An LC/MS method for d8-β-carotene and d4-retinyl esters: β-carotene absorption and its conversion to vitamin A in humans (original) (raw)
Abstract
The intestinal absorption and metabolism of β-carotene is of vital importance in humans, especially in populations that obtain the majority of their vitamin A from provitamin A carotenoids. MS has provided a better understanding of the absorption of β-carotene, the most potent provitamin A carotenoid, through the use of stable isotopes of β-carotene. We report here an HPLC-MS method that eliminates the need for complicated sample preparation and allows us to detect and quantify newly absorbed d8-β-carotene as well as its d4-retinyl ester metabolites in human plasma and chylomicron fractions. Both retinoids and β-carotene were recovered in a single simple extraction that did not involve saponification, thus allowing subsequent quantitation of individual fatty acyl esters of retinol. Separation of d8-β-carotene and its d4-retinyl ester metabolites was achieved using the same C30 reversed-phase liquid chromatography followed by mass spectrometry in selected ion monitoring and negative atmospheric pressure chemical ionization modes, respectively. Total time for the two successive runs was 30 min. This HPLC-MS method allowed us to quantify the absorption of intact d8-β-carotene as well as its extent of conversion to d4-retinyl esters in humans after consumption of a single 5 mg dose of d8-β-carotene.
Keywords: stable isotopes, sample matrix, intestinal absorption, chylomicrons, carotenoid metabolism, retinoid metabolism
Vitamin A deficiency is one of the most prevalent vitamin deficiencies throughout the world that affects over 100 million people and occurs in regions where people obtain most of their vitamin A as provitamin A carotenoids. A better understanding of the mechanisms of absorption and metabolism of provitamin A carotenoids could help alleviate nutritional problems associated with vitamin A deficiency (1).
The absorption of β-carotene involves its release from the food matrix, incorporation into mixed lipid micelles, uptake by the intestinal mucosal cells, packaging into chylomicrons (CMs), and secretion into the lymph. The intestinal uptake of β-carotene may involve facilitated uptake by transport proteins such as scavenger receptor class B, type I, cluster determinant 36, and Niemann-Pick type C1 Like 1 (2). Once β-carotene is taken up by the intestinal mucosal cell, it is either cleaved by β-carotene oxygenase 1 or it is absorbed intact. The β-carotene and its metabolites are incorporated into CMs along with cholesterol esters, triglycerides, apolipoprotein B, and phospholipids. The CMs are then secreted into the lymphatic system and enter the blood via the thoracic duct. CM remnants are then taken up by the liver and other tissues where β-carotene and its metabolites are stored and further metabolized.
In most human studies of β-carotene absorption, subjects have been dosed with stable isotopes of β-carotene such as 13C-β-carotene or [2H8] β-carotene (d8-βC) in order to distinguish the dietary dose from endogenous species. Samples are often saponified to eliminate interference of other lipids in the analysis, thus converting all retinyl esters to retinol and leading to the loss of valuable information. We developed an LC-MS method to study the absorption and metabolism of d8-β-carotene in humans. The method was applied to the analysis of whole plasma or a CM-rich plasma fraction for d4-retinol, individual d4-retinyl esters, and d8-β-carotene. The analytical procedure involved minimal sample handling and avoids saponification, allowing us to distinguish between d4-retinol and individual d4-retinyl esters. We also have only one sample preparation method for both the retinoid derivatives and β-carotene with good recovery of all compounds of interest. Our method allowed us to separate and quantify four retinyl esters (retinyl linoleate, oleate, palmitate, and stearate) and we were able to detect both the native retinyl esters (unlabeled) and the d4-retinyl esters in the CM fraction. The method also allowed us to accurately determine the extent of intestinal conversion of β-carotene to vitamin A metabolites. The method can be used for relatively high-throughput quantification of the absorption of β-carotene and conversion of β-carotene to its vitamin A metabolites in humans and may also be useful for the detection of the retinoids and carotenoids in other biological samples.
Materials and Methods
Chemicals and solutions
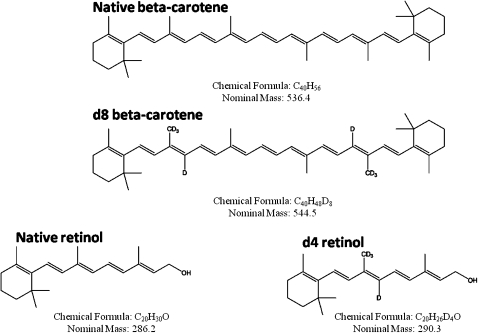
Unless otherwise stated, all chemicals and supplies were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). d8-β-Carotene was purchased from Cambridge Isotopes (Andover, MA) and had 83% all-trans and 17% cis isomers. Isotopomer distribution of d8-β-carotene was 79.44% d8, 17.59% d7, and 2.96% d6. 13C40-β-carotene was purchased from Martek (Columbia, MD) and d4-retinyl acetate was purchased from Cambridge Isotopes. Retinyl linoleate, retinyl oleate, and retinyl stearate were synthesized by Dr. Robert Curley (College of Pharmacy, Ohio State University). The syntheses were patterned after (3) except that retinol was used to make the esters instead of amines to make amides. The structures and masses of the labeled and native β-carotene and retinoids are show in Fig. 1.
Fig. 1.
Chemical structure, formula, and formula weights of the stable isotope-labeled and native β-carotene and retinol analyzed in this study.
Subjects and samples collection
Subjects were recruited from a human study that involved measurement of their cholesterol absorption to participate in a β-carotene absorption study. All procedures were approved by the Johns Hopkins School of Public Health Institutional Review Board and subjects gave written informed consent before participating. All subjects were male smokers and were on a defined diet. Subjects were given a single 5 mg dose of deuterated β-carotene (d8-βC) in 8 ml of corn oil. Subjects were catheterized and 10 ml of blood was drawn into 10 ml Vacutainer tubes containing EDTA (Beckton Dickinson, Franklin Lakes, NJ) every h for nine h. Four milliliters of the plasma was separated into a CM fraction and a nonCM fraction by ultracentrifugation (4). All aliquots of plasma fractions were stored at −80°C until analysis.
Sample extraction
One milliliter of the CM fraction was transferred to an 11 ml glass tube for extraction. Internal standards (ISs; retinyl acetate and 13C40-βC) were added followed by 1 ml of ethanol with 0.1% (w/v) BHT. Next, 5 ml of HEAT (10:6:7:7 Hexane/Ethanol/Acetone/Toluene) was added along with a saturated NaCl solution (200 µL) to facilitate phase separation. Samples were vortexed for 60 s and centrifuged at 5,000 g for 5 min at 4°C. The upper organic layer was removed and the extraction repeated two more times with HEAT/NaCl. The three organic layers were combined and dried under a stream of nitrogen. The residue was reconstituted in 300 µL of 1:1 methyl t-butyl ether (MtBE)/methanol (MeOH) with water bath sonication and filtered through 0.45 µm nylon filter before HPLC injection. An aliquot of whole plasma (1.8 ml) was extracted using the same protocol scaled for starting plasma volume.
HEAT was determined to be the most efficient extraction solvent for both retinoids and β-carotene when compared with several published methods for retinoid and carotenoid extraction. In all extraction methods tested, we added equal volume of ethanol to the plasma/CM-rich fraction and varied the extraction solvents used (i.e., hexane, 2:1 hexane acetone, chloroform, etc.) and the addition of salt and/or acid to facilitate phase separation. HEAT with the addition of NaCl showed the best recovery and consistency of recovery of all compounds of interest.
HPLC
Reversed-phase chromatography with a C30 4.6 × 150 mm, 5 µm column was used to separate retinoids and carotenoids. We used two separate chromatographic separations, one for retinol and retinyl esters and one for β-carotene. Each run utilized the same binary solvent systems: (A) 80:20 MeOH/0.1% formic acid (FA) and (B) 78:20:2 MtBE/MeOH/0.1%FA at 1.8 ml/min and a column temperature of 40°C. UV-vis spectra of eluent were monitored by photodiode array (Waters Acquity PDA). The retinoids separation involved a 25 µL injection with an initial condition of 100%A, a linear gradient to 100%B over 6 min, held at 100%B for 2 min, then returned to 100%A for a total run time of 10 min. The carotenoids LC method used a 50 µL injection starting with 100%A and a linear gradient to 100%B over 16 min, returning to 100%A for a total run time of 18 min. For a given sample, the two separations were run consecutively.
MS
The HPLC eluate was interfaced with a triple quadrupole mass spectrometer (Quattro Ultima, Micromass, UK) via an atmospheric pressure chemical ionization (APcI) probe. For the retinoid MS/MS method, retinol, retinyl acetate, and retinyl esters ionized in APcI positive mode as the M+H-H2O parent ion (m/z 269) and were fragmented by collision-induced dissociation to corresponding product ions of m/z 93 and 107. Comigration with authentic standards and matched relative intensity of MS/MS transitions confirmed identification. The d4 retinol and d4-retinyl esters were monitored as MS/MS transitions m/z 273 > 94, 217 where fragmentation was optimized based on d4-retinyl acetate. The β-carotene HPLC-MS analysis utilized selected ion monitoring (SIM) with m/z 536.4 for native β-carotene, m/z 544.4+543.4 (d8+d7) for d8-βC, and m/z 576.4 for the 13C40-βC (β-carotene IS). It was necessary to have two separate injections from the same extract, one for retinoids and one for β-carotene, to allow the analytes to run under separate modes of ionization. Although it is possible to switch polarity during a single run, there was not enough separation between the retinyl esters and β-carotene in our chromatographic separation to successfully switch polarity without sacrificing run time and peak quality. One advantage of separate injections was that a larger injection could be used for β-carotene, providing greater signal:noise, that would have caused distortion to retinoid peaks. APcI(+) and APcI(−) were the best modes of ionization for retinol/retinyl esters and β-carotene, respectively. Key MS parameters for APcI(−) SIM of β-carotene included 30 µA corona current, 35V cone, 15V RF lens 1, 100°C/500°C block/desolvation temperatures, 100/560 L/hr cone/desolvation nitrogen flow, and 15/15 LM/HM resolution on MS1. For retinoid APcI(+) MS/MS, the same source parameters were used except for the following changes: 17 µA corona, 13V RF lens 1, 12/12 LM/HM resolution on MS1, and 15/15 on MS2. Argon was used as collision gas at 3 × 10−3 mBar with 17eV collision energy.
Standard curves were generated by mixing solutions with varying concentrations of authentic standards with fixed amounts of IS (retinyl acetate for retinol, d4-retinol, retinyl esters and d4-retinyl esters; 13C40-βC for β-carotene and d8-β-carotene). The constant concentration of IS used was chosen to be within the limits of the various concentrations of target compounds. The ISs were used to account for potential changes in response in the presence of biological matrices, thus the importance of using a stable isotope of β-carotene (C13-β-carotene) as an IS. The native β-carotene and d8-β-carotene should have the same degree of matrix suppression as the IS, C13-β-carotene. The matrix suppression was only a problem for β-carotene and retinyl ester MS responses were not affected by the biological matrix under these conditions based on spike experiments.
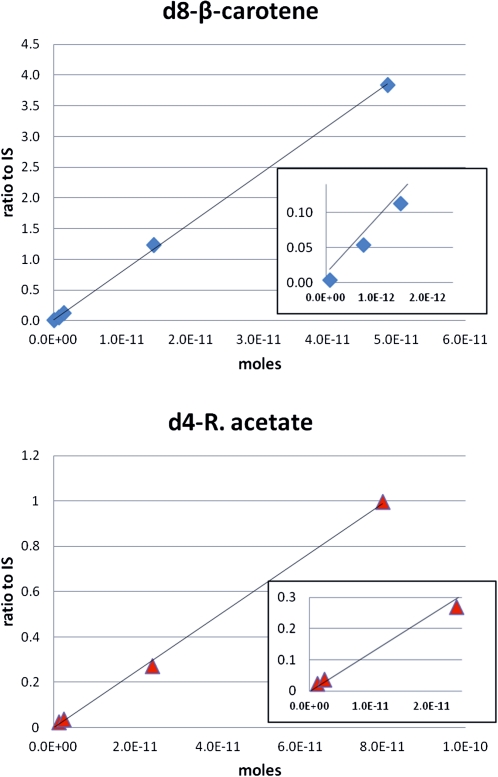
Standard curves for d4-retinyl acetate and d8-β-carotene are shown in Fig. 2. Limits of detection for d4-retinyl acetate and d8-β-carotene were 2.33 × 10−13 and 5.74 × 10−14 moles on column, respectively. In lieu of authentic standard of d4-retinyl palmitate, its MS/MS response was estimated from the d4-retinyl acetate standard curve corrected by UV photo diode array detector response of retinyl palmitate to retinyl acetate standards. The response factor of retinyl palmitate to the retinyl acetate was determined and applied to our calculations of d4-retinyl palmitate and the other d4-retinyl ester concentrations were adjusted according to their ratio of response to retinyl palmitate. d4-Retinol was determined in equivalents of d4-retinyl acetate. Table 1 summarizes the external calibrants and ISs used for each of the analytes. It also shows the multiple reaction monitoring (MRM) transitions and mass ions used for detection of each analyte.
Fig. 2.
HPLC-MS standard curves for d8-β-carotene (m/z 543.5 + 544.5; top) and d4-retinyl acetate (273 > 94,217; bottom). Insets show linearity of curves at the lowest three concentrations used. The y axis represents the ratio of peak area of the analyte to the internal standard. Amounts of internal standards added were 2.6 × 10−11 moles of 13C-β-carotene for the d8-β-carotene analysis and 7.3 × 10−11 moles of 12C- retinyl acetate for the d4-retinyl acetate analysis.
TABLE 1.
Calibration of MS response of retinoids and β-carotene
| Analyte | External Calibrant | Internal Standard | MRM Transition/Mass Ions |
|---|---|---|---|
| retinol | retinol | retinyl acetatea | m/z 269 > 93, 107 |
| d4-retinol | d4-retinyl acetate | retinyl acetate | m/z 273 > 94, 217 |
| retinyl esters | retinyl acetate | retinyl acetate | m/z 269 > 93, 107 |
| d4-retinyl esters | d4-retinyl acetate | retinyl acetate | m/z 273 > 94, 217 |
| β-carotene | β-carotene | 13C40-β-carotenea | m/z 536.4 |
| d8-β-carotene | d8-β-carotene | 13C40-β-carotene | m/z 544.4+543.4 (d8+d7) |
Recovery, matrix effects, and reproducibility
Plasma samples and CM fractions were spiked before or after extraction and HPLC-MS/MS results compared with spike alone to determine recovery as well as possible matrix suppression. Precision was determined from the average of multiple separate extractions (n = 4) and accuracy by comparison of determined amount for a standard to its known quantity in the presence or absence of sample matrix.
Results
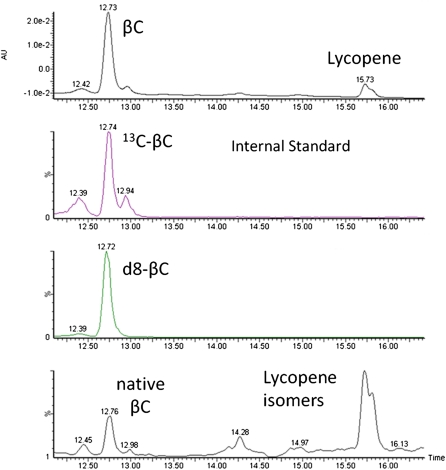
Blood was collected hourly for 9 h and enrichment of chylomicrons with d8-β-carotene and d4-retinyl esters was determined by HPLC-MS. Figures 3 and 4 show chromatograms of retinyl esters and β-carotene in the CM-rich fraction 6 h after dosing in one subject. The four major retinyl esters (linoleate, oleate, palmitate, and stearate) are easily detected and resolved. Lycopene and its isomers are shown in Fig. 3 to show that we had chromatographic separation but these were not quantified. We optimized the method for β-carotene and were able to detect lycopene as the same mass ion in SIM but it is not optimized for lycopene detection and other MS modes are more sensitive for lycopene quantification. The extraction method is efficient in extracting both nonpolar β-carotene and the more polar retinoids but we did not measure the efficiency of lycopene extraction as the study was focused on β-carotene absorption and metabolism.
Fig. 3.
Representative chromatograms of the CM fraction sample at h 6 of one of the subjects analyzed for β-carotene. The four chromatograms, from top to bottom, are the absorbance at 452 nm, m/z 576.5 for 13C40-β-carotene stable isotope internal standard, m/z 543.5 +544.5 for deuterium labeled β-carotene, and m/z 536.5 for native β-carotene and lycopene isomers.
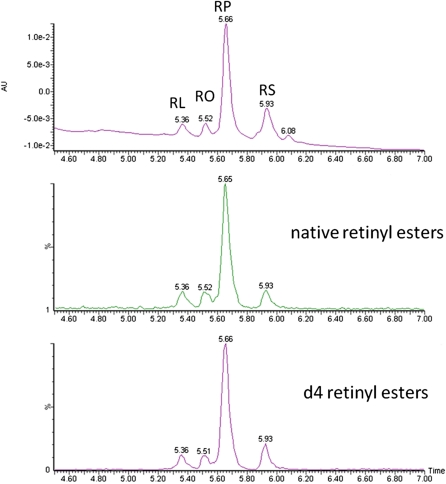
Fig. 4.
Representative chromatograms for retinyl linoleate (RL), retinyl oleate (RO), retinyl palmitate (RP), and retinyl stearate (RS) in the CM fraction. The top chromatogram shows the absorbance at 325 nm, the middle chromatogram shows the native retinyl esters as 269 > 93,107 MS/MS transitions, and the bottom chromatogram shows the d4-retinyl esters as 273 > 94,217 transitions.
A notable methodological issue was suppression of β-carotene MS response by blood sample matrices. This was clearly evident when a stable isotope was added to an extracted sample and response compared with stable isotope alone. This was only a problem for β-carotene response apparently due to high levels of lipid whose elution overlapped with that of β-carotene. Attempts to resolve the analyte from the lipid background were not successful by C30 HPLC. Application of 13C40-β-carotene as an IS was able to correct for this suppression and ensure accurate results where the degree of suppression varied greatly from sample to sample. The use of stable isotopes in this study also provided consistent run-to-run performance of the method and allowed us to avoid extra sample cleanup, which is labor intensive and introduces greater error.
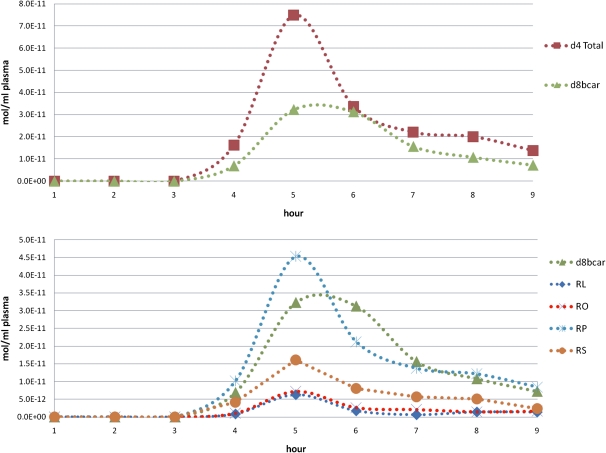
Figure 5 shows concentration of d8-β-carotene and d4-retinyl esters in the CM-rich fraction versus time for a single subject. From these CM fraction plots we were able to calculate the area under the curve (AUC) to give surrogate levels of absorption of the labeled dose of β-carotene. The central cleavage of one mole of β-carotene by β-carotene oxygenase 1 gives two moles of retinal, which are reduced to retinol and esterified to give two moles retinyl esters. Thus, an index of the absorption of the d8-β-carotene dose is represented as:
(Total d4-RE AUC/2) + d8-βC AUC = Total d8-bC equivalents AUC ((µmol/L)*hr)
Fig. 5.
Quantitative HPLC-MS of retinoids in a CM fraction. The top panel shows the total d4-retinyl esters (d4 Total) and d8-βC (d8bcar) whereas the bottom panel contains the individual d4-retinyl esters (RL, retinyl linoleate; RO, retinyl oleate; RP, retinyl palmitate; RS, retinyl stearate) and d8-βC for the same subject. Individual retinyl esters followed the same trend as the total retinyl esters and β-carotene with the peak concentrations of both d4-RE and d8-βC at 5–6 h postdose.
We were also able to estimate the amount of newly absorbed β-carotene that was converted to its retinyl ester metabolites:
(Total d4-RE AUC/2)/Total d8-βC equivalents AUC *100=%Conversion
This particular subject had about a 46% conversion of β-carotene to its retinyl ester metabolites. This is within the previously reported range of 35–75% of the absorbed β-carotene being converted to retinyl esters in the intestine (2, 5, 6).
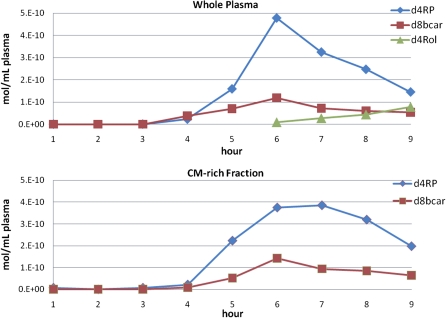
In addition, we compared results of our method using both whole plasma and CM-rich fractions. As seen in Fig. 6, which shows the results for another individual, the results using the whole plasma were similar to those found using the CM-rich fraction. Thus, the AUC (µmol/L × h) for whole plasma d4-retinyl esters was 1.31, whereas for CM-rich d4-retinyl esters, was 1.44. Similarly, the AUCs for whole plasma and CM-rich d8-β-carotene were 0.38 and 0.41, respectively. Thus, the percent conversion of β-carotene to retinyl esters for this subject was 63%. For this individual, the peak appears to be sharper for whole plasma and this may be because the whole plasma β-carotene is less concentrated and may contain more interfering sample matrix than the CM-rich fraction. For this study, we were interested in the absolute absorption of β-carotene and using the CM-rich fraction provided the best sample in terms of matrix suppression and β-carotene enrichment. It also contains only newly absorbed d8-β-carotene, whereas the later time points in the whole plasma may contain recirculated d8-β-carotene. However, whole plasma does give an accurate measure of β-carotene absorption (as evident from the similar AUCs) as well as its percent conversion to d4-retinyl esters.
Fig. 6.
Conversion of d8-β-catotene to d4-retinyl esters as quantified by HPLC-MS. The upper panel shows an extract of human whole plasma and the bottom plot shows the corresponding CM-rich fraction. Both are monitored for d-8-β-carotene (d8bcar), d-4-retinyl palmitate (d4RP), and d4-retinol (d4Rol) by m/z 543.5 + 544.5, and 273 > 94,217 MS/MS transitions.
Our method had sufficient sensitivity to detect d4-retinol regardless of source of the sample, but d4-retinol was not detected in the CM fraction. However, we were able to detect d4-retinol in whole plasma starting at hour 6 as d4-retinyl esters concentrations peaked and were cleared by the liver and d4-retinol was secreted into circulation. Figure 6 shows the whole plasma plots for one individual where it can be seen that d4-retinol levels increase as d4-retinyl esters levels decrease. Therefore, our calculation to determine the conversion of d8-β-carotene to vitamin A using the summed total d4-retinyl esters AUC as a representation of d8-β-carotene that was absorbed as metabolites, excluding the formation of d4-retinol, seems reasonable because the amounts of d4-retinol were small compared with d4-retinyl esters.
As seen in Figs. 3 and 4, we were also able to detect and quantify native levels of β-carotene and retinyl esters as well as native retinol in both whole plasma and CM-rich fraction. This exemplifies the importance of using a stable isotopically labeled dose to distinguish between the newly absorbed dose and native circulating levels. Thus, the method can also be used as is to determine levels of native species with retinyl acetate and 13C40-β-carotene as ISs.
Discussion
A plethora of stable isotope studies of β-carotene absorption has been reported (7–12). Each has examined different aspects of absorption and metabolism and they have different strengths and weaknesses. In most cases, a single pharmacological dose of labeled β-carotene is administered and tracked through the plasma over time. Often, the plasma samples are extracted, saponified, and further purified in complex preparations for analysis. Saponification of the samples converts all of the retinyl esters to retinol and does not allow the individual retinoids to be examined. For example, Dueker et al. (13) administered 73 µmol (40 mg) d8-β-carotene to a human subject and then extracted β-carotene and retinol from plasma. They saponified the lipid extract, which involves reactions with potassium-hydroxide and heat over time, and then it is reextracted, dried, and subjected to a solid phase extraction. Because the samples were saponified, d4-retinol levels included free d4-retinol and d4-retinyl esters. They also further purified β-carotene for MS/MS. Other d8-β-carotene human studies also saponified the plasma samples and detected only d8-β-carotene and d4-retinol (14–17). Human studies using d8-β-carotene that did not saponify the plasma samples still involved complex sample preparation and detection of only d8-β-carotene (18) or d8-β-carotene and d4-retinol (19, 20). The current method allows accurate quantitation of the absorption and conversion of stable isotopically labeled β-carotene while avoiding complex sample preparation and provides shorter chromatographic run times. For example, the gradient HPLC method used for separating individual fatty acyl esters of retinol achieved baseline separation of molecular species in less than a third of the time required for isocratic separations (21). The use of stable isotope ISs was essential to correct for highly variable matrix suppression of β-carotene MS response. The method allows for the distinction between d4-retinol and d4-retinyl esters. It also allows facile distinction of d4-retinyl esters and native retinyl esters. This is useful in understanding the metabolism of both newly absorbed and preexisting retinyl esters. For example, the method might be applied to studies of the synthesis and turnover of retinyl esters in plasma and liver.
Acknowledgments
The authors thank Rachel E. Kopec for her expert assistance and training in the method development and Robert W. Curley for synthesis of retinyl esters.
Footnotes
Abbreviations:
ApcI
atmospheric pressure chemical ionization
AUC
area under the curve
CM
chylomicron
d8-βC
deuterated β-carotene
IS
internal standard
MRM
multiple reaction monitoring
SIM
selected ion monitoring
This work was supported by the National Institutes of Health grants R01-DK044498 and R01-HL049879 to E.H.H. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.During A., Nagao A., Hoshino C., Terao J. 1996. Assay of beta-carotene 15,15′-dioxygenase activity by reverse-phase high-pressure liquid chromatography. Anal. Biochem. 241: 199–205 [DOI] [PubMed] [Google Scholar]
- 2.During A., Harrison E. H. 2006. Digestion and intestinal absorption of dietary carotenoids and vitamin A. In Physiology of the Gastrointestinal Tract, 4th Edition. L. R. Johnson, editor. New York: Raven Press. p. 1735 [Google Scholar]
- 3.Villeneuve G. B., Chan T. H. 1997. A rapid, mild and acid-free procedure for the preparation of acyl chlorides including formyl chloride. Tetrahedron Lett. 38: 6489–6492 [Google Scholar]
- 4.Edwards A. J., You C. S., Swanson J. E., Parker R. S. 2001. A novel extrinsic reference method for assessing the vitamin A value of plant foods. Am. J. Clin. Nutr. 74: 348–355 [DOI] [PubMed] [Google Scholar]
- 5.van Vliet T., Schreurs W. H., van den Berg H. 1995. Intestinal beta-carotene absorption and cleavage in men: response of beta-carotene and retinyl esters in the triglyceride-rich lipoprotein fraction after a single oral dose of beta-carotene. Am. J. Clin. Nutr. 62: 110–116 [DOI] [PubMed] [Google Scholar]
- 6.Edwards A. J., Nguyen C. H., You C. S., Swanson J. E., Emenhiser C., Parker R. S. 2002. Alpha- and beta-carotene from a commercial puree are more bioavailable to humans than from boiled-mashed carrots, as determined using an extrinsic stable isotope reference method. J. Nutr. 132: 159–167 [DOI] [PubMed] [Google Scholar]
- 7.Burri B. J., Clifford A. J. 2004. Carotenoid and retinoid metabolism: insights from isotope studies. Arch. Biochem. Biophys. 430: 110–119 [DOI] [PubMed] [Google Scholar]
- 8.Furr H. C., Green M. H., Haskell M., Mokhtar N., Nestel P., Newton S., Ribaya-Mercado J. D., Tang G., Tanumihardjo S., Wasantwisut E. 2005. Stable isotope dilution techniques for assessing vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutr. 8: 596–607 [DOI] [PubMed] [Google Scholar]
- 9.Novotny J. A., Kurilich A. C., Britz S. J., Clevidence B. A. 2005. Plasma appearance of labeled beta-carotene, lutein, and retinol in humans after consumption of isotopically labeled kale. J. Lipid Res. 46: 1896–1903 [DOI] [PubMed] [Google Scholar]
- 10.Tang G. 2010. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 91: 1468S–1473S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Lieshout M., West C. E., van Breemen R. B. 2003. Isotopic tracer techniques for studying the bioavailability and bioefficacy of dietary carotenoids, particularly beta-carotene, in humans: a review. Am. J. Clin. Nutr. 77: 12–28 [DOI] [PubMed] [Google Scholar]
- 12.Tang G., Qin J., Dolnikowski G. G., Russell R. M., Grusak M. A. 2005. Spinach or carrots can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterated vegetables. Am. J. Clin. Nutr. 82: 821–828 [DOI] [PubMed] [Google Scholar]
- 13.Dueker S. R., Jones A. D., Smith G. M., Clifford A. J. 1994. Stable isotope methods for the study of beta-carotene-d8 metabolism in humans utilizing tandem mass spectrometry and high-performance liquid chromatography. Anal. Chem. 66: 4177–4185 [DOI] [PubMed] [Google Scholar]
- 14.Novotny J. A., Dueker S. R., Zech L. A., Clifford A. J. 1995. Compartmental analysis of the dynamics of beta-carotene metabolism in an adult volunteer. J. Lipid Res. 36: 1825–1838 [PubMed] [Google Scholar]
- 15.Burri B. J., Park J. Y. 1998. Compartmental models of vitamin A and beta-carotene metabolism in women. Adv. Exp. Med. Biol. 445: 225–237 [DOI] [PubMed] [Google Scholar]
- 16.Wang Z., Jiao H., Cao M., Tang G., Zhao X., Yin S. 2003. [Evaluation on intestinal and whole-body conversion of beta-carotene to vitamin A in Chinese adults using a stable isotope reference method]. [Article in Chinese.] Wei Sheng Yan Jiu. 32: 215–221 [PubMed] [Google Scholar]
- 17.Novotny J. A., Harrison D. J., Pawlosky R., Flanagan V. P., Harrison E. H., Kurilich A. C. 2010. Beta-carotene conversion to vitamin A decreases as the dietary dose increases in humans. J. Nutr. 140: 915–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlosky R. J., Flanagan V. P., Novotny J. A. 2000. A sensitive procedure for the study of beta-carotene-d8 metabolism in humans using high performance liquid chromatography-mass spectrometry. J. Lipid Res. 41: 1027–1031 [PubMed] [Google Scholar]
- 19.Tang G., Qin J., Dolnikowski G. G., Russell R. M. 2003. Short-term (intestinal) and long-term (postintestinal) conversion of beta-carotene to retinol in adults as assessed by a stable-isotope reference method. Am. J. Clin. Nutr. 78: 259–266 [DOI] [PubMed] [Google Scholar]
- 20.Tang G., Qin J., Dolnikowski G. G., Russell R. M. 2000. Vitamin A equivalence of beta-carotene in a woman as determined by a stable isotope reference method. Eur. J. Nutr. 39: 7–11 [DOI] [PubMed] [Google Scholar]
- 21.Kim Y-K., Quadro L. 2010. Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol. Biol. 652: 263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]