DTI Tractography and White Matter Fiber Tract Characteristics in Euthymic Bipolar I Patients and Healthy Control Subjects (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 1.
Published in final edited form as: Brain Imaging Behav. 2013 Jun;7(2):129–139. doi: 10.1007/s11682-012-9202-3
Abstract
With the introduction of diffusion tensor imaging (DTI), structural differences in white matter (WM) architecture between psychiatric populations and healthy controls can be systematically observed and measured. In particular, DTI-tractography can be used to assess WM characteristics over the entire extent of WM tracts and aggregated fiber bundles. Using 64-direction DTI scanning in 27 participants with bipolar disorder (BD) and 26 age-and-gender-matched healthy control subjects, we compared relative length, density, and fractional anisotrophy (FA) of WM tracts involved in emotion regulation or theorized to be important neural components in BD neuropathology. We interactively isolated 22 known white matter tracts using region-of-interest placement (TrackVis software program) and then computed relative tract length, density, and integrity. BD subjects demonstrated significantly shorter WM tracts in the genu, body and splenium of the corpus callosum compared to healthy controls. Additionally, bipolar subjects exhibited reduced fiber density in the genu and body of the corpus callosum, and in the inferior longitudinal fasciculus bilaterally. In the left uncinate fasciculus, however, BD subjects exhibited significantly greater fiber density than healthy controls. There were no significant differences between groups in WM tract FA for those tracts that began and ended in the brain. The significance of differences in tract length and fiber density in BD is discussed.
Introduction
Bipolar I disorder (BD) is a serious psychiatric condition in which a patient’s mood vacillates between periodic extremes of joy or depression (Goodwin and Jamison 2007). These mood swings between mania and depression can cycle quickly and intervals of wellness between mood episodes decrease as the illness progresses (http://www.nimh.nih.gov/health/publications/bipolar-disorder/complete-index.shtml).
Despite much research into BD, the underlying neural pathophysiology of BD remains unclear and reliable biomarkers are few. Evaluating WM structure appears to be a promising area of investigation for understanding the potential for altered connectivity between brain regions believed to contribute to BD symptomatology. White matter alterations may be responsible for some of the functional activation deficits found in patients with BD. Several hypotheses regarding white matter (WM) abnormalities in BD have been suggested. A neurodevelopmental etiology has been proposed in which abnormalities of cell migration, chemotechtonic guidance, and other forms of cell-cell interaction could inhibit typical WM formation, hypothetically leading to the development of the disorder (Tkachev, Mimmack et al. 2003; Beyer, Taylor et al. 2005; Regenold, Phatak et al. 2007; Kafantaris, Kingsley et al. 2009; Mahon, Burdick et al. 2010a; Benedetti, Absinta et al. 2011). Recent postmortem studies have also suggested that WM pathology may be etiologically related to bipolar disorder since BD is a heritable disorder and genetically mediated structural differences could play a major role in its genesis (Chaddock, Barker et al. 2009). Researchers have recently begun to suspect that WM may be more dependent on genetic factors than grey matter (GM) (Mahon, Burdick et al. 2010b). Abnormalities in gene expression present in WM (Tkachev, Mimmack et al. 2003) have been reported. Conversely, some investigators have posited that factors related to the disorder could themselves cause alterations in WM (Haznedar, Roversi et al. 2005). For instance, glucocorticoids produced by stress or episodes of the illness could result in not only WM changes but also neuronal loss or shrinkage, affecting the amygdala, hippocampus, and anterior cingulate cortex (Altshuler 1993); (Delaloye, de Bilbao et al. 2009). These gray matter changes could, therefore, directly impact the fidelity of WM architecture.
Given what is known about how emotion is regulated, most models of disrupted networks in BD focus on a deficient modulation of subcortical and limbic structures (Townsend and Altshuler 2012). A breakdown in the architecture of normal WM tracts which connect brain regions involved in emotion regulation (Benedetti, Absinta et al. 2011) has been reported. Studies that have evaluated underlying WM structural deficits have done so by assessing fractional anisotropy (FA). Some investigations have reported significant differences in FA between patients and control subjects in the uncinate fasciculus (UNC) (Houenou, Wessa et al. 2007; Lin, Weng et al. 2011), genu of the corpus callosum (GCC) (Wang, Kalmar et al. 2008; Benedetti, Yeh et al. 2011), rostral corpus callosum (Wang, Kalmar et al. 2008), body of the corpus callosum (BCC) (Wang, Kalmar et al. 2008), the right cingulum (Benedetti, Yeh et al. 2011), and in tracts connecting to the amygdala and to the hippocampus (Houenou, Wessa et al. 2007; Phillips, Ladouceur et al. 2008; Mahon, Burdick et al. 2010b). However, the literature is inconsistent with regard to the direction of the changes, with some studies reporting lower average FA in BD vs. control subjects [e.g. (Pavuluri, Yang et al. 2009)], and others reporting the opposite [e.g. (Benedetti, Yeh et al. 2011)]. Such variation of reported effects across studies could be partly explained by the heterogeneity of the BD sample being studied (e.g. combining subjects with BDI and BDII or combining subjects in different mood states), (Brooks, Bonner et al. 2009), or differences in data acquisition methods or processing approaches (Jones and Cercignani 2010). Given these discrepancies in WM findings between BD and healthy subjects, further investigation using DTI is warranted.
Potential WM neuroimaging biomarkers include FA, relative tract length and tract density (Adler, Holland et al. 2004; Pavuluri, Yang et al. 2009). Disagreement exists about the best way to analyze and compare these metrics between samples (Versace, Almeida et al. 2008; Jones and Leemans 2011; Lin, Weng et al. 2011). Researchers have applied voxel-based morphometry (VBM) (Chaddock, Barker et al. 2009), tract-based spatial statistics (TBSS) (Versace, Almeida et al. 2008), or tractography, though it remains unclear which particular approach returns the most valid or reliable results. The sensitivity of VBM to spatial alignment differences between subjects may render it difficult to isolate the same white matter within each voxel across a subject sample.
Conversely, TBSS requires non-linear registration of the images which can be problematic in cases of brain pathology or disease (Lin, Weng et al. 2011). Therefore, these approaches may be of limited direct clinical utility. DTI tractography, used in conjunction with a set of anatomically defined brain regions obtained from T1 structural brain volumes, can be used to identify, cluster, and characterize fiber pathways in a region-to-region manner. Using this more specific approach, rather than making coordinate-based measurements about WM irregularities between groups, the architecture of specific WM fasciculi and connections can be compared directly between subject groups.
In the current study we sought to 1) characterize tract-specific differences in euthymic BD patients relative to age and gender matched healthy controls and, 2) to further our understanding of the type of WM abnormalities in specific tracts that may be of direct importance in this disorder. We manually identified fiber tracts in 3D space from DTI images, and measured relative WM fiber tract density and length in various fasciculi as well as FA values within the tracts as a whole.
Methods
Patients
The study protocol was approved by the Institutional Review Board at the University of California, Los Angeles (UCLA); each participant gave written consent before participating in the study. Twenty-seven participants (14 males, 13 females, age 44.2 ± 12.9 (mean ± standard deviation (SD)) with a DSM-IV diagnosis of bipolar I disorder (BD), who were euthymic at the time of scanning, were recruited through the UCLA Mood Disorders Outpatient Clinic, local advertising, or from other research projects of the UCLA Mood Disorders Research Program. Twenty-six control participants (12 males, 14 females, age 41.5 ± 12.1 (mean ± SD)) were recruited by advertisements in local newspapers and campus flyers and matched for age and gender to the patients. Both groups had similar average education (14.5 ± 2 years for patients with BD and 15.5 ± 2.3 years for controls). All participants were interviewed using the Structured Clinical Interview (SCID) for DSM-IV (First 1995) to confirm a bipolar diagnosis or absence thereof. Participants with BDI were excluded if they met criteria for any other current Axis I disorder. Control participants were excluded if they had a current or past psychiatric diagnosis (including substance abuse) or were taking medications for any medical reasons. Additional exclusion criteria for all participants included left-handedness, hypertension, neurological illness, metal implants, and a history of head trauma with loss of consciousness for more than 5 minutes.
Mood symptoms were evaluated in all participants on the day of the scan using the Young Mania Rating Scale (YMRS) (Young RC 1978) and the 21-item Hamilton Depression Rating Scale (HDRS) (Hamilton 1959). Patients currently experiencing manic or depressive symptoms were excluded to control for the influence of acute mood state on WM integrity. Bipolar participants were eligible if they had a YMRS score lower than or equal to 7, a 21-item HDRS score lower than or equal to 7, and had been euthymic by self-report and by SCID criteria for at least one month prior to scanning. Patients taking lithium were specifically excluded due to the known effects of lithium treatment on grey matter and limbic regions (Germana, Kempton et al. 2010).
Data acquisition
MRI and DTI volumes were acquired at the Ahmanson-Lovelace Brain Mapping Center at the UCLA David Geffen School of Medicine, using a Siemens Magnetom Trio TIM 3 Tesla MRI scanner. The DTI scan acquired 64 non-colinear directions as well as B0 and B1000 image volumes having a TR of 8,400 ms and a TE of 93ms. Slices were 2 mm thick with a FOV of 190mm giving a voxel size of 2 × 2 × 2 mm.
Image processing
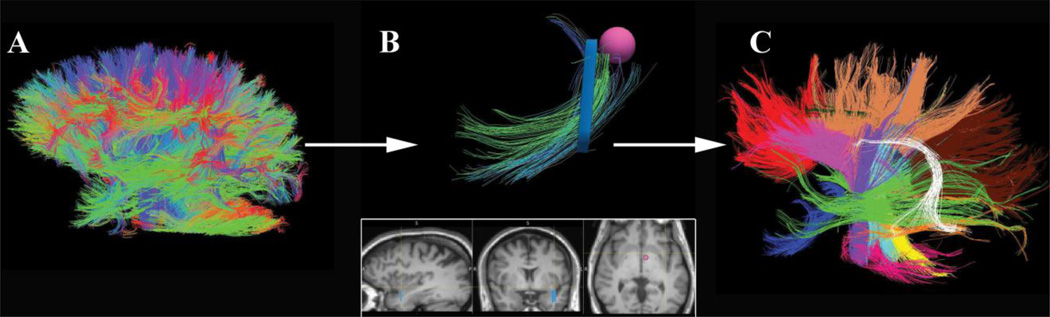
All images underwent eddy current distortion correction, followed by FSL FLIRT for motion correction. Images were reconstructed using the Diffusion Toolkit to create 3D images of the WM fiber tracts in the TrackVis program (Ruopeng Wang, Van J. Wedeen, TrackVis.org, Martinos Center for Biomedical Imaging, Massachusetts General Hospital) (Wedeen, Wang et al. 2008). The TrackVis program uses the FACT approach to reconstruct fiber paths. Tracking is initiated from the center of each voxel, proceeding to follow the direction of water diffusivity to the next appropriate voxel, where directionality is reassessed. Tract end points occur when severe angular orientation changes occur suddenly or, typically, when the underlying value of FA falls below a preset threshold (see also, Mori, Crain et al. 1999). We used an angle threshold of 35 degrees to determine when fiber orientation had changed so greatly as to no longer be part of the same fiber pathway. In the TrackVis program, unlike in other tractography visualization software programs (e.g. DTIStudio, MEDINRIA, etc), no FA threshold is employed. Rather, an image mask is used based on the B0 image. As is customary for the display of WM fiber tract orientation, however, fibers oriented anterior-to-posterior are colored green, superior-to-inferior as blue, and left-to-right as red. Fibers that have directions that are linear combinations of those directionalities are colored with a corresponding combination of colors according to the degree to which they are oriented in each of the standard directions (Figure 1A).
Figure 1.
A) DTI tractography entails propagation of fibers along the path of least diffusivity from each voxel, creating a 3D map of all white matter connections. B) Programs like TrackVis enable an investigator to select voxels and view all fiber orientations that pass through the ROI. Coloration of fibers then allows the investigator to create ROIs (such as the pink sphere) which can turn off all voxels that pass through the original ROI but do not belong to the tract of interest. C) Each tract in this study was then assigned a different color. This panel depicts all of the tracts analyzed.
Regions of Interest (ROIs)
ROIs were manually created by a rater (CMT; trained by JVH) blind to diagnosis using the ICBM DTI-81 Atlas (http://www.loni.ucla.edu/Atlases/Atlas_Methods.jsp?atlas_id=15). The rater first highlighted voxels individually to view each appropriate tract, and then eliminated voxels whose associated fibers were inconsistent with the color of the rest of the tract. Additionally, fibers were excluded from analysis using conditional WM ROIs to eliminate spurious individual fibers inconsistent with the connectivity of the tract (Figure 1B). Next, a second blinded rater (AI) cross-checked the processed image to ensure that the ROIs captured the entirety of the tract and were properly labeled.
In total, 11 tracts in each hemisphere were specifically chosen. That is, bilaterally, 22 tracts per subject were manually delineated and measured (Figure 1C). The study focused on tracts previously reported as abnormal in bipolar disorder, and on tracts connecting brain regions known to be involved in emotion regulation (Houenou, Wessa et al. 2007; Wang, Kalmar et al. 2008; Mahon, Burdick et al. 2010b; Benedetti, Yeh et al. 2011; Lin, Weng et al. 2011). Tracts evaluated to test the hypothesis of differences between patients and healthy controls included the left and right arcuate fasciculi (AF), left and right supra-genual cingulate bundles (CGC), left and right uncinate fasciculi (UNC), left and right inferior longitudinal fasciculi (ILF), left and right optical radiations (OR), left and right tracts from the orbito-frontal cortex to the amygdala, the genu of the corpus callosum (GCC), the body of the corpus callosum (BCC), and the splenium of the corpus callosum (SCC). Additionally, as the inclusion of any constrained set of tracts could preclude examination of the possibility that WM abnormalities in BD may be general and non-specific, several additional tracts were delineated where no differences were expected. These included the cerebral peduncles, and left and right sections of three major corticospinal tracts (CST-L1, L2, L3, R1, R2, and R3). Abbreviations were taken from the closest matching structure in the ICBM DTI-81 Atlas.
White Matter Tract-Based Metrics
Three measurements were obtained using the TrackVis software package for each WM tract: relative tract density, length, and mean FA integrated over the tract. In what follows, we describe each of these metrics:
Relative Fiber Tract Density - Relative fiber tract (bundle) density refers to the number of DTI fibers comprising a tract (the number of distinct tracts that pass through the manually constructed sets of ROIs) greater than the minimally acceptable threshold length of 10mm as a ratio of the total number of all extracted fiber tracts of that length or longer in the whole brain.
Relative Fiber Tract Length - Tract length refers to the distance in millimeters between two end points where the FACT tracking can no longer follow the direction of diffusivity to neighboring voxels within the angle threshold set by the image reconstruction. Tract lengths were normalized with respect to the head circumference of each subject, as measured using the 3D Slicer software package (http://www.slicer.org) developed under the National Alliance for Medical Image Computing (NA-MIC; http://www.na-mic.org).
Mean Fractional Anisotropy (FA) – Mean FA refers to the arithmetic average of FA taken over all voxels comprising each fiber, then averaged over all fibers comprising the entire tract. As Mean FA is already a dimensionless value, it was not standardized within subjects.
Statistical Analyses
Linear discriminant analyses (LDA; http://www.mathworks.com/help/toolbox/stats/classify.html) were performed in order to determine the extent to which mean tract length, mean tract density, and mean FA within each fiber bundle in each of the 22 tracts contributed to the difference between BD and control populations. The LDA classification classes were the two populations involved (BD and healthy control), and the discriminant variables were the FA, tract length and tract density in each of the 22 fiber tracts. An LDA was performed for each of the three measures (mean tract length, mean tract density, mean FA). The Fisher discriminant function of the LDA was computed and used to ascertain the degree to which each variable contributed to classification, with larger absolute values indicating greater importance for categorization. The pooled covariance matrix was used to generate standardized discriminant coefficients and to determine the relative effect of each discriminant variable upon the discriminant function. A posteriori class assignment was found to be accurate for all observations (53 subjects). The explanatory variables with absolute values in the top 10% of the histogram of standardized discriminant coefficients were then selected, and two-tailed Welch t-tests (to account for potential inequalities in variance between groups) were performed for these latent variables to identify which of them exhibited significant differences in means between the two samples. By identifying maximally discriminant latent variables via LDA, the typical problem of multiple comparisons that can arise by performing all possible, fiber tract-based, pair-wise t-tests was greatly minimized because only a proportionally much smaller number of t-tests were performed.
Results
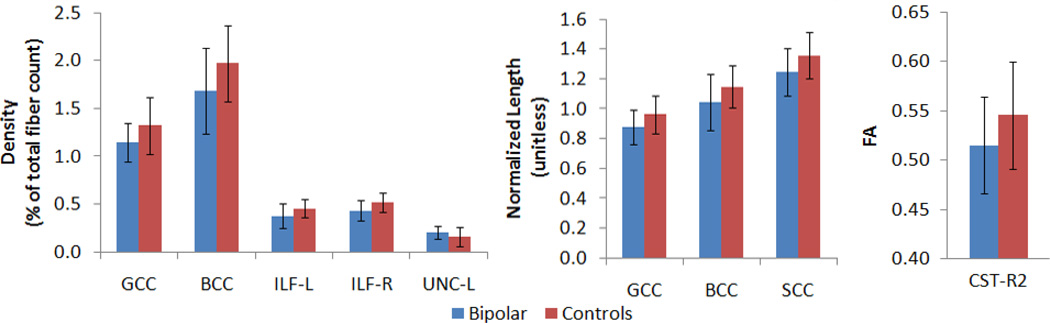
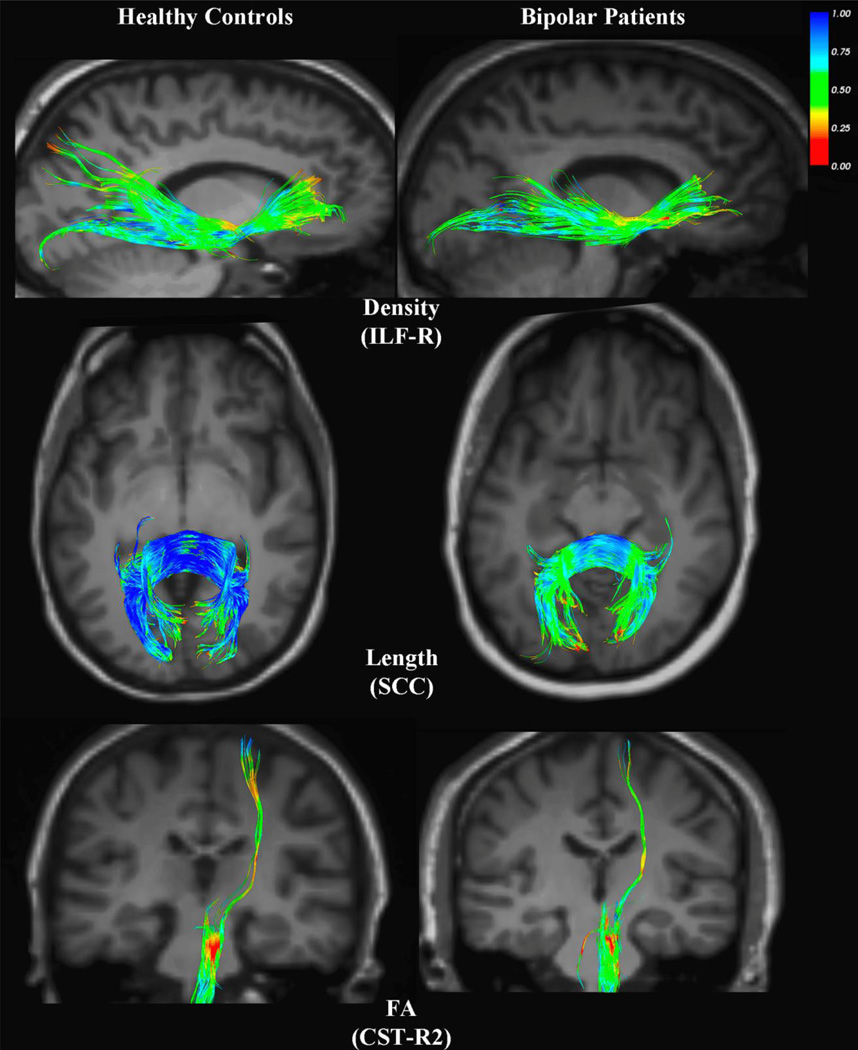
Table 1 displays demographic data for the participants as well as course of illness variables for the bipolar group. There were no significant differences between groups in age (BD = 44.3 ± 12.9, controls = 41.2 ± 12.3; p=0.38), gender (BD = 48% female, controls = 54% female), or years of education (BD = 14.5 ± 2, controls = 15.5 ± 2.3; p=0.11). Table 2 displays the results of the statistical analysis for tract length of all fiber tracts that originated and ended in the brain, as well as for fiber density and FA in all tracts. LDA’s revealed several discriminant variables between groups. At a significance threshold of α ≤ 0.05, _t_-tests revealed significant between-group differences. In BD subjects, the normalized length of tracts in the genu (GCC) (mean=0.877, SD= 0.119), the body (BCC) (mean=1.043, SD=0.185), and the splenium (SCC) (mean=1.246, SD=0.159) of the corpus callosum, were significantly shorter than the corresponding tracts in control subjects (GCC mean=0.962, SD=0.125; BCC mean=1.148, SD= 0.141; SCC mean=1.358, SD=0.157). Furthermore, bipolar subjects exhibited significantly reduced fiber density in the GCC (mean=0.011, SD=0.200), the BCC (mean=0.017, SD=0.449), the ILF-L (mean=0.004, SD=0.130), and the ILF-R (mean=0.004, SD=0.110) compared to control subjects (GCC mean=0.013, SD=0.300, BCC mean=0.020, SD=0.400, ILF-L mean=0.005, SD=0.100, ILF-R mean=0.005, SD= 0.100). The UNC-L of bipolar subjects, however, contained significantly higher fiber density (mean=0.0020, SD=0.066) than the corresponding tract in control subjects (mean=0.0016, SD=0.100). FA values differed significantly in one of the corticospinal tracts, CST-R2, with BD patients exhibiting lower FA (mean=0.515, SD=0.054) than controls (mean=0.546, SD=0.049). Figure 2 depicts graphs of all of the tracts where significant differences between groups were seen in any of the three measures (fiber length, density and FA). Figure 3 shows examples of BD vs. healthy control subjects on some of these measures.
Table 1.
Demographic and Clinical Characteristics of BD Patients and Healthy Controls
| Bipolarn = 27 | HealthyControlsn=26 | GroupDifferenceProbability | |
|---|---|---|---|
| Age (SD) | 44.3 (12.9) | 41.2 (12.3) | 0.38 |
| Number of Females (%) | 13 (48%) | 14 (54%) | 0.78 |
| Years of Education (SD) | 14.5 (2) | 15.5 (2.3)* | 0.11 |
| Illness Duration (SD) | 23.2 (14.5) | - | |
| History of Psychosis (%) | 17 (63%) | ||
| History of Drug Use (%) | 7 (26%) | ||
| History Alcohol Abuse (%) | 13 (48%) | ||
| Years Sober (SD) | 7.7 (5.6) | ||
| HAM-D (SD) | 4.3 (4.0) | - | |
| YMRS (SD) | 2.1 (1.9) | - | |
| Number treated withmedication: | |||
| Antipsychotics (other): | 16 | ||
| Anticonvulsants: | |||
| Divalproex | 8 | ||
| Lamotrigine | 4 | ||
| Carbamazepine | 1 | ||
| Other | 2 | ||
| Antidepressants: | |||
| SSRIs | 7 | ||
| Venlafaxine | |||
| Bupropion | 7 | ||
| Benzodiazepines | 5 |
Table 2.
Significantly different fiber tracts between BD subjects and controls.
| Structure | Measure | Student’s t-score | p |
|---|---|---|---|
| CC-genu | Length | −2.4718 | 0.0169 |
| CC-body | Length | −2.3686 | 0.0221 |
| CC-splenium | Length | −2.5332 | 0.0145 |
| CC-genu | Fiber Density | −2.6062 | 0.0122 |
| CC-body | Fiber Density | −2.2217 | 0.0309 |
| Inf. Long. Fasci.-L | Fiber Density | −2.5608 | 0.0142 |
| Inf. Long. Fasci.-R | Fiber Density | −2.7503 | 0.0083 |
| Uncinate Fasci.-L | Fiber Density | 2.4934 | 0.0162 |
| CST-R2 | FA | −2.2764 | 0.0272 |
Figure 2.
The first graph depicts the significant differences in fiber density. The values were obtained by dividing the number of fibers in the tract of interest by the total number of WM fibers in the subject’s DTI. BD subjects showed lower densities in all significant regions except the UF, in which we found significantly higher fiber density than in healthy controls. The second graph shows all of the significant differences in tract length (after normalizing lengths to head circumference of the subjects, yielding unitless results). BD subjects had shorter tracts in all significant regions. The third graph displays the significant FA differences
Figure 3.
All fiber tracts are colored according to the value of their FA at each point; fibers with an FA close to 0 are shown as red, while increasing values shift toward blue, as elucidated by the colorbar in the upper right. Each row shows a comparison of the control and BD subjects in whom the tract of interest was closest to the median sample value for the group. The first row illustrates the greater fiber density in the ILF-R of controls. The second row depicts the greater length of splenium fibers in control subjects. Finally, the third row demonstrates the higher median FA in the CST-R2 of controls.
Discussion
Our study adds to the few tractography studies that have evaluated WM fiber tract architecture in the bipolar population using whole brain tractography (Pavuluri, Yang et al. 2009; Benedetti, Yeh et al. 2011; Lin, Weng et al. 2011). To our knowledge, our study is among the first to simultaneously evaluate a family of white matter fiber composition measures in BD using DTI and a whole-brain tractography approach with manually defined ROI selection. Our findings demonstrate significant changes in the mean length of WM tracts and in the number of fibers within tracts in subjects with BD compared to controls. Specifically, BD subjects demonstrated 1) significantly reduced tract length in the corpus callosum (genu, body, and splenium); 2) significantly reduced fiber density in the corpus callosum (genu and body) and in the inferior longitudinal fasciculi bilaterally; and 3) significantly increased fiber density in the left uncinate fasciculus. No differences in fiber FA between BD vs. healthy subjects were observed except for reduced FA in one of the corticospinal tracts (CST-R2).
Our observation of reduced tract length and a reduced fiber density in the corpus callosum of BD subjects compared to controls agrees with previous reports of reduced corpus callosum size in several studies (Brambilla, Nicoletti et al. 2003; Atmaca, Ozdemir et al. 2007). In fact, anterior corpus callosum lesions have been reported to cause symptoms similar to BD (Wang, Kalmar et al. 2008) and relative callosal tract length has been shown to be correlated with BD symptoms in adults, but not in children (Barnea-Goraly, Chang et al. 2009). This suggests that callosum axonal myelination during maturation may be altered in BD (Brambilla, Nicoletti et al. 2003) or, alternatively, that myelin loss during normal aging could be accelerated by the disorder. Hong et al., (2011) interpret tract length differences in BD as “an effort to enhance interhemispheric connectivity so that a better functional integration and development could be achieved”. Therefore, it remains likely that relative tract length is a crucial metric to include in analyses of tract-based connectivity in BD, especially in longitudinal examinations of neuroimaging-based biomarkers.
Tract length has been used to examine WM integrity in a number of other patient populations. Deterioration of length due to alcoholism is commonly used as a measure of deficits in alcoholism (Rosenbloom, Sassoon et al. 2008). In studies of autism spectrum disorder (ASD), for example, fiber length is frequently assessed to determine the extent to which long-range network connections have been compromised by the disorder (Sundaram, Kumar et al. 2008; Kumar, Sundaram et al. 2010) - patients with ASD have shorter fibers and tend to lack the longer network connections involved in socio-emotional and language skills. Conversely, greater callosal tract length during development results in greater structural reductions in fiber diameter, amount of myelin, and number of fibers (Lewis, Theilmann et al. 2012). Aberrant patterns of organization may lead to greater conduction delays and cellular costs, such that long-distance connectivity could be reduced in proportion to the degree of brain overgrowth. Thus, mapping and assessing how short or long fibers are topographically distributed with respect to each other can also identify more widespread, whole-brain differences between BD patients and healthy controls.
The inferior longitudinal fasciculus (ILF) had a significantly reduced fiber density in BD compared to healthy control subjects. The ILF connects the occipital lobe with the anterior part of the temporal lobe. Anteriorly, it joins the uncinate fasciculus to relay information to the orbitofrontal region of the brain (Ashtari 2012). The functional role of the ILF is poorly understood, but the ILF as well as the UNC may facilitate language- and emotion-based evaluative processes as well as behavioral control functions that delay gratification (Olson, Collins et al. 2009). Epelbaum et al. (2008) reported that a patient who underwent surgery that disrupted the ILF - and subsequently lost connection to the visual word form area – became alexic, losing the ability to read. The tract is crucial to orthographic processing – the ability to visualize complete words - and accessing their semantic meaning. Lower fiber density bilaterally may account for the issues that some BD patients experience with verbal function (Vandermosten, Boets et al. 2012).
We identified a significantly greater fiber density in the left uncinate fasciculus (UNC-L) of the BD compared to control subjects. Notably, our results concur with those of Houenou et al. (2007) who found a significantly greater fiber density on average in the left uncinate fasciculus in BD patients than controls. The UNC is considered part of the limbic system (Hasan, Iftikhar et al. 2009). This white matter mass connects limbic system structures in the temporal lobe (e.g. amygdala, hippocampus) with regions of the frontal lobe such as the orbitofrontal cortex. Functional imaging techniques such as fMRI have demonstrated abnormal activation in subjects with BD in the ventral affective system, in which the UNC is a critical pathway, (Chaddock, Barker et al. 2009). The UNC also connects the subgenual cingulate gyrus to the amygdala and hippocampus, regions implicated in attention (Wang, Kalmar et al. 2009), memory (Metzler-Baddeley, Jones et al. 2011) and emotion regulation (McCrea 2008). It is possible that the increase in fiber density in BD vs. control subjects may contribute to an overly reactive emotion-based neural system, providing a resultant vulnerability to mood extremes and overall instability of mood. Interestingly, in a study in schizophrenia, no differences were found in the size or symmetry of the UNC in schizophrenia vs. healthy control subjects (Highley, Walker et al. 2002), suggesting a specificity of the current finding to subjects with mood disorders.
In the corticospinal tracts, the CST-R2 yielded a significant difference in average FA between BD patients and healthy controls. We found this result both unexpected and counter-intuitive. We had not set out to suggest that BD patients and healthy controls would be expected to differ in the connectivity of descending motor pathways historically not associated with BD, per se. In attempting to understand this finding, we noted that behavioral studies in euthymic BD have reported their being outperformed by both healthy controls and patients with mild cognitive impairment (MCI) on tasks of motor skill (Osher, Dobron et al. 2011), likely regulated by such large-scale motor networks as the CSTs. Increased FA in these tracts may reflect altered cerebellar-striatal-prefrontal connectivity, influencing mood dysregulation (Mahon, Wu et al. 2009). Serotonin synthesis, transport, and regulation are also dependent upon cortical-subcortical connections, such as the CSTs and corticopontine tracts. In functional neuroimaging studies (Chang, Adleman et al. 2004) increased task-related activation has been found in networks connecting the prefrontal cortex to the limbic structures (insula), striatum (caudate and putamen), and thalamus. Thus, in light of our findings that cortical-subcortical connections may differ between patient and controls populations, future research studies should direct attention toward elucidating differences between bipolar patients and healthy controls involving motor networks.
In our DTI data, measurement of the CSTs ceases at the bottom-most slice in the field-of-view of the volume. Yet, in fact, the CSTs extend outside of the field-of-view. In other words, while we are able to tract the CST from its probable origins in the Betz cells of the primary motor cortices, downward through the posterior limb of the internal capsule, into the mid-brain, and eventually the spinal column, that is where our measurement ceases since that is the bottom-most slice in our volume. However, the CST, strictly speaking, continues downward through the medullary pyramids, basal pons, synapsing in the lower motor neurons of the anterior horn of the spinal cord, and then outward via the dorsal root ganglia toward the limbs. It is these last portions of the full extent of those fibers which comprise the CST that we are unable to measure when only obtaining “full brain coverage”. Therefore, conclusions about the importance of these CST findings are undoubtedly limited. In contrast, however, the cerebral peduncles primarily connect the left and right cerebellum via the pons and, unlike CSTs, exist entirely outside of the cortical envelope. As the peduncles have not been implicated in BD, they serve as truly useful a control region, allowing us to assess the regional specificity of our findings. The peduncles did not show any differences in FA, length or density between groups, suggesting findings were specific to affected areas.
Differences in WM connectivity in the specific brain regions that we report may account for some of the neurocognitive deficits reported in patients with BD. Neuropsychological deficits in BD include impaired working memory, executive function, visual-spatial ability, verbal function, attention, information processing speed, and motor skills (Osher, Dobron et al. 2011). While diminished WM integrity (as measured by FA) has been reported to contribute to age- and disease-related cognitive decline, little is known about the impact of tract length or density on neuropsychological function. Changes in WM FA are related to worse performance on tests that rely on processing speed and executive functioning (Malloy, Correia et al. 2007; Turken, Whitfield-Gabrieli et al. 2008; Perez-Iglesias, Tordesillas-Gutierrez et al. 2010). Reduced FA in the corpus callosum has been associated with cognitive impairment in Alzheimer disease and may play a similar role in the observed working memory deficits of BD patients (Thillainadesan, Wen et al. 2012). Increased WM organization, indicated by higher FA values in the ILF, has been associated with lower discounting rates (increased preference for larger delayed rewards) (Olson, Collins et al. 2009). Regarding neurocognitive function and tract length or density, we are aware of only one study. In a study of alcoholism, the density of fibers between the midbrain and pons was lower in alcoholics than controls and was correlated with an index of cognitive flexibility (Chanraud, Reynaud et al. 2009). While research has yet to demonstrate consistent correlations between WM metrics and neuropsychological symptom presence or severity (Chaddock, Barker et al. 2009), future investigation should perhaps include tract length or fiber density as potential correlates. While the current study did not assess correlations of neurocognition and fiber metrics, future studies may advance the field by coupling behavioral and psychological testing with DTI tractography.
A few studies assessing other psychiatric disorders besides bipolar disorder have used WM metrics of tract length or fiber density. In tractography studies of Aspergers syndrome, no significant differences in FA were seen in patients vs. controls, but significantly greater streamlines were observed bilaterally in Aspergers subjects in the cingulum and inferior longitudinal fasciculus and fewer streamlines were seen in the right uncinate fasciculus (Pugliese, Catani et al. 2009; Kumar, Sundaram et al. 2010). These findings, like ours, suggest that while FA may be normal between patients and controls, other WM metrics may suggest disturbances in WM.
Whether and to what degree WM abnormalities are specific to bipolar disorder remains to be evaluated. Indeed, WM abnormalities are suspected to be involved in many psychiatric disorders. While few studies have assessed WM fiber tract length or density differences between psychiatric vs. control groups, differences in FA have been reported in subjects with many types of psychiatric illness. For example, in subjects with schizophrenia, WM abnormalities (such as FA reductions) have been reported in the dorsolateral prefrontal cortex, prefrontal WM, cingulate bundles, and the splenium of the corpus callosum (Sugranyes, Kyriakopoulos et al. 2012). Patients with obsessive-compulsive disorder (OCD) display lower FA in the body of the corpus callosum (BCC) (Bora, Harrison et al. 2011). In patients suffering from major depressive disorder, FA in the superior longitudinal fasciculus has been found to be negatively correlated with ruminative state (Zuo, Fang et al. 2012). Autism spectrum disorders, with their variety of manifestations, display a range of white matter abnormalities including reduced FA in the subcortical regions of the ventromedial prefrontal cortices, anterior cingulate gyri, and temporoparietal junctions, bilaterally in the superior temporal sulcus, the temporal lobes in the region of the amygdala, the occipitotemporal tracts, and the corpus callosum (Bode, Mattila et al. 2011). ADHD seems to involve disruption of the corticospinal tracts (CSTs), as well as both the inferior and superior longitudinal fasciculi (Konrad and Eickhoff 2010).
Study Limitations
Our study has several limitations. First, as noted above, disagreement exists in the literature about the best way to analyze and compare DTI scan results between samples (Versace, Almeida et al. 2008; Lin, Weng et al. 2011), though it remains unclear which particular approach returns the most valid results (Versace, Almeida et al. 2008). While an advantage of fiber tractography is that it identifies a seed region on a structural map and measures the cluster of fibers that is propagated from the region, this a priori selection of tracts has been criticized, as it may ignore novel regions that are neurobiologically important (Heng, Song et al. 2010). However, computer-generated ROIs based upon regional parcellation schemes are not foolproof - sometimes failing to encapsulate an entire tract, or may combine pieces of neighboring, but disparate tracts. Nevertheless, regionally driven and focused tractographic approaches offer specificity not enjoyed by other methods and are suitable for use in clinical samples.
Second, it has been hypothesized that the corpus callosum interferes with DTI tractography by attracting reconstruction, leading a computer to interpret fibers within two voxels of the corpus callosum as “connected”, and thus causing potential mislabeling or incorrect FA calculation (Houenou, Wessa et al. 2007). Fiber-tracking programs also depend on an FA threshold value used to determine the stopping point of tracking and thus the endpoints of a particular tract. Between-group differences in overall FA, for instance, could alter the measurements of fiber bundle length as a result. That is to say, density and length could be affected by fibers with very low FA values in one group, which would, consequently, not register as being true white matter. Such problems are not a strict limitation of the current investigation, per se, but rather are issues in DTI sample comparisons overall. However, as we found no significant FA differences in tracts beginning and ending in the brain, this would not seem to explain the differences in WM tract length or density found between groups in the current study.
Our study employed multiple measures of white matter structure as determined from DTI data, some of which may be pair-wise co-linear and, thus, their effects not entirely independent. In particular, while mean diffusivity, apparent diffusion coefficient, and FA are crucial to imaging and mapping WM, each depends on a number of other structural components. FA results are often interpreted as indicators of the level of myelination and as relevant to processing speed within fibers, but could also reflect other measures, such as fiber diameter, fiber density, maturation, or tract length. While FA is among the most commonly found metrics of white matter structure in DTI studies, Koraonkar and coworkers (Korgaonkar, Cooper et al. 2012) found through independent components analysis that fiber length has a predictive accuracy of 83% when measured in major depressive disorder vs. control subjects. Furthermore, because of its crucial role in fiber-tracking algorithms and its correlation to other metrics, fiber length is considered a valuable metric for assessing the accuracy of tractography protocols (Andreisek, White et al. 2009; Chen, Ding et al. 2009; Colby, Soderberg et al. 2012; Kristo, Leemans et al. 2012). Moreover, our use of multivariate analysis was conducted in order to specifically account for such covariance and improve on the ability to detect differences between groups.
As in most bipolar populations, a high proportion of subjects had a history of alcohol abuse, thus potentially confounding interpretation of the FA results. Previous research has shown that alcohol and drug dependence are correlated with diminutions in FA in frontal-parietal circuitry and the corpus callosum (Bava, Frank et al. 2009). Abstinence increases FA in multiple WM regions, particularly the corpus callosum (Gazdzinski, Durazzo et al. 2010). Even short-term abstinence is accompanied by re-myelination, but the effect continues to increase as a function of time (Alhassoon, Sorg et al. 2012). Therefore, with an average of 7.7 years sober, our BD cohort is likely to have recovered at least some of the lost WM, perhaps contributing to no significant FA between group differences in tracts that began and ended in the brain. Nevertheless, it is unclear to what extent current or former alcohol-related behavior could influence other diffusion imaging measures (e.g. tract length or fiber density) in our study.
Lastly, we only examined BD I patients in a euthymic mood state. While this reduced the heterogeneity of the patient sample, our findings may not generalize to other BD patient subtypes or BD I subjects in other mood states. Furthermore, our sample size, while relatively large compared to some other BD neuroimaging studies, is still small and may thus limit our statistical power to detect more subtle effects than the ones reported here.
Conclusions
This study found that patients with bipolar disorder have effectively shorter WM fiber tracts and relatively reduced WM fiber density per tract in specific brain regions compared to healthy control subjects. Further, subjects with BD demonstrated an increase in fiber density in a major tract connecting limbic system structures to the frontal lobe. Consequently, several critical memory and emotion regulatory pathways may exhibit altered function in BD patients. While it remains unclear whether these WM abnormalities relate to the pathogenesis of BD, or whether having mood episodes leads to the development of such WM structural differences, this study demonstrates the importance of measuring multiple components of WM integrity, since each reveals unique information regarding brain structure. Future research will be necessary to investigate whether the WM changes seen in euthymic BD patients are stable and present in all mood states, or if WM structural alterations, like those seen in grey matter structures, change as a function of mood state. With these results, and as comparative DTI analyses become more refined, a consensus may begin to emerge about WM structural differences between healthy and BD populations. Our findings, if replicated, could ultimately improve understanding of the underlying WM structural deficits that contribute to functional deficits seen in BD and may aid in the development of effective treatments.
Acknowledgements
This work was supported by grants to LLA from the National Institute of Mental Health (R21MH086104, R21MH085944) and a generous donation from the HF Foundation.
References
- Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, Strakowski SM. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;6(3):197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, Gongvatana A, Grant I. Callosal White Matter Microstructural Recovery in Abstinent Alcoholics: A Longitudinal Diffusion Tensor Imaging Study. Alcoholism, clinical and experimental research. 2012 doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL. Bipolar disorder: are repeated episodes associated with neuroanatomic and cognitive changes? Biol Psychiatry. 1993;33(8–9):563–565. doi: 10.1016/0006-3223(93)90093-s. [DOI] [PubMed] [Google Scholar]
- Andreisek G, White LM, Kassner A, Tomlinson G, Sussman MS. Diffusion tensor imaging and fiber tractography of the median nerve at 1.5T: optimization of b value. Skeletal Radiol. 2009;38(1):51–59. doi: 10.1007/s00256-008-0577-6. [DOI] [PubMed] [Google Scholar]
- Ashtari M. Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Dev Med Child Neurol. 2012;54(1):6–7. doi: 10.1111/j.1469-8749.2011.04122.x. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Ozdemir H, Yildirim H. Corpus callosum areas in first-episode patients with bipolar disorder. Psychol Med. 2007;37(5):699–704. doi: 10.1017/S0033291706009743. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry. 2009;66(3):238–244. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. '2734872:' 2734872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Absinta M, Rocca MA, Radaelli D, Poletti S, Bernasconi A, Dallaspezia S, Pagani E, Falini A, Copetti M, Colombo C, Comi G, Smeraldi E, Filippi M. Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar Disord. 2011;13(4):414–424. doi: 10.1111/j.1399-5618.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Yeh PH, Bellani M, Radaelli D, Nicoletti MA, Poletti S, Falini A, Dallaspezia S, Colombo C, Scotti G, Smeraldi E, Soares JC, Brambilla P. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol Psychiatry. 2011;69(4):309–317. doi: 10.1016/j.biopsych.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Taylor WD, MacFall JR, Kuchibhatla M, Payne ME, Provenzale JM, Cassidy F, Krishnan KR. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30(12):2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- Bode MK, Mattila ML, Kiviniemi V, Rahko J, Moilanen I, Ebeling H, Tervonen O, Nikkinen J. White matter in autism spectrum disorders - evidence of impaired fiber formation. Acta Radiol. 2011;52(10):1169–1174. doi: 10.1258/ar.2011.110197. [DOI] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Fornito A, Cocchi L, Pujol J, Fontenelle LF, Velakoulis D, Pantelis C, Yucel M. White matter microstructure in patients with obsessive-compulsive disorder. Journal of psychiatry & neuroscience : JPN. 2011;36(1):42–46. doi: 10.1503/jpn.100082. '3004974:' 3004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Nicoletti MA, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Magnetic resonance imaging study of corpus callosum abnormalities in patients with bipolar disorder. Biol Psychiatry. 2003;54(11):1294–1297. doi: 10.1016/s0006-3223(03)00070-2. [DOI] [PubMed] [Google Scholar]
- Brooks JO, 3rd, Bonner JC, Rosen AC, Wang PW, Hoblyn JC, Hill SJ, Ketter TA. Dorsolateral and dorsomedial prefrontal gray matter density changes associated with bipolar depression. Psychiatry Res. 2009;172(3):200–204. doi: 10.1016/j.pscychresns.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A, Walshe M, Bramon E, Chitnis XA, Murray R, McDonald C. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry. 2009;194(6):527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61(8):781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Reynaud M, Wessa M, Penttila J, Kostogianni N, Cachia A, Artiges E, Delain F, Perrin M, Aubin HJ, Cointepas Y, Martelli C, Martinot JL. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(5):1223–1232. doi: 10.1038/npp.2008.101. [DOI] [PubMed] [Google Scholar]
- Chen W, Ding Z, Zhang S, MacKay-Brandt A, Correia S, Qu H, Crow JA, Tate DF, Yan Z, Peng Q. A novel interface for interactive exploration of DTI fibers. IEEE Trans Vis Comput Graph. 2009;15(6):1433–1440. doi: 10.1109/TVCG.2009.112. [DOI] [PubMed] [Google Scholar]
- Colby JB, Soderberg L, Lebel C, Dinov ID, Thompson PM, Sowell ER. Along-tract statistics allow for enhanced tractography analysis. Neuroimage. 2012;59(4):3227–3242. doi: 10.1016/j.neuroimage.2011.11.004. '3288584:' 3288584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloye C, de Bilbao F, Moy G, Baudois S, Weber K, Campos L, Canuto A, Giardini U, von Gunten A, Stancu RI, Scheltens P, Lazeyras F, Millet P, Giannakopoulos P, Gold G. Neuroanatomical and neuropsychological features of euthymic patients with bipolar disorder. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2009;17(12):1012–1021. doi: 10.1097/JGP.0b013e3181b7f0e2. [DOI] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Dehaene S, Cohen L. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex; a journal devoted to the study of the nervous system and behavior. 2008;44(8):962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain : a journal of neurology. 2010;133(Pt 4):1043–1053. doi: 10.1093/brain/awp343. '2850577:' 2850577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germana C, Kempton MJ, Sarnicola A, Christodoulou T, Haldane M, Hadjulis M, Girardi P, Tatarelli R, Frangou S. The effects of lithium and anticonvulsants on brain structure in bipolar disorder. Acta Psychiatr Scand. 2010;122(6):481–487. doi: 10.1111/j.1600-0447.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. New York: Oxford University Press; 2007. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. The British Journal of psychiatry & neuroscience : JPN. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain research. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. '2693464:' 2693464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar MM, Roversi F, Pallanti S, Baldini-Rossi N, Schnur DB, Licalzi EM, Tang C, Hof PR, Hollander E, Buchsbaum MS. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57(7):733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Heng S, Song AW, Sim K. White matter abnormalities in bipolar disorder: insights from diffusion tensor imaging studies. J Neural Transm. 2010;117(5):639–654. doi: 10.1007/s00702-010-0368-9. [DOI] [PubMed] [Google Scholar]
- Highley JR, Walker MA, Esiri MM, Crow TJ, Harrison PJ. Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cerebral cortex. 2002;12(11):1218–1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- Hong S, Ke X, Tang T, Hang Y, Chu K, Huang H, Ruan Z, Lu Z, Tao G, Liu Y. Detecting abnormalities of corpus callosum connectivity in autism using magnetic resonance imaging and diffusion tensor tractography. Psychiatry Res. 2011;194(3):333–339. doi: 10.1016/j.pscychresns.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, Poupon C, Martinot JL, Paillere-Martinot ML. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Molecular psychiatry. 2007;12(11):1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23(7):803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Jones DK, Leemans A. Diffusion tensor imaging. Methods Mol Biol. 2011;711:127–144. doi: 10.1007/978-1-61737-992-5_6. [DOI] [PubMed] [Google Scholar]
- Kafantaris V, Kingsley P, Ardekani B, Saito E, Lencz T, Lim K, Szeszko P. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(1):79–86. doi: 10.1097/CHI.0b013e3181900421. '2747245:' 2747245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31(6):904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Cooper NJ, Williams LM, Grieve SM. Mapping inter-regional connectivity of the entire cortex to characterize major depressive disorder: a whole-brain diffusion tensor imaging tractography study. Neuroreport. 2012;23(9):566–571. doi: 10.1097/WNR.0b013e3283546264. [DOI] [PubMed] [Google Scholar]
- Kristo G, Leemans A, de Gelder B, Raemaekers M, Rutten GJ, Ramsey N. Reliability of the corticospinal tract and arcuate fasciculus reconstructed with DTI-based tractography: implications for clinical practice. Eur Radiol. 2012 doi: 10.1007/s00330-012-2589-9. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, Janisse J, Chugani HT, Chugani DC. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cerebral cortex. 2010;20(9):2103–2113. doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Theilmann RJ, Fonov V, Bellec P, Lincoln A, Evans AC, Townsend J. Callosal fiber length and interhemispheric connectivity in adults with autism: Brain overgrowth and underconnectivity. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Weng S, Xie B, Wu G, Lei H. Abnormal frontal cortex white matter connections in bipolar disorder: a DTI tractography study. Journal of affective disorders. 2011;131(1–3):299–306. doi: 10.1016/j.jad.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neuroscience and biobehavioral reviews. 2010a;34(4):533–554. doi: 10.1016/j.neubiorev.2009.10.012. '2847441:' 2847441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010b;34(4):533–554. doi: 10.1016/j.neubiorev.2009.10.012. '2847441:' 2847441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K, Wu J, Malhotra AK, Burdick KE, DeRosse P, Ardekani BA, Szeszko PR. A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology. 2009;34(6):1590–1600. doi: 10.1038/npp.2008.216. '2811531:' 2811531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy P, Correia S, Stebbins G, Laidlaw DH. Neuroimaging of white matter in aging and dementia. Clin Neuropsychol. 2007;21(1):73–109. doi: 10.1080/13854040500263583. [DOI] [PubMed] [Google Scholar]
- McCrea SM. Bipolar disorder and neurophysiologic mechanisms. Neuropsychiatr Dis Treat. 2008;4(6):1129–1153. doi: 10.2147/ndt.s4329. '2646644:' 2646644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O'Sullivan MJ. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. J Neurosci. 2011;31(37):13236–13245. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J Cogn Neurosci. 2009;21(7):1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osher Y, Dobron A, Belmaker RH, Bersudsky Y, Dwolatzky T. Computerized testing of neurocognitive function in euthymic bipolar patients compared to those with mild cognitive impairment and cognitively healthy controls. Psychother Psychosom. 2011;80(5):298–303. doi: 10.1159/000324508. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral EM, Sweeney JA, Zhou XJ. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65(7):586–593. doi: 10.1016/j.biopsych.2008.10.015. '2677389:' 2677389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, de Lucas EM, Rodriguez-Sanchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167(4):451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry. 2008;13(9):829, 833–857. doi: 10.1038/mp.2008.65. '2745893:' 2745893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese L, Catani M, Ameis S, Dell'Acqua F, Thiebaut de Schotten M, Murphy C, Robertson D, Deeley Q, Daly E, Murphy DG. The anatomy of extended limbic pathways in Asperger syndrome: a preliminary diffusion tensor imaging tractography study. Neuroimage. 2009;47(2):427–434. doi: 10.1016/j.neuroimage.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley KC. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry research. 2007;151(3):179–188. doi: 10.1016/j.psychres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sassoon SA, Fama R, Sullivan EV, Pfefferbaum A. Frontal Callosal Fiber Integrity Selectively Predicts Coordinated Psychomotor Performance in Chronic Alcoholism. Brain Imaging Behav. 2008;2(2):74–83. doi: 10.1007/s11682-007-9017-9. '2709859:' 2709859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Dima D, O'Muircheartaigh J, Corrigall R, Pendelbury G, Hayes D, Calhoun VD, Frangou S. Multimodal analyses identify linked functional and white matter abnormalities within the working memory network in schizophrenia. Schizophrenia research. 2012 doi: 10.1016/j.schres.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18(11):2659–2665. doi: 10.1093/cercor/bhn031. '2567426:' 2567426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thillainadesan S, Wen W, Zhuang L, Crawford J, Kochan N, Reppermund S, Slavin M, Trollor J, Brodaty H, Sachdev P. Changes in mild cognitive impairment and its subtypes as seen on diffusion tensor imaging. Int Psychogeriatr. 2012:1–11. doi: 10.1017/S1041610212000270. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362(9386):798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14(4):326–339. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. '2630965:' 2630965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Versace A, Almeida JR, Hassel S, Walsh ND, Novelli M, Klein CR, Kupfer DJ, Phillips ML. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Archives of general psychiatry. 2008;65(9):1041–1052. doi: 10.1001/archpsyc.65.9.1041. '2730162:' 2730162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, Edmiston E, Chepenik LG, Bhagwagar Z, Spencer L, Pittman B, Jackowski M, Papademetris X, Constable RT, Blumberg HP. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry. 2008;64(8):730–733. doi: 10.1016/j.biopsych.2008.06.001. '2586998:' 2586998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66(5):516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D'Arceuil H, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Young RC BJ, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zuo N, Fang J, Lv X, Zhou Y, Hong Y, Li T, Tong H, Wang X, Wang W, Jiang T. White matter abnormalities in major depression: a tract-based spatial statistics and rumination study. PloS one. 2012;7(5):e37561. doi: 10.1371/journal.pone.0037561. [DOI] [PMC free article] [PubMed] [Google Scholar]