Interaction of Voltage-gated Sodium Channel Nav1.6 (SCN8A) with Microtubule-associated Protein Map1b (original) (raw)
Background: Specific sodium channels have unique subcellular localizations within neurons.
Results: We identified a novel interaction of sodium channel Nav1.6 with a microtubule-associated protein.
Conclusion: Trafficking of Nav1.6 to the cell membrane is mediated by interaction with Map1b.
Significance: Correct localization of sodium channels is essential to prevent neurological disorders such as epilepsy and ataxia.
Keywords: Ion Channels, Membrane Trafficking, Microtubules, Neurobiology, Trafficking
Abstract
The mechanism by which voltage-gated sodium channels are trafficked to the surface of neurons is not well understood. Our previous work implicated the cytoplasmic N terminus of the sodium channel Nav1.6 in this process. We report that the N terminus plus the first transmembrane segment (residues 1–153) is sufficient to direct a reporter to the cell surface. To identify proteins that interact with the 117-residue N-terminal domain, we carried out a yeast two-hybrid screen of a mouse brain cDNA library. Three clones containing overlapping portions of the light chain of microtubule-associated protein Map1b (Mtap1b) were recovered from the screen. Interaction between endogenous Nav1.6 channels and Map1b in mouse brain was confirmed by co-immunoprecipitation. Map1b did not interact with the N terminus of the related channel Nav1.1. Alanine-scanning mutagenesis of the Nav1.6 N terminus demonstrated that residues 77–80 (VAVP) contribute to interaction with Map1b. Co-expression of Nav1.6 with Map1b in neuronal cell line ND7/23 resulted in a 50% increase in current density, demonstrating a functional role for this interaction. Mutation of the Map1b binding site of Nav1.6 prevented generation of sodium current in transfected cells. The data indicate that Map1b facilitates trafficking of Nav1.6 to the neuronal cell surface.
Introduction
The voltage-gated sodium channel Nav1.6 is widely expressed in neurons of the central and peripheral nervous system and is highly concentrated at the axon initial segment and nodes of Ranvier (1, 2). Nav1.6 is required for repetitive firing and generation of resurgent currents in cerebellar Purkinje cells (3–5) and sensory neurons in dorsal root ganglia (6) and contributes to firing patterns in other types of neurons (for review, see Ref. 7). Spontaneous mutations of Nav1.6 in the mouse result in neurological disorders including tremor, dystonia, ataxic gait, paralysis, and juvenile lethality (8). Two mutations of human SCN8A have been described, an inherited protein truncation allele in a family with ataxia and cognitive impairment (9), and a de novo gain-of-function mutation in a child with epileptic encephalopathy (10).
Voltage-gated sodium channels interact with multiple binding partners that regulate gating properties and subcellular localization (11). Several protein interaction sites have been mapped to the intracellular loops and C terminus of the channels. Sequence analysis has identified putative protein-protein interacting motifs and sites for post-translational modification. The only previously described interaction of the N terminus with cytoplasmic proteins is the specific interaction of Nav1.8 with the annexin II light chain, which increases channel trafficking to the plasma membrane (12, 13).
We recently characterized the ethylnitrosourea-induced mouse mutant Scn8aataxia3, in which the amino acid substitution S21P results in trapping of the Nav1.6 channel protein in the Golgi (14). The location of this mutation in the N terminus suggested that this region might be involved in protein-protein interactions required for trafficking of the channel protein to the cell surface. To test this prediction, we carried out a yeast two-hybrid screen of a mouse brain cDNA library to identify proteins that interact with the 117-residue N terminus of the channel. We now report the interaction of Nav1.6 with the light chain of Map1b,5 a cytoskeletal protein that binds microtubules and actin (15). The major sites of expression of Map1b are brain and spinal cord. Among other known cargoes, the light chain of Map1b binds and transports two neuronal membrane-bound proteins, the 5-HT3a receptor (16) and the NMDA receptor subunit N3A (17). The work reported here provides evidence that Nav1.6 is subject to microtubular transport to the cell membrane mediated by interaction with the light chain of Map1b.
EXPERIMENTAL PROCEDURES
Yeast Two-hybrid Assay
The cytoplasmic N terminus of Nav1.6 (residues 1–117) was amplified from mouse brain cDNA (strain C57BL/6J) and cloned into the vector pGBKT7 for use as “bait” in the yeast two-hybrid screen. The prey consisted of the mouse brain cDNA library in the vector pGADT7 (630489; Clontech). The yeast two-hybrid screen and directed tests were performed according to recommendations except that yeast were prepared for transformation by placing a 2–3-mm colony into 50 ml of YPDA broth. The culture was incubated at 30 °C for 16–20 h until _A_600 >1.5. The culture was diluted in YPDA to an absorbance of 0.2–0.3 and incubated, with shaking, at 30 °C, until an absorbance = 0.4–0.6 was reached. Transformation of yeast with 0.5 μg of each plasmid was performed using the Clontech Yeastmaker Yeast Transformation System 2 protocol (630439; Clontech). All transformed yeast grew on −Leu/−Trp medium, which selects for the presence of the bait and prey constructs. Interactants were identified by growth on selective −Leu/−Trp/−His/−Ade medium, which requires interaction between the transformed proteins. The cDNA fragment encoding Map1b (residues 1924–2464) was amplified from mouse brain cDNA and cloned into pGADT7. This fragment encodes the 541 C-terminal residues of Map1b, including the ∼374 residues of the light chain (15). The stability of the encoded protein fragment in transfected cells was demonstrated by Western blotting. Hybrid N-terminal constructs were cloned by PCR fusion of cDNA residues 1–54 of Nav1.1 and 55–117 of Nav1.6 (1A/8A), or residue 1–54 of Nav1.6 and 55–117 of Nav1.1 (8A/1A). The fusion products were cloned into pGBKT7. Deletion constructs based on NdeI and EcoRI restriction sites were generated in vector pGBKT7 by Dr. W. Clay Brown at the High Throughput Protein Laboratory, Life Sciences Institute, University of Michigan. Alanine residues were introduced into the N-terminal domain of Nav1.6 by QuikChange XL mutagenesis (Agilent) using the primers listed in supplemental Table S1.
Cloning of Nav1.6-CD74 Fusion Protein
A cDNA fragment encoding the N terminus plus the first transmembrane segment of Scn8a (residues 1–153) was amplified from the Nav1.6 cDNA clone pcDNA3mod-Nav1.6R (18). The pcDNA3-CD74 clone encoding full-length human CD74 (residues 1–232) was provided by Dr. Blanch Schwappach, University of Manchester, UK (19). Residues 1–71 of CD74 were replaced with residues 1–153 of Nav1.6, which removed the cell surface localization signal in the first transmembrane domain of CD74 (19). The ataxia3 mutation p.S21P was introduced into the Nav1.6-CD74 fusion protein by QuikChange XL mutagenesis. The coding regions of all constructs were analyzed by Sanger sequencing at the University of Michigan DNA Sequencing core before use in transfection experiments.
Site-directed Mutagenesis of Nav1.6R cDNA Clone
The V77A/V79A/P80A mutation was introduced into the tetrodotoxin-resistant Nav1.6 cDNA clone Nav1.6R (18) by QuikChange XL mutagenesis using the primers listed in supplemental Table S1. The entire 6-kb open reading frame was sequenced to confirm the absence of additional mutations prior to functional testing.
Immunocytochemistry
HEK293 cells were transfected with 6 μg of DNA using FuGENE 6 (Roche Applied Science). Glass coverslips (12–545-81, 12CIR.-1.5; Fisherband Microscope Coverglass) were prepared for cell culture by coating with poly-l-lysine (0.01% solution, P4832; Sigma) and sterilized under UV light. After 24 h, transfected cells were washed with sterile PBS and fixed with 4% paraformaldehyde-PBS solution (16% paraformaldehyde, product 28908; Thermo Scientific). Cells were blocked in 10% donkey serum in PBS (D9663; Sigma) and incubated at 4 °C overnight with a 1:750 dilution of anti-CD74 (CD74 (C-16) goat polyclonal IgG, sc-5438; Santa Cruz Biotechnology) in 20% donkey serum-PBS. Incubation with DAPI and the secondary antibody, donkey anti-goat (Alexa Fluor 488, Invitrogen, A11055, 1:1000 in 1% donkey serum/PBS) was carried out at room temperature for 1 h. Coverslips were mounted on glass slides (precleaned, 12-550-143; Fisherbrand Superforst Microscope Slides) with Invitrogen ProLong Gold antifade reagent (P36930). Imaging was performed at the University of Michigan Microscopy and Image Analysis Laboratory using an Olympus FluoView 500 Laser Scanning Confocal Microscope mounted on an Olympus IX-71 inverted microscope.
Immunoprecipitation and Western Blotting
HEK293 cells were co-transfected with Nav1.6-CD74 and myc-Map1b as described above. Cell extracts were prepared and immunoprecipitated as described (20). 10-cm plates of confluent cells were lysed in 1 ml of buffer containing 60 mm Tris-HCl, pH 7.5, 180 mm NaCl, 1% Triton X-100, and 6 mm EDTA. Lysates were preincubated for 1 h at 4 °C with 5 μl of IgG and washed protein G-Sepharose beads. After centrifugation, the supernatant was incubated for 1 h at 4 °C with primary antibody, 25 μl of anti-CD74 (sc-5438; Santa Cruz Biotechnology) or 5 μl of monoclonal anti-c-myc (3631206; Clontech). Protein G-Sepharose beads were added and incubated for 1 h at 4 °C. Beads were centrifuged and washed three times; the final wash buffer included 0.1% Triton X-100 and 0.02% SDS. Proteins were eluted into 80 μl of electrophoresis sample buffer (0.125 m Tris-HCl, pH 6.8, 2.5% SDS, 0.025% bromphenol blue, 1 mm β-mercaptoethanol, and 22.5% glycerol in 0.5× PBS). Western blotting was carried out with antibody to CD74 (1:200) and c-myc (1:500) as described previously (14).
Brain membrane fractions were prepared from wild-type and _Scn8amedTg_-null homozygous mice by homogenation in 50 mm Tris-HCl, pH 7.5, containing 10 mm EGTA and 5 tablets of Roche Complete Mini Protease Inhibitor Mixture/50 ml of buffer. After centrifugation at 3,500 rpm, membrane proteins were pelleted from the supernatant by centrifugation at 100,000 × g for 30 min. The membrane pellet was suspended in 0.2 ml of homogenation buffer by tituration, and 25-μl aliquots were stored at −80 °C. For immunoprecipitation, one aliquot of stored membrane protein was diluted to 1 ml in 60 mm Tris-HCl buffer containing 1% Triton X-100 (see above) and incubated with 5 μg of monoclonal pan-neuronal sodium channel antibody (S8809–1MG; Sigma) for 8 h at 4 °C. Western blotting was carried out with polyclonal antibody to Nav1.6 (ASC-009, 1:100; Alomone) or polyclonal antibody to the light chain of Map1b (sc-8971, 1:100; Santa Cruz Biotechnology).
Electrophysiology
The DRG-derived cell line ND7/23 (21) was cultured on 12-mm glass coverslips coated with poly-d-lysine/laminin (BD Biosciences) and transfected using Lipofectamine 2000 (Invitrogen) with 1 μg of DNA/well (0.6 μg of Nav1.6 cDNA, 0.2 μg of pEGFP-C1 (Clontech), and 0.2 μg of either vector pcDNA3 (Clontech) or the Map1b cDNA construct. After 48 h, cells with robust green fluorescence were selected for recording. Whole cell voltage clamp recording was done essentially as described previously (14) with a few modifications: (i) EPC-9 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) was used in this study; (ii) data were filtered at 2.9 kHz and sampled at a rate of 20 kHz; (iii) current-voltage relationship was determined by recording from cells held at −120 mV and stepped to a range of potentials (−80 to 40 mV in 5-mV increments) for 100 ms each; (iv) steady-state fast inactivation was achieved with a series of 500-ms prepulses (−150 to 0 mV in 10-mV increments), and the fraction of noninactivated channels was measured by a 40-ms test pulse to 0 mV; (v) dextrose instead of sucrose was used to adjust the osmolarity of pipette solution (315 mosmol/liter) and external solution (323 mosmol/liter); (vi) data were analyzed using Pulsefit 8.74 software (HEKA Electronics) and OriginPro 8.1 software (Microcal Software, Northampton, MA), and statistical significance was tested using unpaired Student's t test because data followed a normal distribution.
RESULTS
Cell Membrane Localization of CD74 Reporter
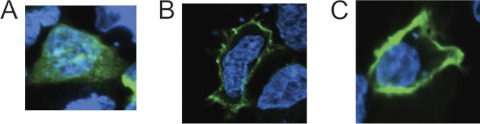
To determine whether the N terminus of Nav1.6 is sufficient to direct protein localization to the cell membrane, we used the extracellular domain of CD74 (residues 72–232) as a cell surface reporter (19). Transfection of HEK293 cells with the CD74 extracellular fragment alone yields diffuse cytoplasmic staining (Fig. 1A). We cloned a hybrid construct containing the N terminus and first transmembrane segment of Nav1.6 (residues 1–153) upstream of the extracellular domain of CD74. This protein is localized to the cell surface (Fig. 1B). Introduction of the ataxia3 mutation p.S21P into the hybrid construct did not prevent surface localization (Fig. 1C). The S21P mutation does prevent surface localization of full-length Nav1.6 in primary cultured neurons; the reason for the lack of effect on the CD74 construct in HEK cells is not clear. The experiments indicate that additional residues in the N terminus are involved in transport of Nav1.6 to the cell surface.
FIGURE 1.
N terminus and first transmembrane segment of Nav1.6 are sufficient to direct a reporter protein to the cell surface. Confocal images of HEK293 cells transfected with the reporter constructs and probed with anti-CD4 antibodies are shown. Green, anti-CD74; blue, DAPI. A, extracellular domain of CD74 lacking membrane-targeting N terminus. B, N terminus and first transmembrane segment of Nav1.6 fused to the extracellular domain of CD74. C, ataxia3 mutation S21P not disrupting surface localization.
Yeast Two-hybrid Screen
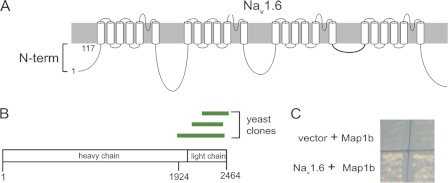
To identify proteins involved in surface localization of Nav1.6, we screened a mouse brain cDNA library using the N-terminal fragment of Nav1.6 (residues 1–117) as bait (Fig. 2A). Growth on selective medium identified three independent overlapping clones containing portions of the light chain of Map1b (Fig. 2B). The interaction was confirmed by a directed yeast two-hybrid assay using a 541-residue Map1b fragment (residues 1924–2464) including the light chain (Fig. 2C).
FIGURE 2.
Yeast two-hybrid screen using the N terminus of Nav1.6 as bait identified Map1b as an interactant. A, schematic of Nav1.6 channel. Residues 1–117 were used as the bait for the yeast two-hybrid screen. B, location of the three independent clones of Map1b identified in the screen. Residues 1924–2426 were cloned into the prey vector for the directed yeast two-hybrid and into pCMV-myc for mammalian cell culture experiments. C, yeast two-hybrid experiments confirming interaction between N terminus of Nav1.6 and Map1b.
Interaction between Nav1.6-CD74 Fusion Protein and Map1b
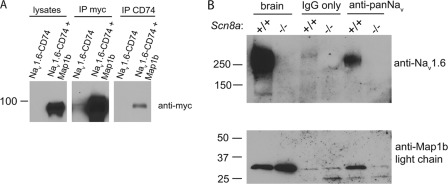
To determine whether the N terminus of Nav1.6 interacts with Map1b in mammalian cells, we cloned the Map1b fragment into the myc-tagged mammalian expression vector pCMV-myc. myc-Map1b and the fusion protein Nav1.6-CD74 were co-transfected into HEK293 cells. Lysates from co-transfected cells were immunoprecipitated with antibody against CD74, and Western blots were probed with anti-myc antibody. A band corresponding to the myc-tagged Map1b was detected in the immunoprecipitate, demonstrating interaction (Fig. 3A). The S21P mutation did not prevent co-immunoprecipitation (data not shown).
FIGURE 3.
Interaction of Nav1.6 with Map1b. A, HEK293 cells co-transfected with Nav1.6-CD74 and myc-Map1b. Map1b light chain co-immunoprecipitates with Nav1.6-CD74 fusion protein from co-transfected cells. B, voltage-gated sodium channels and Map1b light chain co-immunoprecipitated from brain membrane fractions from wild-type (+/+) but not Nav1.6 null (−/−) mice. Lanes contain 50 μg of protein (brain) or 100 μg of protein (immunoprecipitate).
Interaction of Endogenous Full-length Nav1.6 with Light Chain of Map1b
To assess in vivo interaction, we carried out immunoprecipitation of mouse brain membrane protein using a pan-sodium channel antibody followed by Western blotting with an antibody to the light chain of Map1b. Nav1.6 was co-immunoprecipitated with the light chain of Map1b from wild-type brain (Fig. 3B). These results demonstrate that the interaction detected in the yeast two-hybrid system also occurs in vivo with full-length endogenous proteins. Map1b was not co-immunoprecipitated from _Scn8a_-null brain that lacks Nav1.6 (Fig. 3B). Because the null brain extracts contain normal levels of the other major sodium channels Nav1.1 and Nav1.26 (22), the lack of immunoprecipitation of Map1b from null brain suggested that interaction with Map1b might be specific to Nav1.6.
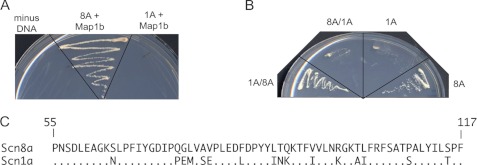
Map1b Does Not Interact with N Terminus of Nav1.1
To evaluate the channel specificity of the interaction directly, we tested the binding of Map1b to the N terminus of Nav1.1, which differs at 37 of 117 residues (30%) from Nav1.6. No interaction of Nav1.1 with Map1b was observed (Fig. 4A). To localize the Map1b binding site of Nav1.6, we constructed hybrid clones consisting of residues 1–54 from one channel and residues 55–117 from the other. The construct containing residues 55–117 of Nav1.6 (1A/8A) interacted with Map1b in the yeast two-hybrid assay, but the reciprocal construct (8A/1A) did not interact (Fig. 4B). This result localized the binding site to residues 55–117 of the N terminus of Nav1.6, which differ at 16 of 62 positions from Nav1.1 (Fig. 4C).
FIGURE 4.
Map1b does not interact with the N terminus of Nav1.1. Co-transformed yeast were plated on selective −Leu/−Trp/−His/−Ade medium. All transformed yeast grew on −Leu/−Trp medium, which selects for presence of the constructs (data not shown). A, yeast two-hybrid interaction of Map1b with the N terminus of Nav1.6 (8A) and Nav1.1 (1A). B, yeast two-hybrid interaction of Map1b with the hybrid constructs 1A(1–54)/8A(55–117) and 8A(1–54)/1A(55–117). C, residues 55–117 of Scn8a and Scn1a differing at 16 of 64 residues.
Localization of Map1b Binding Site in Nav1.6
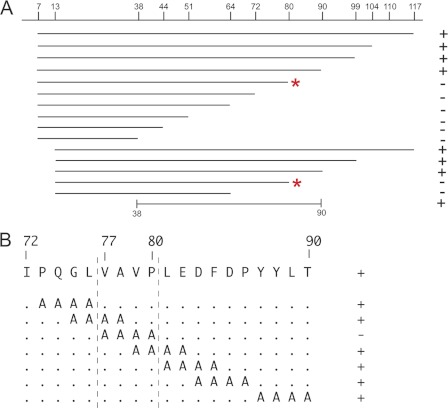
To further define the Map1b binding site in the distal half of the Nav1.6 N terminus, we generated two sets of C-terminal deletion constructs, beginning either at residue 7 or at residue 13 relative to the first methionine in the N terminus (Fig. 5A). In both sets of constructs, deletion of residues 90–117 did not prevent binding of Map1b, but deletion to residue 80 did prevent binding (Fig. 5A, asterisks). The internal fragment containing residues 38–90 was sufficient for interaction with Map1b (Fig. 5A).
FIGURE 5.
Localization of Map1b interaction site within N terminus of Nav1.6. A, 15 deletion constructs assayed for interaction with Map1b using the yeast-2-hybrid assay. C-terminal deletion to residue 80 or beyond prevented interaction with Map1b (asterisks). The internal residue 38–90 was sufficient for interaction. B, alanine-scanning constructs spanning the region between residues 73 and 90. Mutation of residues 77–80 prevented interaction with Map1b. +, growth on stringent selection plates; −, no growth.
To identify the critical amino acids, we generated seven overlapping 4-residue alanine substitution mutations of the Nav1.6 N terminus between residues 73 and 90 of Nav1.6. Six of the 7 alanine substitution constructs retained interaction with Map1b (Fig. 5B). The only noninteracting construct resulted from substitution of AAAA for VAVP (residues 77–80) (Fig. 5B). The corresponding sequence of Nav1.1 (VSEP) differs from Nav1.6 at two residues. These experiments localized the Map1b binding site near the center of the cytoplasmic N terminus of Nav1.6.
The effect of three pathogenic missense mutations was examined: p.S21P (14), p.E82D (23), and p.S107G (23). None of these mutations altered interaction with Map1b, consistent with the mapping data above.
Functional Effect of Map1b on Nav1.6 Current in ND7/23 Cells
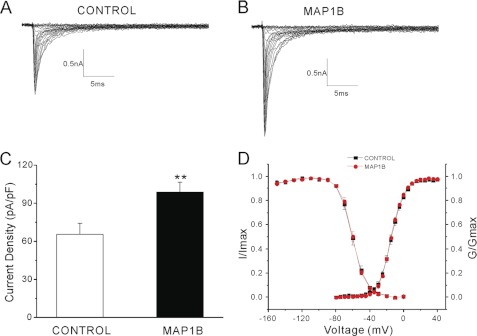
Measurement of sodium current provides a sensitive assay for the presence of functional sodium channels at the cell surface. To test the effect of Map1b on transport of full-length Nav1.6 to the cell surface, we measured sodium current density in neuron-derived cells transfected with Nav1.6 alone or co-transfected with Map1b. The transfected ND7/23 cells were analyzed using whole cell voltage clamp electrophysiology. Endogenous ND7/23 currents were blocked by addition of 300 nm tetrodotoxin to the culture medium (24). ND7/23 cells were transiently transfected with the tetrodotoxin-resistant construct Nav1.6R alone or together with Map1b. Robust sodium currents were detected in both transfections (Fig. 6, A and B). However, co-transfection with Map1b resulted in an increase in current density by 50% (p = 0.008) (Fig. 6C and Table 1). Map1b did not affect the voltage dependence of channel activation and fast inactivation (Table 1 and Fig. 6D) or cell capacitance (control: 22.5 ± 1.7 picofarads, n = 23; Map1b: 20.2 ± 1.0 picofarads, n = 20; p > 0.05).
FIGURE 6.
Co-expression of Map1b increases Nav1.6 peak current density in ND7/23 cells transfected with Nav1.6R. A and B, representative sodium currents were recorded from ND7/23 cells transiently co-transfected with Nav1.6R, EGFP, and vector (n = 23) (A) or Map1b (n = 20) (B). Cells were held at −120 mV, and sodium currents were elicited by a series of step depolarizations from −80 to +40 mV in 5-mV increments. C, co-expression of Map1b significantly increases current density of Nav1.6 in ND7/23 cells (**, p < 0.01). D, Map1b does not alter activation or steady-state fast inactivation of Nav1.6.
TABLE 1.
Map1b increases the amplitude of Nav1.6 current in ND7/23 cells
k represents the slope of the activation or inactivation curve.
| Current density | Activation | Fast inactivation | |||
|---|---|---|---|---|---|
| _V_1/2 | k | _V_1/2 | k | ||
| pA/pF | mV | mV | |||
| Nav1.6 | 65.5 ± 8.7 | −14.2 ± 1.1 | 7.4 ± 0.4 | −59.9 ± 1.6 | 7.0 ± 0.2 |
| (n + 23) | (n + 14) | (n + 14) | (n + 9) | (n + 9) | |
| Nav1.6 and Map1b | 98.7 ± 8.0 | −14.5 ± 0.6 | 7.1 ± 0.2 | −59.7 ± 1.2 | 6.5 ± 0.2 |
| (n + 20)*** | (n + 19) | (n + 19) | (n + 15) | (n + 15) |
The data indicate that interaction with Map1b light chain mediates transport of Nav1.6 to the cell surface. The dependence of Nav1.6 transport on Map1b may be underestimated in this experiment because there is robust expression of endogenous Map1b in untransfected ND7/23 cells (supplemental Fig. S1), and the Nav1.6-transfected cells may include cells that did not co-express the Map1b construct.
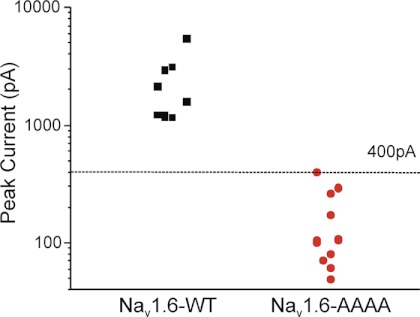
The V77A/V79A/P80A mutation that prevents interaction with Map1b (Fig. 5B) was introduced to the Nav1.6R cDNA by site-directed mutagenesis. The mutated and wild-type cDNAs were co-transfected with Map1b into ND7/23 cells. Sodium current was not detected in cells transfected with the AAAA mutant cDNA (Fig. 7). This observation is consistent with an essential role for Map1b in trafficking of Nav1.6 to the cell surface.
FIGURE 7.
Mutation of the Map1b binding site of Nav1.6R prevents generation of sodium current in transfected ND7/23 cells. Cells were transfected with Map1b and wild-type Nav1.6R or the mutant Nav1.6R-V77A/V79A/P80A and analyzed by whole cell voltage clamp electrophysiology as described in Fig. 6. The threshold of 400 pA for peak current represents the minimum required to construct an unambiguous I-V curve. Peak current amplitude for wild-type channels was >1000 pA (n = 9), whereas cells transfected with the Nav1.6R mutant channel (n = 14) did not produce current above threshold.
DISCUSSION
Novel Nav1.6 Protein Interaction
The functions of the cytoplasmic N terminus of voltage-gated sodium channels are currently not well understood. We demonstrate here that the N terminus of Nav1.6 interacts with the adaptor protein Map1b, resulting in an increase in current density without a change in activation or fast inactivation of the channel. The N terminus in combination with the first transmembrane segment is also sufficient to direct the CD74 extracellular reporter to the cell surface. Interaction between the mature full-length Nav1.6 protein and the light chain of Map1b was demonstrated by co-immunoprecipitation from brain extracts. Co-transfection with Map1b resulted in a 50% increase in the sodium current density generated by transfected Nav1.6, and mutation of the Map1b binding site prevented the generation of sodium currents The data support a model in which interaction with the light chain of Map1b mediates transport of Nav1.6 to the cell surface.
Biological Role of Map1b
Map1b contributes to trafficking of several channel and receptor proteins. It directly interacts with the ligand-gated serotonin channel 5-HT3a to mediate channel desensitization (16) and binds NMDA receptor subunit 3A (NR3A), which indirectly affects the conductance of the receptor (17). GABARAP, a molecule with homology to the light chain of Map1b, interacts with the GABAA receptor and acts as an anchor protein (25). Our work suggests that Nav1.6 is another neuronal protein that is trafficked along the microtubule network to the cell surface.
The nine paralogous mammalian sodium channel genes share a highly conserved tertiary structure and extensive sequence conservation within the transmembrane segments, but their cytoplasmic domains are more divergent (26, 27). Interestingly, the VAVP motif of Nav1.6 required for interaction with Map1b is not conserved in the other channels, consistent with the experimental evidence that this interaction may be specific to Nav1.6. In vertebrate orthologs of Scn8a, the VAVP motif is conserved in reptiles, birds, and marsupials, but not in fish. Further work will be necessary to define a consensus binding motif for the light chain of Map1b.
Consequences of Map1b Deficiency in Mouse
Inactivation of Map1b in targeted knock-out mice results in juvenile lethality of 55% of homozygotes prior to 4 weeks of age (28). The surviving homozygotes have weakness and loss of body weight that resemble the effects of muscle atrophy in Nav1.6 knock-out mice (28). Mice carrying a dominant negative allele of Map1b display a more severe phenotype, with embryonic lethality of homozygotes and a movement disorder in heterozygotes that resembles Scn8a mutants, including ataxia, hind limb tremor, and paralysis (29). Impaired trafficking of Nav1.6 could contribute to the phenotype of these mice as well. Unfortunately, the mice are not available for further testing.
Trafficking and Subcellular Localization of Voltage-gated Ion Channels
The subcellular trafficking of voltage-gated potassium channels in neurons has been studied extensively (30). These channels appear to be selectively transported to their final locations, rather than randomly transported to the cell surface with subsequent selective removal. Vesicles containing dendritically localized potassium channels are trafficked by myosin V and/or dynein, which are unable to enter the axon due to steric or directional constraints. Neuronal activity appears to regulate the trafficking of voltage-gated potassium channels to specific subcellular locations (30).
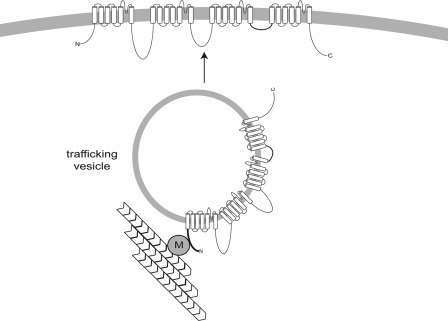
Less is known about the clustering and trafficking of voltage-gated sodium channels. Two alternative models for clustering of sodium channels at the axon initial segment have been considered: transport to the AIS followed by direct insertion or nonspecific transport to the cell surface followed by lateral diffusion to tether points including the AIS (31). Sodium channels are stabilized at the AIS and at nodes of Ranvier by ankyrin G, which interacts with a binding site in cytoplasmic loop II–III (32–34). Adhesion proteins derived from glial cells are also thought to contribute to localization of sodium channels at nodes of Ranvier in myelinated axons. It has been suggested that voltage-gated ion channels are inserted directly into mature nodes, with diffusion limited by myelin and other proteins at the paranode (31). Analysis of axonal transport in transected sciatic nerve suggests that sodium channels reach the nodes by vesicular trafficking, possibly from the cell body (35). However, the molecular mechanism of transport along the axon to the nodes remains unclear. Because microtubules extend along the full length of the axon, Map1b could play a role in localization of Nav1.6 to both the AIS and the nodes of Ranvier. Overall, our data support a model in which microtubular trafficking of Nav1.6 to the cell surface is mediated by interaction with the adaptor protein Map1b (Fig. 8).
FIGURE 8.
Potential role of interaction between Nav1.6 and Map1b in trafficking of Nav1.6 to the cell membrane. According to this model, interaction with Map1b facilitates trafficking of Nav1.6 along axonal microtubules to the AIS or node of Ranvier. At these sites, the channel is stabilized by ankyrin G and other molecules. M, Map1b light chain.
Supplementary Material
Supplemental Data
Acknowledgments
We thank Drs. Luis Lopez-Santiago and Lori Isom for advice and helpful discussions and Dr. W. Clay Brown for cloning the N-terminal deletion constructs.
*
This work was supported, in whole or in part, by National Institutes of Health Research Grant R01 NS34509 through the US Public Health Service (to M. H. M.).
6
J. M. Jones and M. H. Meisler, unpublished data.
5
The abbreviations used are:
Map1b
microtubule-associated protein 1b
AIS
axon initial, segment.
REFERENCES
- 1.Schaller K. L., Caldwell J. H. (2000) Developmental and regional expression of sodium channel isoform NaCh6 in the rat central nervous system. J. Comp. Neurol. 420, 84–97 [PubMed] [Google Scholar]
- 2.Lorincz A., Nusser Z. (2010) Molecular identity of dendritic voltage-gated sodium channels. Science 328, 906–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raman I. M., Sprunger L. K., Meisler M. H., Bean B. P. (1997) Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron 19, 881–891 [DOI] [PubMed] [Google Scholar]
- 4.Khaliq Z. M., Gouwens N. W., Raman I. M. (2003) The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J. Neurosci. 23, 4899–4912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin S. I., Khaliq Z. M., Aman T. K., Grieco T. M., Kearney J. A., Raman I. M., Meisler M. H. (2006) Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (Nav1.6) in cerebellar Purkinje neurons and granule cells. J. Neurophysiol. 96, 785–793 [DOI] [PubMed] [Google Scholar]
- 6.Cummins T. R., Dib-Hajj S. D., Herzog R. I., Waxman S. G. (2005) Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS Lett. 579, 2166–2170 [DOI] [PubMed] [Google Scholar]
- 7.O'Brien J. E., Drews V. L., Jones J. M., Dugas J. C., Barres B. A., Meisler M. H. (2012) Rbfox proteins regulate alternative splicing of neuronal sodium channel SCN8A. Mol. Cell. Neurosci. 49, 120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meisler M. H., Plummer N. W., Burgess D. L., Buchner D. A., Sprunger L. K. (2004) Allelic mutations of the sodium channel SCN8A reveal multiple cellular and physiological functions. Genetica 122, 37–45 [DOI] [PubMed] [Google Scholar]
- 9.Trudeau M. M., Dalton J. C., Day J. W., Ranum L. P., Meisler M. H. (2006) Heterozygosity for a protein truncation mutation of sodium channel SCN8A in a patient with cerebellar atrophy, ataxia, and mental retardation. J. Med. Genet. 43, 527–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veeramah K. R., O'Brien J. E., Meisler M. H., Cheng X., Dib-Hajj S. D., Waxman S. G., Talwar D., Girirajan S., Eichler E. E., Restifo L. L., Erickson R. P., Hammer M. F. (2012) De novo pathogenic SCN8A mutation identified by whole genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am. J. Hum. Genet. 90, 502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dib-Hajj S. D., Waxman S. G. (2010) Isoform-specific and pan-channel partners regulate trafficking and plasma membrane stability and alter sodium channel gating properties. Neurosci. Lett. 486, 84–91 [DOI] [PubMed] [Google Scholar]
- 12.Okuse K., Malik-Hall M., Baker M. D., Poon W. Y., Kong H., Chao M. V., Wood J. N. (2002) Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature 417, 653–656 [DOI] [PubMed] [Google Scholar]
- 13.Poon W. Y., Malik-Hall M., Wood J. N., Okuse K. (2004) Identification of binding domains in the sodium channel Nav1.8 intracellular N-terminal region and annexin II light chain p11. FEBS Lett. 558, 114–118 [DOI] [PubMed] [Google Scholar]
- 14.Sharkey L. M., Cheng X., Drews V., Buchner D. A., Jones J. M., Justice M. J., Waxman S. G., Dib-Hajj S. D., Meisler M. H. (2009) The ataxia3 mutation in the N-terminal cytoplasmic domain of sodium channel Nav1.6 disrupts intracellular trafficking. J. Neurosci. 29, 2733–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riederer B. M. (2007) Microtubule-associated protein 1B, a growth-associated and phosphorylated scaffold protein. Brain Res. Bull. 71, 541–558 [DOI] [PubMed] [Google Scholar]
- 16.Sun H., Hu X. Q., Emerit M. B., Schoenebeck J. C., Kimmel C. E., Peoples R. W., Miko A., Zhang L. (2008) Modulation of 5-HT3 receptor desensitization by the light chain of microtubule-associated protein 1B expressed in HEK 293 cells. J. Physiol. 586, 751–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson M., Samuelsson H., Björklund S., Tortosa E., Avila J., Samuelsson E. B., Benedikz E., Sundström E. (2010) Map1B binds to the NMDA receptor subunit NR3A and affects NR3A protein concentrations. Neurosci. Lett. 475, 33–37 [DOI] [PubMed] [Google Scholar]
- 18.Herzog R. I., Cummins T. R., Ghassemi F., Dib-Hajj S. D., Waxman S. G. (2003) Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons. J. Physiol. 551, 741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuzarte M., Heusser K., Renigunta V., Schlichthörl G., Rinné S., Wischmeyer E., Daut J., Schwappach B., Preisig-Müller R. (2009) Intracellular traffic of the K+ channels TASK-1 and TASK-3: role of N- and C-terminal sorting signals and interaction with 14-3-3 proteins. J. Physiol. 587, 929–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEwen D. P., Meadows L. S., Chen C., Thyagarajan V., Isom L. L. (2004) Sodium channel β1 subunit-mediated modulation of Nav1.2 currents and cell surface density is dependent on interactions with contactin and ankyrin. J. Biol. Chem. 279, 16044–16049 [DOI] [PubMed] [Google Scholar]
- 21.Wood J. N., Bevan S. J., Coote P. R., Dunn P. M., Harmar A., Hogan P., Latchman D. S., Morrison C., Rougon G., Theveniau M. (1990) Novel cell lines display properties of nociceptive sensory neurons. Proc. Biol. Sci. 241, 187–194 [DOI] [PubMed] [Google Scholar]
- 22.Levin S. I., Meisler M. H. (2004) Floxed allele for conditional inactivation of the voltage-gated sodium channel Scn8a (Nav1.6). Genesis 39, 234–239 [DOI] [PubMed] [Google Scholar]
- 23.Kanai K., Hirose S., Oguni H., Fukuma G., Shirasaka Y., Miyajima T., Wada K., Iwasa H., Yasumoto S., Matsuo M., Ito M., Mitsudome A., Kaneko S. (2004) Effect of localization of missense mutations in SCN1A on epilepsy phenotype severity. Neurology 63, 329–334 [DOI] [PubMed] [Google Scholar]
- 24.Wittmack E. K., Rush A. M., Craner M. J., Goldfarb M., Waxman S. G., Dib-Hajj S. D. (2004) Fibroblast growth factor homologous factor 2B: association with Nav1.6 and selective co-localization at nodes of Ranvier of dorsal root axons. J. Neurosci. 24, 6765–6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everitt A. B., Luu T., Cromer B., Tierney M. L., Birnir B., Olsen R. W., Gage P. W. (2004) Conductance of recombinant GABAA channels is increased in cells co-expressing GABAA receptor-associated protein. J. Biol. Chem. 279, 21701–21706 [DOI] [PubMed] [Google Scholar]
- 26.Meisler M. H., O'Brien J. E., Sharkey L. M. (2010) Sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. J. Physiol. 588, 1841–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catterall W. A., Goldin A. L., Waxman S. G. (2005) International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 57, 397–409 [DOI] [PubMed] [Google Scholar]
- 28.Meixner A., Haverkamp S., Wässle H., Führer S., Thalhammer J., Kropf N., Bittner R. E., Lassmann H., Wiche G., Propst F. (2000) Map1B is required for axon guidance and is involved in the development of the central and peripheral nervous system. J. Cell Biol. 151, 1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edelmann W., Zervas M., Costello P., Roback L., Fischer I., Hammarback J. A., Cowan N., Davies P., Wainer B., Kucherlapati R. (1996) Neuronal abnormalities in microtubule-associated protein 1B mutant mice. Proc. Natl. Acad. Sci. U.S.A. 93, 1270–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen C. S., Rasmussen H. B., Misonou H. (2011) Neuronal trafficking of voltage-gated potassium channels. Mol. Cell. Neurosci. 48, 288–297 [DOI] [PubMed] [Google Scholar]
- 31.Leterrier C., Brachet A., Dargent B., Vacher H. (2011) Determinants of voltage-gated sodium channel clustering in neurons. Semin. Cell Dev. Biol. 22, 171–177 [DOI] [PubMed] [Google Scholar]
- 32.Bennett V., Lambert S. (1999) Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J. Neurocytol. 28, 303–318 [DOI] [PubMed] [Google Scholar]
- 33.Dzhashiashvili Y., Zhang Y., Galinska J., Lam I., Grumet M., Salzer J. L. (2007) Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J. Cell Biol. 177, 857–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill A. S., Nishino A., Nakajo K., Zhang G., Fineman J. R., Selzer M. E., Okamura Y., Cooper E. C. (2008) Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet. 4, e1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Bekku Y., Dzhashiashvili Y., Armenti S., Meng X., Sasaki Y., Milbrandt J., Salzer J. L. (2012) Assembly and maintenance of nodes of Ranvier rely on distinct sources of proteins and targeting mechanisms. Neuron 73, 92–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data