Comparative Analysis of Mobilizable Genomic Islands (original) (raw)
Abstract
Mobilizable genomic islands (MGIs) are small genomic islands of less than 35 kbp containing an integrase gene and a sequence that resembles the origin of transfer (oriT) of an integrating conjugative element (ICE). MGIs have been shown to site-specifically integrate and excise from the chromosome of bacterial hosts and hijack the conjugative machinery of a coresident ICE to disseminate. To date, MGIs have been described in three strains belonging to three different Vibrio species. In this study, we report the discovery of 11 additional putative MGIs found in various species of Vibrio, Alteromonas, Pseudoalteromonas, and Methylophaga. We designed an MGI capture system that allowed us to relocate chromosomal MGIs onto a low-copy-number plasmid and facilitate their isolation and sequencing. Comparative genomics and phylogenetic analyses of these mobile genetic elements revealed their mosaic structure and their evolution through recombination and acquisition of exogenous DNA. MGIs were found to belong to a larger family of genomic islands (GIs) sharing a similar integrase gene and often integrated into the same integration site yet exhibiting a different mechanism of regulation of excision and mobilization. We found that MGIs can excise only when an ICE of the SXT/R391 family is coresident in the same cell, while GIs still excise regardless.
INTRODUCTION
The SXT/R391 family of integrating conjugative elements (ICEs) is a well-studied family of self-transmissible mobile genetic elements responsible for the dissemination of antibiotic resistance genes in clinical and environmental vibrios and related gammaproteobacteria, such as Providencia rettgeri, Shewanella putrefaciens, and Photobacterium damselae (1–3). This family currently includes more than 30 members (4, 5) that share a set of 52 conserved genes and the same chromosomal integration site, the 5′ end of prfC, which encodes peptide chain release factor 3. Each ICE of this family also contains variable DNA inserted in specific positions of the backbone (hot spots) and encoding various functions, such as resistance to antibiotics or heavy metals.
Mobilizable genomic islands (MGIs) are small genomic islands (approximately 20 kpb) that rely on an unusual mechanism to transfer at high frequency from one cell to another (6). MGIs carry a conserved sequence that mimics the origin of transfer (oriT) of SXT/R391 ICEs and utilize both the DNA processing and conjugative apparatus encoded by these ICEs to transfer to a new recipient cell. To date, three MGIs have been described, each in a different Vibrio species: MGI_Vvu_Tai1 and MGI_Vfl_Ind1 in the clinical isolates Vibrio vulnificus YJ016 and Vibrio fluvialis H-08942, respectively, and MGI_Vch_USA1 in the environmental isolate Vibrio cholerae RC385 (Table 1). All three MGIs share the same integration site, i.e., the 3′ end of a gene encoding a putative stress-induced protein named yicC in Escherichia coli (6). Besides the _oriT_SXT-like sequence, the three MGIs also share four conserved genes: _int_MGI, which encodes a tyrosine recombinase responsible for MGI excision and integration; rdfM, which encodes a recombination directionality factor necessary for excision; and two putative genes of unknown function (cds4 and cds8) (6, 19). MGI_Vch_USA1 encodes two different putative toxin-antitoxin systems related to the HipA/B system of E. coli, a system that has been shown to promote bacterial multidrug tolerance (20). These toxin-antitoxin systems could also improve the stability of this MGI_Vch_USA1 by acting as postsegregational killing systems (21). MGI_Vvu_Tai1 encodes a type I restriction modification system that could protect its host from bacteriophage infection (22). Potential selective advantages conferred by MGI_Vfl_Ind1 are unknown, as its whole sequence is not available to date (6).
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype_a_ | Reference |
|---|---|---|
| Strains | ||
| Escherichia coli K12 | ||
| CAG18439 | MG1655 lacZU118 lacI42::Tn_10_ (Tcr) | 7 |
| AD157 | CAG18439 Δ_yicC_ (Tcr) | This study |
| AD171 | MG1655 Δ_yicC prfC_::R997 pVB201 (Apr Cmr) | This study |
| AD175 | MG1655 Δ_yicC_ Δ_dapA_::(_erm_-pir) prfC::R997 pVB201 (Emr Apr Cmr) | This study |
| β2163 | (F−) RP4-2-Tc::Mu Δ_dapA_::(_erm_-pir) (Knr Emr) | 8 |
| Alteromonas macleodii Deep ecotype | MGI_Ama_Med1, environmental, 2002, Mediterranean Sea | 9 |
| Shewanella sp. W3-18-1 | ICE_Spu_PO1 GI_Spu_PO1, environmental, Pacific Ocean | 10 |
| Vibrio cholerae | ||
| N16961 | O1 El Tor | 11 |
| RC385 | MGI_Vch_USA1, O135, environmental, 2005, USA | 12 |
| 3AMOZ | ICE_Vch_Moz2 MGI_Vch_Moz2, environmental, 2002, Mozambique | 13 |
| 7AMOZ | ICE_Vch_Moz3 MGI_Vch_Moz3, environmental, 2002, Mozambique | 13 |
| 8AMOZ | ICE_Vch_Moz4 MGI_Vch_Moz4, environmental, 2002, Mozambique | 13 |
| 16AMOZ | ICE_Vch_Moz6 MGI_Vch_Moz6, environmental, 2003, Mozambique | 13 |
| Vibrio fluvialis H-08942 | ICE_Vfl_Ind1 MGI_Vfl_Ind1, clinical, 2002, India | 14 |
| Vibrio mimicus VM573 | GI_Vmi_USA1, clinical, 1990, United States | 15 |
| Vibrio parahaemolyticus 21AMOZ | ICE_Vpa_Moz1 MGI_Vpa_Moz1, environmental, 2002, Mozambique | 13 |
| Vibrio vulnificus YJ016 | MGI_Vvu_Tai1, clinical, 1992, Taiwan | 16 |
| Plasmids | ||
| pBeloBac11 | Cmr | 17 |
| pVB201 | pBeloBac11::_attBVch_N16961 (Cmr) | This study |
| pVI36 | aad7 (Spr), PCR template for one-step chromosomal gene inactivation | 18 |
Unlike the vast majority of genomic islands (GIs) for which the mechanisms of dissemination remain unclear, the regulation of MGI excision, transfer, and integration has recently been elucidated (6, 19). In the absence of an SXT/R391 ICE, an MGI integrated into the chromosome of its host does not excise. If a coresident SXT/R391 ICE is present, the ICE-encoded transcriptional activator SetCD triggers the expression of both rdfM and _int_MGI to promote MGI site-specific excision (19). Once the MGI is excised as a circular covalently closed molecule, _oriT_MGI is recognized by the ICE-encoded relaxase to translocate the MGI into the recipient cell as a single-stranded DNA molecule through the mating bridge encoded by the ICE (6). Mobilization of the MGI to the recipient cell is independent of the cotransfer of the ICE (6, 19). Furthermore, MGI integration into the 3′ end of yicC has been shown to be mediated by IntMGI, which is expressed at low levels in a SetCD-independent manner in the recipient cell (19). Besides promoting genomic plasticity through their own mobilization, MGIs can also mobilize chromosomal DNA located 5′ of their integration site. Chromosomal DNA mobilization is driven by the SXT/R391 ICE through the recognition of the _oriT_SXT-like sequence on the MGI. This process can lead to the mobilization of at least 1 Mbp of chromosomal DNA (6).
Here, we report the characterization of 11 additional putative MGIs in the genome of various gammaproteobacteria. Comparative genomics and phylogenetic analyses of these elements revealed their mosaic structure and their evolution through recombination and acquisition of exogenous DNA. MGIs were found to belong to a larger family of GIs sharing a similar integrase gene and often integrated into the same integration site, yet exhibiting a different mechanism of regulation of excision and mobilization.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains and plasmids used in this study are described in Table 1. The strains were routinely grown in Luria-Bertani (LB) broth at 37°C in an orbital shaker/incubator and were maintained at −80°C in LB broth containing 15% (vol/vol) glycerol. Alteromonas macleodii Deep ecotype was grown in marine broth (Difco, BD). Antibiotics were used at the following concentrations: ampicillin (Ap), 100 μg/ml; chloramphenicol (Cm), 20 μg/ml; erythromycin (Em), 200 μg/ml; kanamycin (Kn), 50 μg/ml; spectinomycin (Sp), 50 μg/ml; tetracycline (Tc), 12 μg/ml.
Bacterial conjugation.
Conjugation assays were used to transfer MGI_Vfl_Ind1 and MGI_Vch_Moz6 into AD175 (intermediate) and AD157 (recipient). Mating assays were carried out by mixing equal volumes of 7-h-grown cultures of donor, intermediate, and recipient strains. The cells were harvested by centrifugation for 5 min at 5,500 × g and resuspended in a 1/20 volume of LB broth. Cell suspensions were poured onto LB agar plates and incubated at 37°C for 15 h. The cells were then resuspended in 1 ml of LB medium, and serial dilutions were plated onto the appropriate selective media to determine the number of donors, intermediates, recipients, and exconjugants. Exconjugants were purified and tested by PCR for the presence of the MGI.
Construction of plasmids and strains.
The plasmids used in this study are described in Table 1. Plasmid pVB201 was constructed by cloning the MGI attB site from V. cholerae N16961 (attB_VchN16961) into the HindIII restriction site of pBeloBac11 (New England BioLabs). attB_VchN16961 was amplified using the primer pair attB-F/attB-R (see Table S1 in the supplemental material) and genomic DNA of V. cholerae N16961 as a template. Deletion of yicC in AD175 and AD157 was constructed by using the one-step chromosomal gene inactivation technique (23) using primer pair attB-WF/attB-WR (see Table S1) and pVI36 as a template. Deletion of dapA was introduced in MG1655 by P1_vir transduction of Δ_dapA::(_erm_-pir) from E. coli β2163 into E. coli AD171 as the recipient.
Molecular biology techniques.
All the enzymes were used according to the manufacturer's instructions (New England BioLabs). Plasmid DNA was prepared with a QIAprep spin miniprep or QIAfilter plasmid midikit (Qiagen), and chromosomal DNA was prepared with a Wizard genomic DNA purification kit (Promega) as described in the manufacturer's instructions. Colony blotting was performed as previously described (6). PCR assays were carried out in 50-μl PCR mixtures with 1 U of Taq DNA polymerase (New England BioLabs). The PCR conditions were as follows: (i) 3 min at 94°C; (ii) 30 cycles of 30 s at 94°C, 30 s at a suitable annealing temperature, and 30 s to 60 s at 72°C; and (iii) 5 min at 72°C. When needed, PCR products were purified using a QIAquick PCR purification kit (Qiagen) according to the manufacturer's instructions. E. coli was transformed by electroporation according to Dower et al. (24), using a Bio-Rad GenePulser Xcell apparatus set at 25 μF, 200 Ω, and 1.8 kV.
Nested PCR experiments on MGIs were performed as previously described (6). For the related GIs in Shewanella sp. W3-18-1 and Vibrio mimicus VM573, the same method was used with primers attPSpu-L1/attPSpu-R1 and attPSpu-L2/attPSpu-R2 and attPVmi-L1/attPVmi-R1 and attPVmi-L2/attPVmi-R2, respectively (see Table S1 in the supplemental material).
Sequencing.
MGI_Vfl_Ind1 (GenBank accession no. KC117176) and MGI_Vch_Moz6 (GenBank accession no. KC117175) were captured in pVB201, and the resulting plasmids were grown in E. coli. The DNA was extracted, and Illumina sequencing libraries were prepared as described previously (25) with the following modifications. The DNA was sheared with 1 unit of double-stranded DNA (dsDNA) shearase (ZymoResearch) at 37°C for 60 min and purified using AMPureXP beads (sheared DNA-to-beads [vol/vol] ratio of 0.7) before proceeding to Illumina adapter ligation. DNA inserts had an average size of approximately 500 bp. Libraries were barcoded and mixed with additional samples and sequenced on an HiSeq2000 instrument at the McGill University and Genome Quebec Innovation Centre. Paired-end reads of 100 bp were generated and demultiplexed using Novobarcode. MGIs were assembled using the Roche gsAssembler version 2.5 software. Gene detection and annotations were carried out using BLAST software (26) and databases available from NCBI.
Bioinformatics analyses.
Phylogenetic trees were generated using the PhyML version 3.0 program (27) with the LG substitution model for the protein sequence-based tree and the HKY85 substitution model for the DNA sequence-based tree. Tree topologies were optimized by PhyML using both the nearest-neighbor interchange (NNI) and subtree pruning and regrafting (SPR) methods, and the starting tree was estimated using BioNJ. Branch support of the phylogenies was estimated using nonparametric bootstrap (100 replicates). Phylogenetic analyses were computed from reliable amino acid or DNA alignments built by MUSCLE multiple sequence alignment software (28). Removal of poorly aligned regions from amino acid alignments was carried out by trimAl version 1.2 software using the automated heuristic approach (29) prior to phylogenetic analyses. Phylogenetic trees were viewed using iTOL version 2 (30).
RESULTS AND DISCUSSION
Five environmental Vibrio strains from Mozambique harbor putative MGIs.
To estimate the prevalence of MGIs in vibrios and other bacterial species, we screened by Southern blotting hybridization a large collection of V. cholerae (O1 and non-O1, non-O139), Vibrio parahaemolyticus, V. vulnificus, V. fluvialis, Vibrio metschnikovii, P. rettgeri, Providencia stuartii, Providencia alcalifaciens, E. coli, Pseudomonas aeruginosa, Salmonella, Aeromonas caviae, and Aeromonas hydrophila. Among 149 strains analyzed were clinical and environmental strains isolated between 1992 and 2004 in Italy, Vietnam, India, Bangladesh, Taiwan, Mexico, Angola, Mozambique, Rwanda, Somalia, Swaziland, and Zimbabwe. Using _int_MGI as a probe, five additional related MGIs were identified in Mozambican environmental strains of V. parahaemolyticus (21AMOZ) and V. cholerae (3AMOZ, 7AMOZ, 8AMOZ, and 16AMOZ) (Table 2).
Table 2.
Properties of MGI-like elements
| Name | Host strain | Type, yr, and site of isolation | % identity to _int_MGI_Vch_USA1 | SXT/R391 ICE | GenBank accession no. | Reference |
|---|---|---|---|---|---|---|
| MGI_Vch_USA1 | V. cholerae RC385 | Environmental, 2005, United States | 100 | ZP_06943583.1 | 12 | |
| MGI_Vvu_Tai1 | V. vulnificus YJ016 | Clinical, 1992, Taiwan | 98.8 | NP_933070.1 | 16 | |
| MGI_Psp_Arc1 | Pseudoalteromonas sp. BSi20311 | Environmental, 2009, Arctic Sea | 98.3 | ZP_09227437.1 | 31 | |
| MGI_Vfl_Ind1 | V. fluvialis H-08942 | Clinical, 2002, India | 97.4 | ICE_Vfl_Ind1 | KC117176 | 14 |
| MGI_Vch_Hai4 | V. cholerae HE45 | Gray Water, External Clinic, 2010, Haiti | 96.9 | EJH66094.1 | 32 | |
| GI_Vch_Hai3 | V. cholerae HE-09 | Environmental, 2010, Haiti | 96.9 | EGS63339.1 | 32 | |
| MGI_Ama_Med1 | A. macleodii Deep ecotype | Environmental, 2002, Mediterranean Sea | 90.9 | YP_004427371.1 | 9 | |
| MGI_Vch_Moz6 | V. cholerae 16AMOZ | Environmental, 2003, Mozambique | 88.5 | ICE_Vch_Moz6 | KC117175 | 13 |
| MGI_Vch_Hai1 | V. cholerae HE39 | Environmental, 2010, Haiti | 87.8 | EGR03948.1 | 32 | |
| MGI_Asp_Kor1 | Alteromonas sp. SN2 | Environmental, 2007, Korea | 83.5 | YP_004469681.1 | 33 | |
| MGI_Mam_Kor1 | Methylophaga aminisulfidivorans MP | Environmental, 2007, Korea | 83.1 | ZP_08537443.1 | 34 | |
| MGI_Vch_Hai5 | V. cholerae HC-43B1 | Clinical, 2010, Haiti | 82.3 | ALDP00000000.1 | 32 | |
| MGI_Vch_Hai2 | V. cholerae HE48 | Environmental, 2010, Haiti | 76.3 | EGR10536.1 | 32 | |
| MGI_Vch_Moz2 | V. cholerae 3AMOZ | Environmental, 2002, Mozambique | ICE_Vch_Moz2 | 13 | ||
| MGI_Vch_Moz3 | V. cholerae 7AMOZ | Environmental, 2002, Mozambique | ICE_Vch_Moz3 | 13 | ||
| MGI_Vch_Moz4 | V. cholerae 8AMOZ | Environmental, 2002, Mozambique | ICE_Vch_Moz4 | 13 | ||
| MGI_Vpa_Moz1 | V. parahaemolyticus 21AMOZ | Environmental, 2002, Mozambique | ICE_Vpa_Moz1 | 13 |
Based on PCR amplification and DNA sequencing, we found that these strains also contain the _oriT_SXT-like sequence, cds4, cds8, and rdfM and are integrated into the 3′ end of the same orthologue of E. coli's yicC. These results suggest that these strains harbor MGI-like elements. Interestingly, all of them also contain an ICE of the SXT/R391 family (Table 1), suggesting that the putative MGIs could be mobilized at relatively high frequency.
Design of an MGI capture system and MGI sequencing.
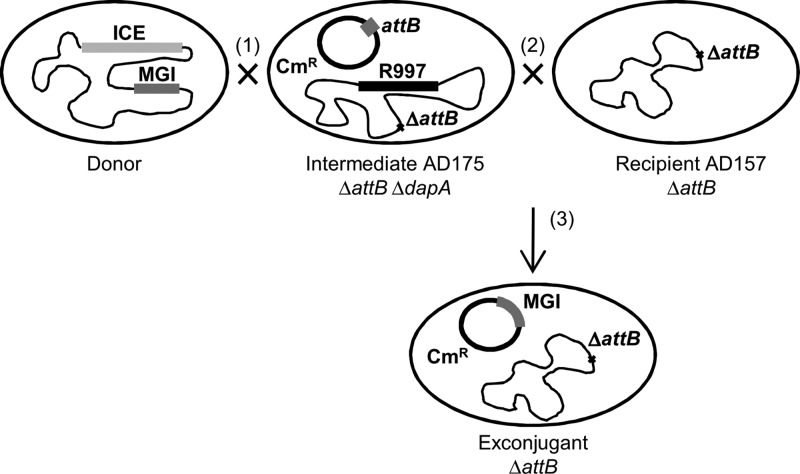
The identification of five putative MGIs in environmental vibrios isolated from the African continent was a great opportunity to take a look at the diversity and variability of MGI structures and sequences. However, since MGIs usually are integrated into the chromosome, excise at low frequency, and do not seem to replicate autonomously, extraction of MGI DNA using standard plasmid preparation protocols was not achievable. To avoid this problem, we devised a strategy allowing us to capture complete MGIs on a low-copy-number vector (pVB201) more suitable for DNA extraction and sequencing. pVB201 is a derivative of pBeloBac11 (17), a single-copy E. coli plasmid that allows the cloning and stable maintenance of large DNA fragments. pBeloBac11 was modified to include a 400-bp fragment that encompasses the MGI attachment site (attB). This modification enabled IntMGI-catalyzed site-specific recombination between attB on pVB201 and attP on an excised and transferred MGI to drive MGI capture and retransfer to a recipient strain (Fig. 1). This strategy was derived from a similar approach developed by Wozniak et al. (4) to capture SXT/R391 ICEs, yet it employs a triparental mating due to the lack of known selectable markers on the putative MGIs to be recovered. Conjugation was carried out between a Vibrio MGI+ ICE+ donor strain and an E. coli intermediate recipient strain (AD175) lacking dapA as well as yicC (and thus chromosomal attB) but harboring pVB201 and R997, the latter being an ICE of the SXT/R391 family conferring resistance to ampicillin. The procedure yielded exconjugant cells containing the transferred MGI integrated into pVB201. The resulting plasmid containing the MGI becomes mobilizable by R997 and can thus be transferred into the final E. coli recipient strain also lacking yicC. Exconjugant colonies containing the transferred MGI integrated into pVB201 were selected using the Cmr antibiotic resistance marker present on the plasmid as well as the Tcr marker of the final E. coli recipient strain (AD157). The Δ_dapA_ mutation ensured the absence of AD175 exconjugants on media not supplemented with diaminopimelic acid (DAP), a phenomenon commonly observed in our initial attempts that we attributed to the Hfr-like mobilization of the Tcr marker from AD157 back into AD175 mediated by R997 (data not shown). Using this capture system, MGI_Vfl_Ind1 of V. fluvialis H-08942 and MGI_Vch_Moz6 of V. cholerae 16AMOZ relocated into pVB201, with the help of the coresident ICEs ICE_Vfl_Ind1 (4, 14) and ICE_Vch_Moz6 (13), respectively. The resulting plasmids were completely sequenced and assembled. Annotation of the sequences led to the identification of 18 open reading frames (ORFs) in the 23,229-bp MGI_Vfl_Ind1 and 17 ORFs in the 19,740-bp MGI_Vch_Moz6.
Fig 1.
MGI capture system. Conjugation between a donor strain bearing an ICE and an MGI with an intermediate strain (AD175) bearing R997 (1) but lacking attB and dapA yields exconjugants that contain the transferred MGI integrated into pVB201. These exconjugants cannot be directly selected because of the lack of a selective marker on the MGI; yet the resulting plasmid becomes mobilizable by R997 and transfer to a Δ_attB_ recipient strain (2), yielding exconjugants harboring pVB201::MGI (3) that can be selected using the chloramphenicol marker present on pVB201 and the ability of the recipient strain (AD157) to grow in the presence of tetracycline and in the absence of diaminopimelic acid (dap).
The MGI family expands and is not restricted to Vibrio strains.
In addition to the MGIs we identified in our strain collection and sequenced, BLAST analysis of IntMGI_Vch_USA1 against the GenBank database (release 191.0) revealed the presence of 8 new MGIs in different environmental strains of V. cholerae (HE-39, HE-45, HE-48, and HC-43B1), in Alteromonas macleodii Deep ecotype, in Alteromonas sp. SN2, in Pseudoalteromonas sp_._
BSi20311, and in Methylophaga aminisulfidivorans MP (see Table 2 for strain details).
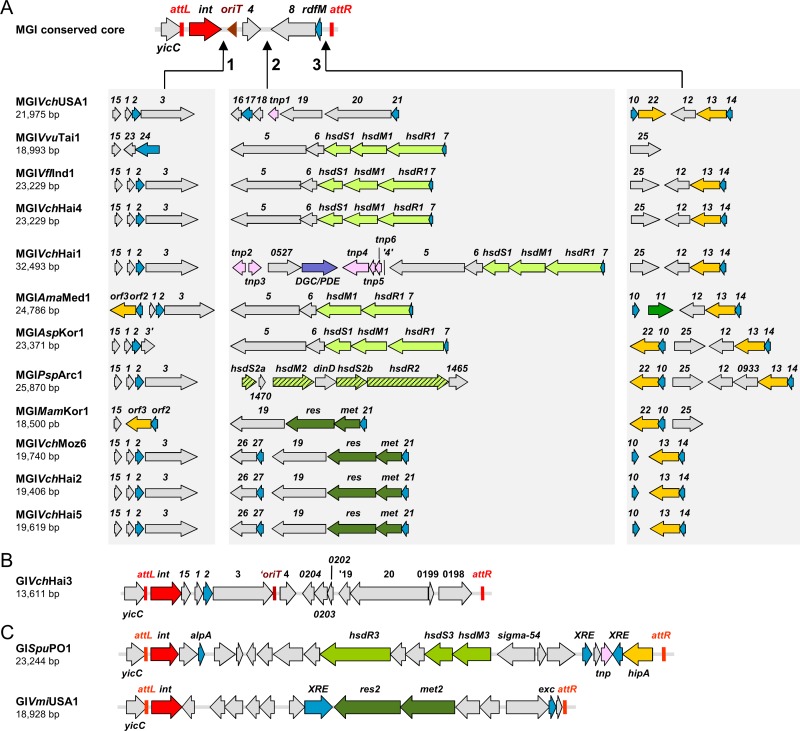
All of these new MGIs share the same conserved features that characterize the family, are integrated into the same site, and exhibit variable DNA regions (Fig. 2A). Interestingly, out of 12 sequenced MGIs, as many as 9 different structures emerge based on the gene content and orientation in each variable region. Only two structures appear in more than one MGI. The first pattern is observed for MGI_Vfl_Ind1 and MGI_Vch_Hai4 found in the Indian V. fluvialis H-08942 and in the Haitian V. cholerae HE45 isolates, respectively. The second conserved pattern is observed for MGI_Vch_Moz6, MGI_Vch_Hai2, and MGI_Vch_Hai5 found in three environmental V. cholerae strains, respectively, isolated from environmental or clinical sources in Mozambique and Haiti. Therefore, the structure of MGIs gives us crucial clues on strain circulation. Indeed, MGI_Vfl_Ind1 and MGI_Vch_Hai4, isolated in two opposite parts of the globe (India and Haiti, respectively) have the same exact gene content and orientation in each variable region. It is also the case for MGI_Vch_Moz6, isolated in Mozambique, and MGI_Vch_Hai2 and MGI_Vch_Hai5, isolated in Haiti.
Fig 2.
General structures of MGIs and phylogenetic analyses of the core genes. (A) Genetic structure of 12 sequenced MGIs; (B) atypical structure of GI_Vch_Hai3, from Vibrio cholerae H-09; (C) genetic content of GI_Spu_PO1 and GI_Vmi_USA1 from Shewanella sp. W3-18-1 and Vibrio mimicus VM573, respectively. Colors of the predicted open reading frames indicate the following putative functions: pink and red, DNA replication, recombination, and repair; purple, diguanylate cyclase/phosphodiesterase; blue, transcription; green, restriction modification systems; orange, putative HipA-like toxin; gray, unknown function. See Table S2 in the supplemental material for predictions of putative function of genes identified in variable regions.
Although the overall genetic diversity of MGIs is rather limited, strains isolated from the same geographical area harbor MGIs exhibiting rather dissimilar variable regions. Out of five Haitian V. cholerae strains collected in 2010 (32), three contain different MGIs (MGI_Vch_Hai1, MGI_Vch_Hai2, and MGI_Vch_Hai4), and a fourth contains an atypical GI, GI_Vch_Hai3, which lacks cds8, rdfM, as well as part of _oriT_MGI (repeats R1, R2, and R3 are missing) and is likely no longer mobilizable by SXT/R391 ICEs (Fig. 2A and B, Table 2). This diversity in a small group of strains isolated during the same period and in the same area suggests frequent exchange of MGIs between strains or frequent recombination events between MGIs or with other mobile genetic elements in the environment.
Our results support the idea that the MGI family is not restricted to vibrios but still appears to be limited to gammaproteobacteria, and more precisely to marine bacteria. Indeed, the most divergent elements of the family, MGI_Ama_Med1, MGI_Asp_Kor1, and MGI_Psp_Arc1, were all found in marine strains of the genera Alteromonas and Pseudoalteromonas (Mediterranean, Yellow, and Arctic seas) (see Fig. 2A and Table 2).
MGI genetic content.
The sizes of MGIs vary from 18,500 bp to 32,493 bp. Based on the analysis of 13 fully and 4 partially sequenced MGIs, we observe that with the exception of the atypical GI_Vch_Hai3, the core structure of MGIs appears to be restricted to _oriT_MGI and 4 conserved genes, _int_MGI, cds4, cds8, and rdfM. We previously showed that IntMGI mediates the site-specific integration and excision of MGIs into and from the chromosome of their host (6, 19). RdfM is required only for the excision process (19). To date, the functions of cds4 and cds8 remain unknown. Comparison of the different _oriT_MGI sequences revealed the conservation of the imperfect direct and inverted repeats that have been previously identified in _oriT_SXT (18) (see Fig. S1 in the supplemental material). Such structures likely play a role in the specific recognition of the oriT by the putative relaxase TraI and/or auxiliary proteins during conjugative transfer initiation. This observation suggests that the _oriT_s of these newly identified MGIs are functional, which is actually supported by the result of our MGI capture approach described above for MGI_Vfl_Ind1 and for MGI_Vch_Moz6. Interestingly, this alignment also highlights two additional regions almost perfectly conserved among SXT and MGIs (see Fig. S1, positions 53 to 67 and 78 to 90). Given their conservation, these regions might also play an important role in transfer initiation for both ICEs and MGIs.
Three variable regions can be observed in the sequenced MGIs, and to date each MGI exhibits the three regions, with different genetic contents from one MGI to the other (Fig. 2A). The genetic content of each region is described in Table S2 in the supplemental material. The first variable region is inserted between _int_MGI and oriT. In this locus, MGIs often contain cds15, cds1, cds2, and cds3. MGI_Ama_Med1 and MGI_Mam_Kor1 harbor a putative toxin-antitoxin system (orf3/orf2) also found in ICEs R391 and ICE_Vch_Mex1 (4). This system is related to the E. coli toxin-antitoxin system _hipA_-hipB, which mediates multidrug tolerance (20). _orf3_-orf2 could also promote MGI maintenance in a manner similar to that of the MosAT system recently identified in the ICE SXT (35).
The second variable region, located between cds4 and cds8, appears to be the most variable one. In all but one MGI, putative R/M systems are found in this region. Two of them are type I host-specific defense (hsd) systems. The hsdRMS1 system is found in MGI_Vvu_Tai1, MGI_Vfl_Ind1, MGI_Vch_Hai4, MGI_Vch_Hai1, MGI_Ama_Med1, and MGI_Asp_Kor1. The hsdRMS2 system is found in MGI_Psp_Arc1 only. At the same locus, MGI_Mam_Kor1, MGI_Vch_Moz6, MGI_Vch_Hai2, and MGI_Vch_Hai5 harbor a putative type III R/M system which is composed of two genes encoding a methyltransferase (met) and a restriction endonuclease (res). R/M systems allow bacteria to distinguish invading foreign DNA from their own genome, conferring resistance to infection by bacteriophages. The prevalence of R/M systems in MGIs could be strongly selected by the pressure exerted by bacteriophages in aquatic environments (36, 37) and confer a selective benefit to strains harboring such elements. Interestingly, MGI_Vch_Hai1 also harbors in variable region 2 a transposon-like structure flanked by multiple putative transposases and encoding a putative diguanylate cyclase/phosphodiesterase. These enzymes are involved in the synthesis and degradation of the second messenger c-di-GMP, which is known to increase biofilm formation and decrease motility in V. cholerae (38, 39). c-di-GMP is also considered an important player in the transition of V. cholerae from a host to persistence in the environment (38, 40). Interestingly, this is not the first report of a diguanylate cyclase/phosphodiesterase encoded on a mobile genetic element. Two diguanylate cyclases have been previously described in ICEs of the SXT/R391 family (41). The presence of such a gene in MGI_Vch_Hai1 could alter the pathway regulating the transition from free-living, motile to biofilm lifestyle of its host, which may provide a potential benefit in specific environmental conditions.
The third variable region is located between rdfM and attR (Fig. 2A). The most common features observed in this region are two distinct toxin-antitoxin systems (_cds10_-cds22 and _cds13_-cds14), all related to _E. coli hipA_-hipB.
Phylogenetic analysis of the MGI backbone.
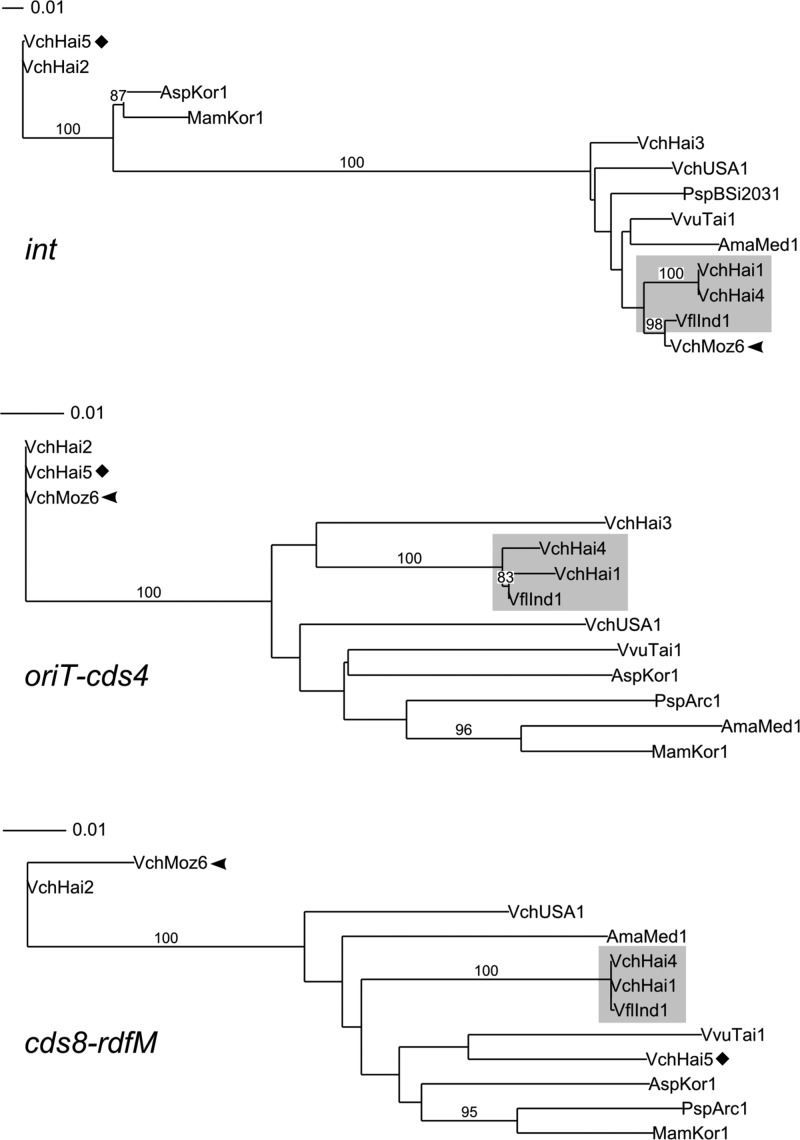
In order to explore the evolution of the conserved backbone, we constructed phylogenetic trees based on nucleotide sequences of the different core regions, including the one containing oriT (Fig. 3). Unlike SXT/R391 ICEs, MGIs have a small core set of conserved sequences, allowing us to construct phylogenetic trees for each region.
Fig 3.
Phylogenetic analysis of the MGI backbone. Phylogenetic trees based on the nucleotide sequences of the indicated core genes or region. Bootstrap values are indicated when over 80%. The individual scale bars represent genetic distances.
Interestingly, each region generated distinct branching patterns, highlighting tree incongruences. Although some MGIs always cluster together, as it is the case for MGI_Vch_Hai1, MGI_Vch_Hai4, and MGI_Vfl_Ind1, others tend to cluster in very different groups depending on the analyzed region. The most striking example is MGI_Vch_Moz6, which has an int gene almost identical to the one of MGI_Vfl_Ind1 but clusters with MGI_Vch_Hai2 for oriT-cds4 and cds8-rdfM regions (Fig. 3). Similar observations can also be made for MGI_Vch_Hai5, which clusters with MGI_Vch_Hai2 for int and _oriT_-cds4 yet has a more distantly related _cds8_-rdfM region. Such tree incongruences likely reflect a model of evolution of MGIs by inter-MGI recombination events.
A closer look at the distribution of variable genes among the MGIs also suggests evidence of recombination in the evolutional history of MGIs. The vast majority of the variable genes are always found in more than one MGI. For example MGI_Vch_USA1, MGI_Vfl_Ind1, MGI_Psp_Arc1, and MGI_Vch_Moz6 have the same genetic content in variable region 1 (Fig. 2A); however, the same MGIs exhibit very different genetic contents in variable regions 2 and 3. The genetic variation between the variable DNA of MGIs indicate that these elements are mosaics and again supports the idea that intra-MGI recombination is an important driver of their evolution in a manner similar to that of the SXT/R391 ICEs (4). It was previously shown that when two SXT/R391 ICEs are present in a cell, they can form tandem arrays by integrating into the same site (42) and recombine to form hybrids using either the host protein RecA or a distant relative of the λ Red recombination system that they encode (43, 44). This phenomenon enhances their diversity and drives their evolution. MGIs do not seem to code for putative homologous recombination functions. However, RecA could be responsible for MGI recombination, and the involvement of the ICE-encoded recombination system is also likely. In the future, it would be interesting to study the ability of MGIs to form tandems and verify whether some MGIs exclude others, as it was reported for the SXT/R391 ICEs (45, 46).
MGIs are part of a larger family of GIs found in various genera.
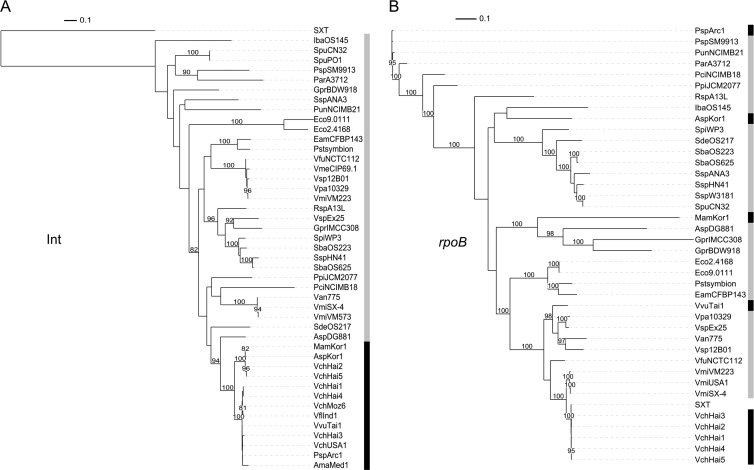
The strong conservation of int among MGIs is an interesting tool to search genomic databases for additional related GIs. To date, proteins that share at least 80% identity with IntMGI_Vch_USA1 have systematically revealed an MGI. However, using this method to screen for new MGIs, we also found many putative integrases related to IntMGI but falling below the 80% threshold. We wondered whether the genes coding for these integrases were part of GIs that could share an evolution history with MGIs. The 31 closest integrases (sharing between 69% and 41% identity with IntMGI_Vch_USA1; see Table S3 in the supplemental material) were included in our analysis. We found that they were encoded by GIs often integrated into the same integration site as the MGIs, as 25 GIs out of 31 (∼81%) are integrated into a gene coding for a putative stress-induced protein related to yicC of E. coli. We constructed phylogenetic trees based on the translation products of these putative int genes or on the nucleotide sequence of rpoB for each of these strains and all the strains bearing an MGI (Fig. 4). Nucleotide sequence of rpoB was chosen to build the tree to provide a better resolution at the species level, while the amino acid sequence of int genes was favored due to the well-known rapid divergence of the nucleotide sequence of genes encoding tyrosine recombinases (47, 48). We found that MGIs form a distinct cluster based on the integrases, even when they originate from the most distant species based on rpoB phylogeny (e.g., MGI_Psp_Arc1 and MGI_Vch_Hai2). This observation suggests frequent loss and horizontal acquisition of MGI by these strains. These results were expected considering the mode of transmission of MGIs and the relative high transfer frequency that we previously observed for MGI_Vfl_Ind1 (6). Interestingly, only MGIs harbor an oriT sequence and cds4 as well as cds8 and rdfM. None of the GIs encoding a related integrase that clusters outside the MGI clade harbor a similar structure. This strongly suggests that the related GIs are not mobilizable by ICEs of the SXT/R391 family. However, as MGIs and their related GIs share the same integration site, MGIs might form tandem arrays with these related GIs. Then recombination events could lead to the acquisition of new genes by MGIs. As such, these related GIs could constitute a significant pool of adaptive genes available for transfer by SXT/R391 ICEs.
Fig 4.
Phylogenetic analysis of MGIs and related GIs. (A) Tree based on protein sequences of Int from SXT, MGIs, and 31 related GIs; (B) phylogenetic tree based on the nucleotide sequences of the rpoB gene from the corresponding strains. Bootstrap values are indicated when over 80%. The individual scale bars represent genetic distances. Refer to Table S3 in the supplemental material for GI and strain details.
Excision of putative MGIs and related GIs.
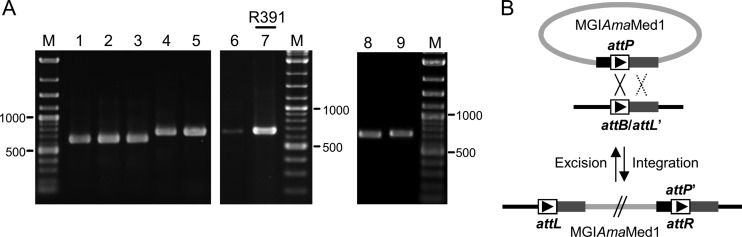
Besides the conservation of oriT in all the putative MGIs identified to date, another way to confirm their functionality as mobile elements is to verify that they can excise in the presence of an SXT/R391 ICE. We tested the 5 Mozambican MGI+ strains by nested PCR to amplify attP sequences resulting from MGIs' excision (Fig. 5A). Two different sets of primers were used due to the polymorphism of the region located on the left side of attR (see Table S1 in the supplemental material). We found that each one of these strains, which all contain an SXT/R391 ICE, yielded a PCR product of expected size (Fig. 5A, lanes 1 to 5 and 7). Sequence analysis of two of the amplicons confirmed the excision event (see Fig. S2 in the supplemental material), indicating that these MGIs are able to form circular intermediates. Surprisingly, MGI_Ama_Med1, from A. macleodii Deep ecotype, an ICE-free strain, was able to excise, yielding a weak PCR product of the expected size (Fig. 5A, lane 6). This result was in contradiction with our previous observations that demonstrated the requirement for SetCD, the main activator of SXT/R391 ICEs, to trigger MGI excision (6, 19). However, sequence analysis of this specific amplicon revealed that excision occurred by homologous rather than site-specific recombination (Fig. 5B). In fact, unlike other MGIs, MGI_Ama_Med1 harbors a 180-bp repeated region on its internal left side and on the right side of its integration site (highlighted in orange in Fig. S3 in the supplemental material); this repeated region seems to be long enough to allow homologous recombination to occur. In order to verify whether despite this particular mechanism of excision MGI_Ama_Med1 was still able to respond to induction by SetCD, we introduced R391, the other prototypical member of the SXT/R391 family originally from P. rettgeri, into A. macleodii Deep ecotype and repeated the same PCR experiment on an R391+ exconjugant. In this case, a strong band corresponding to the amplicon of the expected size was detected, confirming that MGI_Ama_Med1's excision is still triggered by ICEs of the SXT/R391 family (Fig. 5A, lane 7). The anomalous structure of MGI_Ama_Med1, which seems to be flanked by an attL and an attP site, suggests that a related GI, or MGI, was integrated on the right side of MGI_Ama_Med1 in an ancestor of the strain A. macleodii Deep ecotype and was likely lost and supports the idea that two or more MGIs are able to integrate into the same site and form tandem arrays like SXT/R391 ICEs.
Fig 5.
Excision of MGIs and related GIs. (A) Ethidium bromide-stained 1.5% agarose gels of PCR products amplified by nested PCR assays for detection of the excision of the MGIs and related GIs. Lanes: M, 2-log DNA ladder; 1, MGI_Vpa_Moz1; 2, MGI_Vch_Moz2; 3, MGI_Vch_Moz3; 4, MGI_Vch_Moz4; 5, MGI_Vch_Moz6; 6 and 7, MGI_Ama_Med1; 8, GI_Vmi_USA1 from Vibrio mimicus VM573; 9, GI_Spu_PO1 from Shewanella sp. W3-18-1. Lane 7, coresident R391 was present in the cells. (B) Model of excision/integration of MGI_Ama_Med1 by site-specific recombination (solid crossover) and by homologous recombination in the absence of R391 (dotted crossover). Refer to Fig. S3 in the supplemental material for details about the alignment of MGI_Ama_Med1 attachment sites.
We wondered whether the GIs related to MGIs were also able to excise by site-specific recombination from the chromosome of their host. We tested the ability of GI_Spu_PO1 and GI_Vmi_USA1 from Shewanella sp. W3-18-1 and V. mimicus VM573, respectively (Fig. 2C), to form a circular extrachromosomal molecule by amplifying the resulting attP locus by nested PCR (Fig. 5A, lanes 8 and 9). Both GIs yielded PCR products of expected sizes, confirming the excision event. Interestingly Shewanella sp. W3-18-1 bears ICE_Spu_PO1, an SXT/R391 ICE, whereas V. mimicus VM573 does not harbor any SXT/R391 ICE. This finding suggests that, unlike MGIs, the mechanism of excision of these related GIs does not require activation by the SXT/R391 ICE regulator SetCD.
Concluding remarks.
The mechanisms of acquisition of genomic islands are often unknown. There are only few examples of GIs for which the transmission mechanism has been revealed. Nonreplicating Bacteroides units (NBUs) and Tn_4555_ have been shown to be excised and mobilized in trans by Bacteroides conjugative transposons (49–51). The high-pathogenicity island (HPI) found in many Enterobacteriaceae is linked to ICEs and can be mobilized in cis by them (52). In Staphylococcus, transduction has been found to be the mechanism of transfer of Staphylococcus aureus pathogenicity islands (SaPIs). SaPIs are mobilized by helper phages that also induce their excision (53). As for MGIs, their mechanism of mobilization is now well understood (6, 19), and this study gives a better understanding on their characteristics and origins.
Genomic islands are often studied from a strain-centric perspective, and extended studies about families of genomic islands are less frequent. We believe such studies have their importance because they provide a better understanding of the mechanisms involved in the transmission and evolution of genomic islands and the role they play on bacterial genome plasticity.
Supplementary Material
Supplemental material
ACKNOWLEDGMENTS
We are grateful to Steve Vo and Nicolas Carraro for their technical assistance and Dominick Matteau for his contribution to the preparation of Illumina sequencing libraries. We thank Mauro M. Colombo (Università di Roma Sapienza, Rome, Italy), Francisco Rodriguez-Valera (Universidad Miguel Hernandez, Spain), Fabiano Thompson (Federal University of Rio de Janeiro, Brazil), and Alexander S. Beliaev (Pacific Northwest National Laboratory, WA) for the kind gift of strains.
This work was supported in part by a Discovery Grant from the Natural Sciences and Engineering Council of Canada. D.C. was supported by a fellowship from Cenci Bolognetti, Institut Pasteur Foundation, Italy, and part of the work was funded by a grant from PRIN 2007, Italy. V.B. holds a Canada Research Chair in molecular biology, impact and evolution of bacterial mobile elements, and is a member of the FRSQ-funded Centre de Recherche Clinique Étienne-Le Bel. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 30 November 2012
REFERENCES
- 1.Osorio CR, Marrero J, Wozniak RA, Lemos ML, Burrus V, Waldor MK. 2008. Genomic and functional analysis of ICE_Pda_SpaI, a fish-pathogen-derived SXT-related integrating conjugative element that can mobilize a virulence plasmid. J. Bacteriol. 190:3353–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pembroke JT, Piterina AV. 2006. A novel ICE in the genome of Shewanella putrefaciens W3-18-1: comparison with the SXT/R391 ICE-like elements. FEMS Microbiol. Lett. 264:80–88 [DOI] [PubMed] [Google Scholar]
- 3.Boltner D, MacMahon C, Pembroke JT, Strike P, Osborn AM. 2002. R391: a conjugative integrating mosaic comprised of phage, plasmid, and transposon elements. J. Bacteriol. 184:5158–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wozniak RA, Fouts DE, Spagnoletti M, Colombo MM, Ceccarelli D, Garriss G, Dery C, Burrus V, Waldor MK. 2009. Comparative ICE genomics: insights into the evolution of the SXT/R391 family of ICEs. PLoS Genet. 5:e1000786 doi:10.1371/journal.pgen.1000786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrus V, Marrero J, Waldor MK. 2006. The current ICE age: biology and evolution of SXT-related integrating conjugative elements. Plasmid 55:173–183 [DOI] [PubMed] [Google Scholar]
- 6.Daccord A, Ceccarelli D, Burrus V. 2010. Integrating conjugative elements of the SXT/R391 family trigger the excision and drive the mobilization of a new class of Vibrio genomic islands. Mol. Microbiol. 78:576–588 [DOI] [PubMed] [Google Scholar]
- 7.Singer M, Baker TA, Schnitzler G, Deischel SM, Goel M, Dove W, Jaacks KJ, Grossman AD, Erickson JW, Gross CA. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demarre G, Guerout AM, Matsumoto-Mashimo C, Rowe-Magnus DA, Marliere P, Mazel D. 2005. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPalpha) conjugative machineries and their cognate Escherichia coli host strains. Res. Microbiol. 156:245–255 [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Lopez A, Bartual SG, Stal L, Onyshchenko O, Rodriguez-Valera F. 2005. Genetic analysis of housekeeping genes reveals a deep-sea ecotype of Alteromonas macleodii in the Mediterranean Sea. Environ. Microbiol. 7:649–659 [DOI] [PubMed] [Google Scholar]
- 10.Murray AE, Lies D, Li G, Nealson K, Zhou J, Tiedje JM. 2001. DNA/DNA hybridization to microarrays reveals gene-specific differences between closely related microbial genomes. Proc. Natl. Acad. Sci. U. S. A. 98:9853–9858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Umayam L, Gill SR, Nelson KE, Read TD, Tettelin H, Richardson D, Ermolaeva MD, Vamathevan J, Bass S, Qin H, Dragoi I, Sellers P, McDonald L, Utterback T, Fleishmann RD, Nierman WC, White O, Salzberg SL, Smith HO, Colwell RR, Mekalanos JJ, Venter JC, Fraser CM. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, Taviani E, Jeon YS, Kim DW, Brettin TS, Bruce DC, Challacombe JF, Detter JC, Han CS, Munk AC, Chertkov O, Meincke L, Saunders E, Walters RA, Huq A, Nair GB, Colwell RR. 2009. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 106:15442–15447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taviani E, Ceccarelli D, Lazaro N, Bani S, Cappuccinelli P, Colwell RR, Colombo MM. 2008. Environmental Vibrio spp., isolated in Mozambique, contain a polymorphic group of integrative conjugative elements and class 1 integrons. FEMS Microbiol. Ecol. 64:45–54 [DOI] [PubMed] [Google Scholar]
- 14.Ahmed AM, Nakagawa T, Arakawa E, Ramamurthy T, Shinoda S, Shimamoto T. 2004. New aminoglycoside acetyltransferase gene, aac(3)-Id, in a class 1 integron from a multiresistant strain of Vibrio fluvialis isolated from an infant aged 6 months. J. Antimicrob. Chemother. 53:947–951 [DOI] [PubMed] [Google Scholar]
- 15.Thompson CC, Vicente AC, Souza RC, Vasconcelos AT, Vesth T, Alves N, Jr, Ussery DW, Iida T, Thompson FL. 2009. Genomic taxonomy of vibrios. BMC Evol. Biol. 9:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CY, Wu KM, Chang YC, Tsai HC, Liao TL, Liu YM, Chen HJ, Shen AB, LI JC, Su TL, Shao CP, Lee CT, Hor LI, Tsai SF. 2003. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 13:2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim UJ, Birren BW, Slepak T, Mancino V, Boysen C, Kang HL, Simon MI, Shizuya H. 1996. Construction and characterization of a human bacterial artificial chromosome library. Genomics 34:213–218 [DOI] [PubMed] [Google Scholar]
- 18.Ceccarelli D, Daccord A, Rene M, Burrus V. 2008. Identification of the origin of transfer (oriT) and a new gene required for mobilization of the SXT/R391 family of integrating conjugative elements. J. Bacteriol. 190:5328–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daccord A, Mursell M, Poulin-Laprade D, Burrus V. 2012. Dynamics of the SetCD-regulated integration and excision of genomic islands mobilized by integrating conjugative elements of the SXT/R391 Family. J. Bacteriol. 194:5794–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumacher MA, Piro KM, Xu W, Hansen S, Lewis K, Brennan RG. 2009. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science 323:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Melderen L, Saavedra De Bast M. 2009. Bacterial toxin-antitoxin systems: more than selfish entities? PLoS Genet. 5:e1000437 doi:10.1371/journal.pgen.1000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson GG, Murray NE. 1991. Restriction and modification systems. Annu. Rev. Genet. 25:585–627 [DOI] [PubMed] [Google Scholar]
- 23.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dower WJ, Miller JF, Ragsdale CW. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127–6145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigue S, Materna AC, Timberlake SC, Blackburn MC, Malmstrom RR, Alm EJ, Chisholm SW. 2010. Unlocking short read sequencing for metagenomics. PLoS One 5:e11840 doi:10.1371/journal.pone.0011840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59:307–321 [DOI] [PubMed] [Google Scholar]
- 28.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Letunic I, Bork P. 2011. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 39:W475–W478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bian F, Xie BB, Qin QL, Shu YL, Zhang XY, Yu Y, Chen B, Chen XL, Zhou BC, Zhang YZ. 2012. Genome sequences of six Pseudoalteromonas strains isolated from Arctic sea ice. J. Bacteriol. 194:908–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasan NA, Choi SY, Eppinger M, Clark PW, Chen A, Alam M, Haley BJ, Taviani E, Hine E, Su Q, Tallon LJ, Prosper JB, Furth K, Hoq MM, Li H, Fraser-Liggett CM, Cravioto A, Huq A, Ravel J, Cebula TA, Colwell RR. 2012. Genomic diversity of 2010 Haitian cholera outbreak strains. Proc. Natl. Acad. Sci. U. S. A. 109:E2010–E2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Math RK, Jin HM, Kim JM, Hahn Y, Park W, Madsen EL, Jeon CO. 2012. Comparative genomics reveals adaptation by Alteromonas sp. SN2 to marine tidal-flat conditions: cold tolerance and aromatic hydrocarbon metabolism. PLoS One 7:e35784 doi:10.1371/journal.pone.0035784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HG, Doronina NV, Trotsenko YA, Kim SW. 2007. Methylophaga aminisulfidivorans sp nov., a restricted facultatively methylotrophic marine bacterium. Int. J. Syst. Evol. Microbiol. 57:2096–2101 [DOI] [PubMed] [Google Scholar]
- 35.Wozniak RA, Waldor MK. 2009. A toxin-antitoxin system promotes the maintenance of an integrative conjugative element. PLoS Genet. 5:e1000439 doi:10.1371/journal.pgen.1000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breitbart M. 2012. Marine viruses: truth or dare. Annu. Rev. Mar Sci. 4:425–448 [DOI] [PubMed] [Google Scholar]
- 37.Bergh O, Borsheim KY, Bratbak G, Heldal M. 1989. High abundance of viruses found in aquatic environments. Nature 340:467–468 [DOI] [PubMed] [Google Scholar]
- 38.Jenal U, Malone J. 2006. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40:385–407 [DOI] [PubMed] [Google Scholar]
- 39.Romling U, Amikam D. 2006. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 9:218–228 [DOI] [PubMed] [Google Scholar]
- 40.Ryjenkov DA, Simm R, Romling U, Gomelsky M. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281:30310–30314 [DOI] [PubMed] [Google Scholar]
- 41.Bordeleau E, Brouillette E, Robichaud N, Burrus V. 2010. Beyond antibiotic resistance: integrating conjugative elements of the SXT/R391 family that encode novel diguanylate cyclases participate to c-di-GMP signalling in Vibrio cholerae. Environ. Microbiol. 12:510–523 [DOI] [PubMed] [Google Scholar]
- 42.Hochhut B, Beaber JW, Woodgate R, Waldor MK. 2001. Formation of chromosomal tandem arrays of the SXT element and R391, two conjugative chromosomally integrating elements that share an attachment site. J. Bacteriol. 183:1124–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garriss G, Waldor MK, Burrus V. 2009. Mobile antibiotic resistance encoding elements promote their own diversity. PLoS Genet. 5:e1000775 doi:10.1371/journal.pgen.1000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burrus V, Waldor MK. 2004. Formation of SXT tandem arrays and SXT-R391 hybrids. J. Bacteriol. 186:2636–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marrero J, Waldor MK. 2005. Interactions between inner membrane proteins in donor and recipient cells limit conjugal DNA transfer. Dev. Cell 8:963–970 [DOI] [PubMed] [Google Scholar]
- 46.Marrero J, Waldor MK. 2007. The SXT/R391 family of integrative conjugative elements is composed of two exclusion groups. J. Bacteriol. 189:3302–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Argos P, Landy A, Abremski K, Egan JB, Haggard-Ljungquist E, Hoess RH, Kahn ML, Kalionis B, Narayana SV, Pierson LS., III 1986. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 5:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esposito D, Scocca JJ. 1997. The integrase family of tyrosine recombinases: evolution of a conserved active site domain. Nucleic Acids Res. 25:3605–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Shoemaker NB, Wang GR, Salyers AA. 2000. Characterization of a Bacteroides mobilizable transposon, NBU2, which carries a functional lincomycin resistance gene. J. Bacteriol. 182:3559–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li LY, Shoemaker NB, Wang GR, Cole SP, Hashimoto MK, Wang J, Salyers AA. 1995. The mobilization regions of two integrated Bacteroides elements, NBU1 and NBU2, have only a single mobilization protein and may be on a cassette. J. Bacteriol. 177:3940–3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith CJ, Parker AC. 1993. Identification of a circular intermediate in the transfer and transposition of Tn_4555_, a mobilizable transposon from Bacteroides spp. J. Bacteriol. 175:2682–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paauw A, Leverstein-van Hall MA, Verhoef J, Fluit AC. 2010. Evolution in quantum leaps: multiple combinatorial transfers of HPI and other genetic modules in Enterobacteriaceae. PLoS One 5:e8662 doi:10.1371/journal.pone.0008662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mir-Sanchis I, Martinez-Rubio R, Marti M, Chen J, Lasa I, Novick RP, Tormo-Mas MA, Penades JR. 2012. Control of Staphylococcus aureus pathogenicity island excision. Mol. Microbiol. 85:833–845 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material