Heparan Sulfate Facilitates Rift Valley Fever Virus Entry into the Cell (original) (raw)
Abstract
Rift Valley fever virus (RVFV), an emerging arthropod-borne pathogen, has a broad host and cell tropism. Here we report that the glycosaminoglycan heparan sulfate, abundantly present on the surface of most animal cells, is required for efficient entry of RVFV. Entry was significantly reduced by preincubating the virus inoculum with highly sulfated heparin, by enzymatic removal of heparan sulfate from cells and in cells genetically deficient in heparan sulfate synthesis.
TEXT
Rift Valley fever virus (RVFV) belongs to the Phlebovirus genus of the Bunyaviridae family. Its negative-stranded tripartite RNA genome is encapsidated by nucleocapsid protein and is surrounded by a lipid-containing envelope which is derived from the trans-Golgi network (36). Two membrane-anchored viral glycoproteins, Gn and Gc, assemble into capsomers that cover the viral surface, following a T = 12 icosahedral symmetry (12, 19). The glycoproteins mediate host cell attachment of the virus and its subsequent entry into the cell (36). A 78-kDa glycoprotein of unknown function, which is an N-terminally extended version of Gn, has been reported as a third structural glycoprotein, present only in minute amounts in the viral envelope (21, 39).
RVFV is responsible for severe epidemics among ruminants in Africa and on the Arabian Peninsula, manifested by abortion storms and high mortality among young animals. The virus is transmitted by a wide variety of mosquito vectors. After introduction into the body by the bite of an infected mosquito, the virus can spread and infect different organs, including the brain (32). Humans can also be infected, and a small percentage develops severe disease (31, 36). Apart from mosquitoes, ruminants, and humans, a wide variety of animal hosts can be infected with RVFV, including nonhuman primates, rodents, and pets (11, 18). The virus also efficiently infects a large collection of different cell types in vitro (see Fig. S1 in the supplemental material). The broad host, tissue, and cell tropism of RVFV suggests the involvement of a common cell surface attachment factor to be utilized by RVFV to establish infection.
To initiate entry into the cell, viruses need to interact with a cellular receptor, which is sometimes preceded by binding to a primary attachment factor (30). The cell surface structures which facilitate entry of bunyaviruses remain largely unknown, although some receptors have been described. Beta3 integrins and nucleolin have been reported to be involved in attachment of hantavirus and Crimean-Congo hemorrhagic fever virus (genus Nairovirus), respectively (14, 42). DC-SIGN, a C-type lectin primarily restricted to interstitial dendritic cells and certain tissue macrophages (33), has been identified as a receptor for some phleboviruses, including RVFV (29). The broad cell tropism of RVFV, however, suggests that other receptors are important for virus entry into cells that lack DC-SIGN expression.
All eukaryotic cells are covered by a dense and diverse array of carbohydrates. These sugars are essential for many different biological processes (40). It is not surprising that many viruses have evolved to use these ubiquitous and accessible surface glycans as part of their strategy to infect cells (26). Two types of glycans, sialylated glycans (SGs) and glycosaminoglycans (GAGs), have been particularly noted to play a role in virus entry. For example, influenza viruses specifically bind SGs, while dengue virus (7) and adenovirus (34) interact with GAGs to facilitate entry. Merkel cell polyomavirus has been reported to use both SGs and GAGs for entry (37).
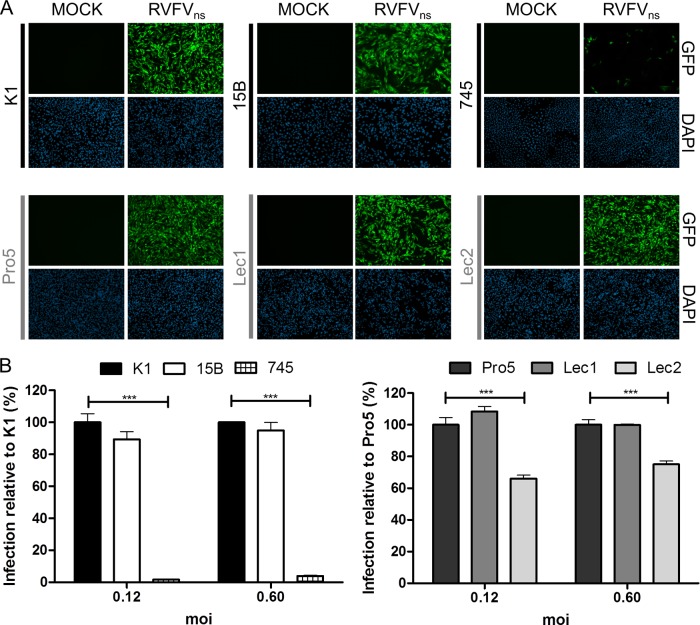
We started to study the involvement of SGs and GAGs in RVFV entry by using a collection of Chinese hamster ovary (CHO) cell mutants with specific genetic deficiencies in glycan synthesis (see Table S1 in the supplemental material) (22). Thus, CHO lec1 and 15B (16, 38) mutants are incapable of synthesizing complex N-linked glycans, while the CHO lec2 mutant cells express sialic acid-free N- and O-linked glycans (9). The CHO pgsA-745 cell mutant (10) is deficient in the synthesis of GAGs. To facilitate our studies, we made use of the recently developed nonspreading RVFV (here referred to as RVFVns) (25). In contrast to wild-type virus, RVFVns can be handled outside biosafety level 3 facilities, while the presence of the enhanced green fluorescent protein (eGFP) gene in the viral genome enables infection to be easily monitored. The mutant lec1 and 15B CHO cells and, to a somewhat lesser extent, the CHO lec2 cells were as efficiently infected with RVFVns as the parental wild-type cells (pro5 and K1), suggesting that N- and O-linked SGs play a minor role in virus infection. In contrast, infection of CHO psgA-745 was dramatically reduced, indicating that GAGs are important for RVFVns infection (Fig. 1).
Fig 1.
RVFVns infection is drastically reduced in the absence of GAGs. The CHO 15B and CHO 745 mutant cells derived from the CHO K1 cell line and the CHO lec1 and CHO lec2 mutant cells derived from the CHO Pro5 cell line were cultured in Ham's F-12K medium (Invitrogen) supplemented with 10% fetal calf serum (FCS). Subconfluent monolayers were infected with RVFVns at different multiplicities of infection (MOIs) (0.12 and 0.6). At 20 h postinfection (p.i.), the cells were washed once with phosphate-buffered saline (PBS) and prepared for fluorescence microscopy (A) or fluorescence-activated cell sorter (FACS) analysis (B). (A) Cells were fixed with 3.7% formaldehyde–PBS for 20 min at room temperature, and representative pictures were taken using an Evos fl microscope (AMG) (magnification, ×4; data shown refer to infections at an MOI of 0.6). Nuclei were counterstained with 4′,6′diamidino-2-phenylindole (DAPI). MOCK, mock-infected cells. (B) Cells were trypsinized and fixed with 3.7% formaldehyde–PBS for 20 min at room temperature, and RVFVns-infected (GFP-positive) cells were quantified by FACS (FACSCalibur). Graphical data shown are normalized to the infectivity of CHO K1 or CHO Pro5 cells and are representative of the results of two independent experiments performed in triplicate. Significant differences between conditions are indicated (analysis of variance [ANOVA]-Bonferroni); ***, P < 0.001. Error bars represent standard deviations (SD).
GAGs are linear polysaccharides that can be attached to proteins to form proteoglycans. There are five classes of GAGs, heparan sulfate (HS), chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate, and hyaluronic acid (28). Of these GAGs, HS has been identified as an attachment factor for a number of viruses and, unlike other GAGs, is abundantly expressed on most cell types (28). We first evaluated whether RVFVns infection could be inhibited by inclusion of soluble heparin, a GAG analogue of HS, as a competitor in the inoculum (23). As a control virus, we used a nonspreading vesicular stomatitis virus (here referred to as VSVns), a VSV-ΔG/GFP recombinant virus pseudotyped with its authentic fusion glycoprotein G (5). Preincubation of RVFVns with heparin reduced infection of CHO K1 cells in a dose-dependent manner, whereas no such effect was observed for VSVns (Fig. 2A). To confirm the involvement of HS in RVFV entry, CHO K1 cells were treated prior to infection for 1 h at 37°C with different heparinases or chondroitinase to remove HS or CS/DS, respectively, from the cell surface (Fig. 2B). Enzymatic treatment of CHO K1 cells with heparinase caused a marked increase of infection with VSVns of more than 2-fold. In contrast, independently of the different heparinases used, infection of heparinase-treated cells with RVFVns was reduced to about 50%. No effect of chondroitinase treatment was observed. The reduced RVFVns infectivity of heparinase-pretreated cells was confirmed using six different cell lines, while the susceptibility of these cells to VSVns was not affected (see Fig. S2 in the supplemental material).
Fig 2.
RVFVns infection is decreased in the presence of heparin and after enzymatic removal of heparan sulfate from the cell surface. (A) RVFVns and VSVns were incubated with different concentrations of soluble heparin (MPBio) for 10 min at room temperature in culture medium, prior to infection of CHO K1 cells. At 8 (VSVns) or 20 (RVFVns) h p.i., infection was quantified by FACS analysis as described for Fig. 1. The data shown correspond to the results of a representative set of two independent experiments performed in triplicate. (B) GAGs were enzymatically removed from the cell surface of CHO K1 cells. Chondroitinase ABC (specific for chondroitin and dermatan sulfate), heparinase I (specific for heparin and highly sulfated domains), heparinase II (specific for heparin and heparan sulfate), and heparinase III (specific for heparan sulfate), all purchased at Sigma, were dissolved in resuspension buffer (20 mM HEPES [pH 7.5], 50 mM NaCl, 4 mM CaCl2, 0.01% bovine serum albumin [BSA]). Dilutions were prepared in digestion buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 4 mM CaCl2, 0.1% BSA). CHO K1 cells were treated for 1 h at 37°C with heparinase I, II, or III, with a combination of them, or with chondroitinase at the indicated concentrations. The cells were washed twice with culture medium and then incubated with RVFVns or VSVns for 30 min at 37°C. The cells were washed twice with culture medium and further incubated in culture medium at 37°C for 8 (VSVns) or 20 (RVFVns) h, after which infection was quantified by FACS analysis as described for Fig. 1. Data were obtained from two independent experiments performed in duplicate. Significant differences between conditions are indicated (ANOVA-Bonferroni); ***, P < 0.001. Error bars represent SD.
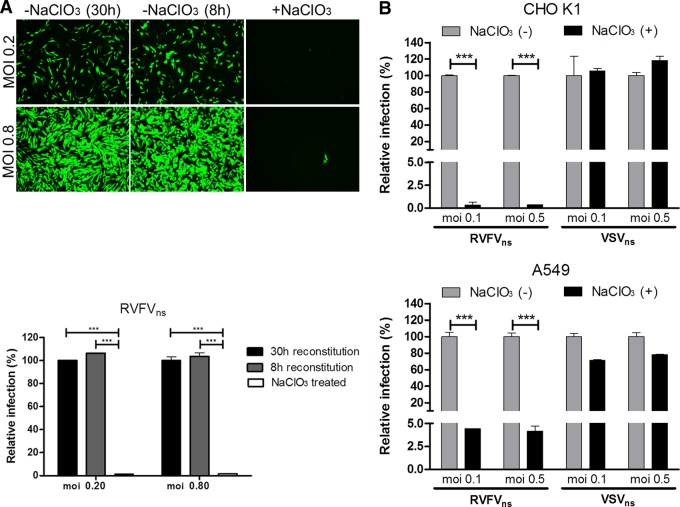
To further characterize the interaction between RVFV and highly sulfated HS polysaccharides, we analyzed RVFVns infection of CHO K1 cells that were subjected to passage in the presence of 50 mM sodium chlorate (NaClO3). NaClO3 is known to inhibit the addition of _O_-sulfate groups to GAGs (1, 35). Importantly, we did not observe any apparent changes in growth rate or cell morphology of CHO K1 or A549 cells cultured for 7 days in the presence of up to 70 mM NaClO3 (data not shown). Infection by RVFVns of CHO K1 or A549 cells maintained in the presence of NaClO3 was dramatically reduced (Fig. 3A and B), in contrast to infection by VSVns, suggesting that _O_-sulfation of HS is necessary for efficient RVFVns infection of both cell lines.
Fig 3.
RVFVns infection strongly depends on sulfation of heparan sulfate. (A) CHO K1 cells were subjected to two passages in culture medium containing 50 mM NaClO3 (Sigma) and subsequently cultured in the presence of 50 mM sodium chlorate, or in chlorate-free culture medium for 30 or 8 h prior to infection, to reverse the chlorate effect. Twenty h postinfection, cells were analyzed by fluorescence microscopy or FACS analysis as described for Fig. 1. Graphical data shown are normalized and are representative of the results of two individual experiments performed in triplicate. (B) A549 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal calf serum (FCS). CHO K1 or A549 cells were subjected to two passages in culture medium containing 50 mM NaClO3 (Sigma) and subsequently cultured in the presence of 50 mM sodium chlorate [NaClO3 (+)] or in chlorate-free culture medium [NaClO3 (−)] for 8 h prior to inoculation with RVFVns or VSVns at the indicated MOI. At 8 (VSVns) or 20 (RVFVns) h p.i., cells were analyzed by fluorescence microscopy or FACS analysis as described for Fig. 1. Significant differences between conditions are indicated (ANOVA-Bonferroni; *** = P < 0.001). Error bars represent SD.
Next we tested the susceptibility of CHO pgsD-677 cells (CHO HS[−]), which are deficient in HS synthesis (27), to RVFVns and VSVns infection. Compared to infection of the parental CHO K1 cells with RVFVns, infection of CHO HS[−] cells was greatly reduced (>97%), whereas VSVns infection of these cells was enhanced (Fig. 4). To confirm HS dependency of RVFV, an autonomously replicating virus was included in this experiment. This virus expresses the eGFP reporter from its genome in a manner similar to that seen with RVFVns and was rescued as previously described (25). Also, this virus displayed significantly reduced infectivity on CHO HS[−] cells. Altogether, the observations strongly support the idea of an important role of HS for RVFV infection.
Fig 4.
Entry of RVFVGFP into GAG-deficient CHO cells is inefficient due to the lack of heparan sulfate. Mutant CHO pgsD-677 cells (HS[−], able to express all GAGs except for heparan sulfate) and pgsA-745 cells (CHO GAG[−], deficient in expression of all GAGs) and the parental CHO K1 cells were inoculated with RVFVns, VSVns, or RVFVGFP. At 8 (VSVns), 10 (RVFVGFP), or 20 (RVFVns) h p.i., cells were analyzed by fluorescence microscopy and GFP-expressing RVFV-infected cells were quantified. Graphical data shown are normalized to the infectivity of CHO K1. Significant differences between conditions are indicated (ANOVA-Bonferroni); ***, P < 0.001. Error bars represent SD.
Many viruses have been reported to utilize HS for host cell attachment (reviewed in reference 28). Interactions of viruses with heparan sulfate are often based on electrostatic contacts between the negatively charged sulfate groups on HS and clusters of basic residues occurring in viral surface proteins. These clusters often comprise a BBXB or a BBBXXB motif (B, basic amino acid; X, any amino acid) (3). In analyzing the complete M segment-encoded polypeptide sequence of the RVFV used in this study (strain 35/74; GenBank accession number JF784387.1), we identified two overlapping BBBXXB HS binding motifs (116-RCERRRDAK-124) in the pre-Gn region of the 78-kDa protein (where the boldface characters represent the first and the underlining represents the second motif), while no HS binding motifs were identified in the Gn or Gc protein sequence. The 78-kDa protein is considered to be a minor structural glycoprotein (39) and is apparently dispensable: RVFV recombinants lacking the pre-Gn region display wild-type growth kinetics in cell culture, calling into question whether the basic amino acid motifs in the protein indeed contribute to HS binding (15, 41). Alternatively, other linear or nonlinear arrangements of basic residues in Gn and/or Gc may create an HS binding motif in the tertiary structures of these glycoproteins (13, 17). Clearly, the identification of the HS binding site on the viral surface requires further study.
HS dependency has for some viruses been shown to be acquired after repetitive virus passage in cell culture through the acquisition of single or multiple amino acid substitutions in the surface glycoproteins, creating a positively charged HS binding motif (6, 8, 20, 24). The RVFV 35/74 strain was isolated from the liver of a sheep that died during an RVFV outbreak in the Free State province of South Africa in 1974. The virus was amplified in suckling mouse brain and subjected to three passages in BHK-21 cells (25). To study the possible acquisition of a HS-binding motif during these procedures, the M segment-encoded polypeptide sequence was aligned with those of four RVFV isolates that had been directly sequenced from serum or organ material of infected animals (2, 4). This analysis did not reveal the presence of additional basic amino acids in the 35/74 sequence (see Table S2 in the supplemental material), indicating that the requirement for HS for efficient entry of the RVFV used in this study is not likely the result of cell culture adaptation.
Although infection of RVFV in the GAG- and HS-deficient CHO cells was dramatically reduced, we observed residual infection of both cell lines. It remains to be determined whether this infection in the absence of HS is explained by the binding of RVFV to another, unidentified attachment factor or receptor present on these cells.
Supplementary Material
Supplemental material
ACKNOWLEDGMENTS
We thank Rianka Vloet, Nadia Oreshkova, Jet Kant, and Paul Wichgers Schreur (Central Veterinary Institute, Lelystad, The Netherlands) for their assistance. We thank Ineke Braakman (Utrecht University, Utrecht, The Netherlands) for providing the CHO 15B cell line. We thank Sean Whelan and Matthijs Raaben (Harvard Medical School, Boston, MA) for providing the VSVΔG-GFP recombinant virus.
This work was supported by the Dutch Ministry of Economic Affairs, Agriculture and Innovation, project codes KB-12-004.02-002 and BO-10-001-211.
Footnotes
Published ahead of print 26 September 2012
REFERENCES
- 1.Baeuerle PA, Huttner WB. 1986. Chlorate—a potent inhibitor of protein sulfation in intact cells. Biochem. Biophys. Res. Commun. 141:870–877 [DOI] [PubMed] [Google Scholar]
- 2.Bird BH, et al. 2008. Multiple virus lineages sharing recent common ancestry were associated with a Large Rift Valley fever outbreak among livestock in Kenya during 2006–2007. J. Virol. 82:11152–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardin AD, Weintraub HJ. 1989. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9:21–32 [DOI] [PubMed] [Google Scholar]
- 4.Carroll SA, et al. 2011. Genetic evidence for Rift Valley fever outbreaks in Madagascar resulting from virus introductions from the East African mainland rather than enzootic maintenance. J. Virol. 85:6162–6167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang A, Masante C, Buchholz UJ, Dutch RE. 2012. Human metapneumovirus (HMPV) binding and infection are mediated by interactions between the HMPV fusion protein and heparan sulfate. J. Virol. 86:3230–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, et al. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866–871 [DOI] [PubMed] [Google Scholar]
- 8.de Haan CA, et al. 2005. Murine coronavirus with an extended host range uses heparan sulfate as an entry receptor. J. Virol. 79:14451–14456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deutscher SL, Nuwayhid N, Stanley P, Briles EI, Hirschberg CB. 1984. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell 39:295–299 [DOI] [PubMed] [Google Scholar]
- 10.Esko JD, Stewart TE, Taylor WH. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 82:3197–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans A, et al. 2008. Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol. Infect. 136:1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freiberg AN, Sherman MB, Morais MC, Holbrook MR, Watowich SJ. 2008. Three-dimensional organization of Rift Valley fever virus revealed by cryoelectron tomography. J. Virol. 82:10341–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandhi NS, Mancera RL. 2008. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 72:455–482 [DOI] [PubMed] [Google Scholar]
- 14.Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. 1998. β3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. U. S. A. 95:7074–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerrard SR, Bird BH, Albarino CG, Nichol ST. 2007. The NSm proteins of Rift Valley fever virus are dispensable for maturation, replication and infection. Virology 359:459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb C, Skinner AM, Kornfeld S. 1974. Isolation of a clone of Chinese hamster ovary cells deficient in plant lectin-binding sites. Proc. Natl. Acad. Sci. U. S. A. 71:1078–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hileman RE, Fromm JR, Weiler JM, Linhardt RJ. 1998. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20:156–167 [DOI] [PubMed] [Google Scholar]
- 18.House JA, Turell MJ, Mebus CA. 1992. Rift Valley fever: present status and risk to the Western Hemisphere. Ann. N. Y. Acad. Sci. 653:233–242 [DOI] [PubMed] [Google Scholar]
- 19.Huiskonen JT, Overby AK, Weber F, Grunewald K. 2009. Electron cryo-microscopy and single-particle averaging of Rift Valley fever virus: evidence for GN-GC glycoprotein heterodimers. J. Virol. 83:3762–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulst MM, van Gennip HG, Moormann RJ. 2000. Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparan sulfate due to a single amino acid change in envelope protein E(rns). J. Virol. 74:9553–9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakach LT, Wasmoen TL, Collett MS. 1988. Rift Valley fever virus M segment: use of recombinant vaccinia viruses to study Phlebovirus gene expression. J. Virol. 62:826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao FT, Puck TT. 1967. Genetics of somatic mammalian cells. IV. Properties of Chinese hamster cell mutants with respect to the requirement for proline. Genetics 55:513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjellén L, Lindahl U. 1991. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 60:443–475 [DOI] [PubMed] [Google Scholar]
- 24.Klimstra WB, Ryman KD, Johnston RE. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357–7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kortekaas J, et al. 2011. Creation of a nonspreading Rift Valley fever virus. J. Virol. 85:12622–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehmann F, Tiralongo E, Tiralongo J. 2006. Sialic acid-specific lectins: occurrence, specificity and function. Cell. Mol. Life Sci. 63:1331–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lidholt K, et al. 1992. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 89:2267–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Thorp SC. 2002. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 22:1–25 [DOI] [PubMed] [Google Scholar]
- 29.Lozach PY, et al. 2011. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe 10:75–88 [DOI] [PubMed] [Google Scholar]
- 30.Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu. Rev. Biochem. 79:803–833 [DOI] [PubMed] [Google Scholar]
- 31.Mundel B, Gear J. 1951. Rift valley fever; I. The occurrence of human cases in Johannesburg. S. Afr. Med. J. 25:797–800 [PubMed] [Google Scholar]
- 32.Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. 2010. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 41:61 doi:10.1051/vetres/2010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puig-Kröger A, et al. 2004. Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin in THP-1 human leukemic cells, monocytes, and macrophages. J. Biol. Chem. 279:25680–25688 [DOI] [PubMed] [Google Scholar]
- 34.Raman S, Hsu TH, Ashley SL, Spindler KR. 2009. Usage of integrin and heparan sulfate as receptors for mouse adenovirus type 1. J. Virol. 83:2831–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Safaiyan F, et al. 1999. Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. J. Biol. Chem. 274:36267–36273 [DOI] [PubMed] [Google Scholar]
- 36.Schmaljohn CS, Nichol ST. 2007. Bunyaviridae, p 1741–1789_In_Knipe DM, et al. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 37.Schowalter RM, Pastrana DV, Buck CB. 2011. Glycosaminoglycans and sialylated glycans sequentially facilitate Merkel cell polyomavirus infectious entry. PLoS Pathog. 7:e1002161 doi:10.1371/journal.ppat.1002161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley P, Caillibot V, Siminovitch L. 1975. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell 6:121–128 [DOI] [PubMed] [Google Scholar]
- 39.Struthers JK, Swanepoel R, Shepherd SP. 1984. Protein synthesis in Rift Valley fever virus-infected cells. Virology 134:118–124 [DOI] [PubMed] [Google Scholar]
- 40.Varki A. 2007. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446:1023–1029 [DOI] [PubMed] [Google Scholar]
- 41.Won S, Ikegami T, Peters CJ, Makino S. 2006. NSm and 78-kilodalton proteins of Rift Valley fever virus are nonessential for viral replication in cell culture. J. Virol. 80:8274–8278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao X, Feng Y, Zhu Z, Dimitrov DS. 2011. Identification of a putative Crimean-Congo hemorrhagic fever virus entry factor. Biochem. Biophys. Res. Commun. 411:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material