Functional Specialization of Mouse Higher Visual Cortical Areas (original) (raw)
. Author manuscript; available in PMC: 2013 Dec 31.
Published in final edited form as: Neuron. 2011 Dec 22;72(6):10.1016/j.neuron.2011.11.013. doi: 10.1016/j.neuron.2011.11.013
SUMMARY
The mouse is emerging as an important model for understanding how sensory neocortex extracts cues to guide behavior, yet little is known about how these cues are processed beyond primary cortical areas. Here, we used two-photon calcium imaging in awake mice to compare visual responses in primary visual cortex (V1) and in two downstream target areas, AL and PM. Neighboring V1 neurons had diverse stimulus preferences spanning five octaves in spatial and temporal frequency. By contrast, AL and PM neurons responded best to distinct ranges of stimulus parameters. Most strikingly, AL neurons preferred fast-moving stimuli while PM neurons preferred slow-moving stimuli. By contrast, neurons in V1, AL, and PM demonstrated similar selectivity for stimulus orientation but not for stimulus direction. Based on these findings, we predict that area AL helps guide behaviors involving fast-moving stimuli (e.g., optic flow), while area PM helps guide behaviors involving slow-moving objects.
INTRODUCTION
All mammals possess a primary visual cortex (V1) that processes a broad range of visual information from the retina via the thalamus (Rosa and Krubitzer, 1999). In carnivores and primates, area V1 is believed to transmit specific information to higher visual areas, each of which is specialized for specific subsets of stimulus attributes (Maunsell and Newsome, 1987; Movshon and Newsome, 1996; Nassi and Callaway, 2009; Orban, 2008). Lesion and anatomical studies in visual cortex of mice and rats have suggested an analogous functional specialization of networks of visual areas involved in distinct aspects of action guidance or object recognition (see below). However, few physiological studies of specific higher visual areas exist in mice (Van den Bergh et al., 2010). Thus, a key question is whether mouse cortical neurons in different higher visual areas are specialized for processing distinct stimulus features (Rosa and Krubitzer, 1999). If strong functional specialization of higher visual areas occurs in the mouse, the experimental advantages of genetic accessibility, small size, and a lissencephalic brain would be of great use in understanding how such specialization comes about.
Within rodent V1, visual response properties of neurons are similar to their counterparts in other mammals, despite an overall increase in receptive field size (e.g., Girman et al., 1999; Niell and Stryker, 2008). However, in contrast to V1 neurons in many carnivores and primates, neighboring neurons in rodent V1 do not show strong functional clustering of orientation preference (Ohki et al., 2005) and ocular dominance (Mrsic-Flögel et al., 2007). Further, recent evidence suggests that functionally intermixed local populations of neurons in mouse V1, particularly those that prefer different ranges of spatial and temporal frequency, may constitute different processing streams (Gao et al., 2010).
Neurons in mouse V1 project to multiple retinotopically organized cortical areas, including areas AL (anterolateral), LM (lateromedial), and PM (posteromedial; Wang and Burkhalter, 2007). The function of different higher visual areas in mice and rats has thus far been inferred largely on the basis of lesion studies (Aggleton et al., 1997; Dean, 1981; Kolb and Walkey, 1987; McDaniel et al., 1982; Prusky and Douglas, 2004; Prusky et al., 2008), together with areal differences in anatomical connectivity and location relative to V1 (Sanderson et al., 1991; Simmons and Pearlman, 1982; Wang et al., 2011). Most recently, Wang et al. (2011) have suggested that mouse area LM may be similar to primate ventrotemporal areas involved in object recognition (Conway et al., 2010; Desimone et al., 1985; Pasupathy and Connor, 1999), while mouse area AL may be more akin to the primate dorsolateral areas (which are involved, for example, in processing of self-motion cues; Andersen et al., 1997; Britten and Van Wezel, 2002; Duffy and Wurtz, 1991). Similar arguments suggest that area PM may be similar to primate dorsomedial areas (which are involved, for example, in processing of external object motion cues; Galletti and Fattori, 2003).
Initial physiological evidence in rodents supporting the notion of functional specialization of target areas downstream of V1 has come from immediate early gene immunohistochemistry and widefield autofluorescence imaging (Montero and Jian, 1995; Tohmi et al., 2009). However, the visual properties of individual neurons within and across higher visual areas remain poorly understood (E. Gao, G. DeAngelis, and A. Burkhalter, 2006, Soc. Neurosci., abstract; M. Roth, F. Helmchen, and B. Kampa, 2010, Soc. Neurosci., abstract; M. Garrett, J. Marshall, L. Nauhaus, and E. Callaway, 2010, Soc. Neurosci., abstract).
While comparatively little is known about visual response properties in unanesthetized mice (Andermann et al., 2010; Niell and Stryker, 2010), cortical neurons in mice and other species may demonstrate visual responses of greater magnitude (Niell and Stryker, 2010), diversity (Qin et al., 2008), and context sensitivity (Pack et al., 2001) in the awake state. Therefore, to determine the degree of functional specialization in mouse higher visual areas, we developed a chronic two-photon imaging system for mapping responses in local volumes of cortical neurons across multiple areas in awake mice. We found striking differences in stimulus preferences across areas, demonstrating distinct functional specialization of different higher visual areas in the mouse.
RESULTS
Identification and Targeting of Functionally Distinct Higher Cortical Areas
We characterized the functional properties of neurons in visual cortical areas of awake mice, using the following approach (see also Experimental Procedures). First, we implanted a 5 mm cranial window over visual cortex. Following recovery, mice were gradually habituated to head restraint (Andermann et al., 2010) while free to walk on a single-axis trackball (Experimental Procedures). We then performed widefield imaging of intrinsic autofluorescence signals to obtain retinotopic maps of multiple visual areas (Figure 1A and Figure S1 available online; Kalatsky and Stryker, 2003; Schuett et al., 2002; cf. Wang and Burkhalter, 2007). We subsequently removed the cranial window under anesthesia and injected adeno-associated virus AAV2/1-_synapsin_-1-GCaMP3 at a depth of 250 µm below the cortical surface to obtain neuron-specific expression of the calcium indicator GCaMP3 (Tian et al., 2009; Dombeck et al., 2010; O’Connor et al., 2010) at approximately matched retinotopic locations in one or two visual cortical areas. Changes in cellular GCaMP3 fluorescence provide an estimate of visually driven increases in calcium influx associated with increases in neural firing rate (Tian et al., 2009; Experimental Procedures). We measured population visual responses across cortical areas using widefield calcium imaging (Figure 1), followed by a more detailed mapping of individual neurons using two-photon calcium imaging (Figures 2–5). Specifically, we assessed tuning of neurons across multiple stimulus dimensions, including spatial and temporal frequency, speed, orientation, and direction of motion. Tuning estimates in Figures 1–5 included all trials, independent of whether the mouse was moving or stationary, as tuning was not strongly affected by locomotion (see Figures 6, S2, and S6, below).
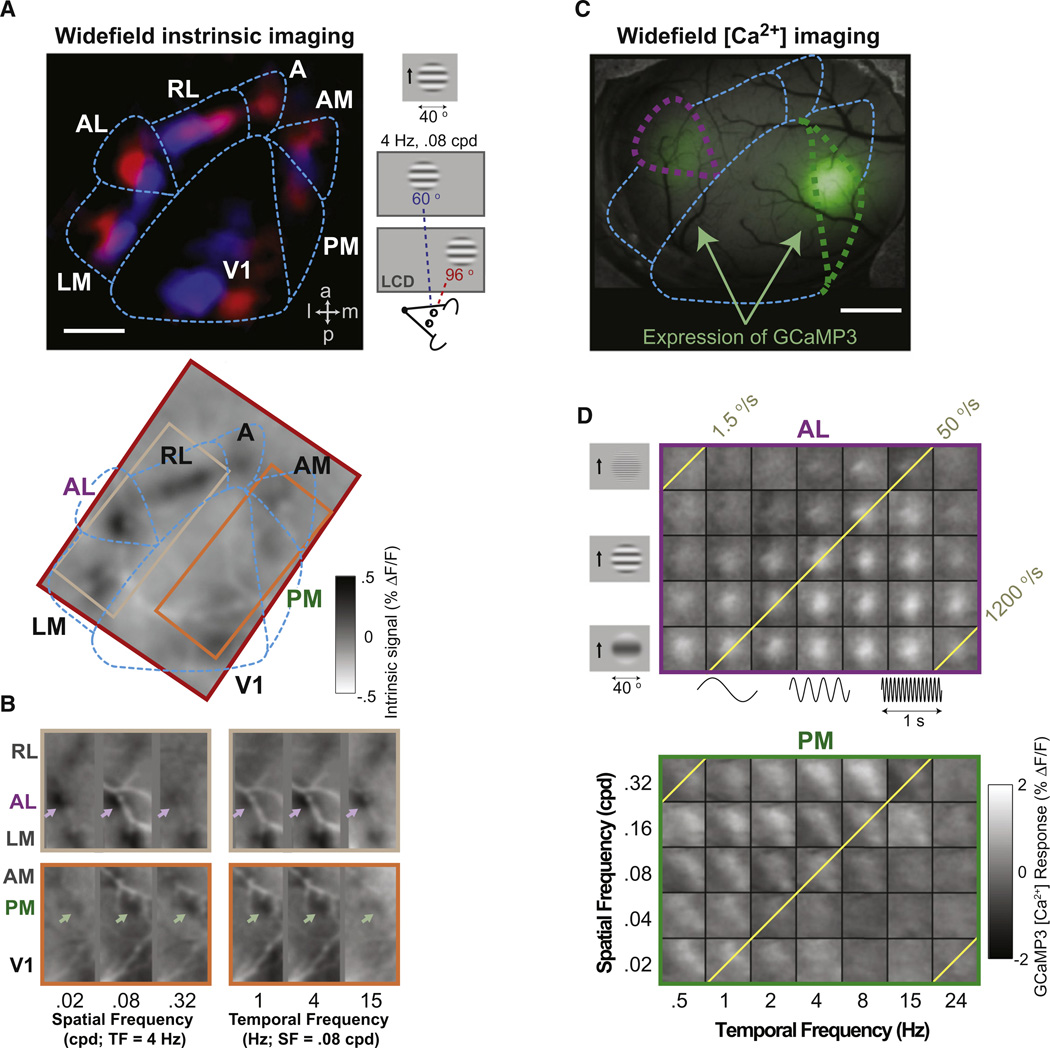
Figure 1. Widefield Functional Imaging in Awake Mice Suggests Differences across Visual Areas.
(A) To target expression of calcium indicators to higher visual areas, we first mapped changes in the intrinsic autofluorescence signal in awake mice during presentation of a local patch (40° diameter, smooth edges) containing upward-drifting sinusoidal gratings, at one of two spatial locations in the upper visual field (14° elevation; 60° or 96° azimuth; darker regions in A, bottom panel, reflect areal responses to stimulation at 96°). Retinotopic maps were generated using blue/ red pseudocolor images of responses to the two stimulus positions (A, upper panel). These maps reliably delineated primary visual cortex (V1) and several higher visual areas (LM: lateromedial; AL: anterolateral; RL: rostrolateral; A: anterior; AM: anteromedial; PM: posteromedial; cf. Wang and Burkhalter, 2007). See Figure S1 for further details.
(B) Differences in intrinsic autofluorescence signal among lateral visual areas (top panels, cf. beige rectangle in A) and among medial visual areas (bottom panels, cf. orange rectangle in A) to stimuli varying in spatial frequency (left panels) and temporal frequency (right panels). Data were averaged across three imaging sessions (same mouse as in A). Note that responses were similar in areas V1, LM, and AM, while responses were distinct in areas AL and PM.
(C) Targeted viral expression of GCaMP3 calcium indicator in areas AL and PM (purple and green dashed regions).
(D) Average calcium responses (lighter spots) using widefield GCaMP3 imaging confirmed the presence of clear differences in spatial and temporal frequency tuning in 600-µm-wide regions of interest encompassing area AL (top panel) versus area PM (bottom panel). Gaussian smoothing: σ = 12 µm. ΔF/F: percent change in fluorescence. Yellow diagonal lines are iso-speed lines. Scale bars in (A) and (C), 1 mm.
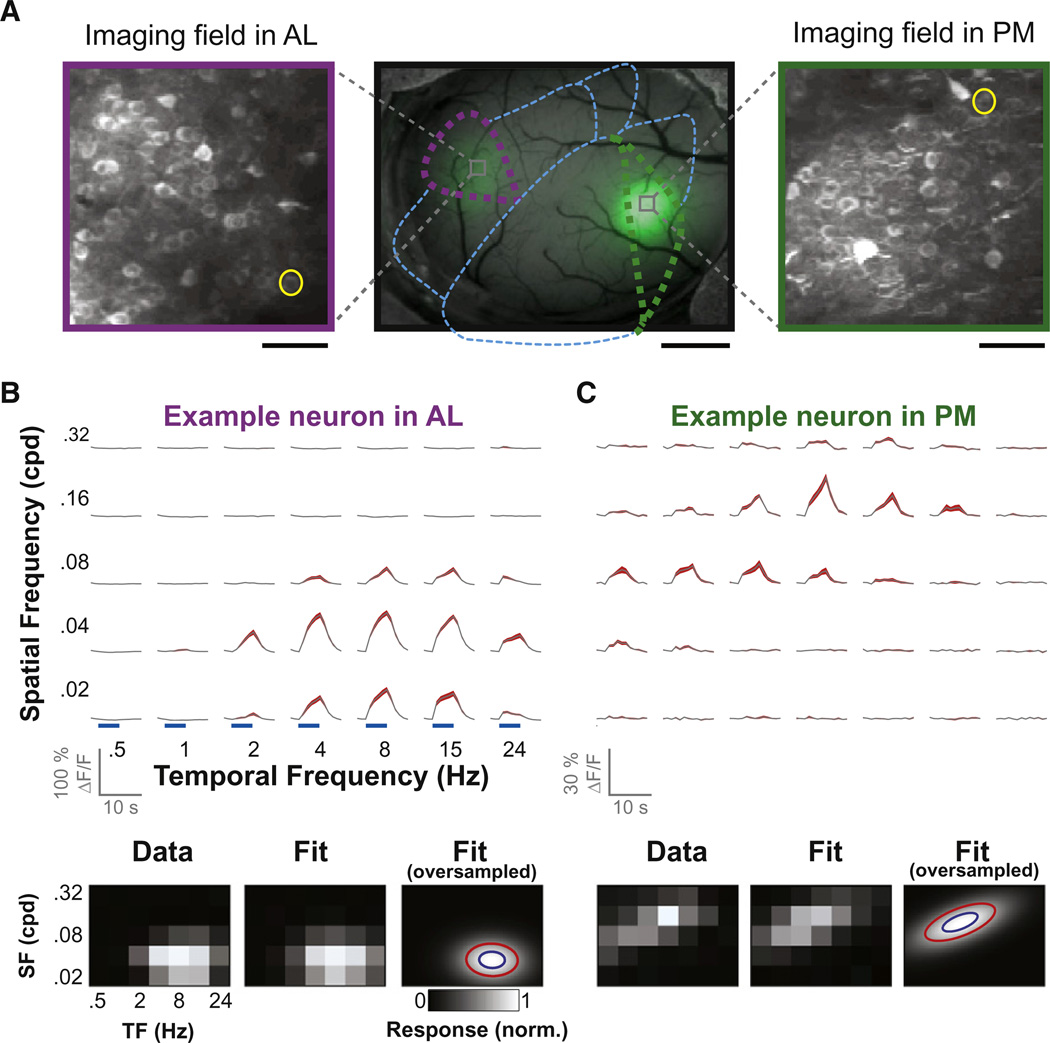
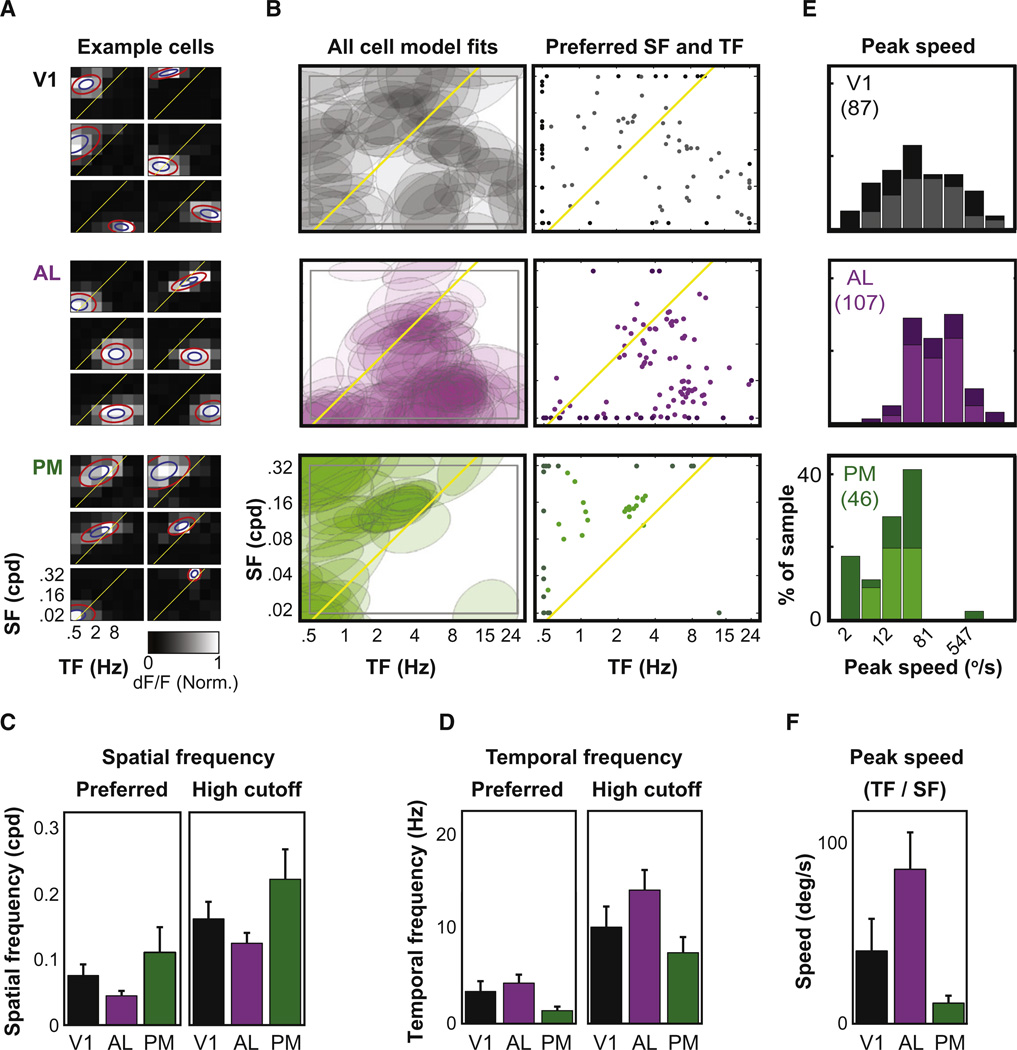
Figure 2. Cellular Imaging of Spatial and Temporal Frequency Responses.
(A) Maximum-intensity projections of baseline two-photon fluorescence volumes (GCaMP3), recorded in area AL (left panel) and PM (right panel) of the same mouse. Scale bars, 50 µm. Middle panel: epifluorescence image of targeted GCaMP3 expression in areas AL and PM (green halos), superimposed on the brightfield image. Scale bar, 1 mm.
(B and C) Average fluorescence time courses of single neurons (yellow circles in A) from areas AL (B) and PM (C) during presentation of stimuli at various spatial and temporal frequencies (top panels). ΔF/F: percent change in fluorescence. Shaded regions are ± SEM. The average change in fluorescence during presentation of each stimulus (blue bars, top panels) was used to generate a response map (bottom left panels) that was then fit to a two-dimensional Gaussian (bottom middle panels). Contours plots of the model fit at 60% of peak (red, halfwidth of σ) and 88% of peak (blue, halfwidth of σ/2), respectively, are overlaid on the same model fit, rendered with higher sampling (bottom right panels; Experimental Procedures). See also Figure S2.
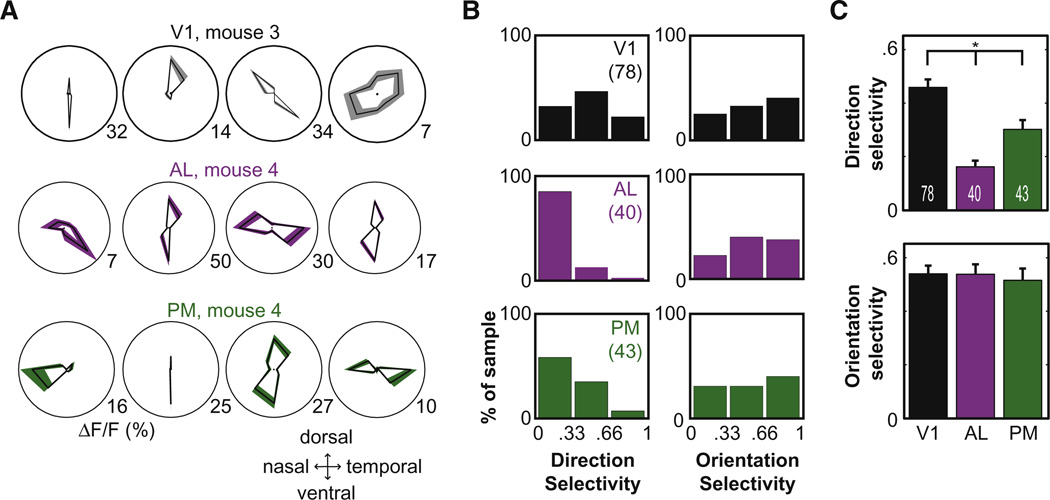
Figure 5. Similarity in Orientation Selectivity, but Not Direction Selectivity, between Visual Areas.
In a second protocol, stimuli were presented at one of 8 directions and 5–6 spatial frequencies.
(A) Top row: polar plots of direction tuning at the neurons’ preferred spatial frequencies, for simultaneously recorded neurons in V1. Shaded regions are ± SEM. Values at lower right are maximum response strength (ΔF/F). Middle/bottom rows: example neurons in AL and PM of the same mouse.
(B) Distributions of direction selectivity (left panels) and orientation selectivity (right panels) across areas.
(C) Mean direction selectivity (top panel) and orientation selectivity (bottom panel) across areas. Values are ± SEM. Numbers of cells: 78 in V1, 40 in AL, and 43 in PM. See also Figure S5.
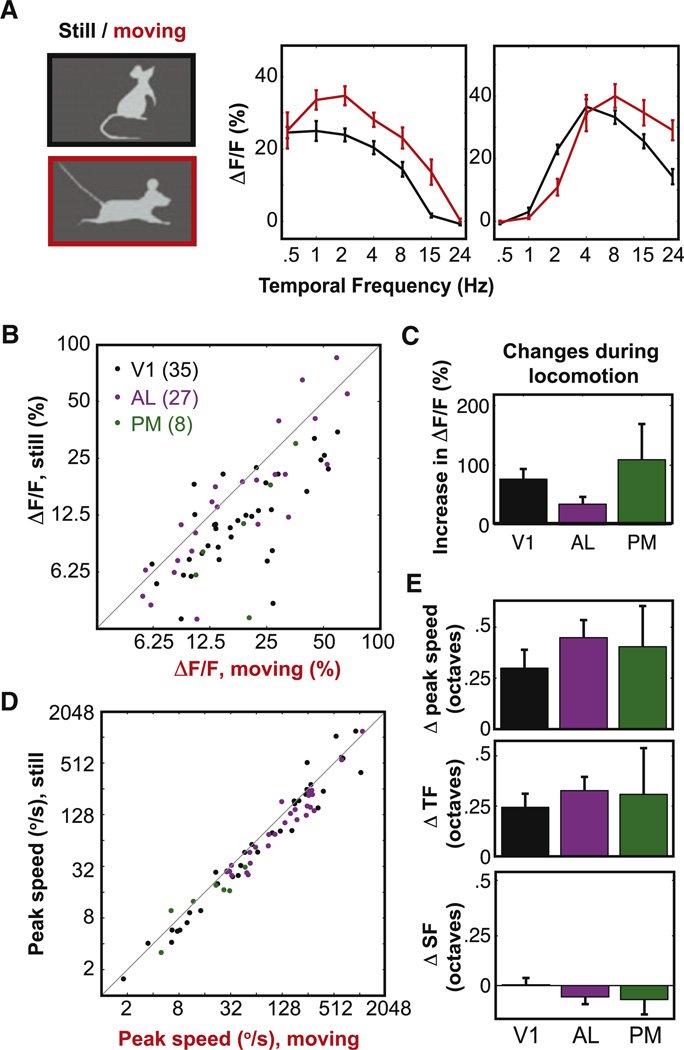
Figure 6. Areal Differences in Peak Speed Are Largely Independent of Locomotion.
(A) Examples of neural responses across temporal frequencies (at the preferred spatial frequency) are shown for a neuron in PM (left panel) and in AL (right panel), illustrating shifts in both response strength (left) and in preferred temporal frequency and speed (right) with locomotion.
(B and C) Increases in response strength were observed in all areas as demonstrated in scatter plots across behavioral conditions (B) and in population averages (C; mean ± SEM; see Results).
(D and E) Small but consistent increases in peak speed were also observed in scatter plots (D) and population averages (E, top panel; mean ± SEM, in octaves; see Results). These increases in peak speed were mainly due to increases in preferred temporal frequency (E, middle panel) but not spatial frequency (E, lower panel). Numberof included neurons:35 in V1, 27in AL, and 8 in PM. See also Figures S6 and S2.
We first asked whether neurons in areas AL, PM, and LM, the major cortical targets of area V1, showed different sensitivity to the spatial and temporal frequencies of upward-drifting, sinusoidal grating stimuli (Figure 1; gratings windowed by a ∼40° patch with smooth edges, centered at 70°–115° eccentricity; see Figure 1A and Experimental Procedures). Intrinsic autofluorescence imaging responses (Figures 1B and S1B) suggested that, of these three higher visual areas, areas AL and PM were strongly driven by different combinations of spatial and temporal frequencies, while area LM demonstrated a response profile more similar to that of area V1 (Figure 1B; see also Van den Bergh et al., 2010; Wang and Burkhalter, 2007). For this reason, we targeted our calcium imaging experiments to areas AL, PM, and V1 (Figures 1C and 1D).
During calcium imaging–both widefield epifluorescence imaging of entire areas (Figures 1C and 1D) and two-photon laser-scanning microscopy of individual neurons (Figures 2–4)–we presented stimuli at one of five spatial frequencies and seven temporal frequencies, corresponding to a range of stimulus speeds of almost three orders of magnitude. Figure 1D illustrates the average visual responses of GCaMP3-labeled neurons within areas AL and PM of an example mouse, using widefield calcium imaging. We observed clear differences in spatial and temporal frequency sensitivity across areas. Specifically, area AL preferred lower spatial and higher temporal frequencies (and thus, higher speeds), while area PM preferred higher spatial and lower temporal frequencies (and lower speeds). While wide-field imaging can reveal such population biases, it cannot assess the diversity of tuning across individual neighboring neurons. Thus, we concentrated our efforts on two-photon cellular imaging of GCaMP3 fluorescence.
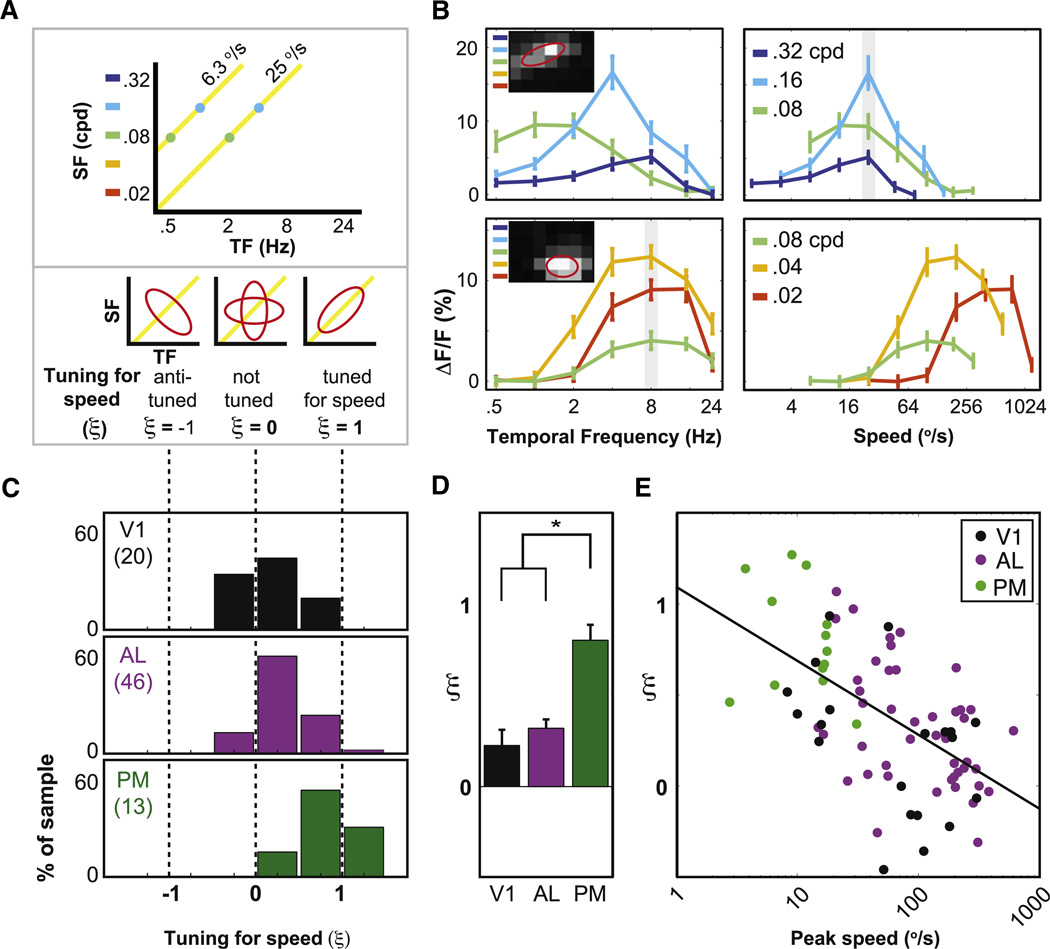
Figure 4. Tuning for Speed in Individual Neurons.
(A) Top panel: yellow iso-speed lines at fixed ratios of temporal:spatial frequency. Bottom panels: neurons with the same peak speed across spatial frequencies (bottom right) are “tuned for speed” (power law relationship between temporal and spatial frequency with exponent ξ ≈ 1 in model fit, see Experimental Procedures).
(B) Top panels: example neuron in PM that is approximately tuned for speed (top right; gray bar at preferred speed; ξ =1.18) but not for temporal frequency (top left). Bottom panels: example neuron in AL that is not tuned for speed (bottom right; ξ= −0.07) but is tuned for temporal frequency (bottom left; gray bar at preferred temporal frequency). Insets in left panels: cell responses across spatial and temporal frequencies (cf. Figure 2).
(C and D) Distributions (C) and average values (D, mean ± SEM) of ξ across areas. Only neurons for which ξ could be accurately estimated are included (i.e., neurons with peak spatial and temporal frequencies contained in our sampling range, and with spatial and temporal frequency bandwidths greater than the sampling resolution of 1 octave).
(E) Scatter plot of tuning for speed (ξ) versus peak speed reveals an inverse relationship, both across and within areas (see Results; black line: least-squares fit across neurons in all areas). Numbers of included cells: 20 in V1, 46 in AL, and 13 in PM. See also Experimental Procedures and Figure S4.
Cellular Imaging of Spatial and Temporal Frequency Tuning in Areas V1, AL, and PM
To determine the diversity in stimulus preferences of neurons within and across areas in awake mouse, we recorded cellular calcium responses using two-photon imaging in layer II/III of cortical areas V1, AL, and PM (Figure 2A). We confirmed the precise location of the imaged volume by comparing surface vasculature in two-photon and widefield images (see Experimental Procedures). We recorded calcium signals simultaneously from several dozen neurons in a volume spanning ∼ 200 µm × 200 µm × 45 µm at a rate of 1 Hz (using a piezoelectric objective Z-scanner; Kerlin et al., 2010). By correcting for slow drifts in neuron location within the imaged volume (<10 µm), we were able to record robust evoked responses from the same neurons for several hours, allowing estimation of the spatial and temporal frequency tuning for individual neurons, as illustrated in Figures 2B and 2C (top panels). Responses in the spatial by temporal frequency plane were fit to oriented two-dimensional Gaussians (Figures 2B and 2C, bottom panels; see Priebe et al., 2006 and Experimental Procedures) to quantify the tuning for spatial and temporal frequency and speed. These estimates were obtained from trials when the mouse was either stationary or walking freely on the trackball.
We observed occasional large horizontal eye movements (>5°) often associated with onset of locomotion (Figure S2). However, the probability of locomotion was not substantially changed by the presentation of any of the visual stimuli (Figures S2D–S2F). In addition, the direction of locomotion-associated eye movements was parallel to the horizontally oriented sinusoidal gratings used in these experiments, suggesting that they should have little impact on tuning. Indeed, response tuning was not different when we removed all trials with blinks or large eye movements for a subset of neurons (Figures S2G and S2H), nor was it strongly affected by locomotion itself (see Figures 6, S2, and S6).
Spatial and temporal frequency tuning estimates were obtained for 241 responsive neurons in areas V1, AL, and PM in six mice (see Table 1). Simultaneously imaged cells in V1 showed dramatically different stimulus preferences (Figure 3A, top). Some response diversity existed across neurons in AL and PM, albeit less than in V1 (Figure 3A, middle and bottom). Contour plots of all model fits in each area (Figure 3B, left) and scatter plots of frequency preferences (Figure 3B, right) revealed that V1 neurons span a broad range of preferred spatial and temporal frequencies, while AL and PM neurons showed less diversity. AL neurons responded best to high temporal and low spatial frequencies, while PM neurons responded best to low temporal and high spatial frequencies (Figures 3B–3D). Indeed, the distributions of preferred spatial and temporal frequencies (and 50%high cutoff frequencies) were all significantly different between pairs of areas (AL versus PM, AL versus V1, and PM versus V1, Kolmogorov-Smirnov [K-S] tests, all p values < 0.01 except preferred temporal frequency in V1 versus AL, p = 0.06; see Table 1 for median values).
Table 1.
Summary of Cells Recorded in the Spatial by Temporal Frequency Protocol
| # Cells | # Sessions | # Mice | Pref. SF(cpd) | Pref. TF(Hz) | Pref. Speed(°/s) | 50% High-CutoffSF (cpd) | 50% High-CutoffTF (Hz) | MaximumΔF/F (%) | |
|---|---|---|---|---|---|---|---|---|---|
| V1 | 87 | 4 | 2 | 0.076 | 3.0 | 39.9 | 0.16 | 9.9 | 11.4 ± 0.8 |
| AL | 107 | 5 | 4 | 0.045 | 3.8 | 84.8 | 0.12 | 13.7 | 14.5 ± 1.4 |
| PM | 46 | 6 | 5 | 0.11 | 1.2 | 10.9 | 0.22 | 7.2 | 9.4 ± 1.2 |
Figure 3. AL and PM Neurons Prefer Nearly Nonoverlapping Ranges of Speeds Spanned by V1.
(A) Example response profiles of subsets of neurons from the same local volume in each cortical area. Simultaneously recorded neurons in V1 often preferred very different combinations of spatial and temporal frequency. By contrast, most neurons in AL preferred low spatial and high temporal frequency, while most neurons in PM preferred high spatial and low temporal frequency. Blue and red ellipses are contours of model fits at 88% and 60% of peak, respectively.
(B) Coverage of the spatiotemporal frequency plane by V1, AL, and PM populations is illustrated by shaded contours (at 88% of peak; left panel) and by scatter plots of neurons’ preferred spatial and temporal frequencies (right panel). Yellow lines in (A) and (B) represent the line in the spatiotemporal frequency plane that best classified response preferences as belonging to AL or PM neurons (an iso-speed line at 41.9°/s).
(C and D) Bar plots of median preferred and high cutoff (50% of peak response) spatial and temporal frequencies across areas.
(E) Distributions of peak speeds (ratio of preferred temporal:spatial frequencies). Darker bars (and darker symbols at edges of scatter plots in right panels of B) indicate neurons with low-or high-pass tuning for spatial or temporal frequency.
(F) Bar plots of median peak speed across areas. Error bars in (C), (D), and (F) are 95% confidence intervals on estimated median values (maximum-likelihood estimate for lognormal distribution). Numbers of cells: 87 in V1,107 in AL, and 46 in PM. Peak % ΔF/F for example neurons in (A), clockwise from top-left, V1:28,9, 12, 27, 11, 8; AL: 56, 14, 74, 22, 34, 41; PM: 7, 4, 8, 8, 15, 13. See also Figure S3.
Neural Populations in AL and PM Have Different Ranges of Peak Speeds
The above results demonstrate clear visual tuning differences across areas AL and PM, when considering either spatial frequency preferences or temporal frequency preferences alone. However, inspection of the scatter plots in Figure 3B also suggested some degree of correlation between neurons’ spatial and temporal frequency preferences. For example, neurons in PM preferring higher temporal frequencies also preferred higher spatial frequencies, while neurons in AL preferring lower temporal frequencies also preferred lower spatial frequencies. In this way, neurons in PM have lower peak speeds (lower ratios of preferred temporal frequency/preferred spatial frequency, which occur in the upper-left triangular portion of the spatiotemporal frequency plane in Figure 3B; see also Figure 1D), while neurons in AL have higher peak speeds (lower-right triangular portion of the plane).
Consistent with these observations, we found that peak speed distinguished neurons in AL from those in PM better than preferred spatial frequency or temporal frequency alone. We used a linear discriminant-based classifier to find the location and tilt of the line in the spatiotemporal frequency plane that best separated AL response preferences from PM preferences (Figure 3B, yellow line). Surprisingly, the optimal classification of AL versus PM peak responses occurred along a line of precisely constant stimulus speed (of 41.9°/s; see Figure 1D and Experimental Procedures), with a classification accuracy of 88% (compared to 79% and 82%, when using only preferred spatial or temporal frequency, respectively). Moreover, neurons with high peak speeds of 80°/s−1000°/s were found almost exclusively in area AL, while neurons with low peak speeds of 1°/s−10°/s were found almost exclusively in area PM (Figure 3E). Neurons in V1, by contrast, demonstrated a much broader range of peak speeds (Figure 3E). These differences were also evident in the median values for peak speed across areas (Figure 3F and Table 1; all areal differences between distributions of peak speed were highly significant, K–S tests, all p values < 10−5).
Areal differences in peak speed could not be explained by differences in the density of responsive neurons or in the strength of responses in different areas (Table S1). The estimated percentages of labeled cells did not differ greatly between areas (range, 63%–70%), and the estimated percentage of labeled cells that were visually driven was significantly but only moderately lower in area PM than in area AL or V1 (PM: 3.5%; AL: 8.5%; V1: 8%; see Table S1 and associated text). Further, peak response strengths of driven cells were not significantly different between areas (Table 1, K–S tests, all p values > 0.05). Although the majority of calcium signals in each area were obtained from confirmed layer II/III cell bodies (range, 72%–77%), a minority of signals were obtained from putative dendrites of local neurons within the same cortical column. Significant differences in peak speed between areas AL and PM were observed when including only confirmed cell bodies (K-S test, p < 10−13; for details, see Figure S3) or only putative dendrites (p < 10−3).
Tuning for Speed versus Tuning for Temporal or Spatial Frequency in Individual Neurons
Given that peak speed was a useful measure for distinguishing AL neurons from PM neurons, we tested whether individual neurons in AL and PM were tuned for speed. A neuron can be considered tuned for speed when a change in stimulus spatial frequency leads to a proportional change in temporal frequency preference, such that responses are always strongest for a common speed (e.g., Priebe et al., 2006). This relationship between spatial and temporal frequency is captured by the power-law exponent, ξ, in the elliptical Gaussian fit for each neuron (Figure 4A and Experimental Procedures). If ξ ≈ 1, the neuron is speed tuned (Figure 4B, top); if ξ ≈ 0, the preferred temporal frequency is constant for all spatial frequencies, so the neuron is not speed tuned (Figure 4B, bottom). Neurons in area PM were significantly more tuned for speed than neurons in V1 and AL (Figures 4C and 4D; all p values < 0.0003, K–S test), though we observed some degree of tuning for speed in all cortical areas (i.e., mean values of ξ > 0, all p values < 10−6 in PM and AL, p < 0.02 in V1, two-tailed t tests; Figures 4C and 4D). Further, tuning for speed was inversely related to peak speed (Figure 4E), both across areas (all neurons in scatterplot, r = −0.57, p < 10−4, Pearson’s correlation of ξ and log2[speed]) and within each area (V1: r = −0.46, p = 0.04; AL: r = −0.46, p = 0.001; similar trend in PM: r = −0.26, p = 0.39). This may partially explain why PM neurons, which tend to have lower peak speeds, also demonstrate greater tuning for speed. Consistent with the inverse relationship between speed tuning and peak speed, speed tuning was also inversely correlated with temporal frequency (all neurons, r = −0.65, p < 0.0001) and positively correlated with spatial frequency (all neurons, r = 0.32, p < 0.02).
Finally, we also considered whether the average output of each visual area, as estimated by the average of all peak-normalized response profiles in each area, was tuned for speed (Figure S4A). We fit the average response profiles in each area (using 2D Gaussian fits, as with single-cell analyses) and found that the average spatiotemporal response in area PM demonstrated considerable speed tuning (ξ = 0.64) while areas AL and V1 did not (AL: ξ = 0.23; V1: ξ = −0.23). Interestingly, the average response profile in area PM had similar tuning for speed and similar shape as the behavioral sensitivity profile obtained by Umino et al. (2008) in experiments estimating optomotor head tracking thresholds in C57BL/6 mice during presentation of sinusoidal gratings at different spatial and temporal frequencies (Figure S4B; ξ for optomotor sensitivity = 0.88; similarity between optomotor behavioral sensitivity and areal neural responses, estimated using linear correlation: roptomotor,PM = 0.88, roptomotor,AL = −0.20, roptomotor,V1 = 0.56, all p values < 10−4).
Similarity in Orientation Selectivity, but Not Direction Selectivity, between Visual Areas
Objects in motion typically consist of multiple spatial frequency components, each moving with similar velocity. Thus, cortical areas involved in processing of moving objects (see Discussion) might be expected to possess neurons with (1) tuning for the same speed across multiple spatial frequencies (see above) and (2) some degree of orientation and direction selectivity (Orban, 2008; Priebe et al., 2006). We therefore characterized selectivity for stimulus orientation and direction for an additional 161 neurons in areas V1, AL, and PM of four mice (see Tables 2 and S1). We presented the same smooth-edged grating patches as in Figures 1–4, but drifting in one of eight directions and one of five or six spatial frequencies (Experimental Procedures; temporal frequency was fixed at 2 Hz for V1 and PM experiments, and at 8 Hz for AL experiments in order to effectively drive neurons).
Table 2.
Summary of Cells Recorded in the Direction Protocol
| # Cells | # Sessions | # Mice | Orientation Selectivity | Direction Selectivity | Maximum ΔF/F (%) | |
|---|---|---|---|---|---|---|
| V1 | 78 | 3 | 3 | 0.54 ± 0.03 | 0.45 ± 0.02 | 15.4 ± 1.0 |
| AL | 40 | 2 | 2 | 0.53 ± 0.04 | 0.16 ± 0.02 | 18.9 ± 2.3 |
| PM | 43 | 3 | 3 | 0.54 ± 0.03 | 0.30 ± 0.03 | 17.6 ± 2.1 |
Example polar plots of responses at the preferred spatial frequency (Figure 5A) illustrate the orientation and direction selectivity of neurons in areas V1, AL, and PM. First, we found no significant difference in orientation selectivity across areas (Figures 5B and 5C and Table 2; K–S tests, all p values > 0.1). The percentages of orientation selective neurons (selectivity index > 0.33, i.e., peak:null response > 2:1) were similar in areas V1 (58/78 = 74%), PM (30/43 = 70%), and AL (31/40 = 78%). Our estimates of orientation selectivity did not depend strongly on stimulus spatial frequency (data not shown) and are not likely to depend on temporal frequency (Moore et al., 2005).
We next considered direction selectivity across areas. Strong direction selectivity (index > 0.33, i.e., peak:null response > 2:1) was evident in 69% of V1 neurons (54/78), as compared to 42% of PM neurons (18/43) and 15% of AL neurons (6/40). V1 neurons were significantly more selective for direction than PM neurons (p < 0.02, K–S test, Figures 5B and 5C and Table 2). Neurons in AL showed less direction selectivity than neurons in V1 (K-S test, p< 10−7)and in PM (p< 0.01). These differences in direction selectivity between V1, PM, and AL cannot be explained by differences in peak response strength, which did not differ across areas (Table 2, K–S tests, all p values > 0.4; see Discussion). However, the lower direction selectivity in AL compared to PM and V1 may be explained by our use of different stimulus temporal frequencies (8 Hz in AL, 2 Hz in PM and V1; see Moore et al., 2005), which were chosen to provide comparable response efficacy in each area (Table S1).
We also investigated whether responses in any of these areas were biased to specific orientations or directions. The average normalized response across all neurons showed a significant bias (to upward and downward drifting stimuli) in area AL (ANOVA across eight directions, p < 0.001; see Figure S5A). Similar results were observed when considering the preferred orientations and directions of individual neurons in area AL (Figures S5B and S5C). Population directional biases were not as clear in areas PM or V1 (all p values > 0.1).
Effects of Locomotion and Eye Movements on Response Strength and Preference
Together, these data indicate strong differences in response tuning between areas AL and PM, which suggests that these areas make distinct contributions to different visual behaviors (see Discussion). We tested whether these differences in response tuning between areas were present both during trials when the mouse was stationary and trials when the mouse was moving on the linear trackball. For this analysis, we selected all neurons in which we obtained robust estimates of spatial and temporal frequency preference both while the mouse was “still” and “moving” (same criteria as in Figure 3; V1: n = 35 neurons, AL: 27, PM: 8; Experimental Procedures).
Temporal frequency tuning curves for two representative neurons, during still and moving conditions, are shown in Figure 6A. Consistent with a previous study (Niell and Stryker, 2010), locomotion led to a significant increase in peak response amplitude in V1 neurons (76%; paired t test, p< 10−4; Figures 6B and 6C). Increases in peak response amplitude with locomotion were also observed in AL(35%, p< 0.01; significantly less than in V1, K–S test, p < 0.02) and in PM (109%, p = 0.12; lack of significance may be due to small sample size of eight neurons in PM). To address the possibility that modulation of neural responses during locomotion could be due to increased eye movements, we monitored eye movements in a subset of these recordings (Figure S2). Removing trials with large eye movements (>5°) or blinks had little effect on the modulation of response amplitude by locomotion (Figures S2G and S2H, bottom panels).
In addition to changes in response amplitude, we found small but significant increases in peak speed during locomotion (Figures 6D and 6E) in V1 (24% or 0.3 octaves, t test, p < 0.003) and AL (36%, 0.4 octaves, p < 10−4) and a similar trend in PM (32%, 0.4 octaves, p = 0.09). The increases in peak speed could be attributed to increases in temporal frequency preference (Figure 6E; t tests, p values < 10−3 in V1 and AL, p = 0.22 in PM) but not spatial frequency preference (all p values > 0.1). These small increases in peak speed are not due to increased incidence of locomotion induced by presentation of high-speed stimuli, as presentation of these localized stimuli did not change the incidence of locomotion (Figures S2D and S2F), nor are they likely due to differences in average eye position between running and nonrunning trials, which were quite small (<1°−2°, Figure S2C). Critically, the changes in frequency preferences were not significantly different across areas (all p values > 0.1), suggesting that area AL responds to very different spatial and temporal frequencies than area PM, whether or not the mouse is in motion. The robustness of areal differences to the effects of locomotion can also be seen in the average normalized response profiles for each area (Figure S6).
DISCUSSION
We used two-photon calcium imaging of local volumes of neurons in alert mice to assess the presence and degree of functional specialization in higher visual areas AL and PM. The two areas had almost entirely nonoverlapping stimulus preferences: AL neurons responded best to low spatial and high temporal frequencies, or fast-moving stimuli, while PM neurons responded best to high spatial and low temporal frequencies, or slowly moving stimuli. By contrast, neurons in area V1, which provide a major source of input to both areas, were sensitive to a broad range of frequencies and speeds. These findings were largely independent of whether the mouse was stationary or running: although responses were enhanced by locomotion across all three areas (cf. Niell and Stryker, 2010), only minor, uniform increases in peak speed were observed. In addition, populations of neurons in PM, AL, and V1 had similar orientation selectivity but differed in direction selectivity. These results show that higher visual areas AL and PM are strongly specialized for processing different kinds of visual information.
Characterization of Visual Cortical Areas in Different Species
Our findings of differential specializations in non-primary sensory neocortical areas in the mouse build on a large number of studies conducted in primates and carnivores, in which segregation of sensory information into parallel cortical streams is a common feature of visual cortex (Nassi and Callaway, 2009; Ungerleider and Mishkin, 1982), as well as auditory (Rauschecker and Tian, 2000) and somatosensory (Renier et al., 2009) cortices. In the visual cortex of primates and carnivores, receptive fields at successive stages in the cortical hierarchy become progressively more specialized (Maunsell and Newsome, 1987; Orban, 2008), and behavioral data suggest that distinct extrastriate areas contribute to different visual abilities such as object recognition (Conway et al., 2010; Desimone et al., 1985; Pasupathy and Connor, 1999) and motion perception (Born and Bradley, 2005; Britten and Van Wezel, 2002). These data, together with a wealth of anatomical evidence (Felleman and Van Essen, 1991), have suggested the presence of distinct ventral and dorsal visual cortical pathways, referred to as “what” and “where” streams (Ungerleider and Mishkin, 1982) or, in a somewhat different formulation, as “object recognition/action guidance” streams (Goodale and Milner, 1992; Nassi and Callaway, 2009). Importantly, the dorsal stream may be classified into multiple subnetworks that are thought to be differentially involved in processing optic flow signals during navigation (including dorsolateral areas; Andersen et al., 1997; Britten and Van Wezel, 2002; Duffy and Wurtz, 1991), or in rapid analysis of external object motion and form to guide motor planning (including dorsomedial areas; Galletti and Fattori, 2003).
Anatomical studies in rodent higher visual areas suggest a parallel organization similar to that in primates and carnivores, although a precise correspondence between individual areas may not exist. Mouse visual areas AL and PM receive strong direct projections from V1 (Wang and Burkhalter, 2007). These areas also receive indirect input from superior colliculus and V1 via higher-order thalamic nuclei (mouse: Simmons and Pearlman, 1982; rat: Caviness and Frost, 1980; Sanderson et al., 1991). Anatomically, area AL is reminiscent of dorsolateral stream areas in primates and carnivores, due to (1) its proximity and projections to anterior and lateral parietal areas, as well as its projections to motor cortex and medial entorhinal cortex (Wang et al., 2011), and (2) its associational inputs from adjacent somatosensory and auditory areas (Sanderson et al., 1991). Area PM, though less well understood, may have more in common with dorsomedial stream areas in higher mammals, due to its more medial location and its strong projections to anterior areas (Sanderson et al., 1991), as well as an absence of amygdalar inputs (compared to ventrotemporal areas; Wang and Burkhalter, 2011, Soc. Neurosci., abstract).
Functional Differences between Rodent Areas AL and PM
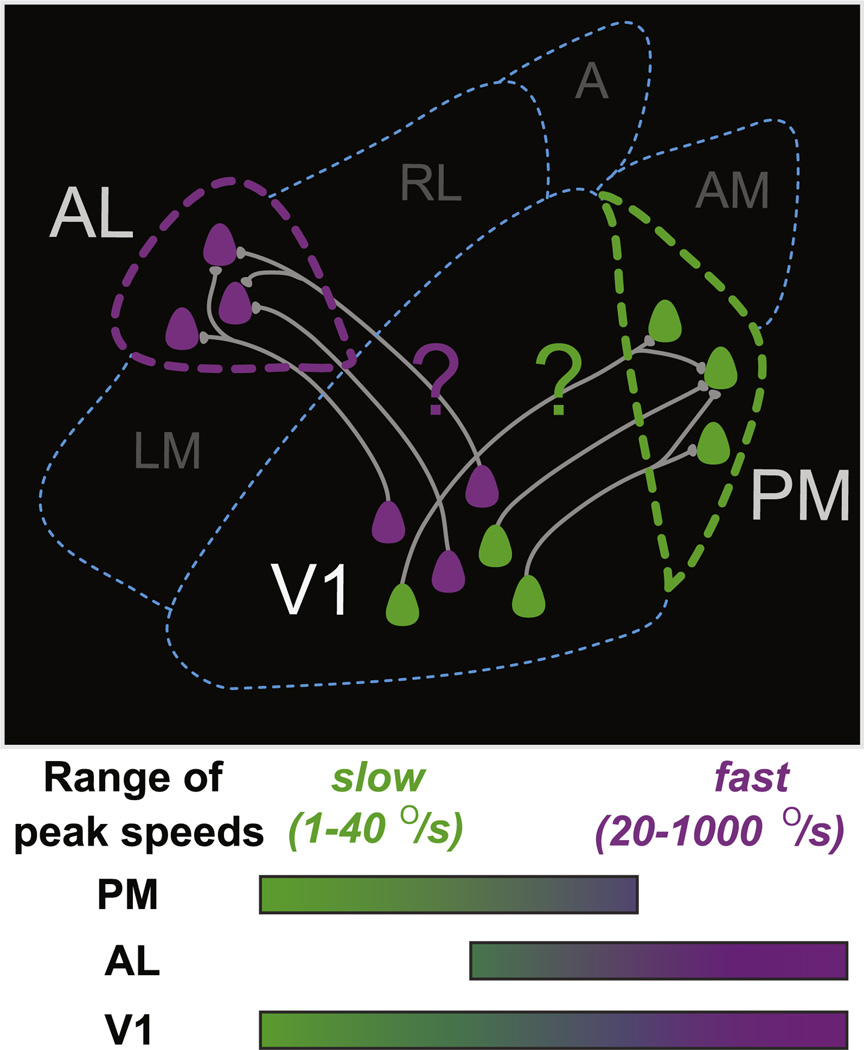
Our main finding was that populations of neurons in areas AL and PM preferred nearly nonoverlapping ranges of fast (20°/s−1000°/s) and slow (1°/s−40°/s) stimulus speeds, respectively, and that V1 spanned both speed ranges (1 °/s−1000°/s; Figures 3 and 7). Classification of neurons by peak speed was more effective than by spatial or temporal frequency alone, as neurons in AL that preferred the lowest temporal frequencies also preferred the lowest spatial frequencies and thus remained responsive to higher speeds (Figure 3B). Conversely, neurons in PM that preferred the highest temporal frequencies also preferred the highest spatial frequencies and thus remained responsive to lower speeds.
Figure 7. Model of Areal Specialization in Awake Mouse Visual Cortex.
Our results reveal intercalated groups neurons in V1 that prefer a broad range of stimulus speeds, while neurons in PM respond to low speeds and neurons in AL respond to high speeds. We predict that this specialization in AL and PM may arise in part from selective input from subsets of V1 neurons whose speed sensitivity matches that of the target area. Differences in peak speed, anatomical location, and connectivity also suggest that area AL may contribute to behaviors involving high-speed stimuli (e.g., optic flow during navigation), while PM may contribute to behaviors involving monitoring of slow-moving objects (see Figure S4).
Our findings of higher peak speeds in AL than PM are consistent with a widefield intrinsic autofluorescence imaging study in anesthetized mice that found stronger responses to higher-speed stimuli (50°/s) than to lower speed stimuli (10°/s) in anterior visual cortical areas including AL, but not in PM (Tohmi et al., 2009). Similarly, a c-fos study in rats found that area AL was robustly activated by moving but not stationary stimuli (Montero and Jian, 1995). Initial findings in area AL and/or PM of anesthetized mice, from several other laboratories, are also generally consistent with our findings regarding spatiotemporal tuning properties (E. Gao, G. DeAngelis, and A. Burkhalter, 2006, Soc. Neurosci., abstract; M. Roth, F. Helmchen, and B. Kampa, 2010, Soc. Neurosci., abstract; M. Garrett, J. Marshall, L. Nauhaus, and E. Callaway, 2010, Soc. Neurosci., abstract).
The upper range of effective stimulus speeds in mouse V1 (1000°/s) is over 20 times higher than in primate area MT (e.g., Perrone and Thiele, 2001; Priebe et al., 2006) but is consistent with an earlier study of neurons (at unknown depths within cortex) in lightly anesthetized mouse V1 (Dräger, 1975). Mouse V1 neurons preferring the highest peak speeds also preferred substantially lower spatial frequencies than in primate visual cortical neurons (e.g., Priebe et al., 2006). High-speed visual cues may be useful to mice during navigation. For example, when mice run across floors or along walls at high speeds (typical speeds of 10–50 cm/s at distances of 2–4 cm; Lipkind et al., 2004; Harvey et al., 2009), the resulting optic flow patterns are dominated by speeds up to ∼1000°/s. Despite these considerations, the dimension of stimulus speed may not be the computationally relevant variable for all neurons in our study. Indeed, while most neurons with low peak speeds were tuned for the same speed across spatial frequencies (Figure 4; Priebe et al., 2006), this was not the case for neurons with higher peak speeds.
Response Properties of Neurons in the Awake Mouse
We observed higher median values and broader ranges of spatial and temporal frequency preferences in layer II/III of awake mouse V1 (Figure 3; spatial and temporal frequency preferences > 0.1 cycles per degree and/or > 4 Hz, respectively) compared to several recent studies in anesthetized mouse V1 (Gao et al., 2010; Kerlin et al., 2010; Niell and Stryker, 2008). Similar increases in spatial and temporal frequency preferences have been observed in alert versus anesthetized primate LGN (Alitto et al., 2011). Thus, while many factors may contribute to the higher frequency preferences observed in our study, the absence of anesthesia may be an important factor. Anesthesia might influence several other aspects of visual processing, including increased retinal response latency (Guarino et al., 2004) and increased inhibition and/or response adaptation in thalamus and cortex (Campagna et al., 2003; Castro-Alamancos, 2004). Our pilot studies also suggested that V1 responses to fullfield gratings may be less effective in awake mice (data not shown) compared to anesthetized mice (Kerlin et al., 2010), presumably due to surround suppression. This led us to use localized 40° patches of drifting gratings in the current study. These effects may be even greater in higher visual areas than in V1 (Heinke and Schwarzbauer, 2001), underscoring the importance of studying higher visual areas in the absence of anesthesia.
Given the increased diversity of visual receptive field properties in awake mice, it is not surprising that our estimate of the percentage of neurons significantly responsive to sinusoidal stimuli (∼10%; Table S1) was considerably lower in this study than in previous imaging studies in anesthetized mouse V1 (Kerlin et al., 2010; Smith and Häusser, 2010; Sohya et al., 2007). These previous studies also used synthetic calcium indicators, which exhibit larger changes in fluorescence than GCaMP3 at low firing rates. Thus, cells in our experiments that were driven at low peak firing rates may have gone undetected due to background neuropil activation, especially given the strong and dense expression of GCaMP3 (cf. O’Connor et al., 2010). Finally, our inability to stimulate at multiple directions, spatial and temporal frequencies, retinotopic locations, and patch sizes within the same stimulus protocol certainly contributed to the low percentage of responsive cells.
Despite these considerations, the estimated percentages of significantly responsive neurons in PM, AL, and V1 were relatively similar (except somewhat lower PM responsiveness in the spatial frequency by temporal frequency protocol, see Results and Table S1), indicating that effectiveness of our stimulus set in driving responses was similar across areas. GCaMP3 fluorescence increases monotonically with firing rate (Tian et al., 2009), so the peak GCaMP3 responses used to estimate response preferences (Figures 3, 4, 6, and S5) should reflect the peak spiking response. However, the supralinear relationship between the size of GCaMP3 fluorescence transients and number of spikes (Borghuis et al., 2011; Tian et al., 2009) may influence absolute estimates of orientation and direction selectivity (Figure 5) by disproportionately underestimating weaker responses. Nevertheless, given that neither peak GCaMP3 response (Table 2) nor orientation selectivity (Figure 5) differed significantly between cortical areas, this nonlinearity is unlikely to affect our results regarding the relative degree of selectivity across areas.
Roles of Higher Visual Areas in Behavior
Previous behavioral and lesion studies in rats have suggested that, as in primates (Nassi and Callaway, 2009), specific higher visual areas may be differentially involved in specific aspects of action guidance or object recognition (Aggleton et al., 1997; Dean, 1981; Kolb and Walkey, 1987; McDaniel et al., 1982). It is clear that mice can also rely on visual cues for action guidance and object recognition during various natural behaviors, including navigation (Harvey et al., 2009; Mather and Baker, 1980), escaping predators (Edut and Eilam, 2004), and optomotor head-tracking (Umino et al., 2008). It is possible that cortical areas such as AL are involved in estimating self-motion, due to the sensitivity of neurons in AL to very high-speed stimuli that would arise during locomotion. By contrast, cortical area PM may be more involved than AL in cortically dependent aspects of object tracking, based on anatomical arguments described above, together with the following considerations: (1) the behavioral sensitivity for head-tracking of visual stimuli of varying spatial and temporal frequencies (Umino et al., 2008) is well matched to the neural sensitivity profile that we observed in area PM (Figure S4); (2) objects consist of multiple spatial frequencies moving with similar speed, and tuning for speed across different spatial frequencies is more common in PM (Figure 4); and (3) lesions of posterior visual cortex abolish experience-dependent optomotor learning (Prusky et al., 2008).
We also considered the possibility that a very simple behavior, locomotion, may modulate the responses of neurons in different visual areas. We found that response strength increased with locomotion across all cortical areas (Figure 6), consistent with a recent electrophysiological study in mouse V1 (Niell and Stryker, 2010). In addition, we observed small but significant increases in speed and temporal (but not spatial) frequency preference (< ½ octave) in V1 and AL neurons. Critically, these modest effects of locomotion did not alter our principal finding of large differences in the range of peak speeds between areas AL and PM (Figures 3, 6D, and Figure S2G and S2H). Similar increases in temporal frequency preference have been observed in rabbit LGN during arousal (Bezdudnaya et al., 2006), and even in Drosophila visual neurons during both locomotion and flight (Chiappe et al., 2010; Jung et al., 2011; Maimon et al., 2010).
How Might Functional Specialization in Rodent Areas AL and PM Emerge?
We found that the nearly nonoverlapping ranges of peak speeds in areas AL and PM were contained within a broader range of peak speeds observed in V1 neurons (Figure 3). We therefore propose that the distinct functional properties of AL and PM neurons may emerge, at least in part, from selective routing of axonal projections of functionally distinct subsets of V1 layer II/III neurons (Figure 7). Segregation of information streams may also come about via local competition between sets of V1 neurons preferring low versus high speeds (Figure 7), through recurrent excitation between neurons with similar preferences (Ko et al., 2011) and/or nonspecific inhibition across neurons regardless of preference (Bock et al., 2011; Fino and Yuste, 2011; Kerlin et al., 2010; Kapfer et al., 2007; Swadlow and Gusev, 2002).
While hypotheses regarding interareal functional connectivity can be tested using antidromic stimulation and electrophysiological recordings (e.g., Movshon and Newsome, 1996; Swadlow, 1998), an increasing number of complementary anatomical, imaging, and genetic techniques are becoming available, particularly in the mouse (Berezovskii et al., 2011; Molyneaux et al., 2009; Osakada et al., 2011; Sato and Svoboda, 2010). Our findings provide a conceptual and technical framework for combining these tools with cellular imaging to dissect interareal circuitry in the visual cortex of behaving mice (Andermann et al., 2010).
EXPERIMENTAL PROCEDURES
Cranial Window Implant, Habituation, and Targeted Expression of Calcium Indicator
All procedures were conducted in accordance with the ethical guidelines of the National Institutes of Health and were approved by the IACUC at Harvard Medical School. Eight male and female adult mice (2–6 months old; various strains, C57BL/6 primary background) were used in this study. Of these, 5 mice were crosses of the Pvalb-IRES-Cre line (Hippenmeyer et al., 2005; Jax no. 008069) and the Rosa-CAG-LSL-tdTomato-WPRE:: deltaNeo line (Madisen et al., 2010; Jax no. 007914). The labeling of parvalbumin-expressing neurons via red tdTomato fluorescence in these mice was not used in the current study. For cranial window implant surgery, animals were anesthetized with isoflurane (1.2%–2% in 100% O2). Dexamethasone was administered on the day prior to surgery (3.2 mg/kg, IM) and atropine at surgery onset (0.2 mg/kg, IP). Using aseptic technique, a headpost and EEG leads were secured in place using cyanoacrylate, dental acrylic, and C&B Metabond (Parkell), and a 5 mm craniotomy was made over the left visual cortex (center ∼2.8 mm lateral, 0.5 mm anterior to lambda) as described previously (Andermann et al., 2010). We implanted a 5 mm glass cranial window consisting of an 8 mm coverslip cured to two 5 mm coverslips (Warner #1; total thickness: ∼0.5 mm; thickness below skull: ∼200 µm) using index-matched adhesive (Norland #71). The window was secured in place using cyanoacrylate and dental acrylic, and the mice were allowed to recover for at least 4 days.
Habituation consisted of water scheduling so that water was delivered only during and immediately after head restraint training. Sessions of head restraint increased in duration over the course of 1–2 weeks, from 3 min to 2 hr (Andermann et al., 2010). At this point, retinotopic mapping of visual cortical areas was conducted in awake mice using widefield intrinsic autofluorescence imaging (see below). Targeted expression of calcium indicator GCaMP3 was achieved as follows: mice were anesthetized (isoflurane, 1.5%–2%) and the cranial window was sterilized with alcohol and the coverslip removed. We then used a volume injection system (100 µl/min, Stoelting) to inject 100–1000 nl (depending on batch titer) of a 7:3 mixture of AAV2/1.hSynap. GCaMP3.3.SV40 (Tian et al., 2009; Penn Vector Core) and D-mannitol (Mastakov et al., 2001). Using the blood vessel pattern observed during widefield imaging as a guide, we made either one injection in the posterior/medial part of area V1 (temporal/superior visual field) or two injections in the retinotopically matched regions of areas AL and PM. All injections were at a depth of 200–300 µm below the pial surface. After injections, a new cranial window was sealed in place and the mouse was recovered. Experiments were conducted 10 days-6 weeks after injections.
Widefield Imaging
To map visual cortical areas, we used epifluorescence imaging (Husson et al., 2007; Tohmi et al., 2009) to measure changes in the intrinsic autofluorescence signal. Autofluorescence produced by blue excitation (470 nm center, 40 nm band, Chroma) was measured through a green/red emission filter (longpass, 500 nm cutoff). Images were collected using a CCD camera (Sensicam, Cooke, 344 × 260 pixels spanning 4×3 mm; 2 Hz acquisition rate) through a 5× air objective (0.14 NA, Mitituyo) using ImageJ acquisition software. For retinotopic mapping, we stimulated at 2–6 retinotopic positions for 5 s each, with 15 s of blank monitor screen (mean luminance) between trials. Autofluorescence visual responses consist of a weak positive signal (flavoprotein oxidization during increased metabolism; Tohmi et al., 2009) followed by a stronger negative signal (increased light absorption due to delayed increase in blood volume and deoxyhemoglobin concentration, Schuett et al., 2002). Thus, the response to a stimulus was computed as the fractional change in fluorescence between the average of all frames from 0–3 s after stimulus onset and the average from 9–19 s after stimulus onset (Figures 1A and 1B). For widefield imaging of GCaMP3 (Figures 1C and 1D), an identical procedure was used except total trial duration was reduced to 10 s, and changes in fluorescence were calculated as the fractional change in average fluorescence from [−2 s, 0 s] to [0 s, 5 s] after stimulus onset. See Figure S1, legend, for additional details.
Two-Photon Calcium Imaging
Imaging was performed with a custom-built two-photon microscope controlled by a modified version of ScanImage (Pologruto et al., 2003), as described previously (Andermann et al., 2010; Kerlin et al., 2010). Excitation light from a Mai Tai DeepSee laser (Newport Corp.) with group delay dispersion compensation was scanned by galvanometers (Cambridge Technology) through a 25× 1.05 NA objective (Olympus). Three-dimensional imaging was achieved by trapezoidal scanning of the microscope objective at 1 Hz using a piezo Z-scanner (P-721.LLQ, Physik Instrumente), while acquiring frames at 16 Hz (128 × 128 pixel frames, bidirectional scanning, pixel dwell time ∼3 µs; a total of 15 frames were used per volume, 3 µm depth between frames; one frame during objective flyback was discarded). Volumes were typically 200 µm × 200 µm × 45 µm. Laser power exiting the objective ranged from 12–60 mW and was continuously adjusted depending on instantaneous focal depth. GCaMP3 was excited at 960 nm and emission was collected with a green 2″ filter (542 nm center; 50 nm band; Semrock) via GaAsP photomultiplier tubes (Hamamatsu). Neurons were confirmed to be within a particular cortical area by comparison of two-photon images of surface vasculature above the imaging site with surface vasculature from widefield (intrinsic autofluorescence signal) retinotopic mapping. Recording sessions were 3–5 hr in duration. Viral expression of GCaMP3 permitted recording from neurons across multiples cortical areas in the same mice on different days (Andermann et al., 2010; Dombeck et al., 2010; Mank et al., 2008; O’Connor et al., 2010; Tian et al., 2009). When recording from the same cortical region on multiple days, previously imaged neurons were relocated and an adjacent volume was selected to ensure that all neurons in the sample were unique.
During imaging, mice were placed on a 6″ foam trackball (Plasteel) that could spin noiselessly on ball bearings (McMaster-Carr). We monitored trackball revolutions using a custom photodetector circuit. In a subset of experiments, we recorded eye movements using a CMOS camera (Mightex; 20 Hz) and infrared illumination (720–900 nm bandpass filters, Edmund).
Visual Stimulation
To achieve accurate stimulation at temporal frequencies of 0.5–24 Hz, we used a 120 Hz LCD monitor (Samsung 2233RZ, 22″) calibrated (at each stimulus frequency) using a spectrophotometer (Photoresearch PR-650; see also Wang and Nikolić, 2011). Waveforms were also confirmed to be sinusoidal by measuring luminance fluctuations of a full-field sinusoidally modulated stimulus (using a photomultiplier tube, Hamamatsu). The monitor was positioned so that the stimulus patch was 21 cm from the contralateral eye. Stimuli were centered at monocular locations of 70° to 115° eccentricity and −5° to 14° elevation (which provided maximal separation of responsive regions across visual cortical areas, Figure 1A). For cellular imaging, local 40° Gabor-like circular patches (sigmoidal 10%–90% falloff in 10°) containing sine-wave drifting gratings (80% contrast) were presented for 5 s, followed by 5 s of uniform mean luminance (46 cd/m2). In the spatial frequency × temporal frequency protocol (Figure 2), we presented upward-drifting gratings at 5 spatial frequencies (0.02, 0.04, 0.08, 0.16, and 0.32 cycles per degree, cpd) and 7 temporal frequencies (0.5,1,2, 4, 8,15, and 24 Hz) for a total of 35 stimulus types plus 10% blank trials. In the spatial frequency × direction protocol (Figure 5), we presented up to 6 spatial frequencies (0.02,0.04,0.08, 0.16, and 0.32 and sometimes 0.64 cpd) and 8 directions of motion (45° spacing) plus 10% blanks. In this protocol, the temporal frequency of gratings was 2 Hz in areas V1 and PM, but 8 Hz in AL in order to drive a comparable fraction of cells. All stimuli in a given protocol were randomized (sampling without replacement), and presented 9–28 times (median of 20 and 15 trials per stimulus for spatial frequency × temporal frequency and spatial frequency × direction protocols, respectively).
Data Analysis
Data analyses were performed in Matlab (MathWorks) and ImageJ (NIH). Two-photon imaging stacks were aligned (using rigid-body transformation) volume-by-volume to correct for slow drifts, as described previously (Kerlin et al., 2010). Evoked responses for each stimulus type were defined for each pixel in the imaging volume as the fractional change in fluorescence (ΔF/F) between [−2 s, 0 s] and [0 s, 5 s] after onset of the 5 s stimulus, averaged across trials. Because baseline fluorescence was sometimes dim, three-dimensional cell masks were obtained by taking the maximum fractional change in fluorescence (ΔF/F) across average response volumes for all stimulus types, and using custom semi-automated segmentation algorithms (see Figure S3, legend, for additional details).
Cellular fluorescence time courses were generated by averaging all pixels in a cell mask. Neuropil signals were removed by first selecting a spherical neuropil shell surrounding each neuron (excluding adjacent cell masks; Kerlin et al., 2010), estimating the common time course of all such shells in the volume (1st principal component), and removing this component from each cell’s time course (scaled by the baseline fluorescence of the surrounding shell). For subsequent analyses, only cells that were significantly driven by at least one stimulus type were included (t tests with Bonferroni correction, p < (0.05/n), where n = 35–48 depending on the stimulus protocol).
For the spatial frequency × temporal frequency protocol (Figure 2), responses were well fit by a two-dimensional elliptical Gaussian (Priebe et al., 2006):
R(sf,tf)=Aexp(−(log2sf−log2sf0)22(σsf)2)exp(−(log2tf−log2tfp(sf))22(σtf)2)
where A is the neuron’s peak response, sf_0 and tf_0 are the neuron’s preferred spatial and temporal frequencies, and σ_sf and σ_tf are the spatial and temporal frequency tuning widths. The dependence of temporal frequency preference on spatial frequency is captured by a power-law exponent î, such that log2 tfp (sf) = ξ(log2 sf – log2 _sf_0) + log2 tf0. For this protocol, we estimated upper and lower confidence bounds for _sf_0 and _tf_0 by performing 500 Monte-Carlo simulations (random sampling of trials of each stimulus type with replacement). Only neurons with 95% confidence intervals less than 1.5 octaves for both sf_0 and tf_0 were included in subsequent analyses. This strict criterion eliminated an additional 37%, 20% and 20% recordings in PM, AL, and V1, respectively (results were very similar without this criterion, data not shown). For analyses in Figure 4, only estimates of î with (1) 95% confidence intervals < 1; (2) preferred frequencies contained within our sampling range; and (3) estimates of σ_Sf and σ_tf both exceeding 1 octave were included. Estimates of 50% high-cutoff values for spatial and temporal frequency (Figures 3C and 3D) were also obtained from the model fit (from cross-sections at R(_sf, tf_0) and R(_sf_0, tf), respectively).
For estimation of the optimal linear classifier of frequency preferences, (_sf_0, _tf_0), between AL and PM, we performed linear discriminant analysis and found that the optimal classifier line described was given by log2(_sf_0) = −5.39 + 0.997*log2(_tf_0), which corresponds approximately to an iso-speed line given by speed = _tf/sf_= 41.9°/s (yellow line, Figure 3B).
For the spatial frequency × direction protocol, we first found the preferred orientation (averaged across spatial frequencies), and estimated the peak spatial frequency (at the neuron’s preferred orientation). We then computed orientation and direction selectivity indices as (Rpeak −Rnull) / (Rpeak + Rnull) at the neuron’s preferred spatial frequency (for direction estimates, Rpeak = preferred direction, Rnull = response at 180° from preferred; for orientation estimates, Rpeak = preferred orientation, Rnull = response at 90° from preferred; Kerlin et al., 2010; Niell and Stryker, 2008).
For analyses of influences of locomotion on spatial and temporal frequency responses (Figures 6, S2, and S6), we divided trials for each stimulus type into those in which any wheel motion was observed in the 5 s of stimulus presentation (“moving” trials) and those that lacked any movement (“still” trials). In a subset of experiments (Figure S2), we analyzed eye position using custom Matlab implementation of a previously described algorithm for pupil tracking (Zoccolan et al., 2010).
Supplementary Material
01
ACKNOWLEDGMENTS
We thank Glenn Goldey for surgical contributions, Anthony Moffa and Paul Serrano for behavioral training, and Sergey Yurgenson for technical contributions and eye-tracking code. Aleksandr Vagodny, Adrienne Caiado, and Derrick Brittain provided valuable technical assistance. We also thank John Maunsell, Bevil Conway, Jonathan Nassi, Christopher Moore, Rick Born, and members of the Reid Lab–especially Vincent Bonin–for advice, suggestions, and discussion. This work was supported by NIH (R01 EY018742) and by fellowships from the Helen Hay Whitney Foundation (M.L.A. and L.L.G.), the Ludcke Foundation and Pierce Charitable Trust (M.L.A.), and the Sackler Scholar Programme in Psychobiology (A.M.K.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes one table and six figures and can be found with this article online at doi:10.1016/j.neuron.2011.11.013.
REFERENCES
- Aggleton JP, Keen S, Warburton EC, Bussey TJ. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res. Bull. 1997;43:279–287. doi: 10.1016/s0361-9230(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Alitto HJ, Moore BD, 4th, Rathbun DL, Usrey WM. A comparison of visual responses in the lateral geniculate nucleus of alert and anaesthetized macaque monkeys. J. Physiol. 2011;589:87–99. doi: 10.1113/jphysiol.2010.190538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann ML, Kerlin AM, Reid RC. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front. Cell. Neurosci. 2010;4:3. doi: 10.3389/fncel.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu. Rev. Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Berezovskii VK, Nassi JJ, Born RT. Segregation of feedforward and feedback projections in mouse visual cortex. J. Comp. Neurol. 2011;519:3672–3683. doi: 10.1002/cne.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Bock DD, Lee WC, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;471:177–182. doi: 10.1038/nature09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Tian L, Xu Y, Nikonov SS, Vardi N, Zemelman BV, Looger LL. Imaging light responses of targeted neuron populations in the rodent retina. J. Neurosci. 2011;31:2855–2867. doi: 10.1523/JNEUROSCI.6064-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. Annu. Rev. Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Britten KH, Van Wezel RJ. Area MST and heading perception in macaque monkeys. Cereb. Cortex. 2002;12:692–701. doi: 10.1093/cercor/12.7.692. [DOI] [PubMed] [Google Scholar]
- Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron. 2004;41:455–464. doi: 10.1016/s0896-6273(03)00853-5. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr., Frost DO. Tangential organization of thalamic projections to the neocortex in the mouse. J. Comp. Neurol. 1980;194:335–367. doi: 10.1002/cne.901940205. [DOI] [PubMed] [Google Scholar]
- Chiappe ME, Seelig JD, Reiser MB, Jayaraman V. Walking modulates speed sensitivity in Drosophila motion vision. Curr. Biol. 2010;20:1470–1475. doi: 10.1016/j.cub.2010.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BR, Chatterjee S, Field GD, Horwitz GD, Johnson EN, Koida K, Mancuso K. Advances in color science: from retina to behavior. J. Neurosci. 2010;30:14955–14963. doi: 10.1523/JNEUROSCI.4348-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. Visual pathways and acuity hooded rats. Behav. Brain Res. 1981;3:239–271. doi: 10.1016/0166-4328(81)90050-4. [DOI] [PubMed] [Google Scholar]
- Desimone R, Schein SJ, Moran J, Ungerleider LG. Contour, color and shape analysis beyond the striate cortex. Vision Res. 1985;25:441–452. doi: 10.1016/0042-6989(85)90069-0. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 2010;13:1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dräger UC. Receptive fields of single cells and topography in mouse visual cortex. J. Comp. Neurol. 1975;160:269–290. doi: 10.1002/cne.901600302. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large-field stimuli. J. Neurophysiol. 1991;65:1329–1345. doi: 10.1152/jn.1991.65.6.1329. [DOI] [PubMed] [Google Scholar]
- Edut S, Eilam D. Protean behavior under barn-owl attack: voles alternate between freezing and fleeing and spiny mice flee in alternating patterns. Behav. Brain Res. 2004;155:207–216. doi: 10.1016/j.bbr.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti C, Fattori P. Neuronal mechanisms for detection of motion in the field of view. Neuropsychologia. 2003;41:1717–1727. doi: 10.1016/s0028-3932(03)00174-x. [DOI] [PubMed] [Google Scholar]
- Gao E, DeAngelis GC, Burkhalter A. Parallel input channels to mouse primary visual cortex. J. Neurosci. 2010;30:5912–5926. doi: 10.1523/JNEUROSCI.6456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girman SV, Sauvé Y, Lund RD. Receptive field properties of single neurons in rat primary visual cortex. J. Neurophysiol. 1999;82:301–311. doi: 10.1152/jn.1999.82.1.301. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Guarino I, Loizzo S, Lopez L, Fadda A, Loizzo A. A chronic implant to record electroretinogram, visual evoked potentials and oscillatory potentials in awake, freely moving rats for pharmacological studies. Neural Plast. 2004;11:241–250. doi: 10.1155/NP.2004.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–946. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinke W, Schwarzbauer C. Subanesthetic isoflurane affects task-induced brain activation in a highly specific manner: a functional magnetic resonance imaging study. Anesthesiology. 2001;94:973–981. doi: 10.1097/00000542-200106000-00010. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson TR, Mallik AK, Zhang JX, Issa NP. Functional imaging of primary visual cortex using flavoprotein autofluorescence. J. Neurosci. 2007;27:8665–8675. doi: 10.1523/JNEUROSCI.2156-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SN, Borst A, Haag J. Flight activity alters velocity tuning of fly motion-sensitive neurons. J. Neurosci. 2011;31:9231–9237. doi: 10.1523/JNEUROSCI.1138-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalatsky VA, Stryker MP. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron. 2003;38:529–545. doi: 10.1016/s0896-6273(03)00286-1. [DOI] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjöström PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Walkey J. Behavioural and anatomical studies of the posterior parietal cortex in the rat. Behav. Brain Res. 1987;23:127–145. doi: 10.1016/0166-4328(87)90050-7. [DOI] [PubMed] [Google Scholar]
- Lipkind D, Sakov A, Kafkafi N, Elmer GI, Benjamini Y, Golani I. New replicable anxiety-related measures of wall vs center behavior of mice in the open field. J. Appl. Physiol. 2004;97:347–359. doi: 10.1152/japplphysiol.00148.2004. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat. Neurosci. 2010;13:393–399. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- Mank M, Santos AF, Direnberger S, Mrsic-Flogel TD, Hofer SB, Stein V, Hendel T, Reiff DF, Levelt C, Borst A, et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat. Methods. 2008;5:805–811. doi: 10.1038/nmeth.1243. [DOI] [PubMed] [Google Scholar]
- Mastakov MY, Baer K, Xu R, Fitzsimons H, During MJ. Combined injection of rAAV with mannitol enhances gene expression in the rat brain. Mol. Ther. 2001;3:225–232. doi: 10.1006/mthe.2001.0246. [DOI] [PubMed] [Google Scholar]
- Mather JG, Baker RR. A demonstration of navigation by small rodents using an orientation cage. Nature. 1980;284:259–262. doi: 10.1038/284259a0. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Newsome WT. Visual processing in monkey extrastriate cortex. Annu. Rev. Neurosci. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- McDaniel WF, Coleman J, Lindsay JF., Jr. A comparison of lateral peristriate and striate neocortical ablations in the rat. Behav. Brain Res. 1982;6:249–272. doi: 10.1016/0166-4328(82)90027-4. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Fame RM, MacDonald JL, MacQuarrie KL, Macklis JD. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J. Neurosci. 2009;29:12343–12354. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero VM, Jian S. Induction of c-fos protein by patterned visual stimulation in central visual pathways of the rat. Brain Res. 1995;690:189–199. doi: 10.1016/0006-8993(95)00620-6. [DOI] [PubMed] [Google Scholar]
- Moore BD, 4th, Alitto HJ, Usrey WM. Orientation tuning, but not direction selectivity, is invariant to temporal frequency in primary visual cortex. J. Neurophysiol. 2005;94:1336–1345. doi: 10.1152/jn.01224.2004. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Newsome WT. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J. Neurosci. 1996;16:7733–7741. doi: 10.1523/JNEUROSCI.16-23-07733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flögel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hu¨ bener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J. Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron. 2010;67:1048–1061. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- Orban GA. Higher order visual processing in macaque extrastriate cortex. Physiol. Rev. 2008;88:59–89. doi: 10.1152/physrev.00008.2007. [DOI] [PubMed] [Google Scholar]
- Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, Callaway EM. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011;71:617–631. doi: 10.1016/j.neuron.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack CC, Berezovskii VK, Born RT. Dynamic properties of neurons in cortical area MT in alert and anaesthetized macaque monkeys. Nature. 2001;414:905–908. doi: 10.1038/414905a. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Responses to contour features in macaque area V4. J. Neurophysiol. 1999;82:2490–2502. doi: 10.1152/jn.1999.82.5.2490. [DOI] [PubMed] [Google Scholar]
- Perrone JA, Thiele A. Speed skills: measuring the visual speed analyzing properties of primate MT neurons. Nat. Neurosci. 2001;4:526–532. doi: 10.1038/87480. [DOI] [PubMed] [Google Scholar]
- Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. 2003:13. doi: 10.1186/1475-925X-2-13. Online2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Lisberger SG, Movshon JA. Tuning for spatiotem-poral frequency and speed in directionally selective neurons of macaque striate cortex. J. Neurosci. 2006;26:2941–2950. doi: 10.1523/JNEUROSCI.3936-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Characterization of mouse cortical spatial vision. Vision Res. 2004;44:3411–3418. doi: 10.1016/j.visres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Silver BD, Tschetter WW, Alam NM, Douglas RM. Experience-dependent plasticity from eye opening enables lasting, visual cortex-dependent enhancement of motion vision. J. Neurosci. 2008;28:9817–9827. doi: 10.1523/JNEUROSCI.1940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wang J, Sato Y. Heterogeneous neuronal responses to frequency-modulated tones in the primary auditory cortex of awake cats. J. Neurophysiol. 2008;100:1622–1634. doi: 10.1152/jn.90364.2008. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc. Natl. Acad. Sci. USA. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. Multisensory integration of sounds and vibrotactile stimuli in processing streams for “what” and “where”. J. Neurosci. 2009;29:10950–10960. doi: 10.1523/JNEUROSCI.0910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MG, Krubitzer LA. The evolution of visual cortex: where is V2? Trends Neurosci. 1999;22:242–248. doi: 10.1016/s0166-2236(99)01398-3. [DOI] [PubMed] [Google Scholar]
- Sanderson KJ, Dreher B, Gayer N. Prosencephalic connections of striate and extrastriate areas of rat visual cortex. Exp. Brain Res. 1991;85:324–334. doi: 10.1007/BF00229410. [DOI] [PubMed] [Google Scholar]
- Sato TR, Svoboda K. The functional properties of barrel cortex neurons projecting to the primary motor cortex. J. Neurosci. 2010;30:4256–4260. doi: 10.1523/JNEUROSCI.3774-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuett S, Bonhoeffer T, Hübener M. Mapping retinotopic structure in mouse visual cortex with optical imaging. J. Neurosci. 2002;22:6549–6559. doi: 10.1523/JNEUROSCI.22-15-06549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons PA, Pearlman AL. Retinotopic organization of the striate cortex (area 17) in the reeler mutant mouse. Brain Res. 1982;256:124–126. doi: 10.1016/0165-3806(82)90105-5. [DOI] [PubMed] [Google Scholar]
- Smith SL, Häusser M. Parallel processing of visual space by neighboring neurons in mouse visual cortex. Nat. Neurosci. 2010;13:1144–1149. doi: 10.1038/nn.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohya K, Kameyama K, Yanagawa Y, Obata K, Tsumoto T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. J. Neurosci. 2007;27:2145–2149. doi: 10.1523/JNEUROSCI.4641-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Neocortical efferent neurons with very slowly conducting axons: strategies for reliable antidromic identification. J. Neurosci. Methods. 1998;79:131–141. doi: 10.1016/s0165-0270(97)00176-3. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. Receptive-field construction in cortical inhibitory interneurons. Nat. Neurosci. 2002;5:403–404. doi: 10.1038/nn847. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohmi M, Takahashi K, Kubota Y, Hishida R, Shibuki K. Transcranial flavoprotein fluorescence imaging of mouse cortical activity and plasticity. J. Neurochem. 2009;109(Suppl 1):3–9. doi: 10.1111/j.1471-4159.2009.05926.x. [DOI] [PubMed] [Google Scholar]
- Umino Y, Solessio E, Barlow RB. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J. Neurosci. 2008;28:189–198. doi: 10.1523/JNEUROSCI.3551-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two Cortical Visual Systems. In: Ingle D, Goodale M, Mansfield R, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. [Google Scholar]
- Van den Bergh G, Zhang B, Arckens L, Chino YM. Receptive-field properties of V1 and V2 neurons in mice and macaque monkeys. J. Comp. Neurol. 2010;518:2051–2070. doi: 10.1002/cne.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Burkhalter A. Area map of mouse visual cortex. J. Comp. Neurol. 2007;502:339–357. doi: 10.1002/cne.21286. [DOI] [PubMed] [Google Scholar]
- Wang P, Nikolić D. An LCD Monitor with Sufficiently Precise Timing for Research in Vision. Front Hum Neurosci. 2011;5:85. doi: 10.3389/fnhum.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Gao E, Burkhalter A. Gateways of ventral and dorsal streams in mouse visual cortex. J. Neurosci. 2011;31:1905–1918. doi: 10.1523/JNEUROSCI.3488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolan D, Graham BJ, Cox DD. A self-calibrating, camera-based eye tracker for the recording of rodent eye movements. Front Neurosci. 2010;4:193. doi: 10.3389/fnins.2010.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01