus (TSWV): RNA binding, interaction with the TSWV N protein, and identification of interacting plant proteins. Proc. Natl Acad. Sci. U S A. 2000;97:2373–2378. doi: 10.1073/pnas.030548397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyev AG, Savenkov EI, Agranovsky AA, Morozov SY. Comparisons of the genomic cis-elements and coding regions in RNA beta components of the hordeiviruses barley stripe mosaic virus, lychnis ringspot virus, and poa semilatent virus. Virology. 1996a;219:9–18. doi: 10.1006/viro.1996.0217. [DOI] [PubMed] [Google Scholar]
- Solovyev AG, Zelenina DA, Savenkov EI, Grdzelishvili VZ, Morozov SY, Lesemann DE, Maiss E, Casper R, Atabekov JG. Movement of a barley stripe mosaic virus chimera with a tobacco mosaic virus movement protein. Virology. 1996b;217:435–441. doi: 10.1006/viro.1996.0137. [DOI] [PubMed] [Google Scholar]
- Solovyev AG, Zelenina DA, Savenkov EI, Grdzelishvili VZ, Morozov SY, Maiss E, Casper R, Atabekov JG. Host-controlled cell-to-cell movement of a hybrid barley stripe mosaic virus expressing a dianthovirus movement protein. Intervirology. 1997;40:1–6. doi: 10.1159/000150514. [DOI] [PubMed] [Google Scholar]
- Solovyev, A.G., Stroganova, T. A., Zamyatnin, A.A., Fedorkin, O.N., Schiemann, J., and Morozov S. Yu. (2000) Subcellular sorting of small membrane-associated triple gene block proteins: TGBp3-assisted targeting of TGBp2. Virology 269, 113–127. [DOI] [PubMed]
- Sparkes I, Runions J, Hawes C, Griffing L. Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell. 2009;21:3937–3949. doi: 10.1105/tpc.109.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavolone L, Villani ME, Leclerc D, Hohn T. A coiled-coil interaction mediates cauliflower mosaic virus cell-to-cell movement. Proc. Natl Acad. Sci. U S A. 2005;102:6219–6224. doi: 10.1073/pnas.0407731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storms MMH, Kormelink R, Peters D, van Lent JWM, Goldbach RW. The nonstructural NSm protein of tomato spotted wilt virus induces tubular structures in plant and insect cells. Virology. 1995;214:485–493. doi: 10.1006/viro.1995.0059. [DOI] [PubMed] [Google Scholar]
- Su SZ, Liu ZH, Chen C, Zhang Y, Wang X, Zhu L, Miao L, Wang XC, Yuan M. Cucumber mosaic virus movement protein severs actin filaments to increase the plasmodesmal size exclusion limit in tobacco. Plant Cell. 2010;22:1373–1387. doi: 10.1105/tpc.108.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szécsi J, Ding XS, Lim CO, Bendahmane M, Cho MJ, Nelson RS, Beachy RN. Development of tobacco mosaic virus infection sites in Nicotiana benthamiana. Mol. Plant–Microbe Interact. 1999;12:143–152. [Google Scholar]
- Tagami Y, Watanabe Y. Effects of brefeldin A on the localization of tobamovirus movement protein and cell-to-cell movement of the virus. Virology. 2007;361:133–140. doi: 10.1016/j.virol.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Tamai A, Meshi T. Cell-to-cell movement of potato virus X: the role of p12 and p8 encoded by the second and third open reading frames of the triple gene block. Mol. Plant–Microbe Interact. 2001;14:1158–1167. doi: 10.1094/MPMI.2001.14.10.1158. [DOI] [PubMed] [Google Scholar]
- Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ. Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol. 2008;6:180–190. doi: 10.1371/journal.pbio.0060007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilsner J, Amari K, Torrance L. Plasmodesmata viewed as specialised membrane adhesion sites. Protoplasma. 2011;248:39–60. doi: 10.1007/s00709-010-0217-6. [DOI] [PubMed] [Google Scholar]
- Tilsner J, Cowan GH, Roberts AG, Chapman SN, Ziegler A, Savenkov E, Torrance L. Plasmodesmal targeting and intercellular movement of potato mop-top pomovirus is mediated by a membrane anchored tyrosine-based motif on the lumenal side of the endoplasmic reticulum and the C-terminal transmembrane domain in the TGB3 movement protein. Virology. 2010;402:41–51. doi: 10.1016/j.virol.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Tremblay D, Vaewhongs AA, Turner KA, Sit TL, Lommel SA. Cell wall localization of Red clover necrotic mosaic virus movement protein is required for cell-to-cell movement. Virology. 2005;333:10–21. doi: 10.1016/j.virol.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Tyulkina LG, Karger EM, Sheveleva AA, Atabekov JG. Binding of monoclonal antibodies to the movement protein (MP) of Tobacco mosaic virus: influence of subcellular MP localization and phosphorylation. J. Gen. Virol. 2010;91:1621–1628. doi: 10.1099/vir.0.018002-0. [DOI] [PubMed] [Google Scholar]
- Ueda T, Yamaguchi M, Uchimiya H, Nakano A. Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J. 2001;20:4730–4741. doi: 10.1093/emboj/20.17.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki S, Citovsky V. To gate, or not to gate: regulatory mechanisms for intercellular protein transport and virus movement in plants. Mol. Plant. 2011;4 doi: 10.1093/mp/ssr060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki S, Spektor R, Natale DM, Citovsky V. ANK, a host cytoplasmic receptor for the tobacco mosaic virus cell-to-cell movement protein, facilitates intercellular transport through plasmodesmata. PLoS Path. 2010;6 doi: 10.1371/journal.ppat.1001201. e1001201. doi:10.1371/journal.ppat.1001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lent J, Storms M, van der Meer F, Wellink J, Goldbach R. Tubular structures involved in movement of cowpea mosaic virus are also formed in infected cowpea protoplasts. J. Gen. Virol. 1991;72:2615–2623. doi: 10.1099/0022-1317-72-11-2615. [DOI] [PubMed] [Google Scholar]
- Verchot-Lubicz J, Torrance L, Solovyev AG, Morozov SY, Jackson AO, Gilmer D. Varied movement strategies employed by triple gene block-encoding viruses. Mol. Plant–Microbe Interact. 2010;23:1231–1247. doi: 10.1094/MPMI-04-10-0086. [DOI] [PubMed] [Google Scholar]
- Vilar M, Sauri A, Monne M, Marcos JF, von Heijne G, Perez-Paya E, Mingarro I. Insertion and topology of a plant viral movement protein in the endoplasmic reticulum membrane. J. Biol. Chem. 2002;277:23447–23452. doi: 10.1074/jbc.M202935200. [DOI] [PubMed] [Google Scholar]
- von Bargen S, Salchert K, Paape M, Piechulla B, Kellmann JW. Interactions between the tomato spotted wilt virus movement protein and plant proteins showing homologies to myosin, kinesin and DnaJ-like chaperones. Plant Physiol. Biochem. 2001;39:1083–1093. [Google Scholar]
- Waigmann E, Zambryski P. Tobacco mosaic virus movement protein-mediated protein transport between trichome cells. Plant Cell. 1995;7:2069–2079. doi: 10.1105/tpc.7.12.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waigmann E, Chen MH, Bachmaier R, Ghoshroy S, Citovsky V. Regulation of plasmodesmal transport by phosphorylation of tobacco mosaic virus cell-to-cell movement protein. EMBO J. 2000;19:4875–4884. doi: 10.1093/emboj/19.18.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Ogawa T, Okada Y. In vivo phosphorylation of the 30-kDa protein of tobacco mosaic virus. FEBS Lett. 1992;313:181–184. doi: 10.1016/0014-5793(92)81440-w. [DOI] [PubMed] [Google Scholar]
- Wei T, Wang A. Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J. Virol. 2008;82:12252–12264. doi: 10.1128/JVI.01329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Zhang C, Hong J, Xiong R, Kasschau KD, Zhou XP, Carrington JC, Wang AM. Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N-PIPO. PLoS Pathog. 2010a;6:e1000962. doi: 10.1371/journal.ppat.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei TY, Huang TS, McNeil J, Laliberté J-F, Jong J, Nelson RS, Wang AM. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J. Virol. 2010b;84:799–809. doi: 10.1128/JVI.01824-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Deom CM, Beachy RN, Lucas WJ. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989;246:377–379. doi: 10.1126/science.246.4928.377. [DOI] [PubMed] [Google Scholar]
- Wright KM, Cowan GH, Lukhovitskaya NI, Tilsner J, Roberts AG, Savenkov EI, Torrance L. The N-terminal domain of PMTV TGB1 movement protein is required for nucleolar localization, microtubule association, and long-distance movement. Mol. Plant–Microbe Interact. 2010;11:1486–1497. doi: 10.1094/MPMI-05-10-0105. [DOI] [PubMed] [Google Scholar]
- Wright KM, Wood NT, Roberts AG, Chapman S, Boevink P, MacKenzie KM, Oparka KJ. Targeting of TMV movement protein to plasmodesmata requires the actin/ER network: evidence from FRAP. Traffic. 2007;8:21–31. doi: 10.1111/j.1600-0854.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- Wu, C.-H., Lee, S.-C., and Wang, C.-W. (2011). Viral protein targeting to the cortical endoplasmic reticulum is required for cell-cell spreading in plants. J. Cell Biol. 193, 521–535. [DOI] [PMC free article] [PubMed]
- Xiong, R., Wu, J., Zhou, Y., and Zhou, X. (2008). Identification of a movement protein of the tenuivirus Rice stripe virus. J. Virol. 82, 12304–12311. [DOI] [PMC free article] [PubMed]
- Yamanaka T, Ohta T, Takahashi M, Meshi T, Schmidt R, Dean C, Naito S, Ishikawa M. TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein. Proc. Natl Acad. Sci. U S A. 2000;97:10107–10112. doi: 10.1073/pnas.170295097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Matsushita Y, Kasahara M, Konagaya K-I, Nyunoya H. Interaction of Tomato mosaic virus movement protein with Tobacco RIO kinase. Mol. Cells. 2004;17:223–229. [PubMed] [Google Scholar]
- Yuan, Z., Chen, H., Chen, Q., Omura, T., and Xie, L., Wu, Z., and Wei, T. (2011). The early secretory pathway and an actin-myosin VIII motility system are required for plasmodesmatal localization of the NSvc4 protein of Rice stripe virus. Virus Res. 159, 62–68. [DOI] [PubMed]
- Zamyatnin AA, Solovyev AG, Bozhkov PV, Valkonen JPT, Morozov SY, Savenkov EI. Assessment of the integral membrane protein topology in living cells. Plant J. 2006;46:145–154. doi: 10.1111/j.1365-313X.2006.02674.x. [DOI] [PubMed] [Google Scholar]
- Zamyatnin AA, Solovyev AG, Savenkov EI, Germundsson A, Sandgren M, Valkonen JPT, Morozov SY. Transient coexpression of individual genes encoded by the triple gene block of Potato mop-top virus reveals requirements for TGBp1 trafficking. Mol. Plant–Microbe Interact. 2004;17:921–930. doi: 10.1094/MPMI.2004.17.8.921. [DOI] [PubMed] [Google Scholar]