Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein (original) (raw)
. Author manuscript; available in PMC: 2013 Aug 1.
SUMMARY
Background & aim
Protein-energy supplementation is routinely employed to combat muscle loss. However, success is often compromised by increased satiety, poor palatability, high costs and low compliance.
Methods
For 2-weeks we supplemented meals of older individuals with leucine (4 g/meal; 3 meals/day; days 2–14). Metabolic studies were performed prior to (Day 1) and following (Day 15) supplementation. Leucine was not provided on metabolic study days. Venous blood and vastus lateralis muscle biopsies were obtained during a primed constant infusion of L-[ring-13C6] phenylalanine. Mixed muscle fractional synthesis rate (FSR), body composition and markers of nutrient signaling (mTOR, 4E-BP1 and p70S6K1 phosphorylation) were measured before and after a low protein/carbohydrate simulated meal.
Results
The meal modestly increased FSR on Day 1 (postabsorptive: 0.063 ± 0.004 vs. postprandial: 0.075 ± 0.006%/h; p = 0.03), however, two weeks of leucine supplementation increased postabsorptive FSR (p = 0.004) and the response to the meal (p = 0.01) (postabsorptive: 0.074 ± 0.007 vs. postprandial: 0.10 ± 0.007%/h). Changes in FSR were mirrored by increased phosphorylation of mTOR, 4E-BP1 and p70S6K1 (p ≤ 0.1). No change in fat free mass was observed (p > 0.05).
Conclusions
In older adults, leucine supplementation may improve muscle protein synthesis in response to lower protein meals.
Keywords: Aging, Sarcopenia, Nutrition, Protein requirements
1. Introduction
Aging is commonly associated with the progressive loss of skeletal muscle tissue and functional capacity. Illness and injury notwithstanding, sarcopenia is facilitated by factors secondary to the adoption of a more sedentary lifestyle and consumption of a less than optimal diet. While maintaining or increasing physical activity is clearly desirable, establishing a basic nutrition foundation focusing on adequate protein and energy consumption is necessary if other interventions are to succeed.
It is generally accepted that aging is associated with a blunted protein synthetic response to meals containing less than approximately 15–20 g of protein or the equivalent essential amino acid content.1–3 While this deficiency may be partially or wholly overcome by simply increasing protein intake, obstacles such as total energy consumption, palatability, cost, satiety and habitual practices must be considered.4,5 Consequently, the practical validity of prescribed supplementation regimens must also be critically examined. For example, in controlled metabolic studies it is easy to demonstrate acute increases in muscle protein synthesis or markers of anabolism following ingestion of a protein or amino acid supplement.6–8 However, in practice, there is a risk that acute responses will not translate into positive chronic adaptations in muscle mass or function. The reasons for a lack of success are varied, but we suspect they more often reflect compliance-related issues or consumer choice, rather than a temporally diminished physiological capacity.9,10 To this end, we propose that an effective supplement should produce a robust anabolic response, and be i) low-volume and easily incorporated into existing menu plans, ii) palatable, and iii) cost effective.
From a practical and mechanistic perspective, the branch chain amino acid leucine is an attractive supplement. Leucine has many well described effects on the regulatory mechanisms controlling translation initiation and muscle protein synthesis.2,6,11–18 Nevertheless, aging muscle appears to be less responsive to the stimulatory effects of normal/typical post-prandial concentrations of leucine.11 Several studies in both animals19,20 and humans21–23 suggest that increasing the leucine content of regular mixed nutrient meals may normalize or even increase protein synthesis in older populations. Specifically, Rieu et al.,20 reported that following a mixed meal, muscle protein synthesis in an older rat population was blunted compared to younger rats. However, the addition of supplemental leucine to the meal restored/normalized muscle protein synthesis in the older animals.20 In a follow-up study in older adults (70 ± 1 yr), muscle protein synthesis was acutely increased in response a leucine supplemented meal (0.053 ± 0.009%/h vs. 0.083 ± 0.008%/h, p < 0.05).21 Of note, however, is the fact that for 4 days preceding the metabolic study, the older adults consumed a controlled diet that limited protein intake to 0.8 g/kg/day.21 As described below, we propose that habitual protein intake is a key determinant of the efficacy of leucine/protein supplementation regimens.
While the headline result from the leucine studies mentioned above are encouraging, an important emerging caveat is that leucine supplementation is not unconditionally effective. Specifically, ingestion of supplemental leucine appears to be of little benefit to: i) younger adults,6 and/or, ii) individuals who habitually consume a protein/leucine-sufficient diet.24 For example, in two well-designed studies, no change in skeletal muscle mass or strength was reported in a cohort of healthy24 (n = 15; 71 ± 4 yrs) or diabetic older adults25 (n = 29; 71 ± 1 yrs) who received 2.5 g of supplemental leucine per meal for 3- and 6 months respectively. In both cases, the average daily protein intake was approximately 1.0 g/kg body weight/day and regular daily exercise was described as moderate. Consequently, we concur with the authors’ assertion that a habitual moderate-to-high dietary protein intake may provide sufficient leucine to facilitate a maximal meal-induced protein synthetic response.
The purpose of this study was to determine whether the increase in protein synthesis following a small, low protein and carbohydrate simulated meal could be chronically increased by 2-weeks of low-volume leucine supplementation in healthy, community-dwelling older adults habitually consuming close to the recommended daily allowance (RDA) for protein. We hypothesized that leucine supplementation would improve mixed muscle protein synthesis by stimulating the mTOR signaling pathway and increasing muscle protein synthesis via a more efficient use of meal-derived amino acids.
2. Materials and methods
2.1. Participants
Eight healthy but sedentary older adults participated in this project. The study was approved by the Institutional Review Board of the University of Texas Medical Branch. Written informed consent was obtained prior to participation. Volunteers were screened at the University Of Texas Medical Branch (UTMB) Institute for Translational Sciences Clinical Research Center (ITS-CRC) to determine eligibility. Inclusion criteria included a habitual protein intake (assessed via a 3-day dietary recall questionnaire) at or near the current RDA for protein (range: 0.75–0.85 g protein/kg/day). Exclusion criteria included: a history of regular physical exercise or training (>30 min, 3 times/wk) cardiac, liver, kidney or autoimmune disease; hypo- or hypercoagulation disorders; diabetes; cancer; uncontrolled hypertension, infectious disease, a BMI >30; recent history of anabolic steroid or corticosteroid use or recent participation in a weight loss diet. During the experimental period, subjects were instructed to continue all regular activities of daily living and maintain their usual diet.
2.2. Experimental protocol
Each subject completed two inpatient visits at the ITS-CRC (days 1 and 15) with an intervening two-week outpatient period involving leucine supplementation (4 g per meal; 3 meals per day; days 2–14) (Fig. 1). Subjects reported to the ITS-CRC at noon the day before each metabolic study at which time total body fat and lean mass were determined via Dual Energy X-Ray Absorptiometry (DEXA, Hologic, Inc., Natick, MA). On the morning of the metabolic study, following an overnight fast, polyethylene catheters were inserted into the antecubital vein of both arms for infusion of the stable isotope and arterialized venous blood sampling. Baseline blood samples were drawn for the analysis of hormones and background amino acid enrichment. A primed (2 μmol/kg), continuous infusion of L-[ring-13C6] phenylalanine (Phe) (0.08 μmol kg−1 min−1) was started (time 0) and continued uninterrupted until the conclusion of each metabolic study (Fig. 1). Blood samples were obtained at hours 2 and 3 (fasting) and every 20 min for the hour prior to and following ingestion of the simulated meal. To simulate a mixed meal, a standardized beverage containing 7 g of essential amino acids and 10 g carbohydrates (sucrose) (Table 1) dissolved in approximately 300 mL of diet soda was ingested at hour 4. 0.05 g of L-[ring-13C6] phe was added to maintain an isotopic steady state. The amino acid content of this meal was consistent with a 80–90 g serving of most animal proteins (e.g., beef, chicken, fish) and while the digestion/absorption kinetics of amino acids would certainly differ from protein-rich foods,8 this relatively small simulated meal has been previously shown to elicit only a modest anabolic response in older adults.2
Fig. 1. Stable isotope infusion protocol.
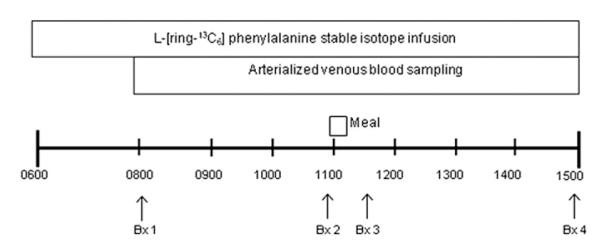
Stable isotope infusion studies were performed prior to (Day 1) and following (Day 15) leucine supplementation. Arterialized blood samples were obtained at 20-min intervals during an infusion of L-[ring-13C6] phenylalanine. Basal muscle biopsies from the vastus lateralis were obtained at 120 and 240 min. Postprandial muscle biopsies were obtained 30 and 180 min after consuming a simulated meal containing 7 g EAA and 10 g sugar.
Table 1.
Composition of the standardized beverage used as the simulated meal on Day 1 and 15.
| Essential amino acids | Weight (g) |
|---|---|
| His | 0.3 |
| Ile | 0.8 |
| Leu | 1.7 |
| Lys | 1.4 |
| Met | 0.3 |
| Phe | 0.5 |
| Thr | 0.9 |
| Val | 0.7 |
| Table sugar | 10 |
| L-[ring-13C6] phe | 0.05 |
A total of four muscle biopsies (~100–200 mg) were taken from the mid-portion of the vastus lateralis muscle during each stable isotope study to capture data from the postabsorptive (biopsy 1 & 2) and postprandial (biopsy 3 & 4) periods (Fig. 1). Samples were snap frozen in liquid nitrogen to abruptly stop all enzymatic reactions and frozen at −80 °C for later analysis.
2.3. Analytical methods
Dietary analysis from a 3-day diet recall period (pre-study screening) and daily diet logs (14 day supplementation period) were analyzed using Nutrition Data System for Research software version 2006, (Nutrition Coordinating Center Minneapolis, MN).
Phenylalanine enrichments in arterialized venous blood samples were determined after deproteinization with sulfosalicylic acid, extraction with cation exchange chromatography (Dowex AG 50W-8X, 100–200 mesh H+ form; BioRad Laboratories, Inc., Richmond, CA), dried under vacuum (Savant Speedvac, Thermo Fisher Scientific, Inc., Waltham, MA), and _tert_-butyldimethylsilyl (_t_-BDMS) derivatization using gas-chromatography mass-spectrometry (GCMS) in electron impact mode (GC HP 5890, MSD HP 5989, Hewlett Packard, Palo Alto, CA).26
Muscle samples were weighed and the proteins precipitated with 800 μl of 10% sulfosalicylic acid. Tissue homogenization and centrifugation were performed on two occasions, and the pooled supernatant was collected. Intracellular amino acids were purified using cation exchange chromatography (Dowex AG 50W-8X, 200–400 mesh H+ form; BioRad Laboratories, Inc., Richmond, CA) and _t_-BDMS derivatization using GCMS in electron impact mode.27 The remaining pellet containing bound mixed-muscle proteins was repeatedly washed, dried at 50 °C overnight and hydrolyzed in 3 ml of 6 N HCL at 110 °C for 24 h. Amino acids in the hydrolyzate were extracted and derivatized, as previously described, by monitoring the ions 238 and 240 and using the external standard curve approach.28
2.4. Calculations
Mixed muscle fractional synthesis rate (FSR) was calculated by directly measuring the incorporation of L-[ring-13C6]-phenylalanine into muscle protein using the precursor-product model:
FSR=[(EBP2−EBP1)∕(EP×t)]×60×100
where _E_BP1 and _E_BP2 are the enrichments of bound L-[ring-13C6]-phenylalanine in sequential muscle biopsies, t is the time interval between biopsies, and _E_P is the mean L-[ring-13C6]-phenylalanine enrichment in the muscle intracellular or plasma precursor pools.
Muscle phenylalanine concentrations (C) (μmol/L) in total muscle tissue fluid was determined using a chloride correction29 and calculated as:
where _Q_IS (μmol) is the amount of internal standard added to the sample, V is the volume of muscle tissue fluid and _E_IS is the tracer-to-tracee ratio of internal standard in total muscle water. Measured values of intracellular phe concentrations relative to total tissue water were corrected using the chloride correction method.29 The chloride method is based on the premise that chloride is freely diffusible across the skeletal muscle fiber membrane and is distributed according to Nernst’s equation and assuming a normal resting membrane potential of muscle in man to be −87.2 mV.
2.5. Immunoblot analysis
Biopsy samples from the postabsorptive (biopsy 1) and post-prandial (biopsy 3 and 4) periods were homogenized in tissue lysis buffer, and protein concentrations were determined. A total of 80 μg of protein was loaded per lane and separated by SDS-PAGE. Following SDS-PAGE, proteins were transferred to polyvinylidene difluoride membranes (PVDF) (Hybond-P, Amersham Biosciences, Piscataway, NJ) and blocked for 1 h. Blots were serially washed and incubated with primary antibody overnight at 4 °C with constant agitation. The next day, the blots were washed and incubated with secondary antibody for 1 h at room temperature with constant agitation. Blots were serially washed and incubated for 5 min with enhanced chemiluminescence reagent (ECL Advanced Western Blotting Detection System, Amersham Biosciences, Piscataway, NJ). Optical density measurements were obtained with a ChemiDoc XRS imaging system (BioRad, Hercules, CA). Densitometric analysis was performed by using Quantity One 1-D analysis software (Ver 4.5.2) (BioRad, Hercules, CA). Data are expressed as the phosphorylation divided by total protein content (in arbitrary units) normalized to a rodent standard.
2.6. Antibodies
Primary antibodies were purchased from Cell Signaling (Beverly, MA): phosphor-mTOR (Ser2448), phosphor-p70S6K1 (Thr389), phospho-4E-BP1 (Thr37/46), total mTOR, total p70S6K1 and total 4E-BP1. Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (Piscataway, NJ).
2.7. Amino acid analysis
Amino acids were analyzed by a solid phase extraction followed by derivatization and a liquid/liquid extraction (EZ:faast Amino Acid Analysis Kit, Phenomenex, Inc., Torrance, CA). Briefly, calibration standards were prepared at concentration of 50, 100 and 200 nmol/mL from 2 standard amino acid solutions. Standard 1 contained a mixture of AAA (α-aminoadipic acid), ABA (α-aminobutyric acid), aIle (allo-isoleucine), Ala, Asp, βAIB (β-aminoisobutyric acid), C–C, Glu, Gly, His, Hyp (4-hydroxyproline), Ile, Leu, Lys, Met, Orn, Phe, Pro, Sar, Ser, Thr, Tyr, and Val. Standard 2 contained Asn, Gln and Trp, which were unstable in acid solution. Calibration standards and serum samples were passed through a sorbent packed tip, binding the amino acids while allowing interfering compounds to flow through. The amino acids on sorbent were then extruded and quickly derivatized in an aqueous solution to facilitated concomitant migrate of the amino acids to the organic layer for additional separation from interfering compounds. The organic layer was then removed, evaporated, and re-suspended in redissolution solvent and analyzed using gaschromatography mass-spectrometry (GCMS: GC HP 5890, MSD HP 5989, Hewlett Packard, Palo Alto, CA).
2.8. Insulin and glucose
Serum insulin concentrations were measured by using an IMMULITE ®2000 (Siemens Healthcare Diagnostics, Deerfield, IL, USA). Plasma glucose concentrations were measured by using an automated glucose analyzer (YSI, Yellow Springs, OH, USA). Four blood draws were taken prior to ingestion of a test meal, and these samples were averaged and reported as the basal value. All post-prandial blood draws were analyzed and reported individually.
2.9. Statistical analysis
Each subject served as his or her own control. All values are expressed as means ± SEM. Comparisons were performed by using ANOVA with repeated measures, the effects being time and day. For post hoc testing we used Bonferroni when appropriate. Significance was set at P ≤ 0.05. Descriptive data are presented as means ± SEM.
3. Results
3.1. Subject characteristics
Eight healthy volunteers (5 male, 3 female) completed the study. Subjects characteristics were: age: 68 ± 2 yr, height: 168 ± 4 cm, weight: 76 ± 2 kg, BMI: 27 kg/m2, body fat: 30 ± 3%. There was no change in lean (Day 1: 47.3 ± 3.7 kg vs. Day 15: 47.0 ± 3.7 kg) or fat mass (Day1: 21.5 ± 2.6 vs. Day 15 = 22.6 ± 3.3 kg) during the 2-week intervention (p > 0.05). Habitual protein consumption during the study period was 0.81 ± 0.04 g protein/kg/day, or approximately 62 g protein/day. Daily energy consumption from dietary record analysis was 1840 ± 125 kcal/day. No compliance issues were noted during the study.
3.2. Plasma and muscle enrichments
Plasma L-[ring-13C6] phenylalanine enrichments are depicted in Fig. 2. There were minor fluctuations in postprandial phenylalanine enrichment, however, no differences were observed from day 1 to day 15. Muscle phenylalanine enrichments and concentrations are also presented in Table 2. Muscle phenylalanine concentrations remained constant throughout the study with no differences from Day 1 to Day 15. Muscle free phenylalanine enrichments increased over time with no differences from Day 1 to Day 15.
Fig. 2. Plasma phenylalanine enrichments.
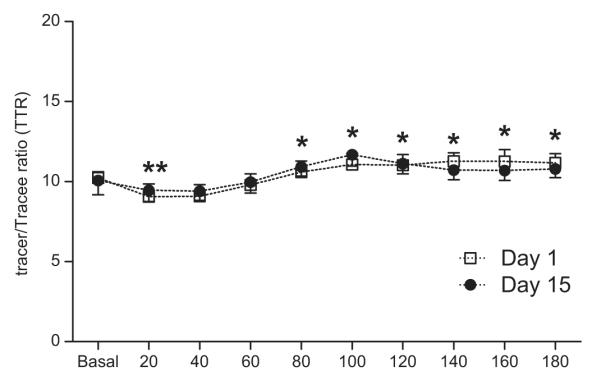
Plasma L-[ring-13C6] phenylalanine enrichments before and after the simulated meal on Day 1 and Day 15. Values are expressed as means ± SEM. * Significant difference from postabsorptive values.
Table 2.
Muscle phenylalanine concentrations, tracer enrichments and mixed muscle fractional synthesis rate.
| Day 1 | Day 15 | |||||
|---|---|---|---|---|---|---|
| M1 | M2 | M4 | M1 | M2 | M4 | |
| Muscle phe concentrations, μmol/L | 103 ± 18 | 95 ± 13 | 90 ± 10 | 102 ± 16 | 92 ± 12 | 97 ± 13 |
| Muscle free phe enrichments (TTR) | 0.0541 ± 0.0066 | 0.0772 ± 0.0057* | 0.0987 ± 0.0085* | 0.0530 ± 0.0047 | 0.0721 ± 0.0052* | 0.0852 ± 0.0039* |
| Muscle bound phe enrichment (TTR) | 0.00029 ± 0.00007 | 0.00037 ± 0.00007* | 0.00060 ± 0.00009* | 0.00066 ± 0.00007 | 0.00075 ± 0.00007* | 0.0010 ± 0.00007* |
| Δ Muscle protein enrichment | 0.00008 ± 0.00001 | 0.00023 ± 0.00003 | 0.00009 ± 0.00001 | 0.00025 ± 0.00002 |
3.3. Muscle protein synthesis
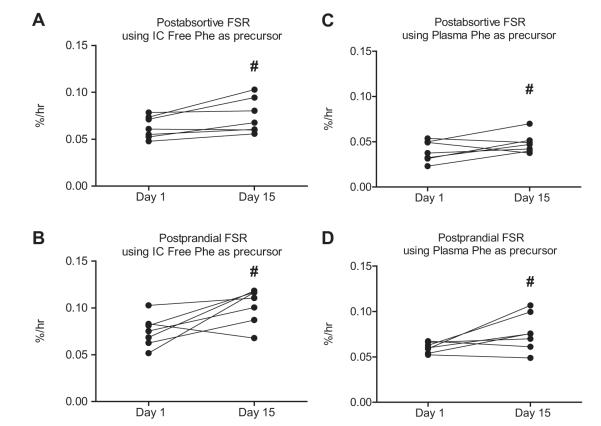
Two weeks of leucine supplementation increased post-absorptive muscle protein synthesis (Day 1: 0.063 ± 0.004 vs. Day 15: 0.074 ± 0.007%/h; p = 0.004). The response to the simulated meal was also increased by leucine supplementation (Day 1: 0.075 ± 0.006 vs. Day 15: 0.10 ± 0.007%/h; p = 0.01), (Fig. 3). A similar pattern was observed using plasma phenylalanine as the precursor (see calculations) for postabsorptive (Day 1: 0.040 ± 0.004 vs. Day 15: 0.048 ± 0.004%/h; p = 0.02) and post-prandial (Day 1: 0.060 ± 0.002 vs. Day 15: 0.077 ± 0.008%/h; p = 0.001) muscle protein synthesis (Fig. 4).
Fig. 3. Mixed muscle fractional synthesis rate (FSR).
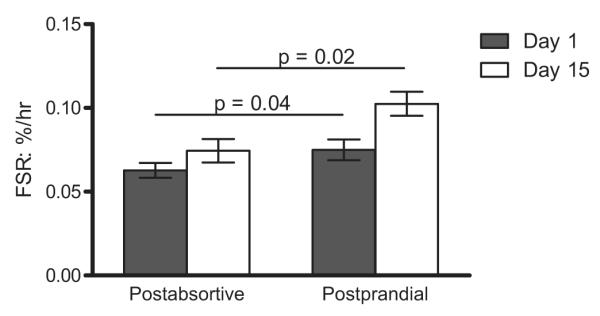
Comparison of the mixed muscle FSR measured before (black bars) and after (white bars) ingestion of the simulated meal on Day 1and again on Day 15. Values are expressed as means ± SEM.
Fig. 4. Intercellular and plasma mixed muscle fractional synthesis rate (FSR).
Comparison of the mixed-muscle postabsorptive (A) and postprandial (B) FSR measured on Day 1 and again on Day 15 utilizing muscle free phenylalanine as the precursor pool. Comparison of the mixed-muscle postabsorptive (C) and postprandial (D) FSR measured on Day 1 and again on Day 15 utilizing plasma phenylalanine as the precursor pool. Individual values are presented. Group values are expressed as means ± SEM.
3.4. Nutrient anabolic signaling
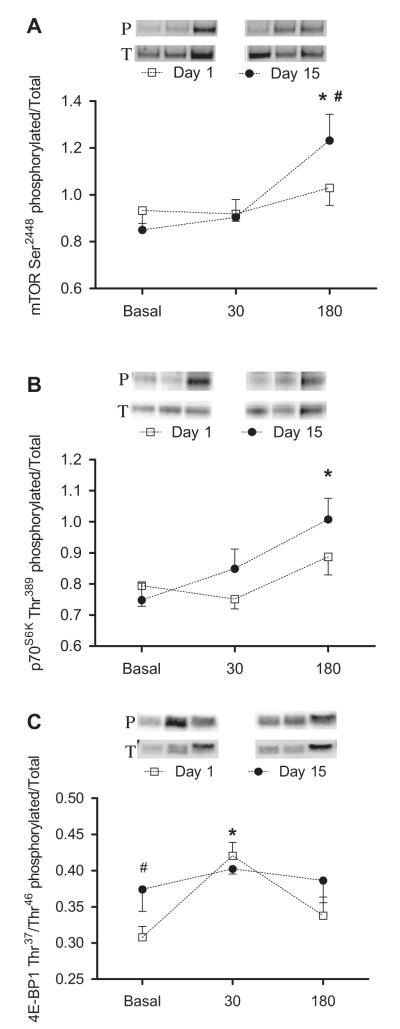
The simulated meal increased the phosphorylation of mTOR, p70S6K1, and 4E-BP1 (p < 0.05; Fig. 5). Two weeks of leucine supplementation increased mTOR (Day 1: 1.03 ± 0.07 vs. Day 15: 1.23 ± 0.11 AU; p = 0.01) and _p_70S6K1 (Day 1: 0.89 ± 0.06 vs. Day 15: 1.01 ± 0.07 AU) phosphorylation in response to the simulated meal; (p ≤ 0.05). We also noted an increase in postabsorptive 4E-BP1 phosphorylation (Day 1: 0.30 ± 0.02 vs. Day 15: 0.37 ± 0.03 AU; p = 0.03). Total protein did not significantly change in response to the simulated meal or leucine supplementation.
Fig. 5. Effects of chronic leucine supplementation on markers of nutrient signaling.
Comparisons of the phosphorylation state of mTOR (A), p70S6K (B), and 4E-BP1 (C) obtained on Day 1 (□) and Day 15 (•). Protein phosphorylation and total protein were determined before and again at 30 and 180 min after ingestion of the simulated meal. A representative blot of the phosphorylation on Day 1 and Day 15 is shown above each graph. Values are expressed as means ± SEM. * significantly different from basal and # significantly different from Day 1; P < 0.05.
3.5. Amino acid analysis
Postabsorptive plasma concentrations of essential and non-essential amino acids did not change following 14 days of leucine supplementation (Table 3).
Table 3.
Mean serum amino acid concentrations before and after 14 days of leucine supplementation.
| Day 1 | Day 15 | |
|---|---|---|
| Essential amino acids | ||
| Leucine | 127 ± 9 | 132 ± 10 |
| Isoleucine | 69 ±7 | 66 ± 7 |
| Valine | 278 ± 19 | 250 ± 19 |
| Histidine | 87 ±7 | 80 ± 5 |
| Lysine | 182 ± 10 | 196 ± 15 |
| Methionine | 21 ±2 | 24 ± 2 |
| Phenylalanine | 71 ±3 | 82 ± 6 |
| Threonine | 144 ± 14 | 140 ± 13 |
| Non-essential amino acids | ||
| Alanine | 512 ± 51 | 483 ± 35 |
| Asparagine | 49 ±5 | 56 ± 6 |
| Aspartic acid | 27 ±5 | 28 ± 4 |
| Glutamic acid | 115 ± 13 | 119 ± 16 |
| Glutamine | 728 ± 58 | 645 ± 43 |
| Glycine | 336 ± 49 | 330 ± 44 |
| Proline | 305 ± 43 | 278 ± 34 |
| Serine | 115 ± 14 | 114 ± 12 |
| Tyrosine | 68 ±7 | 76 ± 4 |
| Tryptophan | 62 ±4 | 67 ± 4 |
| Sarcosine | 631 ± 63 | 596 ± 43 |
| a-Aminobutyric acid | 14 ± 2 | 16 ± 23 |
| b-Aminoisobutyric acid | 89 ± 0.3 | 89 ± 0.3 |
| Hydroxyproline | 19 ±2 | 22 ± 2 |
| a-Aminoadipic acid | 7 ± 0.4 | 5 ± 0.9 |
| Ornithine | 97 ± 7 | 96 ± 8 |
| Cystine | 51 ± 2 | 52 ± 3 |
3.6. Serum insulin and glucose concentrations
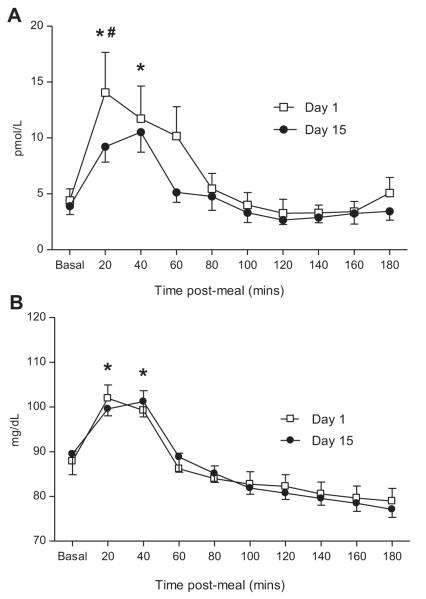
No changes in postabsorptive serum insulin concentrations following 2-weeks of leucine supplementation were noted (P < 0.05). However, on Day 15, subjects experienced a 33% lower insulin response 20 min following the simulated meal (Day 1: 17 ± 3 pmol/L vs. Day 15: 10 ± 2 pmol/L; p = 0.04), (Fig. 6A). Glucose concentrations were elevated for approximately 60 min following the simulated meal on days 1 and 15 (p = 0.02), however, no differences attributable to leucine supplementation were noted (P < 0.05; Fig. 6B).
Fig. 6. Insulin and glucose concentrations.
Plasma insulin (A) and glucose (B) concentrations obtained at Day 0 (□) and Day 14 (•) of leucine supplementation. Measurements were made before and every 20 min after ingestion of the simulated meal. Values are expressed as means ± SEM. * significantly different from basal and # significantly different from Day 1; P < 0.05.
4. Discussion
Our data suggest that leucine supplementation may be an energetically efficient and practical means of chronically improving muscle protein synthesis in response to a low protein meal in older adults who habitually consume close to the RDA for protein. In the context of preventing or slowing sarcopenic muscle loss, the rationale for the use of a dietary supplement should take into account one or more of the following assumptions: i) supplementation will improve net muscle protein anabolism above that afforded by regular meals alone, ii) the supplement will not negatively influence consumption of normal daily meals and iii) use of the supplement will not be compromised or restricted by a lack of compliance, complex preparation, high cost or poor palatability. To this end, we demonstrated that supplementing regular daily meals with a relatively small amount of leucine improves both mixed-muscle protein synthesis and anabolic signaling in older adults. Further, while we did not conduct a specific assessment of practical issues such as longer duration compliance, ease of use or palatability, our low-volume approach is intuitively less likely to be burdensome than most traditional higher-volume amino acid supplement regimens.
A key finding of this study was the postabsorptive and post-prandial improvement in muscle protein synthesis after 2-weeks of leucine supplementation. Speed of digestion and absorption notwithstanding, the study meal containing 7 g of essential amino acids and 10 g carbohydrates was designed with two goals in mind. First, we wanted it to mimic the amino acid and carbohydrate content of a typical small meal or snack consumed by older adults (e.g., approximately a half of a chicken breast and a half cup of cooked rice). Second, we wanted to provide a meal that would initially produce a marginal protein synthetic response; a characteristic of meals ingested by older adults at risk of, or experiencing sarcopenic muscle loss. Data from Day 1 clearly show that the simulated meal had a minimal effect on muscle protein synthesis. However, the response to the same meal after 2-weeks of leucine supplementation was quite robust, despite the fact that leucine supplementation was not consumed during the metabolic study days (Day 1 and 15). Conceptually, this suggests that leucine supplementation is able to chronically up regulate the potential for muscle protein synthesis thereby making an otherwise insufficient or marginal quantity of protein more biologically available and efficient. Notably, we saw no differences in plasma amino acid concentrations during the metabolic studies on Day 1 and 15. In some instances, elevated plasma leucine concentrations have been associated with a reduction in the plasma or intracellular concentrations of other essential amino acids, particularly isoleucine and valine.30
The anabolic potential of leucine has been attributed to its capacity to stimulate translation initiation both dependently and independently of the mTOR signaling pathway.13,31 As noted, aging has been associated with impairment of nutrient-sensing signaling pathways.11 However, our data show that the postprandial phosphorylation of mTOR, and its downstream effector p70S6K, was more pronounced following chronic leucine supplementation.13,15,31 Further, leucine supplementation increased post-absorptive 4E-BP1 phosphorylation, suggesting that leucine supplementation effects muscle protein synthesis through increased eIF4E availability and constitutive hyperphosphorylation of 4E-BP1.
In addition to its effects on muscle protein synthesis, there is some evidence suggesting that leucine augments glucose-induced insulin secretion. In animal models, chronic leucine supplementation improved insulin sensitivity despite the consumption of a high-fat diet.32 However, recently Verhoeven et al.24 reported no such benefit in healthy older individuals. Furthermore, in a group of type 2 diabetic older men no changes were observed in glycemic control with leucine supplementation.25 Consistent with these findings, basal glucose and insulin concentrations in the present study did not change. However, we did detect a decrease in insulin concentrations immediately after the consumption of the simulated meal. This subtle improvement suggests a contribution of leucine to the enhancement of insulin-mediated glucose disposal in normal healthy aging.
While our results are encouraging, there are clearly limitations both in the design of this study and its generalizability. This was a small, non-randomized study with a limited cohort and no direct measure of muscle protein breakdown Participants were not overtly sarcopenic, but rather representative of a population at increased risk of muscle loss. Despite the lack of a traditional control group, our pre-post-test design demonstrated that leucine supplementation was more effective than the status quo. Further, there is no mechanism or previous data to suggest that an isoenergetic control could provide a comparable result. Similarly, it is possible, but unlikely that an alternate, low-volume isonitrogenous supplement (e.g., 4 g BCAA or EAA/meal) would have produced a similar result. Enthusiasm for an amino acid cocktail is further diminished by palatability, cost and compliance issues.
Given the right circumstances it seems clear that leucine supplementation can acutely increase muscle protein synthesis.16–18,33 Unfortunately however, there is too often a disconnect between positive acute metabolic changes and longer-term improvement in outcomes that really matter (e.g. muscle mass, strength).24 In the current study, 2-weeks is obviously a very short period of time to observe a measurable change in muscle mass. However, the magnitude of the increase we observed in basal and postabsorptive muscle protein synthesis during this period should have mathematically increased muscle mass by approximately 4% – conservatively assuming no change in muscle protein breakdown. Moving forward, it is clear that additional steps must be taken to clarify and define groups of individuals who will, and importantly, will not benefit from longer-term leucine supplementation.
In conclusion, we have shown that leucine supplementation increases the potential for muscle protein synthesis in older adults and may make an otherwise insufficient or marginal quantity of meal-derived protein more biologically available for muscle tissue growth and repair.
Acknowledgments
This research was supported by a pilot grant (DPJ) from the UTMB Claude D. Pepper Older Americans Independence Center # P30 AG024832. Additional supports was provided by NIH/NCI grant 5R01 CA127971 (MSM), NIH grant T32HD007539, the National Cattlemen’s Beef Association and the National Space Biomedical Research Institute grant NNJ08ZSA002N (DPJ). Studies were conducted in the Institute for Translational Sciences – Clinical Research Center at UTMB funded by grant 1UL1RR029876-01 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Statement of authorship All authors read and approved the final manuscript. SLC carried out the studies, performed sample and data analysis, and drafted the manuscript. MSM participated in the design of the study. SJH performed analysis of the 3-day diet recall period and daily diet logs. DPJ conceived the study, participated in its design and coordination, and assisted with drafting the manuscript.
Conflict of interest DPJ has participated on Scientific Advisory Panels for the National Dairy Council, the American Egg Board, the National Cattlemens Beef Association and Abbott Nutrition.
References
- 1.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85(12):4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 3.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19(3):422–4. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 4.Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA. Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. Am J Physiol Endocrinol Metab. 2005;288(4):E761–7. doi: 10.1152/ajpendo.00291.2004. [DOI] [PubMed] [Google Scholar]
- 5.Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87(5):1562S–6S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- 6.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, et al. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 140(11):1970–6. doi: 10.3945/jn.110.127647. Epub 2010/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286(3):E321–8. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 8.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86(2):451–6. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 9.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 10.Morley JE. Anorexia of aging: physiologic and pathologic. Am J Clin Nutr. 1997;66(4):760–73. doi: 10.1093/ajcn/66.4.760. [DOI] [PubMed] [Google Scholar]
- 11.Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130(11):2630–5. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- 12.Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr. 2001;131(3):856S–60S. doi: 10.1093/jn/131.3.856S. [DOI] [PubMed] [Google Scholar]
- 13.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130(2):139–45. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 14.Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, et al. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282(5):E1092–101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]
- 15.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130(10):2413–9. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 16.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 17.Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, et al. Coingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006;84(3):623–32. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 18.Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, et al. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288(4):E645–53. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- 19.Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, et al. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J Physiol. 2005;569(Pt 2):489–99. doi: 10.1113/jphysiol.2005.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rieu I, Sornet C, Bayle G, Prugnaud J, Pouyet C, Balage M, et al. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J Nutr. 2003;133(4):1198–205. doi: 10.1093/jn/133.4.1198. [DOI] [PubMed] [Google Scholar]
- 21.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575(Pt 1):305–15. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Layman DK. Role of leucine in protein metabolism during exercise and recovery. Can J Appl Physiol. 2002;27(6):646–63. doi: 10.1139/h02-038. [DOI] [PubMed] [Google Scholar]
- 23.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136(1 Suppl.):319S–23S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- 24.Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89(5):1468–75. doi: 10.3945/ajcn.2008.26668. Epub 2009/03/27. [DOI] [PubMed] [Google Scholar]
- 25.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, et al. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr. 141(6):1070–6. doi: 10.3945/jn.111.138495. Epub 2011/04/29. [DOI] [PubMed] [Google Scholar]
- 26.Rosenblatt J, Chinkes D, Wolfe M, Wolfe RR. Stable isotope tracer analysis by GC-MS, including quantification of isotopomer effects. Am J Physiol. 1992;263(3 Pt 1):E584–96. doi: 10.1152/ajpendo.1992.263.3.E584. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe RR. Principles and practice of kinetic analysis. Wiley-Liss; New York: 1992. Radioactive and stable isotope tracers in biomedicine. [Google Scholar]
- 28.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6(7):421–4. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 29.Bergstrom J, Furst P, Nore LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J App Physiol. 1974;36:693–7. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- 30.Hagenfeldt L, Eriksson S, Wahren J. Influence of leucine on arterial concentrations and regional exchange of amino acids in healthy subjects. Clin Sci (Lond) 1980;59(3):173–81. doi: 10.1042/cs0590173. Epub 1980/09/01. [DOI] [PubMed] [Google Scholar]
- 31.Stipanuk MH. Leucine and protein synthesis: mTOR and beyond. Nutr Rev. 2007;65(3):122–9. doi: 10.1111/j.1753-4887.2007.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56(6):1647–54. doi: 10.2337/db07-0123. Epub 2007/03/16. [DOI] [PubMed] [Google Scholar]
- 33.Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11(3):222–6. doi: 10.1097/MCO.0b013e3282fa17fb. [DOI] [PMC free article] [PubMed] [Google Scholar]