Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial (original) (raw)
Abstract
Aims
We evaluated the impact of diabetes mellitus (DM) and diabetic therapy on outcomes in patients with reduced ejection fraction (EF) after hospitalization for heart failure (HF). DM is prevalent in patients hospitalized with HF, yet inconclusive data exist on the post-discharge outcomes of this patient population.
Methods and results
Post-hoc analysis was performed on the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan) study, a randomized trial of patients hospitalized with HF (n = 4133) with median follow-up of 9.9 months. DM status was determined from intake questionnaires and cross-verified by medication history. Univariate relationships were examined using χ2 test, _t_-test, and Wilcoxon tests. The two primary outcomes of (i) all-cause mortality (ACM) and (ii) cardiovascular mortality or HF hospitalization (CVM + HFH) were assessed for those with and without DM and by diabetic treatment strategy using log rank tests and multivariable Cox regression models. DM was present in 40% of participants. Patients with DM were more likely to have hypertension, coronary artery disease, and chronic kidney disease. Diabetes was associated with ACM and CVM + HFH (both P < 0.001). Following multivariate risk adjustment, DM was associated with ACM, but this estimate was imprecise [hazard ratio (HR) 1.16; 95% confidence interval (CI) 1.00–1.34] and remained associated with CVM or HFH (HR 1.17; 95% CI 1.04–1.31). Diabetic control strategy did not independently affect outcomes.
Conclusion
Diabetes is common in patients hospitalized for heart failure with a reduced EF. These patients have a higher post-discharge CVM and higher HF hospitalizations compared with patients with no diabetes. Different diabetic treatment regimens did not appear to influence post-discharge outcomes.
Keywords: Heart failure, Diabetes mellitus, Outcomes, Insulin
Introduction
Approximately 40% of patients hospitalized with heart failure (HF) and low ejection fraction (EF) have diabetes mellitus (DM).1 While DM is associated with increased cardiovascular morbidity and mortality in ambulatory patients with chronic systolic HF,2,3 its influence as an independent predictor of long-term outcomes after hospitalization for HF is not consistently apparent. The OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) registry showed that diabetic patients admitted for HF had slightly longer lengths of stay than non-diabetic patients (5.9 days vs. 5.5 days, P < 0.0001) but had no differences in in-hospital or post-discharge mortality at 90-day follow-up.4 Similarly, in the ADHERE (Acute Decompensated Heart Failure National Registry) database, there was a similar risk of in-hospital mortality in HF patients with and without DM. However, ADHERE did not address the effect of DM on post-discharge cardiovascular events as post-discharge data were not collected.5 While findings from both the OPTIMIZE-HF and ADHERE registries suggest that DM patients hospitalized with HF do not fare worse than their non-DM counterparts with regards to in-hospital or short-term post-discharge mortality, only 10% of patients in the OPTIMIZE-HF registry had follow-up at 90 days, limiting observation of longer term effects of diabetes on adverse outcomes. In addition, other registries of hospitalized HF patients with diabetes have shown conflicting results, with worse outcomes in diabetic patients post-discharge suggesting the need for further studies.6,7 To our knowledge, the effect of diabetes on outcomes after hospitalization for HF with reduced EF has not been studied in a large randomized trial setting which would provide more thorough follow-up and comprehensive determination of clinical outcomes compared with registry analysis. We investigated the clinical characteristics and long-term outcomes of DM patients vs. non-DM patients in the setting of the large, international EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan) study; an trial of hospitalized HF patients with high use of contemporary HF therapies, long-term follow-up, and blindly adjudicated endpoints.
Methods
We performed a post-hoc analysis of the EVEREST trial. The design and primary results of EVEREST have been described previously.8 Briefly, from October 2003 to February 2006, 4133 patients with chronic systolic dysfunction (EF <40%) hospitalized for HF exacerbations in 359 centres across 20 countries were randomized in a double-blind, placebo-controlled manner to receive either tolvaptan, a vasopressin receptor blocker, or placebo, and were followed for a median of 9.9 months with maximum follow-up of 2.5 years. Study physicians were given recommendations for guideline-based HF therapy as part of the study protocol. Patients were assessed clinically at the time of randomization, hospital day 7, or day of discharge, and scheduled clinic visits at 1, 4, and 8 weeks, and every 8 weeks thereafter. The two primary outcomes of the trial were all-cause mortality (ACM) and a combined endpoint of cardiovascular mortality or HF hospitalization (CVM + HFH), measured as time to first event and adjudicated by a blinded clinical events committee. CVM was an aggregate of HF, myocardial infarction (MI), stroke, or sudden cardiac deaths. Secondary endpoints included cardiovascular mortality or hospitalization and clinically worsening HF (death, hospitalization, or unscheduled outpatient HF visit).
Participants were identified as diabetic by trial intake questionnaires, which were obtained by study site coordinators from patient interviews and medical records. Duration of diabetes or haemoglobin A1c% was not documented. Patients receiving insulin or oral hypoglycaemic agents for diabetes were also categorized as diabetic, and patients who were reported as diabetic but not on antidiabetic therapy were classified as ‘diet controlled’. Other co-morbid conditions at the time of study entry including history of hypertension, coronary artery disease (CAD), and chronic kidney disease (CKD) were also documented on initial intake questionnaires. Daily medication regimens starting 7 days prior to entrance into the study until the end of the study were recorded.
The relationship between diabetes and potential confounders was examined using χ2 tests for categorical confounders, Student's _t-_tests for normally distributed variables, or Wilcoxon rank sum tests for continuous variables for non-normally distributed variables. We created Cox regression models to evaluate the association between diabetes and ACM and CVM or HFH over time and performed log-rank tests to evaluate differences between individuals with DM and without DM. Covariates used for adjustment of baseline variables were similar to those of previouslyr published reports9 using the EVEREST database, and included sex, age, body mass index, EF, sodium, blood uirea nitrogen (BUN), baseline systolic blood pressure, QRS duration, brain natriuretic peptide (BNP)/N-terminal pro BNP (NT-proBNP), region, presence of atrial fibrillation or flutter on baseline electrocardiogram (ECG), baseline medication use [angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, beta-blockers, aldosterone blockers, digoxin, and intravenous inotropes), and co-morbidities (history of hypertension, CKD, stroke, and smoking history). In addition to ACM and CVM + HFH endpoints, rates of re-hospitalization for stroke, MI, and arrhythmia, and incidence of clinically worsening HF were also compared using Cox regression models.
Participants were further subdivided into three groups by their diabetic treatment strategy after reviewing the medication regimens of all patients with documented diabetes: (i) diet controlled; (ii) oral hypoglycaemic use; and (iii) insulin use, either as monotherapy or in conjunction with oral medications. Multivariate Cox regression models with adjustment for the baseline covariates listed above were fit to compare time to ACM and CVM + HFH between the three groups. Statistical analyses were performed using SAS v9.2 (Cary, NC, USA).
Results
Diabetes mellitus status was available for 4131 (>99%) of EVEREST participants, of whom 40% (n = 1657) had DM. The baseline characteristics of DM and non-DM patients are summarized in Table 1. Patients with DM were more likely than patients without DM to have hypertension (80% vs. 65%; P < 0.001), CAD (78% vs. 65%; P < 0.001), and CKD (36% vs. 21%; P < 0.001), but less likely to be current smokers (9.8% vs. 14.1%; P < 0.001). Admission weights (88 kg vs. 80 kg; P < 0.001) and systolic blood pressure (123 mmHg vs. 119 mmHg; P < 0.001) were higher in DM patients. In addition, those patients with DM tended to have higher baseline levels of serum BUN and creatinine and shorter QRS intervals. There were no between-group differences in baseline EF or BNP/NT-proBNP. Medication regimens were generally similar between both groups. with the exception of beta-blocker use (74% vs. 68%; P < 0.001), which was higher in participants with DM, and aldosterone antagonist use, which was lower (49% vs. 57%; P < 0.001) in participants with DM. In the peri-hospitalization period, there was no difference in dyspnoea scores or jugular venous distension elevation but an increase in pedal oedema in diabetics compared with non-diabetics (8.56% vs. 12.57%; P < 0.001) at 7 days of follow-up.
Table 1.
Baseline characteristics of patients by diabetes mellitus status
| Diabetes (n = 1657) | No diabetes (n = 2474) | _P_-value | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) [range] | 66.5 (10.3) [28–93] | 65.3 (12.8) [18–94] | 0.001 |
| Male, n (%) | 1197 (72.2) | 1876 (75.8) | 0.01 |
| Race, n (%) | 0.05 | ||
| Caucasian | 1408 (85.0) | 2123 (85.8) | |
| Black | 144 (8.7) | 166 (6.7) | |
| Hispanic | 70 (4.2) | 131 (5.3) | |
| Other | 34 (2.1) | 54 (2.2) | |
| Weight, kg (SD) | 88.1 (19.2) | 80.0 (17.9) | <0.001 |
| Height, cm (SD) | 170.2 (9.4) | 170.2 (9.4) | 0.78 |
| Never smoked | 544 (32.8) | 880 (35.6) | <0.001 |
| Smoker | 163 (9.8) | 348 (14.1) | |
| Ex-smoker | 950 (57.3) | 1242 (50.3) | |
| Region | <0.001 | ||
| Eastern Europe | 558 (33.7) | 1061 (42.9) | |
| North America | 649 (39.2) | 601 (24.3) | |
| South America | 210 (12.7) | 489 (19.8) | |
| Western Europe | 240 (14.5) | 323 (13.1) | |
| Physical and laboratory findings | |||
| Systolic BP, mmHg (SD) | 123 (20) | 119 (19) | <0.001 |
| Diastolic BP, mmHg (SD) | 72 (13) | 73 (13) | 0.22 |
| Heart rate, b.p.m. (SD) | 79 (15) | 80 (16) | 0.08 |
| JVD ≥10 cm, n (%) | 450 (28.2) | 632 (26.2) | 0.16 |
| Rales, n (%) | 1301 (80.5) | 1994 (82.0) | 0.21 |
| Peripheral oedema, n (%) | 1497 (92.6) | 2127 (87.5) | <0.001 |
| Ejection fraction, mean (SD) | 28 (8) | 27 (8) | 0.40 |
| BUN, mg/dL (25th, 75th) | 28 (20, 39) | 25 (19, 32) | <0.001 |
| Creatinine mg/dL (25th, 75th) | 1.3 (1.1, 1.7) | 1.2 (1.0, 1.5) | <0.001 |
| Sodium, mEq/L (25th, 75th) | 140 (137, 142) | 140 (137, 143) | <0.001 |
| BNP, pg/mL (25th, 75th) | 656 (291, 1412) | 739 (292, 1560) | 0.05 |
| NT-proBNP, pg/mL (25th, 75th) | 4369 (1924, 8569) | 4877 (2338, 10 116) | 0.08 |
| QRS interval, ms (25th, 75th) | 122 (96, 147) | 123 (98, 153) | 0.01 |
| AF/flutter on ECG, n (%) | 421 (25.4) | 774 (31.3) | <0.001 |
| Medical history | |||
| Previous HF hospitalization, n (%) | 1360 (82.5) | 1890 (76.7) | <0.001 |
| CAD, n (%) | 1294 (78.2) | 1617 (65.4) | <0.001 |
| Previous MI, n (%) | 954 (57.6) | 1133 (45.8) | <0.001 |
| Hypertension, n (%) | 1327 (80.1) | 1605 (64.9) | <0.001 |
| Hypercholesterolaemia, n (%) | 992 (60.1) | 1011 (41.1) | <0.001 |
| Previous CABG, n (%) | 446 (26.9) | 416 (16.8) | <0.001 |
| Chronic kidney disease, n (%) | 599 (36.2) | 508 (20.5) | <0.001 |
| Peripheral vascular disease, n (%) | 432 (26.1) | 434 (17.6) | <0.001 |
| PCI, n (%) | 381 (23.0) | 357 (14.4) | <0.001 |
| ICD, n (%) | 287 (17.3) | 313 (12.7) | <0.001 |
| Previous stroke, n (%) | 226 (13.8) | 245 (10.0) | <0.001 |
| Medications | |||
| Tolvaptan, n(%) | 855 (51.6) | 1217 (49.2) | 0.13 |
| Diuretics, n (%) | 1607 (97.0) | 2395 (96.8) | 0.75 |
| Beta-blockers, n (%) | 1225 (73.9) | 1678 (67.8) | <0.001 |
| Calcium channel blockers, n (%) | 222 (13.4) | 218 (8.8) | <0.001 |
| ACE inhibitors/ARBs, n (%) | 1381 (83.3) | 2098 (84.8) | 0.21 |
| Aldosterone-blocking agents, n (%) | 816 (49.3) | 1421 (57.4) | <0.001 |
| Digoxin, n (%) | 694 (41.9) | 1121 (45.3) | 0.03 |
| Nitrates, n (%) | 661 (39.9) | 856 (34.6) | 0.01 |
| Hydralazine, n (%) | 70 (4.2) | 51 (2.1) | <0.001 |
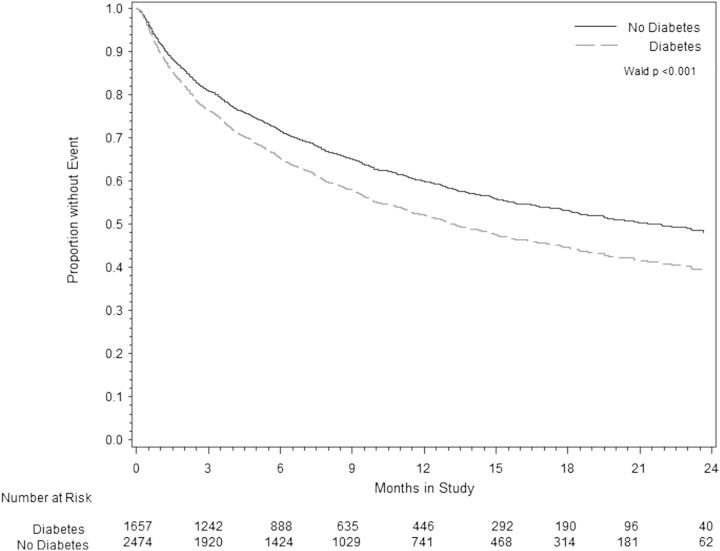
Over the study period, 1080 participants died; 478 (29%) with DM and 614 (24%) without DM. DM was significantly associated with time to ACM [hazard ratio (HR) 1.18; 95% confidence interval (CI) 1.05–1.33] and CVM + HFH (HR 1.28; 95% CI 1.17–1.41), over a median follow-up period of 9.9 months and maximum follow-up 2.5 years (Table 2). After adjusting for baseline covariates, medication use, and co-morbidities, these associations were minimally attenuated for ACM (HR 1.16; 95% CI 1.00–1.34) and CVM + HFH (HR 1.17; 95% CI 1.04–1.31) (age-adjusted survival is presented in Figure 1). DM was associated with an increased risk for the secondary endpoints of HF hospitalization (HR 1.19; 95% CI 1.04–1.36) and clinically worsening HF (HR 1.17; 95% CI 1.04–1.32) (Table 2), even after multivariate adjustment. There was no association between DM and the combined endpoint of CVM, hospitalization for MI, arrhythmia, or other cardiovascular causes.
Table 2.
Primary and secondary outcomes by diabetes mellitus status
| Event rate for DM patients | Event rate for non-DM patients | Univariate HR (95% CI) | Multivariate HR (95% CI) | |
|---|---|---|---|---|
| Primary outcomes | ||||
| All-cause mortality | 478 (29%) | 614 (24.2%) | 1.18 (1.05–1.33) | 1.16 (1.00–1.34) |
| Cardiovascular mortality or HF hospitalization | 745 (46%) | 954 (37.7%) | 1.28 (1.17–1.41) | 1.17 (1.04–1.31) |
| Secondary outcomes | ||||
| Cardiovascular mortality | 349 (21.8%) | 480 (19.0%) | 1.15 (1.00–1.32) | 1.15 (0.97–1.35) |
| Heart failure death | 246 (9.9%) | 197 (11.9%) | – | – |
| Sudden cardiac death | 167 (6.8%) | 115 (6.9%) | – | – |
| Stroke death | 10 (0.4%) | 14 (0.8%) | – | – |
| Acute MI death | 16 (0.6%) | 12 (0.7%) | – | – |
| Other cardiovascular mortality | 30 (1.2%) | 24 (1.4%) | – | – |
| Cardiovascular mortality/morbidity | 846 (52.9%) | 1117 (44.1%) | 1.24 (1.14–1.36) | 1.14 (1.02–1.27) |
| Clinically worsening HF | 666 (44%) | 829 (32.7%) | 1.32 (1.19–1.46) | 1.17 (1.04–1.32) |
| HF hospitalization | 575 (36%) | 711 (28.1%) | 1.33 (1.19–1.49) | 1.19 (1.05–1.36) |
| MI hospitalization | 29 (1.8%) | 38 (1.5%) | 1.14 (0.69–1.85) | 0.89 (0.47–1.66) |
| Arrhythmia hospitalization | 72 (4.5%) | 120 (4.7%) | 0.91 (0.68–1.21) | 0.76 (0.54–1.06) |
| Stroke hospitalization | 33 (2.1%) | 36 (1.4%) | 1.42 (0.88–2.28) | 1.68 (0.96–2.95) |
Figure 1.
Cox proportional hazards model comparing rates of age-adjusted cardiovascular mortality or heart failure re-hospitalization in patients with and without diabetes.
Diabetes treatment effects
Diabetic patients were further subdivided into three groups by diabetic control strategy: (i) diet controlled; (ii) oral hypoglycaemic medications only; and (iii) insulin use, either as monotherapy or in conjunction with oral agents. The baseline characteristics of the three groups are shown in Table 3. Of the 1598 with diabetes, 319 patients (20%) were diet controlled, 572 (36%) were taking oral hypoglycaemics, and 766 patients (48%) required insulin. Diabetic patients treated with insulin were more likely to have CAD, hypertension, and CKD than patients treated with oral hypoglycaemics or diet alone (all P < 0.001). In addition, patients treated with insulin weighed more and had higher blood glucose on admission and discharge. There were no significant differences in EF, BNP, or New York Heart Association functional class between groups (data for functional class not shown). In general, the medication regimens were similar between groups, with the exception of beta-blocker, aspirin, and nitrate use, which was slightly higher in diabetic patients requiring insulin. There was no association between type of diabetic control strategy and ACM. There was an increased risk of CVM + HFH for patients treated with insulin, but this was attenuated after adjusting for baseline covariates (Table 4).
Table 3.
Baseline characteristics by diabetes mellitus status
| No diabetes (n = 2474) | Diabetes, no treatment (n = 319) | Diabetes, oral antidiabetic (n = 572) | Diabetes, insulin treated (n = 766) | _P_-value | |
|---|---|---|---|---|---|
| Demographic parameters | |||||
| Age, mean (SD) | 65.3 (12.8) | 66.9 (10.7) | 67.5 (10.6) | 65.2 (9.8) | <0.001 |
| Weight, mean (SD) | 80.0 (17.9) | 84.8 (19.5) | 86.7 (19.3) | 90.5 (18.8) | <0.001 |
| Physical and laboratory findings | |||||
| Systolic BP (mmHg), mean (SD) | 119.9 (19.3) | 120.9 (19.6) | 122.9 (20.4) | 122.9 (20.0) | <0.001 |
| Ejection fraction, %, mean (SD) | 27.4 (8.0) | 26.9 (8.3) | 27.9 (8.2) | 27.8 (8.2) | 0.227 |
| BUN (mg/dL) (25th, 75th) | 25 (19, 32) | 25 (19, 36) | 27 (20, 36) | 31 (22, 43) | <0.001 |
| Creatinine (mg/dL) (25th, 75th) | 1.2 (1, 1.5) | 1.2 (1, 1.6) | 1.2 (1, 1.6) | 1.3 (1.1, 1.8) | <0.001 |
| BNP (pg/mL) (25th, 75th) | 738.9 (291.5, 1559.5) | 711 (267.2, 1585) | 625.3 (292, 1341) | 668.8 (297.3, 1408.5) | 0.183 |
| Baseline serum glucose (random) (mg/dl) (25th, 75th) | 110 (95, 130) | 139 (113, 168) | 161 (122, 205) | 171 (124, 225) | <0.001 |
| Day 7 or discharge serum glucose (random) (25th, 75th) | 111.5 (95, 138) | 140 (113, 177.5) | 163 (122, 216) | 173 (124, 232) | <0.001 |
| Medical history, n (%) | |||||
| Previous HF hospitalization | 1890 (76.7) | 263 (83.5) | 461 (81.0) | 636 (83.3) | <0.001 |
| CAD | 1617 (65.4) | 231 (72.4) | 431 (75.4) | 632 (82.7) | <0.001 |
| Previous MI | 1133 (45.8) | 167 (52.4) | 310 (54.3) | 477 (62.3) | <0.001 |
| Hypertension | 1605 (64.9) | 244 (76.5) | 454 (79.4) | 629 (82.1) | <0.001 |
| Hypercholesterolaemia | 1011 (41.1) | 163 (51.3) | 322 (56.4) | 507 (66.5) | <0.001 |
| Chronic kidney disease | 508 (20.5) | 90 (28.2) | 176 (30.8) | 333 (43.5) | <0.001 |
| Medication use, n (%) | |||||
| Tolvaptan | 1217 (49.2) | 166 (52.0) | 290 (50.7) | 399 (52.1) | 0.460 |
| Diuretics | 2395 (96.8) | 304 (95.3) | 558 (97.6) | 745 (97.3) | 0.272 |
| ACE inhibitors/ARBs | 2098 (84.8) | 264 (82.8) | 482 (84.3) | 635 (82.9) | 0.542 |
| Beta-blockers | 1678 (67.8) | 221 (69.3) | 408 (71.3) | 596 (77.8) | <0.001 |
| Aldosterone-blocking agents | 1421 (57.4) | 169 (53.0) | 296 (51.8) | 351 (45.8) | <0.001 |
| Digoxin | 1121 (45.3) | 151 (47.3) | 242 (42.3) | 301 (39.3) | 0.013 |
| Nitrates | 856 (34.6) | 119 (37.3) | 204 (35.7) | 338 (44.1) | <0.001 |
| Hydralazine | 51 (2.1) | 11 (3.5) | 23 (4.0) | 36 (4.7) | 0.001 |
| Aspirin | 1287 (52.0) | 178 (55.8) | 319 (55.8) | 483 (63.1) | <0.001 |
Table 4.
Primary outcomes by diabetes mellitus treatment type
| Univariate HR (95% CI) | Multivariate HR (95% CI) | |
|---|---|---|
| All-cause mortality | ||
| Diet only | Reference | Reference |
| Oral antidiabetic medications | 0.85 (0.66–1.10) | 0.92 (0.70–1.24) |
| Insulin | 0.97 (0.76–1.23) | 1.09 (0.82–1.44) |
| CV mortality or HF hospitalization | ||
| Diet only | Reference | Reference |
| Oral antidiabetic medications | 1.00 (0.81–1.24) | 1.13 (0.90–1.43) |
| Insulin | 1.27 (1.04–1.55) | 1.25 (1.00–1.57) |
Discussion
The results of this analysis demonstrate that hospitalized HF patients with DM tend to have more co-morbidities and worse long-term outcomes after hospitalization, specifically increased rates of CVM or HFH after discharge, than those without DM. Although haemoglobin A1c% levels and details of duration of diabetes were not available, the current investigation of the impact of DM status in acute HF is strengthened by data collection in the setting of a large, international randomized trial of >4000 patients with high use of contemporary HF therapies and long-term follow-up, blindly adjudicated outcomes, and an improved ability to adjust for apparent confounders to estimate the hazard associated with DM more precisely. Even after adjusting for baseline risk factors and medications, DM was associated with a 17% increased risk for CVM or HFH over a median follow-up of 9.9 months. In addition, diabetic control strategy was not independently associated with outcomes; specifically, patients treated with insulin or oral hypoglycaemic agents had similar rates of ACM and CVM or HFH compared with diabetic patients controlled on diet alone.
Previous studies analysing the impact of DM on post-discharge outcomes in HF patients were either performed using registry data with partial follow-up or were small, randomized trials with limited use of contemporary HF therapies.4,6,7,10,11 Although 40% of hospitalized patients were diabetic in the OPTIMIZE-HF registry, there was no independent association between DM and the risk of death or HF readmission after hospitalization. The registry drew its conclusions on post-discharge outcomes from a pre-specified follow-up cohort representing ∼10% of the initial registry entrants at 60–90 days, a shorter period than our study which followed patients for a median of 9.9 months, potentially explaining the discrepancy in findings. In contrast, the EVEREST database provides a large randomized sample of hospitalized HF patients with complete follow-up of all enrolled patients over an average of 9.9 months. The independent association of DM and adverse outcomes in systolic HF in our analysis may point to a role for aggressive treatment of co-morbid conditions as an opportunity to reduce post-discharge event rates.
Similar to trends in the general population, the prevalence of DM in those with HF is increasing. Up to 24% of patients with HF carry a concomitant diagnosis of diabetes, underscoring the importance of managing co-morbid conditions in HF patients.12 Diabetes is known to cause alterations in cardiac function, affecting intracellular calcium releaseand myocardial lipid metabolism, and impairing endothelial cell function.13 In addition, patients with DM are more likely to have hypertension and kidney disease.14 Studies of ambulatory diabetic patients with chronic systolic dysfunction found a significantly higher likelihood of all-cause mortality, cardiovascular death, and HF re-hospitalization compared with non-diabetics, with HRs ranging between 1.60 and 1.79 over 3–5 years of follow-up.2,15–17 When comparedwith patients with chronic systolic dysfunction, the interaction between DM and outcomes in patients who are hospitalized with HF appears to be less robust. This may be partially explained by a temporal impact on disease progression. In our study, the association between DM and CVM + HFH increased with time, consistent with the trends seen in outpatients over longer follow-up periods. Accrual of advanced glycation end-products or persistent insulin resistance could be speculative causal mechanisms leading to progressive haemodynamic dysfunction and increased adverse cardiovascular events over time.
The attenuated association between DM and post-discharge outcomes may also suggest that co-morbidities have less impact on all-cause mortality in the peri-hospitalization period. Instead prognosis during this period may be largely determined by the severity of HF disease burden. Patients admitted with acute HF syndromes are at higher risk for death and represent a sicker subset of HF patients, with rates of mortality of 30% after 1 year and increasing 20% with each subsequent re-hospitalization.18,19 The effect of co-morbidites in this setting may be less meaningful than in ambulatory patients with chronic HF with regards to ACM, but probably still plays a role in HF re-hospitalization and CVM. The high mortality associated with re-hospitalizations underscores the importance of further studies identifying modifiable factors that may reduce readmission rates. Our findings show that while DM was significantly associated with increased likelihood of a combined outcome of CVM or HFH, the increased rates of clinically worsening HF and HF hospitalization were key contributors to the primary outcome (Table 2). DM patients had a 20% increased rate of HF hospitalization compared with non-DM patients, highlighting the need to identify post-discharge strategies tailored specifically to DM patients with systolic dysfunction to reduce re-hospitalization rates.
Predictors of post-discharge outcome have gained attention due to the need to reduce high readmission rates and post-discharge CV mortality. Patients with a recent HF hospitalization represent a vulnerable population, and strategies to reduce post-discharge outcomes and re-hospitalization have focused on a wide range of initiatives, from monitoring volume status to dietary and medication compliance. Identification of clinical predictors of poor post-discharge outcomes may help further risk-stratify certain subgroups of patients and lead to better targeted therapies. From our study it is not readily apparent if improving standard medical therapy for HF alone will be successful, as a large majority of patients were already taking beta-blockers and ACE inhibitors. Interestingly, DM patients were less likely to be on aldosterone-blocking agents (49.3% vs. 57.4%). Increased utilization of mineralocorticoid antagonists in a diabetic population may improve microvascular dysfunction in addition to aiding blood pressure control, two known contributors to HF progression.20
Multiple co-morbidities may overlap in some patients, and in our analysis diabetic patients were more likely to carry concomitant diagnoses of common cardiac risk factors including CAD, hypertension, and CKD. While aggressive treatment of traditional cardiac risk factors, including hypertension and hyperlipidaemia, in these patients may improve post-discharge outcomes, there is considerable uncertainty in the optimal diabetic management in patients who are hospitalized with HF. A number of commonly used categories of oral antidiabetic drugs have come under scrutiny for potentially harmful cardiac effects.21,22 In the presence of HF, peroxisome proliferator-activated receptor (PPAR) agonists such as rosiglitazone and pioglitazone are contraindicated for use in patients with moderate to severe symptoms.23,24 Metformin, a commonly available biguanide, had a relative contraindication in patients with HF when the drug was first introduced out of concern for lactic acidosis, but recent studies showing a mortality benefit led to revision of the package label.25,26 The effects of sulfonylureas appear to be neutral.27
There are few studies examining the effect of insulin use on outcomes in HF. In a single-centre study of 554 consecutive patients evaluated with advanced HF, of which 132 were diabetic, insulin was associated with a four-fold increase in mortality when compared with a reference group of non-diabetic patients (HR 4.30; 95% CI 1.69–10.94) in contrast to diabetic patients not treated with insulin (HR 0.95; 95% CI 0.31–2.93).28 Similarly, an analysis of the SAVE (Survival and Ventricular Enlargement) trial, a double-blind placebo-controlled randomized trial of captopril in patients post-MI with EF <40%, found that 168 of 496 patients with diabetes taking insulin had a higher risk of death compared with patients on non-insulin regimens over a median 3.5-year follow-up (HR 1.66; 95% CI 1.20–2.31).29 However, a retrospective study of >16 000 diabetic Medicare patients discharged with a primary diagnosis of HF did not demonstrate an association between insulin use and increased mortality at 1 year.30 In our analysis, patients requiring insulin were not at higher risk of either ACM or CVM + HFH comparedwith patients not taking insulin after adjusting for baseline risk factors. Diabetic patients controlled on oral hypoglycaemic agents similarly did not have an increased incidence of adverse cardiac events, suggesting that commonly used antidiabetic strategies, whether insulin based or non-insulin based, are not associated with worse post-discharge outcomes after acute HF hospitalization.
The results of these analyses should be interpreted in the context of several limitations. First, the definition of DM was based on initial intake questionnaires, and cross-verified by medication history. The ability to distinguish between type 1 and type 2 DM was not possible. Haemoglobin A1c% and fasting blood sugars were also not recorded, limiting insight into glucose control, which may affect the analysis of the effects of insulin therapy on outcomes. Secondly, the duration of diabetes prior to study enrolment was not known, which might reflect the severity of diabetes and associated co-morbid conditions, a potential confounder in risk adjustment for patients taking insulin. Thirdly, the EVEREST study was a randomized trial with specific inclusion and exclusion criteria that may limit generalization to broader hospitalized patient populations. For instance, EVEREST was comprised predominantly of men. EVEREST also did not include patients with HF with preserved EF, and it is well established that DM is a risk factor for development of HF with preserved EF. Fourthly, while the adjusted analysis incorporated covariates that have previously been shown to be clinically relevant, it is possible that additional measured and unmeasured variables may have confounded these results.
Despite these limitations, we believe our findings are strengthened by the fact that data were collected prospectively by clinicians in the controlled setting of a randomized trial and provide valuable insight into the role of diabetes in post-discharge outcome in patients with HF. Due to the high prevalence of DM in HF patients, particularly those who are hospitalized for HR, a better understanding of the clinical profiles of diabetic patients should lead to more aggressive management of traditional cardiac risk factors in addition to a renewed focus on identifying optimal antidiabetic regimens.
In conclusion, diabetes is common in patients hospitalized for heart failure with a reduced EF. These patients have a higher post-discharge CVM and higher HF hospitalizations compared with patients with no diabetes. Different diabetic treatment regimens did not appear to influence post-discharge outcomes.
Funding
This work was supported by Otsuka Inc. (Rockville, MD, USA) which provided financial and material support for the EVEREST trial. The Center for Cardiovascular Innovation (Northwestern University Feinberg School of Medicine, Chicago, IL, USA) provided funding and support for statistical analysis.
Conflict of interest: F.Z., M.K., A.P.M., and K.S., have received funding from Otsuka. M.G. has received funding from Abbott Laboratories, Astellas, AstraZeneca, Bayer Schering Pharma AG, Cardiorentis Ltd, CorThera, Cytokinetics, CytoPherx Inc., DebioPharm S.A., Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Intersection Medical, INC, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Ono Parmaceuticals USA, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Sticares InterACT, Takeda Pharmaceuticals North America, Inc and Trevena Therapeutics; and has received signficant (>$10 000) support from Bayer Schering Pharma AG, DebioPharm S.A., Medtronic, Novartis Pharma AG, Otsuka Pharmaceuticals, Sigma Tau, Solvay Pharmaceuticals, Sticares InterACT and Takeda Pharmaceuticals North America, Inc. All other authors have no conflicts to declare.
References
- 1.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 3.De Groote P, Lamblin N, Mouquet F, Plichon D, McFadden E, Van Belle E, Bauters C. Impact of diabetes mellitus on long-term survival in patients with congestive heart failure. Eur Heart J. 2004;25:656–662. doi: 10.1016/j.ehj.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg BH, Abraham WT, Albert NM, Chiswell K, Clare R, Stough WG, Gheorghiade M, O'Connor CM, Sun JL, Yancy CW, Young JB, Fonarow GC. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2007;54(277):e1–e8. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 6.Harjola VP, Follath F, Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Hochadel M, Komajda M, Lopez-Sendon JL, Ponikowski P, Tavazzi L. Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail. 2010;12:239–248. doi: 10.1093/eurjhf/hfq002. [DOI] [PubMed] [Google Scholar]
- 7.Parissis JT, Rafouli-Stergiou P, Mebazaa A, Ikonomidis I, Bistola V, Nikolaou M, Meas T, Delgado J, Vilas-Boas F, Paraskevaidis I, Anastasiou-Nana M, Follath F. Acute heart failure in patients with diabetes mellitus: clinical characteristics and predictors of in-hospital mortality. Int J Cardiol. 2012;157:108–113. doi: 10.1016/j.ijcard.2011.11.098. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Orlandi C, Burnett JC, Demets D, Grinfeld L, Maggioni A, Swedberg K, Udelson JE, Zannad F, Zimmer C, Konstam MA. Rationale and design of the multicenter, randomized, double-blind, placebo-controlled study to evaluate the Efficacy of Vasopressin antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) J Card Fail. 2005;1:260–269. doi: 10.1016/j.cardfail.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Wang NC, Maggioni AP, Konstam MA, Zannad F, Krasa HB, Burnett JC, Jr, Grinfeld L, Swedberg K, Udelson JE, Cook T, Traver B, Zimmer C, Orlandi C, Gheorghiade M. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299:2656–2666. doi: 10.1001/jama.299.22.2656. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson I, Brendorp B, Seibaek M, Burchardt H, Hildebrandt P, Kober L, Torp-Pedersen C. Influence of diabetes and diabetes–gender interaction on the risk of death in patients hospitalized with congestive heart failure. J Am Coll Cardiol. 2004;43:771–777. doi: 10.1016/j.jacc.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Burger AJ, Tsao L, Aronson D. Prognostic impact of diabetes mellitus in patients with acute decompensated heart failure. Am J Cardiol. 2005;95:1117–1119. doi: 10.1016/j.amjcard.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 12.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;29:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 13.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 14.Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update. Hypertension. 1995;26:869–879. doi: 10.1161/01.hyp.26.6.869. [DOI] [PubMed] [Google Scholar]
- 15.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodeheffer RJ, Roger VL. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 17.Domanski M, Krause-Steinrauf H, Deedwania P, Follmann D, Ghali JK, Gilbert E, Haffner S, Katz R, Lindenfeld J, Lowes BD, Martin W, McGrew F, Bristow MR. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J Am Coll Cardiol. 2003;42:914–922. doi: 10.1016/s0735-1097(03)00856-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012. 125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005;28:2106–2112. doi: 10.2337/diacare.28.9.2106. [DOI] [PubMed] [Google Scholar]
- 21.Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, Hansen ML, Fosbøl EL, Køber L, Norgaard ML, Madsen M, Hansen PR, Torp-Pedersen C. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32:1900–1908. doi: 10.1093/eurheartj/ehr077. [DOI] [PubMed] [Google Scholar]
- 22.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 23.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, LeWinter M, Porte D, MD, Semenkovich CF, Smith SC, Jr, Young L, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 24.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 25.Inzucchi SE, Masoudi FA, McGuire DK. Metformin in heart failure. Diabetes Care. 2007;30:e129. doi: 10.2337/dc07-1686. [DOI] [PubMed] [Google Scholar]
- 26.Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Changes in labelling for metformin use in patients with type 2 diabetes and heart failure: documented safety outweighs theoretical risks. Open Med. 2011;5:e33–e34. [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson C, Gislason GH, Jorgensen CH, Hansen PR, Vaag A, Sorensen R, Mérie C, Olesen JB, Weeke P, Schmiegelow M, Norgaard ML, Køber L, Torp-Pedersen C. Comparable long-term mortality risk associated with individual sulfonylureas in diabetes patients with heart failure. Diabetes Res Clin Pract. 2011;94:119–125. doi: 10.1016/j.diabres.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J. 2005;149:168–174. doi: 10.1016/j.ahj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Murcia AM, Hennekens CH, Lamas GA, Jimenez-Navarro M, Rouleau JL, Flaker GC, Goldman S, Skali H, Braunwald E, Pfeffer MA. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med. 2004;164:2273–2279. doi: 10.1001/archinte.164.20.2273. [DOI] [PubMed] [Google Scholar]
- 30.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–590. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]