Therapeutic Targeting of CD47 to Modulate Tissue Responses to Ischemia and Radiation (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 6.
Published in final edited form as: J Genet Syndr Gene Ther. 2011 Sep 26;2(2):1000105. doi: 10.4172/2157-7412.1000105
Abstract
CD47 is a widely expressed cell surface receptor that serves as a counter-receptor for signal regulatory protein-α and as a receptor for the secreted matricellular protein thrombospondin-1. Thrombospondin-1 signaling through CD47 regulates cellular signaling pathways that control cell survival, growth, motility, mitochondrial biogenesis, arterial vasoactive responses to physiologic vasodilators and blood flow, and responsiveness to growth factors. Studies employing mice lacking either thrombospondin-1 or CD47 have revealed an important role for this receptor-ligand interaction in tissue responses to injury and stress. These null mice show enhanced recovery from soft tissue fixed ischemic injuries, ischemia reperfusion injuries, and radiation injuries. These studies have led to development of antisense strategies to locally or globally suppress CD47 gene expression. A translation-blocking CD47 morpholino improves tissue survival in skin flap and hindlimb fixed ischemia models, full thickness skin grafts, and a liver ischemia/reperfusion model of organ transplantation in mice. Furthermore, the benefits of morpholino treatment extend to aged mice and mice with dysregulated fat metabolism that characteristically exhibit impaired recovery from ischemic injuries. Activity of the morpholino was also demonstrated for treatment of ischemic injury in miniature pigs. Treatment with the CD47 morpholino protects mice from major effects of ionizing radiation including alopecia, deterioration of muscle function, soft tissue and cutaneous fibrosis, and loss of hematopoietic stem cells in bone marrow. Remarkably, the same treatment does not protect tumors but instead enhances their ablation by irradiation. We discuss prospects for further development of CD47 antisense therapeutics for clinical applications including reconstructive surgery, organ transplantation, angioplasty, and cancer.
Keywords: Morpholino oligonucleotides, Cancer radiotherapy, CD47, Reperfusion injury
Introduction
Cell and tissue responses to stress
Tissue injury results in a complex array of physiological responses that limit blood loss, monitor for infection, eliminate nonviable cells, and initiate regeneration of tissue architecture and function. With the development of modern antibiotics and surgical procedures, limitations to tissue regeneration have become a focus of research to improve recovery from traumatic injuries and reconstructive surgery. Several of the key elements that have emerged from these studies are the importance of maintaining blood flow, limiting inflammatory responses and the accompanying formation of reactive oxygen species (ROS), and limiting programmed cell death responses in tissues that are temporarily deprived of oxygen and nutrients. Correspondingly, nitric oxide (NO) and NO donor drugs have been used to increase blood flow, anti-inflammatory agents to limit recruitment or activation of neutrophils, antioxidants to scavenge ROS, and anti-apoptotic agents to minimize loss of viable cells in ischemic tissues [1–4]. Unfortunately, such approaches to date have demonstrated limited success in clinical use [1,2]. Therefore, a deeper understanding is needed of the signaling pathways that limit tissue survival under such conditions.
We have been studying stress responses using several tissue injury models in mice wherein each of the above processes that limit tissue survival can be examined. Partial and total fixed ischemic injuries result in acute loss of blood flow to a tissue. Cells in the injury are thus deprived of oxygen and nutrients, and the ischemic tissue will survive to the extent that it can obtain these essential nutrients by diffusion from adjacent uninjured tissues until vascular remodeling and angiogenesis can restore perfusion and before the ischemic tissue undergoes necrosis. Ischemia/reperfusion (I/R) injuries differ from fixed ischemia in that reperfusion initiates an inflammatory cascade and ROS stress that can result in tissue necrosis despite adequate perfusion [5]. In I/R injuries, loss of blood flow may occur secondary to such inflammatory reactions. Radiation injuries differ from the above in that perfusion is initially uninterrupted, but cell survival is limited by the balance between DNA damage repair pathways, ROS responses, and cell death pathways that are triggered by radiation-induced DNA double strand breaks. Damage-associated molecular patterns (DAMPs) expressed on irradiated cells can also trigger an inflammatory response by the innate immune system that can compromise tissue survival [6].
TSP1/CD47 signaling
We are using these injury models to study the role of the matricellular protein thrombospondin-1 and its receptor CD47 in injury responses. Our previous studies identified TSP1/CD47 signaling as a global regulator of the NO/cGMP cascade in vascular cells [7–9]. The NO/cGMP pathway is a central regulator of several processes involved in tissue injury responses. Several growth factors produced by damaged tissue activate nitric oxide synthases (NOS) to increase NO production, and nitrite is reduced to NO by several enzymes in hypoxic areas of the tissue [10,11]. The resulting increase in NO levels has a number of beneficial effects on tissue repair. NO acts as a chemical sink for ROS by rapidly reacting with superoxide [12]. NO/cGMP signaling via sGC is a proangiogenic signal in endothelial cells, relaxes arterial vascular smooth muscle cells (VSMC) to dilate arteries and arterioles to increase tissue perfusion, inhibits platelet activation to limit thrombosis, and limits activation of inflammatory and immune responses [7]. Endogenous levels of TSP1 physiologically limit these activities of NO in endothelial cells, VSMC, and platelets by limiting the ability of NO to activate soluble guanylyl cyclase (sGC) and the ability of cGMP produced by sGC to activate cGMP-dependent protein kinase, and limiting the ability of upstream signals to increase NO biosynthesis by activating eNOS [8–9,13–15]. In the whole animal the TSP1-CD47 axis modulates blood pressure, with CD47 null mice demonstrating significantly lower systolic, diastolic and mean arterial blood pressure compared to control [13,16]. Conversely, activating CD47 with antibodies that mimic TSP1 leads to a sustained elevation of blood pressure in control animals. In ischemic tissues, and especially after an I/R injury, tissue TSP1 levels can become elevated and effectively disable NO/cGMP signaling by redundantly inhibiting this signaling pathway [17,18]. Thus, TSP1, as an activator of CD47, may be a major cause of the NO-insufficiency that has been described in such injuries and in cardiovascular diseases and could explain why administration of NO donors, pharmacological sGC activators, or NO-stimulating growth factors such as VEGF has achieved only limited success for treating ischemic injuries in animal models and in treating peripheral vascular disease and hypertension in human clinical trials.
Based on the above studies, we predicted that therapies targeting TSP1 or CD47 could improve tissue responses to ischemic stress. To date we have validated TSP1 antibodies that block TSP1 binding to CD47, CD47 antibodies that block TSP1 binding, and antisense approaches that suppress CD47 expression [17,19–23]. We chose to target CD47 for the latter approach because some cells, particularly platelets, contain stores of preformed TSP1, which would limit the effectiveness of antisense strategies to control TSP1 levels. Furthermore, TSP1 is a secreted protein, and the resulting ability to act at a distance would limit the effectiveness of organ-targeted gene therapy approaches. Activities of TSP1 and CD47 antibodies to improve tissue survival have been demonstrated in mouse, rat, and pig injury models [21–23], but the present review will focus on an antisense strategy employing a morpholino oligonucleotide.
Antisense Morpholinos
Antisense gene therapy usually employs chemical modifications of the phosphorodiester-deoxyribosyl backbone to increase the stability of antisense oligonucleotides. Phosphorodiamidate morpholino oligonucleotides (morpholinos) are neutral antisense oligomers where the deoxyribose moiety is replaced with a substituted morpholine, which maintains the base-specific hybridization of the parent oligonucleotide [24,25]. Morpholinos are widely used as a research tool in developmental biology and are currently the standard reagent to temporarily but efficiently suppress expression of target genes in developing zebrafish embryos [26]. Morpholinos are designed to hybridize with complementary nucleotide sequences in the mRNA of a target gene, but unlike RNA interference approaches, they do not generally induce RNA degradation. Morpholinos can be designed to prevent gene expression by two mechanisms. Translation-blocking morpholinos hybridize with the 5′ region of mature mRNA to prevent its efficient translation. Splice-blocking morpholinos hybridize with an exon-intron junction in a specific nuclear pre-mRNA to prevent the splicing required to generate a functional mature mRNA.
In addition to their utility as research tools, morpholinos are advancing as potential therapeutics [25]. Preclinical studies of morpholinos involving a range of cell and animal models have shown efficacy with minimal toxicity [24,27–29]. Morpholinos have demonstrated antiviral activities in animal models against Hepatitis C virus, herpes simplex virus type-2, West Nile virus, Influenza A virus, SARS virus, Dengue virus, Marburg hemorrhagic fever virus, Japanese encephalitis, and Ebola virus [28,30–34]. Phase I human trials for hepatitis C, influenza A, Ebola, and Marburg virus are ongoing or completed [35–38].
Morpholinos have also been tested in several on-going or completed human clinical trials for non-infectious diseases. Phase I and II trials were recently completed involving systemic administration of a morpholino oligonucleotide targeted to modify splicing of exon 51 in the human dystrophin pre-mRNA transcript, excluding exon 51 from the mature mRNA [39–41]. The morpholino in these studies, Eteplirsen, was delivered by intravenous injection to treat patients with Duchenne muscular dystrophy. Another completed phase I trial examined delivery of a morpholino targeting c-Myc for treating patients with prostate cancer [42]. Other clinical trials employing morpholinos have tested their use to enhance patency of saphenous vein bypass grafts [43] and angioplasty stents [44] and to cross the blood brain barrier to target cancers [45].
Ischemic injury models
Ischemia is a process that involves partial or complete loss of blood flow to a tissue region or organ. This process results in a loss of sufficient nutrients and oxygen to supply the metabolic needs of cells and tissues. The process of ischemia can be acute, that may occur secondary to trauma or thrombosis, or it may be a gradual and unremitting loss of blood flow as occurs in peripheral vascular disease. Acute ischemia may also overlap and compound chronic process as in a thrombosis or embolic event in the face of pre-existing peripheral vascular disease. Ischemia, both acute and chronic, is a major component of cardiovascular disease including myocardial infarction and secondary heart failure, stroke, kidney failure, and chronic limb ischemia with subsequent amputation. Ischemia is also a major contributor to the recognized limited wound healing capacity that is associated with aging and contributes to chronic wounds including pressure ulcers.
We have employed several well validated models of ischemia in mice to mimic human disease including random soft tissue flaps and hind limb ischemia. The random soft tissue flap model is based upon extensive experimental and clinical work that has quantified the soft tissue and skin perfusion and blood flow patterns of mammals and in people. Skin capillary networks are supplied by arcades of subdermal and dermal arterials. The territories of these networks are perfused by perorating arteries that arise in a segmental fashion from the underlying major skeletal muscle groups. Taken together this entire vascular system is termed an angiosome by anatomists and vascular biologists. Some overlap and intercommunication occurs between adjacent angiosomes, allowing for retrograde perfusion of surrounding areas should a primary feeding vessel be occluded or lost. However, the degree that this can meet the physiologic needs of a tissue area is dependent upon how many adjacent angiosomes are lost. In the experimental model, surgical incisions transect a substantial number of angiosomes such that retrograde perfusion cannot provide blood flow to the distal aspect of the tissue flap. Using this basic principal, one can define the physical dimension of a random flap in terms of width versus length to achieve a predictable degree of ischemia and tissue necrosis. Mutant murine strains such as ApoE null animals that develop atherosclerotic type changes in blood vessels or aged animals over 12 months old can mimic the ischemia responses of human subjects with chronic vascular and metabolic disease.
The hind limb ischemia model represents a more complex and severe ischemic stress. The stress can be modeled in mice by surgical ligation or ligation and transection of the femoral artery to the hind limb. Mice receive the vast majority of blood supply to the hind limb through the femoral artery. Acute ligation of this vessel imposes substantial ischemia distal to point of vascular ligation. Some degree of limb perfusion can be maintained via rapid redirection of blood flow through small vessels around the hip and acetabulum of the pelvis. Also, over the first 7 to 14 days post artery ligation new vessel formation, termed angiogenesis, is stimulated. These same processes participate in maintaining blood flow in random soft tissue flaps. In both models of ischemia, one can follow immediate effects (those occurring within minutes to hours) via laser Doppler imaging to assess real time blood flow and tissue perfusion, and long term effects occurring over one to two weeks with laser Doppler and immunohistochemistry of flap tissue sections.
Application of antisense gene suppression technology to these models has been utilized successfully by our group in murine and porcine models in young and aged animals (mice > 12 months old) and in mutant ApoE null mice that have baseline peripheral vascular disease. We chose to use gene suppression of the cell surface receptor CD47, as the necessary component of the inhibitory TSP1/CD47 ligand receptor nexus, for several reasons. First, studies in CD47 null mice demonstrated enhanced tissue blood flow to ischemia compared to both wild type and TSP1 null animals [21]. Thus, loss of the necessary receptor CD47 provides greater blood flow and tissue protection than absence of the ligand TSP1 alone, suggesting that CD47 activation may occur in part through non-TSP1 based interactions, and additional CD47 activators may remain to be identified. Second, platelet alpha granules represent a depot location of preformed TSP1 that is released with platelet activation, which can be expected with ischemia, making TSP1 a less attractive target for gene suppression.
In a murine model of random soft tissue flap ischemia a CD47 morpholino oligonucleotide provided dramatic tissue protection when injected subcutaneously into the flap soft tissues in normal saline as a vehicle 48 h before surgery [21]. Whereas control flaps experienced ~50% tissue loss, CD47 morpholino treated tissue units enjoyed over 90% tissue survival. Importantly, in these and other experiments we used the morpholino with or without the manufacturer’s proprietary delivery agent and achieved similar tissue protection, which correlated with decreased CD47 on immunohistochemistry. Cell delivery of morpholino in injured tissues without the proprietary delivery agent may be facilitated by the trauma and hydrostatic forces developed by regional subcutaneous injection. However, in other studies we have obtained effective systemic delivery in mice in the absence of such stress.
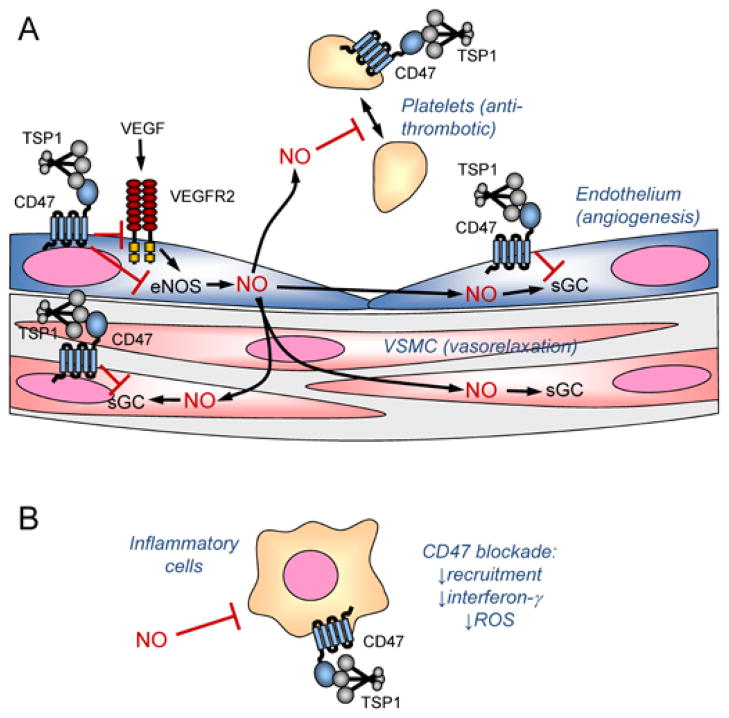
Similar success in terms of maintaining blood flow and perfusion were found when we applied CD47 morpholino to ischemic flaps in ApoE null mice fed a high fat diet and aged wild type mice (14–18 months of age) [19]. Laser Doppler imaging of ischemic skin flaps and hind limbs in these mice also revealed that CD47 blockade confers an immediate advantage in maintaining perfusion of the ischemic tissue. This is consistent with the increased levels of cGMP measured in both uninjured and ischemic tissue lacking CD47 and indicates that CD47 blockade increases ischemic injury survival via the NO/cGMP pathway to increase blood flow by relaxing vascular smooth muscle in arterioles supplying the site of injury (Figure. 1A). To extend the technology to mammals other than rodents, we treated random ischemic flaps in miniature pigs with the CD47 morpholino and achieved CD47 protein suppression in tissue units on immunohistology and increased flap blood flow and tissue survival [22].
Figure 1.
Regulation of vascular cell signaling by thrombospondin-1 and CD47. A, Angiogenic factors such as VEGF stimulate NO synthesis in endothelial cells by endothelial nitric oxide synthase (eNOS). TSP1 binding to CD47 inhibits activation of the VEGF receptor VEGFR2 and inhibits calcium-dependent activation of the VEGFR2 target eNOS. Within endothelial cells, NO activates soluble guanylate cyclase (sGC) to increase angiogenesis, and this enzyme is another target of inhibitory signaling through CD47. NO can also diffuse into underlying vascular smooth muscle where it activates sGC to cause relaxation and increase blood flow. TSP1 in the vessel wall can antagonize this signal via CD47 on vascular smooth muscle cells. NO diffusing into the vascular lumen limits platelet activation, and TSP1 signaling via CD47 blocks this signal to promote platelet aggregation. B, NO can also act on inflammatory cells to inhibit their activation. TSP1 signaling through this pathway may regulate inflammation, and additional signals through CD47 regulate recruitment and interferon-γ and ROS production.
Absolute loss of all blood flow in a tissue unit can be mimicked in mice through skin grafts. Under the extreme conditions of grafting, tissues are nearly completely anoxic until angiogenesis reestablishes a vascular flow between the graft and the wound bed. Treatment with a CD47 morpholino provided complete survival of the skin graft compared to almost complete non-healing of control grafts [20]. Again the morpholino was delivered in advance of grafting by subcutaneous injection. Examination of the wound beds of treated grafts showed increased vascular density when CD47 was absent in null mice or suppressed by the morpholino in wild type mice. This confirmed that angiogenesis is an important target of CD47, and this delayed response complements the immediate perfusion and survival advantage of CD47 blockade, which is mediated by dilation of and subsequent increased blood flow through the existing vasculature (Figure. 1A).
I/R transplant model
I/R injuries inflict substantially more injury to cells and tissues than simple ischemia through dysregulated oxygen metabolism with pathologic generation of ROS that then induce damage to key proteins and hasten cell death [5]. The clinical correlate is found in myocardial infarction, stroke and trauma. All elective organ and composite tissue transplant surgery and emergent re-plantation of amputated parts, by definition, also induces I/R in the tissue units or organs. Transplant associated I/R is one of the leading causes of organ loss, and at present mitigation of I/R is achieved either by organ cooling [46] or continued flow systems using portable pumps [47–48]. Experimental I/R consists of a defined interval of ischemia established by temporary occlusion of a major in-flow artery to a soft tissue unit or visceral organ, followed by release of the obstruction and restoration of tissue perfusion. Data from cultured cells indicated the CD47 morpholino provided gene silencing at 48 h post-treatment, hence our protocol of treating prior to surgical initiation of soft tissue ischemia. To consider the potential for a gene suppressing CD47 morpholinos to provide enhanced blood flow and tissue survival following injury/stress we employed an I/R surgical model [17]. Mutant mice that lacked CD47 demonstrated an advantage in terms of organ survival in response to liver I/R. Additionally, studies in rats showed that functional blockade of CD47 using an antibody could provide near complete tissue survival to I/R even 30 min after reperfusion [23]. However, it is not clear if morpholino technology can be applied after the initial insult, a situation more widely applicable to clinical practice. We have recently treated mice with a CD47 morpholino at the time of kidney I/R injury and demonstrated end organ protection (Isenberg unpublished data) providing in vivo evidence that gene suppression via morpholinos could be tissue protective even after the point of injury.
In addition to aiding the rapid restoration of perfusion through enhanced NO signaling, the absence of CD47 in null mice or CD47 blockade in wild type mice dramatically reduced circulating levels of liver enzymes 6 h after reperfusion [17]. This suggested that CD47 also regulates the inflammatory process associated with I/R. This was confirmed by the reduced number of inflammatory leukocytes seen at 6 h post reperfusion in the null livers and livers of wild type mice treated with CD47 antibody. CD47 blockade significantly decreased neutrophil recruitment in the rat soft tissue I/R injury model, and consequently less ROS damage occurred when CD47 was blocked as indicated by reduced tissue malondialdehyde levels 3 days after surgery [23]. Furthermore, circulating levels of the inflammatory cytokine interferon-γ were significantly reduced in treated rats. Therefore, CD47 blockade appears to enhance survival of I/R injuries through combined effects on the vasculature and inflammatory responses to reperfusion injury (Figure. 1A and 1B). It is not clear at present whether decreased neutrophil recruitment in this model depends primarily on elevated tissue levels of anti-inflammatory NO or on suppression of CD47 expression on the infiltrating inflammatory cells. Consistent with the latter mechanism, loss of CD47 is known to impair neutrophil recruitment in both infection and inflammatory models [49,50].
Tissue radioprotection
The protective effects of CD47 blockade in a variety of ischemic and I/R injury models combined with many reports that CD47 ligation can induce programmed cell death [51–62] suggested that CD47 blockade might improve cell or tissue survival of exposure to ionizing radiation. TSP1-null and CD47-null mice were remarkably resistant to high dose regional irradiation of the hind limb at 25 Gy [63]. In the irradiated skin, alopecia and wet desquamation were decreased in TSP1 null mice compared to that observed in wild type mice and essentially absent in CD47 null mice. Histological examination after 2 months confirmed preservation of skin architecture and function in the irradiated null mice. Remarkably, underlying hindlimb skeletal muscle in TSP1-null and CD47-null mice showed essentially no signs of necrosis or fibrosis two months after irradiation. Not only was muscle mitochondrial function preserved, but the null mice tended to display greater muscle mass in the irradiated hindlimb compared to nonirradiated control limbs [63].
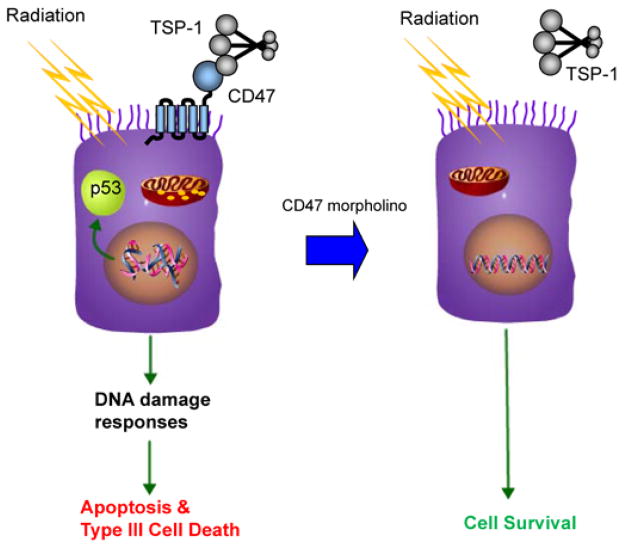
The acute radioprotection conferred by deletion of TSP1 or CD47 is cell autonomous. Vascular cells cultured from the null mice showed enhanced cell-survival and proliferative capacity after irradiation [63]. Irradiated cells show decreased apoptosis when CD47 is blocked. The mechanism remains to be determined but may include effects on the p53 damage response pathway that is initiated by ionizing radiation and/or loss of the pro-apoptotic effects of CD47 mediated through mitochondria (Figure. 2). The role of mitochondria as a CD47 target is reinforced by our recent report that CD47 limits mitochondrial number, size and ROS production in skeletal muscle [64]. Treatment with a CD47-binding peptide, a function blocking CD47 antibody, or antisense suppression with the CD47 morpholino protected human endothelial cells in vitro, and the morpholino protected soft tissue, bone marrow, and tumor-associated leukocytes in irradiated hind limbs of mice [65]. Hematopoietic stem cells are the most radiosensitive cells in bone marrow, but treatment with CD47 morpholino preserved the colony forming activity for all hematopoietic lineages. Loss of these cells and the resulting neutropenia and thrombocytopenia are dose limiting side effects for whole body radiotherapy of cancer, suggesting that the CD47 morpholino could be used in conjunction with radiotherapy to prevent neutropenia and possibly permit delivery of higher radiation doses to improve tumor clearance. Furthermore, the CD47 morpholino is a candidate drug for radioprotection of workers exposed to hazardous levels of radiation associated with nuclear accidents, space travel, and first responders following a nuclear or radiological terrorist attack.
Figure 2.
CD47 signaling controls radiosensitivity. Ionizing radiation induces double strand breaks in DNA, which trigger p53 activation and other damage response pathways that lead to cell death. In the presence of CD47 this involves caspase-dependent apoptosis as well as type III programmed cell death via mitochondria. Down regulation of CD47 by morpholino treatment blocks these death pathways and allows cell survival and continued growth following repair of the radiation damage.
Cancer and radiation therapy
CD47 was first identified as the ovarian tumor marker OA3 [66–68]. Subsequent studies concluded that CD47 is a pan-ovarian carcinoma antigen [69]. Chemical carcinogenesis in rats was associated with increased CD47 expression in kidney, and elevated CD47 was associated with high grade renal cell carcinomas and their lung metastases [70]. Increased CD47 expression was also reported in rat prostate cancer cell lines [71], human multiple myeloma [72], T-cell acute lymphoblastic leukemia versus T-cell lymphoblastic lymphoma [73], oral squamous cell carcinoma [74], human acute myeloid leukemia-associated leukemia stem cells [75], and CD44+ tumor initiating bladder carcinoma cells [76].
The functional importance of this widespread elevated CD47 expression in evasion of host antitumor immunity was first revealed when Kim et al reported that elevated expression of CD47 on head and neck squamous carcinoma cells inhibited natural killer (NK) cell-mediated cytotoxicity and that a function blocking CD47 antibody enhanced NK-mediated cytotoxicity [77]. Subsequent studies using the same CD47 antibody showed increased macrophage-mediated phagocytosis of acute myeloid leukemia (AML) stem cells and bladder carcinoma tumor initiating cells [76,78]. This anti-human CD47 antibody inhibited human AML xenograft growth in immune deficient mice lacking T cells, B cells and NK cells and expressing a mutant SIRPα that can recognize human CD47 [75]. Thus, two independent groups have shown that elevated CD47 expression in cancer protects against against innate immune surveillance. However, the practical utility of using such CD47 antibodies to treat human cancers is unclear given the levels of CD47 present on normal cells that would adsorb such antibody and probably result in toxic side effects [68].
In addition to inhibiting host immunity, several pathways regulated by CD47 may be involved in carcinogenesis and tumor progression [68]. CD47 regulates cell death in several cell types including cancer cells [55,56,61,79,80]. Antibody binding to CD47 enhances Fas-dependent apoptosis in Jurkat T lymphoma cells and in primary mouse T cells. Treatment with immobilized or soluble CD47 monoclonal antibody or TSP1 directly induces apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cell clones from patients [60,80]. Electron microscopy analysis showed swelling of the mitochondria, indicating an increase in permeability of the mitochondrial membrane, indicating regulation of mitochondrial function to control cell survival. In activated T-cells, treatment with the CD47 antibody B6H12 disrupted mitochondrial transmembrane potential, followed by the release of reactive oxygen species [58]. One possible mediator of CD47-dependent cell death is BNIP3, a member of the Bcl2-interacting proteins family that translocates to the mitochondria to induce apoptosis [59,80]. CD47 ligation also regulates dynamin-related protein 1 (Drp1), a mayor regulator of type III programmed cell death. CD47 ligation induces Drp1 translocation from the cytosol to mitochondria [53]. Once in mitochondria, Drp1 blocks the mitochondrial electron transport chain, which dissipates the mitochondrial transmembrane potential, increases reactive oxygen species generation, and produces a drop in ATP levels. A peptide derived from the C-terminal domain to TSP1, 4N1, and an agonist to CD47 exhibited anti-apoptotic activity in thyroid carcinoma cells and protected cells from doxorubicin ceramide, and camptothecin treatment, suggesting that CD47 could be a pharmacological target to overcome cancer drug resistance [81,82]. In the case of ceramide, inhibition of cell death by CD47 ligation was associated with decreased caspase-3 processing. On the other hand, ligation of CD47 by TSP1, the modified TSP1 peptide 4N1K and the CD47 antibody IF7 induced death of four different types of breast cancer cell lines [55]. Inhibition of breast cancer cell survival was mediated by regulation of cAMP levels and G-couple receptor signaling.
The CD47 ligand TSP1 also regulates several processes involved in tumor progression, but the most studied anti-cancer role for this molecule is as an angiogenesis inhibitor [10]. This anti-angiogenic activity results in part from the ability of physiological concentrations of TSP1 binding to CD47 to control NO signaling, which is necessary for signaling by several pro-angiogenic factors [10]. In addition, TSP1 inhibits VEGF receptor-2 (VEGFR2) activation through engaging its receptor CD47 to mediate a broader anti-angiogenic activity that is independent of the NO/cGMP pathway [15,83]. In addition to regulating tumor angiogenesis, TSP1 signaling via CD47 can acutely control tumor perfusion via its vasopressor activity [84]. In addition to modulating tumor vascular responses, TSP1 signaling through CD47 may modulate adaptive tumor immunity. TSP1 is a potent inhibitor of T cell receptor signaling [85,86]. Therefore, blocking CD47 by antibodies or antisense suppression would be expected to increase T cell responses to tumor antigens. These studies suggest that targeting CD47 could have several beneficial effects in cancer therapy.
Since therapeutic irradiation is employed in treating a majority of cancer patients, the radioprotected status of CD47 and TSP1 null mice prompted us to examine the effects of CD47 suppression using the CD47 morpholino in mice bearing syngeneic tumors. This allowed us to study tumor responses in immune competent mice that better reflect human cancers. Suppression of CD47 in was first studied in B16 melanomas grown in C57Bl/6 mice in combination with radiation treatment [65]. Treatment with radiation alone at 10 Gy resulted in the expected tumor regrowth delay. Morpholino treatment alone did not significantly alter tumor growth, but CD47 morpholino treatment followed by radiation dramatically delayed tumor regrowth. A similar tumor growth delay was observed in C3H mice bearing SCC VII squamous carcinoma tumors.
In vitro, CD47 blockade conferred minimal radioprotection to tumor cells, suggesting that suppression of CD47 indirectly enhances tumor growth delay after irradiation. One possibility is that hemodynamic regulation observed with blockade of CD47 could be responsible for regulating tumor hypoxia, and morpholino suppression of CD47 may cause some normalization of the tumor vasculature. This could increase oxygenation to the treated tumors, which is a well established way to increase tumor sensitivity to ionizing radiation and reduce tumor growth [87,88]. Consistent with evidence from other studies that TSP1/CD47 signaling regulates inflammatory responses including immune cell activation and migration, we demonstrated that blockade of CD47 expression with antisense morpholino was associated with an increased density of tumor-associated macrophages that may be cytotoxic to the tumor [65]. Therefore, blockade of CD47 may enhance anti-tumor immunity by protecting imnmune cells associated with the tumor from the cytotoxic effects of ionizing radiation, thereby enhancing tumor immunosurveillance (Figure. 3) Simultaneously, radiation damage to the tumor cells may cause them to present DAMPs that increase their recognition by host anti-tumor immunity [89–91]. The mechanisms of these effects are currently being explored, but it is clear that the expression of CD47 is a critical regulator of survival for several cell types.
Figure 3.
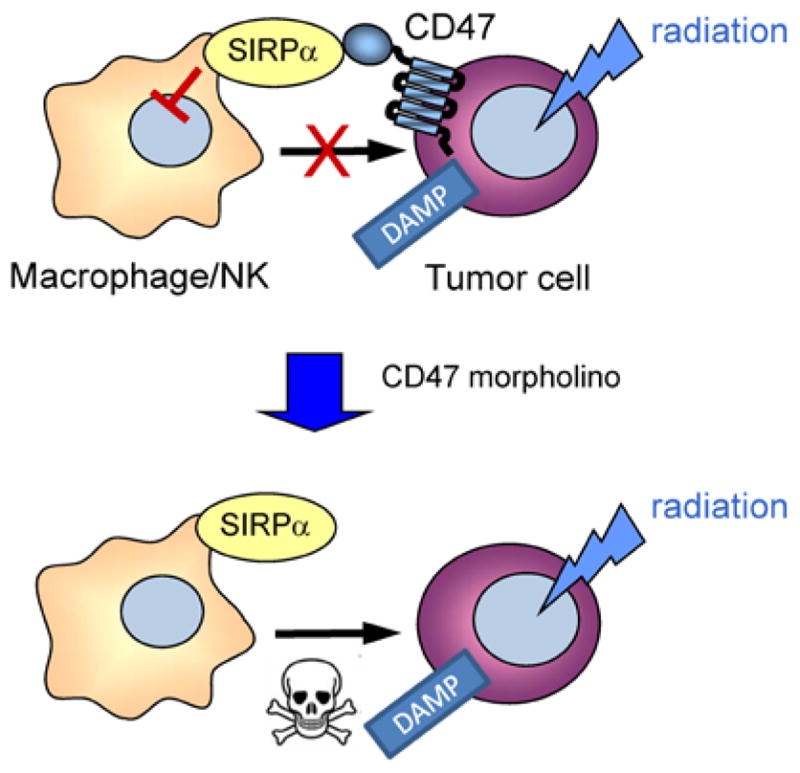
Model for radiosensitization of tumors by CD47 blockade. Radiation of tumors induces expression or surface exposure of damage-associated molecular patterns (DAMP) that can lead to phagocytic clearance by macrophages of killing by cytotoxic cells. High expression of CD47 on tumor cells limits this clearance. Suppression of CD47 by morpholino treatment removes this “don’t eat me” signal and allows destruction of irradiated tumor cells by macrophages or NK cells.
Future prospects
Preclinical studies clearly demonstrate the important role of CD47 in cell and tissue survival. Its regulation of cell death makes CD47 an attractive target for the treatment of tissue injuries and neoplastic lesions. CD47 signaling redundantly controls both NO synthesis and downstream effector pathways, enabling it to control both intracellular and intercellular signaling between vascular cells (Figure. 1). This signaling has physiological roles in angiogenesis, cardiovascular dynamics, and hemostasis [68]. Chronic or acute loss of blood flow plays a significant role in many diseases that affect aging populations in Western societies. However, therapeutic approaches to improve tissue blood flow and cardiovascular dynamics in this aging population remain elusive. Elevated TSP1 levels in these patients may limit the effectiveness of existing therapeutics that are designed to enhance NO levels, activate sGC, or inhibit cGMP phosphodiesterases. The central role that NO plays in vascular physiology and its regulation by TSP1 via CD47 indicates the tremendous potential for therapeutics targeting this pathway in aging populations and those affected by acute and chronic ischemic injuries.
Radioprotection of cells by blocking TSP1/CD47 occurs largely in a NO-independent manner. TSP1 signaling via CD47 is known to induce caspase-independent type III cell death; the absence of CD47 may prevent radiation-induced cell death through a similar mechanism. This combined with enhancement of prosurvival NO/cGMP signaling could account for the cell-autonomous radioprotection of normal tissue. The preservation of hematopoietic stem cells indicates that the CD47 morpholino may also protect against the onset of the radiation induced hematopoetic and gastrointestinal syndrome after whole body radiation exposure. We are currently examining radioprotective activity of the morpholino in other radiosensitive organs and to enhance survival of whole body radiation. These results could benefit the management of accidental or military radiation exposure and in therapeutic settings such as cancer.
Whereas blockade of CD47 resulted in the radioprotection of normal tissue, it also causes increased radiation induced tumor growth delay in two in vivo tumor models. Potential mechanisms for radiosensitization of tumors include the enhancement of antitumor immunity and re-oxygenation of the tumor causing cancer cell radiosensitization. Cells are most radiosensitive at mitosis, when chromatin is subject to strong torsional stress and when double strand DNA breaks have low chance of repair before chromosome segregation [92]. Cells are more radioresistant in early G1 and late S phase [92]. Thus, further studies are needed to determine whether blockade of CD47 alters radiosensitivity through its effects on cell cycle regulation.
Several approaches have been validated to disrupt CD47 signaling in vitro including antibodies, peptides, and CD47 morpholino. In contrast to CD47 antibodies, which require humanization, and peptides that have yet to demonstrate in vivo efficacy, the CD47 morpholino has the most immediate potential for use in patients. The morpholino that was validated in mice and pigs is known to suppress CD47 expression in human cells and so could be used in its present form in human clinical trials. The success of a recent human phase II trial employing the morpholino Eteplirsen to treat Duchenne muscular dystrophy is encouraging for further development of CD47 morpholinos as therapeutics [41].
Acknowledgments
This work was supported by the Intramural Research Program of the NIH/NCI (DDR) and by NIH grants K22 CA128616 and R01 HL-108954, American Heart Association grant 11BGIA7210001, the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (JSI).
Abbreviations
DAMP
Damage-Associated Molecular Patterns
I/R
Ischemia/Reperfusion
NO
Nitric Oxide
NOS
Nitric Oxide Synthase
ROS
Reactive Oxygen Species
SIRP-α
Signal Regulatory Protein-α
TSP1
Thrombospondin-1
VEGF
Vascular Endothelial Growth Factor
VSMC
Vascular Smooth Muscle Cells
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Disclosures
JSI is Chair of the Scientific Advisory Board of Vasculox, Inc. (St. Louis, MO) and Founder and Chair of the Scientific Advisory Board of Radiation Control Technologies, Inc. (Rockville, MD).
References
- 1.Wijnen MH, Vader HL, Roumen RM. Multi-antioxidant supplementation does not prevent an increase in gut permeability after lower torso ischemia and reperfusion in humans. Eur Surg Res. 2002;34:300–305. doi: 10.1159/000063072. [DOI] [PubMed] [Google Scholar]
- 2.Meade MO, Granton JT, Matte-Martyn A, McRae K, Weaver B, et al. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am J Respir Crit Care Med. 2003;167:1483–1489. doi: 10.1164/rccm.2203034. [DOI] [PubMed] [Google Scholar]
- 3.Kim DS, Ha KC, Kwon DY, Kim MS, Kim HR, et al. Kaempferol protects ischemia/reperfusion-induced cardiac damage through the regulation of endoplasmic reticulum stress. Immunopharmacol Immunotoxicol. 2008;30:257–270. doi: 10.1080/08923970701812530. [DOI] [PubMed] [Google Scholar]
- 4.Cheadle C, Watkins T, Ehrlich E, Barnes K, Osama Gaber A, et al. Effects of anti-adhesive therapy on kidney biomarkers of ischemia reperfusion injury in human deceased donor kidney allografts(1,2) Clin Transplant. 2010 doi: 10.1111/j.1399-0012.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaczorowski DJ, Tsung A, Billiar TR. Innate immune mechanisms in ischemia/reperfusion. Front Biosci (Elite Ed) 2009;1:91–98. doi: 10.2741/E10. [DOI] [PubMed] [Google Scholar]
- 6.Lambros MP, Parsa C, Mulamalla H, Orlando R, Lau B, et al. Identifying cell and molecular stress after radiation in a three-dimensional (3-D) model of oral mucositis. Biochem Biophys Res Commun. 2011;405:102–106. doi: 10.1016/j.bbrc.2010.12.135. [DOI] [PubMed] [Google Scholar]
- 7.Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65:728–742. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, et al. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, et al. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas DD, Ridnour LA, Espey MG, Donzelli S, Ambs S, et al. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem. 2006;36:25984–25993. doi: 10.1074/jbc.M602242200. [DOI] [PubMed] [Google Scholar]
- 13.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, et al. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res. 2010;88:471–481. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006;71:785–793. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, et al. Thrombospondin-1 inhibits vascular endothelial growth factor receptor-2 signaling by disrupting its association with CD47. J Biol Chem Res. 2010;285:38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, et al. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, et al. Treatment of ischemia/reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery. 2008;144:752–761. doi: 10.1016/j.surg.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, et al. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest. 2005;115:3451–3459. doi: 10.1172/JCI25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, et al. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol. 2007;27:2582–2588. doi: 10.1161/ATVBAHA.107.155390. [DOI] [PubMed] [Google Scholar]
- 20.Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, et al. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg. 2008;247:180–190. doi: 10.1097/SLA.0b013e31815685dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, et al. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 22.Isenberg JS, Romeo MJ, Maxhimer JB, Smedley J, Frazier WA, et al. Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: implications for human disease. Ann Surg. 2008;247:860–868. doi: 10.1097/SLA.0b013e31816c4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1-CD47 blockade following ischemia reperfusion injury is tissue protective. Plast Reconstr Surg. 2009;124:1880–1889. doi: 10.1097/PRS.0b013e3181bceec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isenberg JS, Frazier WA, Roberts DD. In vivo applications of morpholino oligonucleotides. In: Templeton NS, editor. Cell and Gene Therapy. CRC Press; Boca Raton, FL: 2008. pp. 487–96. [Google Scholar]
- 25.Muntoni F, Wood MJ. Targeting RNA to treat neuromuscular disease. Nat Rev Drug Discov. 2011;10:621–637. doi: 10.1038/nrd3459. [DOI] [PubMed] [Google Scholar]
- 26.Bill BR, Petzold AM, Clark KJ, Schimmenti LA, Ekker SC. A primer for morpholino use in zebrafish. Zebrafish. 2009;6:69–77. doi: 10.1089/zeb.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu B, Xiao B, Cloer C, Shaban M, Sali A, et al. One-year treatment of morpholino antisense oligomer improves skeletal and cardiac muscle functions in dystrophic mdx mice. Mol Ther. 2010;19:576–583. doi: 10.1038/mt.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein, David A. Inhibition of RNA virus infections with peptide-conjugated morpholino oligomers. Curr Pharm Des. 2008;14:2619–2634. doi: 10.2174/138161208786071290. [DOI] [PubMed] [Google Scholar]
- 29.Sazani P, Ness KP, Weller DL, Poage DW, Palyada K, et al. Repeat-Dose Toxicology Evaluation in Cynomolgus Monkeys of AVI-4658, a Phosphorodiamidate Morpholino Oligomer (PMO) Drug for the Treatment of Duchenne Muscular Dystrophy. Int J Toxicol. 2011;30:313–321. doi: 10.1177/1091581811403505. [DOI] [PubMed] [Google Scholar]
- 30.Warfield KL, Swenson DL, Olinger GG, Nichols DK, Pratt WD, et al. Gene-specific countermeasures against Ebola virus based on antisense phosphorodiamidate morpholino oligomers. PLoS Pathog. 2006;2:e1. doi: 10.1371/journal.ppat.0020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eide K, Moerdyk-Schauwecker M, Stein DA, Bildfell R, Koelle DM, et al. Reduction of herpes simplex virus type-2 replication in cell cultures and in rodent models with peptide-conjugated morpholino oligomers. Antivir Ther. 2010;15:1141–1149. doi: 10.3851/IMP1694. [DOI] [PubMed] [Google Scholar]
- 32.Nazmi A, Dutta K, Basu A. Antiviral and neuroprotective role of octaguanidinium dendrimer-conjugated morpholino oligomers in Japanese encephalitis. PLoS Negl Trop Dis. 2010;4:e892. doi: 10.1371/journal.pntd.0000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Opriessnig T, Patel D, Wang R, Halbur PG, Meng XJ, et al. Inhibition of porcine reproductive and respiratory syndrome virus infection in piglets by a peptide-conjugated morpholino oligomer. Antiviral Res. 2011;91:36–42. doi: 10.1016/j.antiviral.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Neuman BW, Bederka LH, Stein DA, Ting JP, Moulton HM, et al. Development of peptide-conjugated morpholino oligomers as pan-arenavirus inhibitors. Antimicrob Agents Chemother. 2011;55:4631–4638. doi: 10.1128/AAC.00650-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AVI BioPharma I. ClinicalTrials gov. National Institutes of Health; Bethesda: 2006. Pharmacokinetic study of a single dose of AVI-4065 in cerebral spinal fluid. [Google Scholar]
- 36.AVI BioPharma I. ClinicalTrials gov. National Institutes of Health; Bethesda: 2011. Safety study of single administration Iintravenous treatment for influenza. [Google Scholar]
- 37.AVI BioPharma I. ClinicalTrials gov. National Institutes of Health; Bethesda: 2011. Safety study of single administration post-exposure prophylaxis treatment for Ebola virus. [Google Scholar]
- 38.AVI BioPharma I. ClinicalTrials gov. National INstitutes of Health; Bethesda: 2011. Safety study of single administration post-exposure prophylaxis treatment for Marburg virus. [Google Scholar]
- 39.Sazani P, Weller DL, Shrewsbury SB. Safety pharmacology and genotoxicity evaluation of AVI-4658. Int J Toxicol. 2010;29:143–156. doi: 10.1177/1091581809359206. [DOI] [PubMed] [Google Scholar]
- 40.Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iversen PL, Arora V, Acker AJ, Mason DH, Devi GR. Efficacy of antisense morpholino oligomer targeted to c-myc in prostate cancer xenograft murine model and a Phase I safety study in humans. Clin Cancer Res. 2003;9:2510–2519. [PubMed] [Google Scholar]
- 43.AVI BioPharma I. ClinicalTrials.gov. National Institutes of Health; Bethesda: 2005. A Phase 1b Study Evaluating RESTEN-MP in Subjects With Focal de Novo Stenosis. [Google Scholar]
- 44.AVI BioPharma I. ClinicalTrials.gov. National Institutes of Health; Bethesda: 2005. Safety and efficacy of RESTEN-MP when used in conjunction with a bare metal stent in coronary arteries. [Google Scholar]
- 45.AVI BioPharma I. ClinicalTrials.gov. National Institutes of Health; Bethesda: 2006. Pharmacokinetic study of a single dose of AVI-4126 (RESTEN-NG®) in cerebral spinal fluid. [Google Scholar]
- 46.Fuller BJ, Lee CY. Hypothermic perfusion preservation: the future of organ preservation revisited? Cryobiology. 2007;54:129–145. doi: 10.1016/j.cryobiol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Monbaliu D, Brassil J. Machine perfusion of the liver: past, present and future. Curr Opin Organ Transplant. 2010;15:160–166. doi: 10.1097/MOT.0b013e328337342b. [DOI] [PubMed] [Google Scholar]
- 48.Taylor MJ, Baicu SC. Current state of hypothermic machine perfusion preservation of organs: The clinical perspective. Cryobiology. 2010;60:S20–35. doi: 10.1016/j.cryobiol.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, et al. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 50.Su X, Johansen M, Looney MR, Brown EJ, Matthay MA. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J Immunol. 2008;180:6947–6953. doi: 10.4049/jimmunol.180.10.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing C, Lee S, Kim WJ, Jin G, Yang YG, et al. Role of oxidative stress and caspase 3 in CD47-mediated neuronal cell death. J Neurochem. 2009;108:430–436. doi: 10.1111/j.1471-4159.2008.05777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xing C, Lee S, Kim WJ, Wang H, Yang YG, et al. Neurovascular effects of CD47 signaling: promotion of cell death, inflammation, and suppression of angiogenesis in brain endothelial cells in vitro. J Neurosci Res. 2009;87:2571–2577. doi: 10.1002/jnr.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bras M, Yuste VJ, Roue G, Barbier S, Sancho P, et al. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol. 2007;27:7073–7088. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manna PP, Dimitry J, Oldenborg PA, Frazier WA. CD47 augments Fas/ CD95-mediated apoptosis. J Biol Chem. 2005;280:29637–29644. doi: 10.1074/jbc.M500922200. [DOI] [PubMed] [Google Scholar]
- 55.Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.can-03-1708. [DOI] [PubMed] [Google Scholar]
- 56.Saumet A, Slimane MB, Lanotte M, Lawler J, Dubernard V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphavbeta3 in promyelocytic leukemia NB4 cells. Blood. 2005;106:658–667. doi: 10.1182/blood-2004-09-3585. [DOI] [PubMed] [Google Scholar]
- 57.Johansson U, Higginbottom K, Londei M. CD47 ligation induces a rapid caspase-independent apoptosis-like cell death in human monocytes and dendritic cells. Scand J Immunol. 2004;59:40–49. doi: 10.1111/j.0300-9475.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 58.Roue G, Bitton N, Yuste VJ, Montange T, Rubio M, et al. Mitochondrial dysfunction in CD47-mediated caspase-independent cell death: ROS production in the absence of cytochrome c and AIF release. Biochimie. 2003;85:741–746. doi: 10.1016/s0300-9084(03)00129-9. [DOI] [PubMed] [Google Scholar]
- 59.Lamy L, Ticchioni M, Rouquette-Jazdanian AK, Samson M, Deckert M, et al. CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J Biol Chem. 2003;278:23915–23921. doi: 10.1074/jbc.M301869200. [DOI] [PubMed] [Google Scholar]
- 60.Mateo V, Lagneaux L, Bron D, Biron G, Armant M, et al. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med. 1999;5:1277–1284. doi: 10.1038/15233. [DOI] [PubMed] [Google Scholar]
- 61.Pettersen RD, Hestdal K, Olafsen MK, Lie SO, Lindberg FP. CD47 signals T cell death. J Immunol. 1999;162:7031–7040. [PubMed] [Google Scholar]
- 62.Uno S, Kinoshita Y, Azuma Y, Tsunenari T, Yoshimura Y, et al. Antitumor activity of a monoclonal antibody against CD47 in xenograft models of human leukemia. Oncol Rep. 2007;17:1189–1194. [PubMed] [Google Scholar]
- 63.Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, et al. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol. 2008;173:1100–1112. doi: 10.2353/ajpath.2008.080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, et al. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol. 2011;30:154–161. doi: 10.1016/j.matbio.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, DeGraff WG, et al. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poels LG, Peters D, van Megen Y, Vooijs GP, Verheyen RN, et al. Monoclonal antibody against human ovarian tumor-associated antigens. J Natl Cancer Inst. 1986;76:781–791. [PubMed] [Google Scholar]
- 67.Mawby WJ, Holmes CH, Anstee DJ, Spring FA, Tanner MJ. Isolation and characterization of CD47 glycoprotein: a multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem J. 1994;304:525–530. doi: 10.1042/bj3040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frazier WA, Isenberg JS, Kaur S, Roberts DD. Nature Signaling Gateway. 2010. CD47. [Google Scholar]
- 69.Van Niekerk CC, Ramaekers FC, Hanselaar AG, Aldeweireldt J, Poels LG. Changes in expression of differentiation markers between normal ovarian cells and derived tumors. Am J Pathol. 1993;142:157–177. [PMC free article] [PubMed] [Google Scholar]
- 70.Nishiyama Y, Tanaka T, Naitoh H, Mori C, Fukumoto M, et al. Overexpression of integrin-associated protein (CD47) in rat kidney treated with a renal carcinogen, ferric nitrilotriacetate. Jpn J Cancer Res. 1997;88:120–128. doi: 10.1111/j.1349-7006.1997.tb00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vallbo C, Damber JE. Thrombospondins, metallo proteases and thrombospondin receptors messenger RNA and protein expression in different tumour sublines of the Dunning prostate cancer model. Acta Oncol. 2005;44:293–298. doi: 10.1080/02841860410002806. [DOI] [PubMed] [Google Scholar]
- 72.Rendtlew Danielsen JM, Knudsen LM, Dahl IM, Lodahl M, Rasmussen T. Dysregulation of CD47 and the ligands thrombospondin 1 and 2 in multiple myeloma. Br J Haematol. 2007;138:756–760. doi: 10.1111/j.1365-2141.2007.06729.x. [DOI] [PubMed] [Google Scholar]
- 73.Raetz EA, Perkins SL, Bhojwani D, Smock K, Philip M, et al. Gene expression profiling reveals intrinsic differences between T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Pediatr Blood Cancer. 2006;47:130–140. doi: 10.1002/pbc.20550. [DOI] [PubMed] [Google Scholar]
- 74.Suhr ML, Dysvik B, Bruland O, Warnakulasuriya S, Amaratunga AN, et al. Gene expression profile of oral squamous cell carcinomas from Sri Lankan betel quid users. Oncol Rep. 2007;18:1061–1075. [PubMed] [Google Scholar]
- 75.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim MJ, Lee JC, Lee JJ, Kim S, Lee SG, et al. Association of CD47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumour Biol. 2008;29:28–34. doi: 10.1159/000132568. [DOI] [PubMed] [Google Scholar]
- 78.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kikuchi Y, Uno S, Kinoshita Y, Yoshimura Y, Iida S, et al. Apoptosis inducing bivalent single-chain antibody fragments against CD47 showed antitumor potency for multiple myeloma. Leuk Res. 2005;29:445–450. doi: 10.1016/j.leukres.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 80.Sagawa M, Shimizu T, Fukushima N, Kinoshita Y, Ohizumi I, et al. A new disulfide-linked dimer of a single-chain antibody fragment against human CD47 induces apoptosis in lymphoid malignant cells via the hypoxia inducible factor-1alpha pathway. Cancer Sci. 2011;102:1208–1215. doi: 10.1111/j.1349-7006.2011.01925.x. [DOI] [PubMed] [Google Scholar]
- 81.Rath GM, Schneider C, Dedieu S, Rothhut B, Soula-Rothhut M, et al. The C-terminal CD47/IAP-binding domain of thrombospondin-1 prevents camptothecin- and doxorubicin-induced apoptosis in human thyroid carcinoma cells. Biochim Biophys Acta. 2006;1763:1125–1134. doi: 10.1016/j.bbamcr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Rath GM, Schneider C, Dedieu S, Sartelet H, Morjani H, et al. Thrombospondin-1 C-terminal-derived peptide protects thyroid cells from ceramide-induced apoptosis through the adenylyl cyclase pathway. Int J Biochem Cell Biol. 2006;38:2219–2228. doi: 10.1016/j.biocel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Kaur S, Roberts DD. CD47 applies the brakes to angiogenesis via vascular endothelial growth factor receptor-2. Cell Cycle. 2011;10:10–12. doi: 10.4161/cc.10.1.14324. [DOI] [PubMed] [Google Scholar]
- 84.Isenberg JS, Hyodo F, Ridnour LA, Shannon CS, Wink DA, et al. Thrombospondin-1 and vasoactive agents indirectly alter tumor blood flow. Neoplasia. 2008;10:886–896. doi: 10.1593/neo.08264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z, He L, Wilson KE, Roberts DD. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J Immunol. 2001;166:2427–2436. doi: 10.4049/jimmunol.166.4.2427. [DOI] [PubMed] [Google Scholar]
- 86.Kaur S, Kuznetsova SA, Pendrak ML, Sipes JM, Romeo MJ, et al. Identification of CD47 and amyloid precursor-like protein-2 as the major heparan sulfate proteoglycans on T lymphocytes and this isoform of CD47 as the signaling receptor for thrombospondin-1. J Biol Chem. 2011;286:14991–15002. doi: 10.1074/jbc.M110.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McGee MC, Hamner JB, Williams RF, Rosati SF, Sims TL, et al. Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys. 2010;76:1537–1545. doi: 10.1016/j.ijrobp.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karar J, Maity A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol Ther. 2009;8:1994–2001. doi: 10.4161/cbt.8.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 90.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, et al. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res. 2009;15:597–606. doi: 10.1158/1078-0432.CCR-08-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gudkov AV, Komarova EA. Radioprotection: smart games with death. J Clin Invest. 2010;120:2270–2273. doi: 10.1172/JCI43794. [DOI] [PMC free article] [PubMed] [Google Scholar]