Modular Evolution of TnGBSs, a New Family of Integrative and Conjugative Elements Associating Insertion Sequence Transposition, Plasmid Replication, and Conjugation for Their Spreading (original) (raw)
Abstract
Integrative and conjugative elements (ICEs) have a major impact on gene flow and genome dynamics in bacteria. The ICEs Tn_GBS1_ and Tn_GBS2_, first identified in Streptococcus agalactiae, use a DDE transposase, unlike most characterized ICEs, which depend on a phage-like integrase for their mobility. Here we identified 56 additional Tn_GBS_-related ICEs by systematic genome analysis. Interestingly, all except one are inserted in streptococcal genomes. Sequence comparison of the proteins conserved among these ICEs defined two subtypes related to Tn_GBS1_ or Tn_GBS2_. We showed that both types encode different conjugation modules: a type IV secretion system, a VirD4 coupling protein, and a relaxase and its cognate oriT site, shared with distinct lineages of conjugative elements of Firmicutes. Phylogenetic analysis suggested that Tn_GBS_s evolved from two conjugative elements of different origins by the successive recruitment of a transposition module derived from insertion sequences (ISs). Furthermore, Tn_GBS_s share replication modules with different plasmids. Mutational analyses and conjugation experiments showed that Tn_GBS1_ and Tn_GBS2_ combine replication and transposition upstream promoters for their transfer and stabilization. Despite an evolutionarily successful horizontal dissemination within the genus Streptococcus, these ICEs have a restricted host range. However, we reveal that for Tn_GBS1_ and Tn_GBS2_, this host restriction is not due to a transfer incompatibility linked to the conjugation machineries but most likely to their ability for transient maintenance through replication after their transfer.
INTRODUCTION
Mobile genetic elements (MGEs) play a profound role in the evolution of both eukaryotes and prokaryotes. In bacteria, they are major contributors to gene flow within and among species and they may represent a significant proportion of their genomes. For example, Escherichia coli O157H7 strain EC 4115 carries 18 integrated prophages totaling 786 kb, covering 15% of its genome (1). In the obligate intracellular bacterium Orientia tsutsugamushi, 30% of the genome corresponds to multiple copies of a 33-kb integrative and conjugative element (ICE) (2). These elements may carry genes which provide a selective advantage for their hosts, such as specific metabolic functions, drug or heavy metal resistance, and virulence factors. Indeed, MGEs were initially described as being responsible for spreading antibiotic resistance (3). However, systematic sequencing of bacterial genomes revealed a much broader diversity of MGEs, some without obvious selective function for the host.
Among the different MGEs identified in bacterial genomes, there is a growing interest in ICEs (4). These elements combine two properties, being conjugative like conjugative plasmids and having the capacity to integrate into the bacterial host chromosome like temperate phages. Integration into a host chromosome ensures the vertical transmission of these MGEs, whereas the capacity to be transferred by conjugation allows their horizontal spreading. A recent systematic search for conjugation machineries related to type IV secretion systems (T4SS) in complete genome sequences showed that ICEs are the most abundant conjugative elements among prokaryotes (5). In addition, ICEs have been shown to promote the conjugative transfer of chromosomal DNA among bacterial cells by the so-called HfR mechanism (6). Such chromosomal DNA transfers have been shown to contribute to chromosomal recombination among natural isolates of Streptococcus agalactiae (7), Enterococcus faecalis (8), and Escherichia coli (9).
The conjugative transfer of an ICE requires its excision from the chromosome as a circular molecule. This reaction is catalyzed in most ICEs by a phage-like integrase in some cases associated with an excisionase. Then, as for conjugative plasmids, the DNA transfer is initiated by a relaxase, which performs a single-strand nick of the circularized ICE at the oriT site. The single-stranded DNA covalently bound to the relaxase is then recruited to a pore-forming T4SS by the T4 coupling protein (T4CP) VirD4, where it is actively pumped into the recipient cell (10, 11). In the recipient cell, the relaxase catalyzes the circularization of the ICE, and the second strand is resynthesized, leading to a double-strand circular molecule, which is inserted into the chromosome of the recipient cell by the integrase. However, for S. agalactiae strain NEM316, we recently described two ICEs, Tn_GBS1_ and Tn_GBS2_, that rely on a DDE transposase for circularization and integration into the chromosome (12). Tn_GBS1_ is 47 kb long, carries 49 protein-encoding genes, and is present in three copies in strain NEM316, whereas Tn_GBS2_ is 33.5 kb long and carries 36 genes. In addition, Tn_GBS2_ shows a unique insertion specificity among bacterial transposable elements 15 or 16 bp (bp) upstream of the −35 region of σA-dependent promoters. Homologs of its transposase have been shown to belong to a new family of insertion sequences (ISs). These ISs show the same insertion specificity upstream of σA-dependent promoters (12). At the time we characterized these two ICEs, no similar element had been identified for any publicly available bacterial genomes, and it was unknown how common and diverse these related elements would be.
In the present study, we took advantage of the increase in genomic sequences publicly available and expanded our previous screen (13) for the presence and distribution of Tn_GBS_-like elements. Indeed, this revealed a large number of ICEs similar to Tn_GBS1_ and Tn_GBS2_. However, they are almost exclusively found in streptococci. Using comparative analyses and phylogeny, we propose an evolutionary model for the emergence of Tn_GBS_ elements that involve the successive recruitment of the same transposition module by two unrelated conjugation machineries. Using in silico prediction and experimental analyses, we show that the Tn_GBS_ ICE family used a highly efficient strategy for spreading, involving both genome integration by transposition and replication as a plasmid. The requirement for both transient replication and stable insertion into the chromosome is probably responsible for the observed host restriction to the genus Streptococcus. The Tn_GBS_ family therefore represents a paradigm to study the coupling of transposition, conjugation, and replication.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this work are listed in Table S1 in the supplemental material. Escherichia coli strains DH5α (Invitrogen) and XL2-Blue (Stratagene) were used for subcloning. Streptococcus agalactiae, S. gallolyticus, S. pyogenes, Enterococcus faecalis, and Bacillus subtilis were grown in Todd-Hewitt (TH) broth or agar (Difco Laboratories, Detroit, MI) and E. coli in Luria-Bertani medium. Antibiotics were used at the following concentrations: for E. coli, 100 mg · liter−1 ampicillin and 150 mg · liter−1 erythromycin; for S. agalactiae, 10 mg · liter−1 erythromycin, 100 mg · liter−1 rifampin, 10 mg · liter−1 fusidic acid, and 10 mg · liter−1 tetracycline; for S. gallolyticus, 10 mg · liter−1 erythromycin and 10 mg · liter−1 tetracycline; for S. pyogenes and E. faecalis, 10 mg · liter−1 erythromycin, 100 mg · liter−1 rifampin, and 10 mg · liter−1fusidic acid; and for B. subtilis, 10 mg · liter−1 erythromycin.
General DNA techniques and sequencing.
Plasmid DNA was prepared by alkaline lysis using the Qiagen miniprep plasmid kit, and genomic DNA was extracted using the Qiagen DNeasy kit (Qiagen, Hilden, Germany). The oligonucleotide primers used for PCRs were designed with the Primer3 software (http://primer3.sourceforge.net). To determine insertion sites of Tn_GBS_s and of derived recombinant transposons, sequencing reactions were directly performed on genomic DNA. Complete genome sequences were determined by using Illumina technology (GAIIX). The 36 nucleotide reads were assembled using Velvet software (14). Contigs corresponding to Tn_GBS_s elements were identified by BLASTN and TBLASTN searches, with the sequences of Tn_GBS1_ and Tn_GBS2_ and the sequences of the proteins encoded by these two ICEs as queries, respectively. Sequences between contigs were determined by Sanger sequencing after PCR amplification of the corresponding regions. The four newly identified ICEs were annotated manually by similarity with Tn_GBS2_ and are available from DDBJ/GenBank/EMBL under the following accession numbers: KC492040 (Tn_GBS2.3_), KC492041 (Tn_GBS2.4_), KC460338 (T_nGBS2.5_), and KC492042 (Tn_GBS2.6_).
Plasmid and strain construction.
Oligonucleotides used in this work are listed in Table S2 in the supplemental material. Mobilizable plasmids carrying the putative oriT regions of Tn_GBS1_ and Tn_GBS2_ are derived from the broad-spectrum vector pTCV_erm_ (15). The Tn_GBS1_ and Tn_GBS2 oriT_ regions were amplified by PCR using the primer pairs O-3/O-4 and O-5/O-6, respectively. The PCR products were subsequently cloned into pTCV_erm_ after digestion of the vector and inserts by EcoRI and BamHI. For pSU-erm::MiniTn_GBS1_ construction, a fragment containing both inverted repeats (IRs) and gbs0410 (the transposase gene of Tn_GBS1_) was amplified from Tn_GBS1_ circular forms and by using the oligonucleotide pair O-1/O-2. The PstI-BamHI amplimer was cloned into the pSU18-erm plasmid. pSU18-erm was obtained by cloning the Tn_1545_ erythromycin resistance gene (16) into the pACYC184 derivative pSU18 (17). pSU-erm::MiniTn_GBS2_ was constructed by cloning the HindIII-SalI DNA fragment from pMUTIN::MiniTn_GBS2_ (12) and encompassing the transposase gene and the two IRs into the pSU18-erm vector. The resulting plasmids are unable to replicate in S. agalactiae. DNA fragments encompassing the repE and repA homologs were amplified by using the oligonucleotide pairs O-29/O-30 and O-27/O-28 and cloned as the NotI-SacII fragment into pJIM2246-Ptet for complementation of the repE and repA insertion mutants, respectively. The constructed plasmids were verified by sequencing and introduced into S. agalactiae strains by electroporation using electrocompetent cells prepared as described previously (18).
Strain GMP205 is a derivative of S. agalactiae strain A909RF carrying Tn_GBS1_ with an inserted copy of pSU-erm::MiniTn_GBS2_ in the gbs0398-gbs0399 intergenic region. The insertion of the pSU derivative was obtained through a mating experiment between an NEM316 derivative carrying pSU-erm::MiniTn_GBS2_ inserted into the core genome and strain BM110. Tn_GBS1_::erm was subsequently transferred by conjugation to strain A909RF, and the insertion of the ICE into the chromosome was obtained after several passages on TH plates supplemented with erythromycin. The insertion position of Tn_GBS1_ in strain GMP205 upstream of rpsJ was identified by direct sequencing on chromosomal DNA. Insertional inactivation of repE and repA genes was carried out by a single crossover of pG+host5 (pG1) plasmid derivatives encoding resistance to erythromycin (18b) as described by Brochet et al. (7). Briefly, 492- and 521-bp-long internal fragments of Tn_GBS1 repE_ and Tn_GBS2 repA_ homologs were amplified by using the primers pairs O-25/O-26 and O-23/O-24 and cloned as EcoRI-BamHI fragments into the pG1 vector. The resulting plasmids were integrated into strain NEM316, leading to strains GMP206 and GMP207. The mutated copy of Tn_GBS1_ was subsequently transferred by conjugation into strain BM110, leading to strain GMP208. Similarly, 526- and 496-bp-long DNA fragments amplified by using the primer pairs O-35/O-36 and O-33/O-34 and encompassing the 3′ ends of Tn_GBS1 repE_ and Tn_GBS2 repA_ homologs were cloned into pG1. Integration of the resulting plasmids into strain NEM316 led to strains GMP209 and GMP210, respectively (see Table S1 in the supplemental material). Finally, the mutated copy of Tn_GBS1_ was transferred by conjugation from strain GMP209 into strain BM110, where it remains as a circular form (strain GMP211).
Bacterial conjugation.
The donor and recipient strains were mixed together on a 3-mm hydrophobic-edge membrane filter (Millipore, Billerica, MA) with a donor-to-recipient ratio of 1:1. Plates were incubated overnight at 37°C. The bacterial cells were resuspended in TH broth and spread on TH agar plates containing the appropriate antibiotics or, in the case of B. subtilis strain 168, at 44°C to select for transconjugants (TCs). To quantify conjugative transfer efficiencies, dilutions were plated on TH media containing antibiotics for the selection of transposon-containing donor cells. The frequency of transfer was determined by dividing the number of TCs by the number of donor cells. All conjugation assays were performed in triplicate. Control conjugation experiments were performed with the donor or the recipient strain only, and no spontaneous resistant colonies were observed.
Quantitative PCR (qPCR).
Circular forms of Tn_GBS1_ and Tn_GBS2_ were quantified by comparing the amounts of circular ICE DNA (primer pairs O-11/O-12 and O-19/O-20, amplifying the coupling sequences of Tn_GBS1_ and Tn_GBS2_, respectively) with the amounts of circular and integrated forms of the ICE (primers O-13/O-14 and O-21/O-22, located in the transposase gene of Tn_GBS1_ and Tn_GBS2_, respectively). The relative quantity of specific DNA was normalized by the relative amplification of the two primer pairs using pSU::MiniTn_GBS1_ and pSU::MiniTn_GBS2_ as a template in which these two regions are in equal amounts and using the polA gene as control (primers O-17/O-18). DNA fragments were amplified with the ABI PRISM 7900 SDS system and SYBR green PCR kits (Applied Biosystems, Foster City, CA). All measurements were performed at least in triplicate.
Phylogenetic analysis.
For phylogenetic reconstruction, all protein sequences, except the Tn_GBS_ sequences obtained in this work, were retrieved by BLASTP from the NCBI nr protein database using proteins of the Tn_GBS1_ or Tn_GBS2_ prototype as query. The different orthologous proteins were aligned using the MUSCLE algorithm (19), and blocks of poorly aligned positions and divergent regions of the alignment were removed using the Gblock tool (improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments). Maximum likelihood (ML) using PhyML on the phylogeny.fr platform (20) was used to infer phylogenetic relationships. Prior to ML analysis, a protein substitution model for each data set was selected using Prottest3 and the Akaike information criterion (21). ML bootstrap support was determined using 100 bootstrap replicates.
RESULTS
Tn_GBS1_ and Tn_GBS2_ belong to a broad and diverse family of streptococcal ICEs.
In order to evaluate the diversity of Tn_GBS_s, we systematically searched for Tn_GBS_-related ICEs using the published bacterial genome sequences at NCBI. We first searched for genes encoding proteins similar to the transposase of Tn_GBS2_ (Gbs1118), and subsequently, we analyzed the flanking regions for conjugation-related proteins and for the presence of the imperfect inverted repeats (IRs). At the time of writing this work, we identified 52 putative new ICEs encoding a transposase of the Tn_GBS_ type. Interestingly, all except one, which is inserted in the genome of Lactobacillus salivarius strain ATCC 11741, were in streptococcal genomes (see Table S3 in the supplemental material). Among the 47 streptococcal species for which one or more genome sequences are available, 21 contain at least one Tn_GBS_-related ICE. Seven strains contain, like NEM316, two different ICEs of this family and one strain contains three in its genome. Thus, ICEs related to Tn_GBS_ are a large and diverse family of mobile genetic elements, specific for the genus Streptococcus.
Previously, we had shown by DNA/DNA hybridization analysis that Tn_GBS_-related ICEs are common among S. agalactiae isolates from animal origin (12). Based on these results, we selected three bovine and one human strain for which DNA/DNA hybridization indicated the presence of a Tn_GBS_-like element for whole-genome sequencing by Illumina technology. Indeed, assembly and analyses of these genomes allowed us to determine the sequences of four new Tn_GBS_-like ICEs (Tn_GBS2.3_, Tn_GBS2.4_, Tn_GBS2.5_, and Tn_GBS2.6_) (see Table S3 in the supplemental material). In Streptococcus dysgalactiae subsp. equisimilis, we also identified a 6.3-kb transposon bordered by two IRs and expressing a putative transposase that is 91% identical to the transposase expressed by Tn_Sict2-1_ (see Table S3). This transposon probably derived from a Tn_GBS_ element, as it expresses proteins similar to proteins identified in other MGEs but is devoid of functions related to conjugation (data not shown).
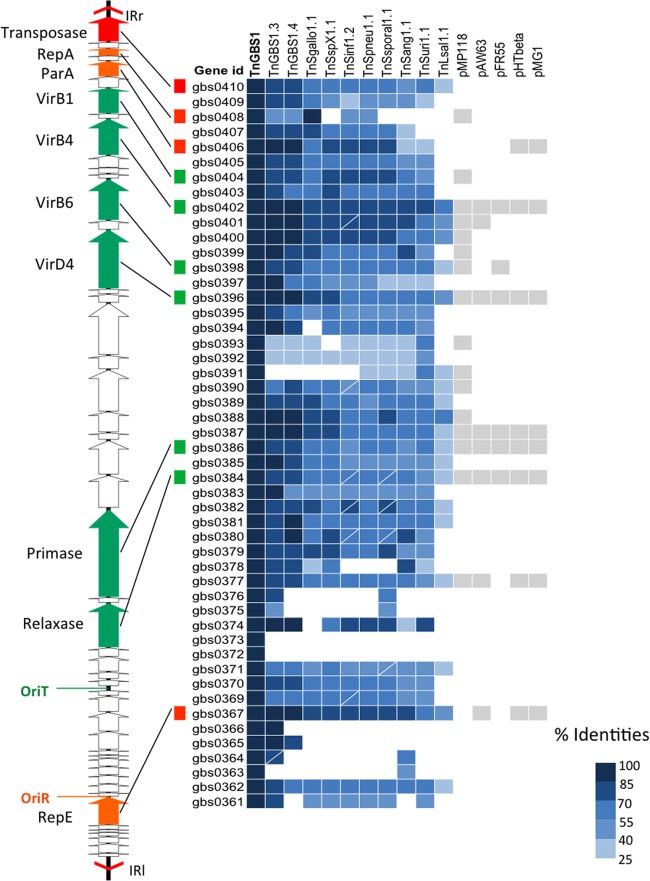
Systematic comparisons of the protein sequences of the newly identified ICEs with Tn_GBS1_ and Tn_GBS2_ CDSs showed that these ICEs were related to either Tn_GBS1_ (23 ICEs) or Tn_GBS2_ (35 ICEs) (Fig. 1 and 2). Accordingly, we named the newly identified ICEs on the basis of their relatedness to Tn_GBS1_ or Tn_GBS2_ (see Table S3 in the supplemental material). To precisely characterize the shared features of each Tn_GBS_ subtype, we selected 33 ICEs representative of the diversity of all identified ICEs for further analyses. Sequence comparison of these ICEs with Tn_GBS1_ and Tn_GBS2_ identified 33 proteins shared by the 10 streptococcal Tn_GBS1_-related elements and 14 proteins shared by the 22 Tn_GBS2_-related elements. The Tn_GBS1_-related ICE identified in L. salivarius shows only 20 proteins similar to Tn_GBS1_ proteins with a systematically lower score than streptococcal Tn_GBS1_-related ICEs. Further analyses showed that these core sets of genes defining each subtype encode proteins likely required for the mobility of these ICEs, as several of the most conserved proteins are similar to proteins involved in the conjugation of different Firmicutes plasmids. For the Tn_GBS1_ and Tn_GBS2_ subtypes, we identified genes encoding the relaxase, the coupling protein VirD4, and components of the T4SS with similarities to two different families of conjugative elements as depicted in Fig. 1 and 2. We did not identify any obvious cargo genes, such as antibiotic resistances or metabolic functions. However, global alignment of ICEs representative of the diversity of the family revealed genes specific for one ICE or a few ICEs that may encode accessory proteins or may represent cargo genes with functions not known yet (see Fig. S1 and S2 in the supplemental material). In summary, the Tn_GBS_ family of transposons comprises, to date, 37 complete and 21 partial ICE sequences that are nearly exclusively present in streptococcal genomes (see Table S3 in the supplemental material).
Fig 1.
Distribution of conserved proteins among Tn_GBS1_-like ICEs and related conjugative plasmids. On the right, the presence of a gene encoding a protein similar to a Tn_GBS1_ protein is indicated by a filled box. The intensity of the blue color corresponds to the percent identities according to the indicated scale. For the related plasmids, the presence of a gene coding for a similar protein or a protein showing a similar domain is indicated by a gray box. The CDS map of Tn_GBS1_ is shown on the left. Genes are indicated by arrows. The gene encoding the DDE transposase, proteins involved in conjugation, and proteins involved in replication are colored in red, green, and orange, respectively. Boxes marked with a white line correspond to genes with a frameshift mutation or a sequencing error. ICE accession numbers are indicated in Table S3 in the supplemental material. Plasmid accession numbers are as follows: pMP118, NC_007930; pAW63, NC_010599; pFR55, NC_010283; pHTβ, AB183714; and pMG1, NC_011364.
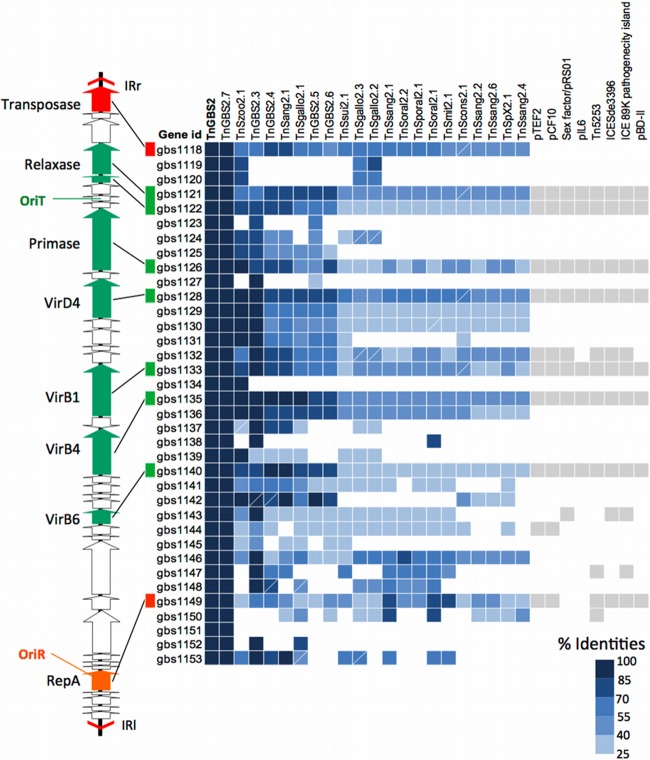
Fig 2.
Distribution of conserved proteins among Tn_GBS2_-like ICEs and related conjugative plasmids and ICEs depending on a phage-like integrase. On the right chart, the presence of a gene encoding a protein similar to a Tn_GBS2_ protein is indicated by a filled box. The intensity of the blue color corresponds to the percent identities according to the indicated scale. For the related plasmids, the presence of a gene coding for a similar protein or a protein showing a similar domain is indicated by a gray box. The CDS map of Tn_GBS2_ is shown on the left. Genes are indicated by arrows. The gene encoding the DDE transposase, proteins involved in conjugation, and proteins involved in replication are colored in red, green, and orange, respectively. Boxes marked with a white line correspond to genes with a frameshift mutation or a sequencing error. Tn_GBS2_-related ICE accession numbers are indicated in Table S3 in the supplemental material. Plasmid and ICE accession numbers are as follows: pTEF2, NC_004671.1; pCF10, AY855841.2; sex factor, NC_009004; pIL6, HM021331.1; Tn_5253_, EU351020.1; ICESde3396, EU142041; 89K pathogenicity island of S. suis, EU589333.1; and pBD-II, NC_017476.
Tn_GBS_s emerged through the assembly of the same transposition module with different conjugation modules.
In order to determine the evolutionary history of these ICEs, we performed phylogenetic analyses of their different components. The common feature unifying the Tn_GBS_ ICE family is a closely related DDE transposase. Phylogeny of the 44 complete transposase sequences of Tn_GBS_-like ICEs and of the transposases of related ISs showed that the transposases of the streptococcal ICEs represent a monophyletic group within a widespread family of ISs, indicating a common IS origin for this transposase (Fig. 3). All transposases of the Tn_GBS1_ group, except the ill-characterized Tn_Lsal1.1_, represent a monophyletic group branching within the transposases of the Tn_GBS2_-like ICEs. Therefore, all Tn_GBS_ transposases seem to have a common origin of a Tn_GBS2_ type except Tn_Lsal1.1_, as well as streptococcal Tn_GBS1_s that have emerged among the Tn_GBS2_-like ICEs.
Fig 3.
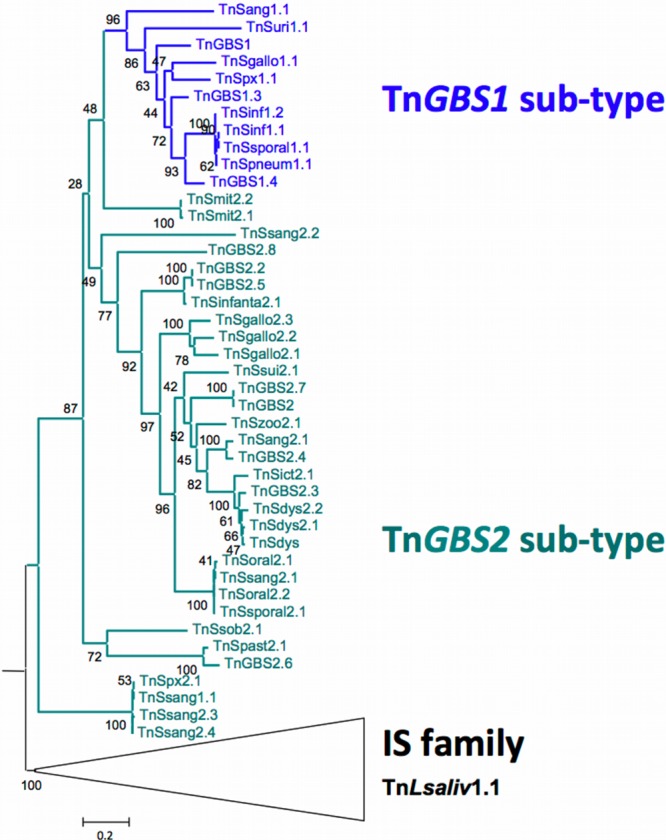
Phylogenetic tree of the Tn_GBS_ transposases. Maximum likelihood (ML) using PhyML on the phylogeny.fr platform (20) was used to infer phylogenetic relationships. ML bootstrap support was determined using 100 bootstrap replicates. The sequence of the transposases of Lactobacillus crispatus, Lactobacillus helveticus, and Bacillus coagulans ISs were used to root the tree. The names correspond to the ICE name given in Table S3 in the supplemental material.
Similarly, the comparison of the IRs of 41 ICEs identified two well-resolved groups showing two different but related consensus sequences corresponding to either Tn_GBS1_- or Tn_GBS2_-related ICEs (see Fig. S3 in the supplemental material). The differences between the IRs of the two subtypes may prevent the cross-recognition of the IRs by the transposases of the two subtypes. Indeed, we have previously shown that strain GMP202, which is a derivative of NEM316, in which the transposase gene of Tn_GBS_2 has been inactivated, does not produce a circular form of Tn_GBS2_, although it expresses three copies of the Tn_GBS1_ transposase (12). This may also explain why we identified five other strains that carry Tn_GBS_s of the two subtypes. Following the emergence of the Tn_GBS1_ subtype, the absence of cross-recognition by Tn_GBS2_-related transposases might therefore have been selected to increase the fitness of these elements.
To further understand the evolution of these ICEs, we also performed a phylogenetic analysis of the VirB4, VirD4, and relaxase proteins, the most conserved proteins of the Tn_GBS_ conjugation machinery, and their counterparts from related conjugative plasmids and ICEs. The phylogenetic trees obtained for the three Tn_GBS1_ proteins showed a perfect congruence, suggesting a single origin and coevolution of these conjugation proteins (see Fig. S4 in the supplemental material). The same result was obtained for these three proteins of Tn_GBS2_-related ICEs (Fig. S5). Therefore, the proteins implicated in conjugation seem to represent genetic modules that have been inherited from different families of conjugative elements en bloc. Interestingly, the conjugation module of Tn_Lsal1.1_ is closely related to conjugative modules of other Tn_GBS1_ proteins, whereas its transposase clusters with IS transposases. This suggests a distinct event of recruitment of an IS-derived transposition module by a conjugative module related to Tn_GBS1_.
Tn_GBS1_ and Tn_GBS2_ transpose upstream σA-dependent promoters but with different preferential sites.
Tn_GBS2_ can be efficiently transferred by conjugation between S. agalactiae strains (12). In order to test whether Tn_GBS1_ is also autonomously conjugative, we used strain GMP205 carrying the nonreplicative plasmid pSU-erm::MiniTn_GBS2_ inserted in the gbs0398-gbs0399 intergenic region of Tn_GBS1_. Indeed, the marked Tn_GBS1_ was transferred efficiently to strain BM110 (Tetr) and retransferred subsequently to strain A909RF. Transconjugants (TCs) were obtained with a frequency of 10−5 TCs per recipient strain, a value similar to the efficiency of the transfer of Tn_GBS2_ (Table 1).
Table 1.
Transfer efficiencies of Tn_GBS1_ and Tn_GBS2_
| Recipient strain | Transfer efficiency of_a_: | |
|---|---|---|
| Tn_GBS1b_ | Tn_GBS2c_ | |
| S. agalactiae BM110 | 7.2 · 10−5 (1.4) | 2.4 · 10−5 (2.5) |
| S. gallolyticus UCN34 | 3.2 · 10−8 (1) | 7 · 10−8 (5.9) |
| S. pyogenes BM137 | <10−9_d_ | 3.5 · 10−5 (3) |
| E. faecalis JH2-2 | <10−9 | <10−9 |
| B. subtilis 168 | <10−9 | <10−9 |
In order to test whether the putative Tn_GBS1_ transposase encoded by gbs0410 is sufficient for transposition, we constructed a nonreplicative plasmid mimicking the circular form of Tn_GBS1_ (pSU-erm::MiniTn_GBS1_) (see Fig. S6 in the supplemental material) and tested its transposition. After electrotransformation into the S. agalactiae strains BM110 and NEM316, Eryr transformants were obtained at a frequency of 30 to 80 transformants per μg of DNA. These transformants indeed harbored a copy of pSU-erm::MiniTn_GBS1_ inserted into the chromosome as shown by direct sequencing of the plasmid-chromosome junctions (see below). Thus, gbs0410 encodes the transposase that is, with the two IRs, sufficient for the insertion of the transposon. Using PCR, we then searched the strains containing Tn_GBS1_ (NEM316 and GMP205) or pSU-erm::MiniTn_GBS1_ (BM110), which was inserted into the chromosome, for the presence of a circular form of the ICE. Using the divergent primers O-11 and O-12, located at both ends of the ICE as shown in Fig. S6, we amplified junction sequences of Tn_GBS1_ and pSU-erm::MiniTn_GBS1_. These sequences included the two IRs separated by 7 to 10 nucleotides derived from the sequence duplicated at the insertion site (data not shown). Thus, Gbs0410 together with the two IRs is also sufficient to generate a circular form of pSU-erm::MiniTn_GBS1_ and Tn_GBS1_. These results showed that, as for Tn_GBS2_, the Tn_GBS1_ transposition process involves two steps catalyzed by the transposase Gbs0410: the formation of a circular form in the donor strain and the integration into the genome of the recipient strain.
We then characterized the insertion specificity of the Tn_GBS1_ transposase. We first attempted to determine the insertion site of Tn_GBS1_ in BM110 TCs. However, in the 25 TCs analyzed directly after conjugation, the DNA sequences obtained corresponded to the circular form of the ICE (see below). Only after up to 7 serial passages on TH-erythromycin agar plates, we identified 16 clones with a copy of Tn_GBS1_ inserted in the genome. Sequence-based analyses showed that in all 16 strains, Tn_GBS1_ was inserted upstream of σA promoters in the same relative orientation as that observed previously for Tn_GBS2_ (12): the IR close to the 3′ end of the transposase gene (IR-right) was located 15 to 17 bp upstream of the −35 region of the promoters (see Fig. S7 in the supplemental material). In order to further analyze this insertion specificity, we determined the insertion sites of a larger number of pSU-erm::MiniTn_GBS1_ transformants. Indeed, all 84 additional insertion sites tested were located upstream of 50 different putative promoters (see Fig. S7). Among the 100 sites analyzed here, 56 were different, indicating only a low level of preference for specific promoters (see Fig. S7). Strikingly, we identified only one promoter, upstream of gbs0411, targeted by Tn_GBS1_ and Tn_GBS2_ and we did not identify any insertion of pSU-erm::MiniTn_GBS1_ upstream of nrdF, the preferred insertion site of Tn_GBS2_ (12). Furthermore, all newly reported ICEs whose insertion site was identified share the same insertion specificity upstream of the promoters of 39 different genes (see Table S3 in the supplemental material). A great majority of targeted genes are conserved housekeeping genes. In particular, 21 ICEs are inserted upstream genes encoding ribosomal proteins.
Tn_GBS1_- and Tn_GBS2_-like ICEs encode different replication modules.
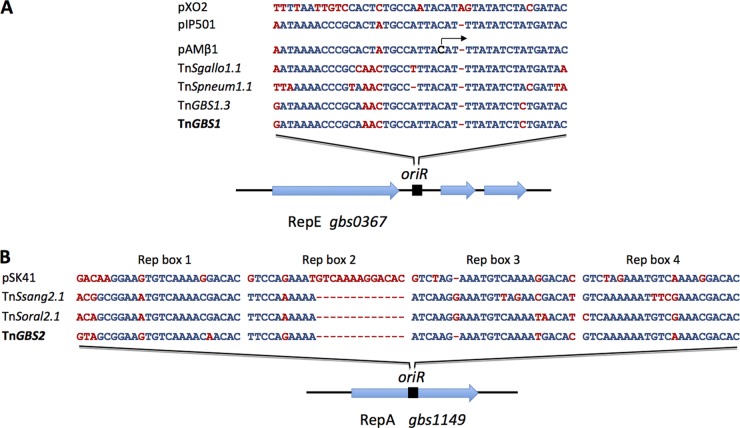
Strikingly, in all Tn_GBS1_- and Tn_GBS2_-like elements, we identified conserved proteins related to plasmid replication (Fig. 1 and 2). This prompted us to further characterize their origins and their role in the propagation of the ICE. All Tn_GBS1_-like ICEs encode a protein homolog of the RepE protein. RepE is a replication initiator protein harboring a characteristic PriCT-1 domain initiating the θ-replication of diverse plasmids of Firmicutes (22). RepE binds to the origin of replication located downstream of the repE gene of the broad-host-range plasmid pAMβ1 (23). Analysis of the DNA region surrounding the repE_-like genes in Tn_GBS1_-like elements identified a conserved sequence similar to the origin of replication characterized in plasmids pAMβ1, pIP501, and pXO2 (23, 24) (Fig. 4A). In contrast, we did not identify the seg locus involved in the stability of these plasmids (25). However, in all Tn_GBS1_-like elements and also in eight Tn_GBS2_-like elements, a gene encoding a protein similar to the ParA protein from the Lactococcus lactis plasmid pCI2000, which is involved in its stability, was present (25). Tn_GBS1 also encodes a putative replication initiator of the RepA_N family (Gbs0408). However, in Tn_GBS1_, this protein is 127 amino acids (aa) long and thus shorter than plasmidic RepA_N proteins, and it is present only in 7 of the 11 Tn_GBS1_-like elements analyzed (Fig. 1). In contrast, all Tn_GBS2_-like ICEs encode a full-length RepA_N protein. RepA_N proteins initiate the replication of a broad range of plasmids from different Firmicutes like pCF10, pTEF2, pSK41, and pBD-II (26). The origin of replication targeted by RepA_N in these plasmids lies within the coding sequence of repA (26). By analyzing the Tn_GBS2 repA_ nucleotide sequence, we identified a sequence similar to the oriR region of the Staphylococcus aureus plasmid pSK41 (27) (Fig. 4B). This sequence represents a putative origin of replication recognized by the Tn_GBS2_ RepA protein. Accessory proteins (RepB and RepC) shown to be involved in plasmid stability (28) are missing in Tn_GBS2_ and in related ICEs. In summary, we identified different replicator proteins and their cognate putative origin of replication in both Tn_GBS1_ and Tn_GBS2_, suggesting that these replication modules are functional.
Fig 4.
Sequence of the putative origins of replication of Tn_GBS1_ and Tn_GBS2_. (A) The putative origins of replication located downstream of the Tn_GBS1 repE_ homologs were aligned with validated or hypothetical origins of replication of Gram-positive plasmids. The validated replication initiation site of pAMβ1 is indicated by an arrow (23). (B) The putative origins of replication located within the repA coding region of Tn_GBS2_, Tn_Ssang2.1_, and Tn_Soral2.1_ were aligned with the oriR site of the S. aureus plasmid pSK41. The four directly repeated sequences (Rep box 1 to Rep box 4) correspond to Rep protein binding sites identified in the S. aureus plasmid pSK41 oriR region (27). Conserved bases are indicated in blue and nonconserved bases in red.
Replication of Tn_GBS1_ and Tn_GBS2_ promotes their transfer.
Sequence-based analyses identified Tn_GBS1_ TCs carrying a circular form of the ICE and the absence of an integrated copy. This result provided a first hint that this ICE replicates in the recipient cell before its insertion. To test whether replication of Tn_GBS1_ and Tn_GBS2_ takes place in the recipient strain, we analyzed the sequence between the two IRs of the circular form, which derives from the duplicated sequence at the insertion site. Indeed, before insertion in the recipient bacterium, the coupling sequence contains the nucleotides duplicated at the insertion site in the donor strain, whereas the circular form generated from an inserted copy in the recipient strain should carry the sequence corresponding to the new insertion site. We thus PCR amplified, cloned, and sequenced the coupling sequences of TCs from Tn_GBS1_ and Tn_GBS2_ (Table 2). In all 151 Tn_GBS1_ sequences analyzed, the coupling sequence corresponded to the insertion site in the donor strain. This result confirms that Tn_GBS1_ replicates in the recipient strain. In the case of Tn_GBS2_, 97.5% (279 sequences) of the circular forms corresponded to the transferred circular Tn_GBS2_ (Table 2). However, we also observed seven coupling sequences that corresponded to ICEs inserted in the recipient. Five corresponded to insertions at nrdF, the preferential site of Tn_GBS2_. Thus, Tn_GBS2_ also replicates in the recipient strain.
Table 2.
Sequence of the coupling sequences in BM110 transconjugants
| Target gene_a_ | Coupling sequence (no. of sequences) | Tn_GBS1_ |
|---|---|---|
| Tn_GBS2_ | ||
| Expt 1 | Expt 2 | |
| gbs1117b | CATTTAAA (108) | CCATTTAAA (171) |
| nrdF | ATTTTTCAA (2) | TTTTTCAA (3) |
| gbs1262 | TTATAACGC (1) | |
| Unknown | GATTTTTAAA (1) | |
| rpsJc | AAATAAA (151) |
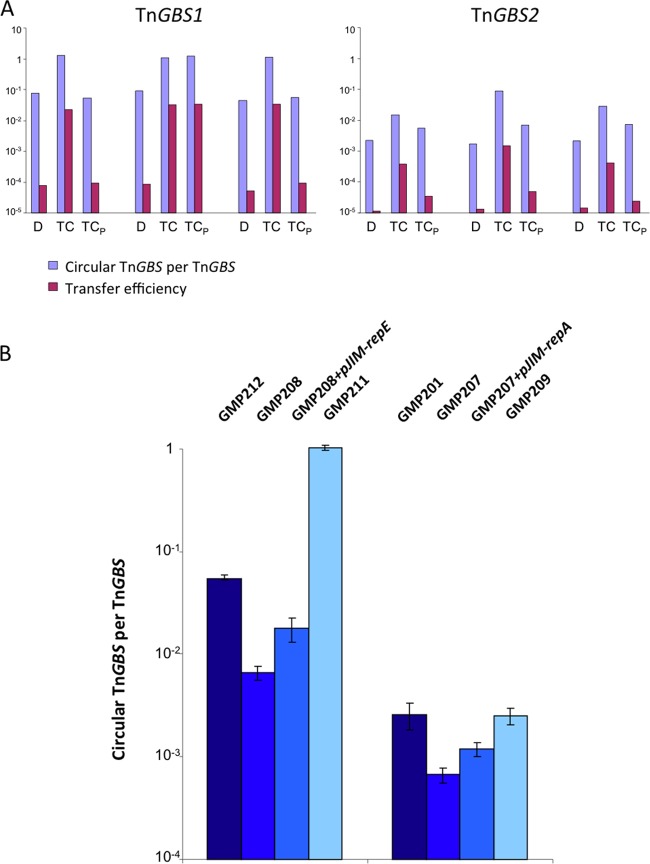
To evaluate the relative amount of the replicating circular form of Tn_GBS1_ and Tn_GBS2_ in the donor strains and in TCs just after the conjugative transfer, we used qPCR (Fig. 5A). We analyzed three independent TCs in detail for Tn_GBS1_ and Tn_GBS2_. For Tn_GBS2_, the ratio between circular and inserted forms was about 2 · 10−3 in the donor strain GMP201, as reported previously (12). Surprisingly, for Tn_GBS1_, the ratio was 35 times higher (7 · 10−2) in strain GMP205. However, for both ICEs, a strong increase in the copy number of circular forms in the TCs was observed. In the case of Tn_GBS1_, one circular form per element was present in the three TCs, a value in agreement with a replicative maintenance of Tn_GBS1_ without insertion. In the three Tn_GBS2_ TCs, the ratio of the circular form ranged from 1 to 10%, values 10 to 100 times higher than the basal ratio observed in the donor. The increase in the copy number of the circular forms of Tn_GBS2_ is in agreement with a transient replication of this ICE after conjugation. In addition, the variable ratios in the three TCs probably reflect a variable delay in the insertion of Tn_GBS2_ in the TC after transfer. Thus, we show here that a transient replication of Tn_GBS_ elements occurs in the recipient cell and that Tn_GBS2_ inserts faster into the recipient's genome than Tn_GBS1_.
Fig 5.
Replication of Tn_GBS1_ and Tn_GBS2_ correlates with its transfer efficiency. (A) The amount of circular forms and the efficiency of transfer were quantified in three independent experiments for Tn_GBS1_ and Tn_GBS2_. Ratios of circular forms to the total amount of ICEs (indicated with blue bars) were quantified by qPCR in the initial donor strain (D) in transconjugants just after selection (TC) and in transconjugants after serial passages on plates to allow the integration of the ICE into the chromosome (TCP). Purple bars represent the conjugation efficiency measured by the ratio of TC colonies to donor colonies using D, TC, and TCP as donor strains. (B) Quantification of the circular forms of Tn_GBS1_ and Tn_GBS2_. Tn_GBS1_ was quantified in strains GMP212 (BM110 Tn_GBS1_::erm), GMP208 (BM110 Tn_GBS1-repE_::pG1), GMP208 pJIM-repE (BM110 Tn_GBS1-repE_::pG1, complemented), and GMP211 (a BM110 Tn_GBS1_-repE+::pG1 transconjugant). Tn_GBS2_ was quantified in strains GMP201 (NEM316 Tn_GBS2_::erm), GMP207 (NEM316 Tn_GBS2-repA_::pG1), GMP207 pJIM-repA (NEM316 Tn_GBS2-repA_::pG1, complemented), and GMP209 (NEM316 Tn_GBS2-repA_+::pG1). Quantifications were performed in triplicate.
To determine whether the increased number of circular forms impacts the transfer efficiency, we performed conjugation assays and quantification of the circular forms in (i) the original donor strains GMP205 and GMP201, (ii) the TCs just after their selection, and (iii) the TCs after serial passages on TH agar to select for integration of the Tn_GBS_s. For both ICEs, we observed a strong correlation between the conjugation efficiency and the number of circular forms present in the donor strain (Fig. 5A). In particular, TCs show an increase in donor efficiency up to 2 logs just after their selection. Due to the integration of Tn_GBS1_ and Tn_GBS2_ into the host chromosome after serial passages (determined by direct sequencing of Tn_GBS_ IR-chromosome junctions), the efficiency of transfer returns to its basal level. We observed only one exception, a Tn_GBS1_ TC in which the ICE remained in its replicative state (Fig. 5A, experiment 2). These results show that the frequency of transfer depends on the number of circular forms in the donor strains and that replication after conjugation transiently increases the efficiency of transfer in subsequent conjugation experiments.
RepE and RepA homologs are required for Tn_GBS1_ and Tn_GBS2_ replication, respectively.
To determine the role of the RepE and RepA homologs in ICE replication, we inactivated the two structural genes by inserting the thermosensitive replication plasmid pG1. Quantification of the relative amounts of the circular forms of Tn_GBS1-repE_::pG1 and Tn_GBS_2-repA::pG1 in the mutated strains (GMP208 and GMP207) showed 8- and 3.7-fold reductions of the circular form of Tn_GBS1_ and Tn_GBS2_, respectively (Fig. 5B). In both mutant strains, the number of circular forms was partially restored by complementation with the repE and repA genes, respectively (Fig. 5B). In order to further ascertain that the reduction of the circular form was not due to a polar effect of the insertion of pG1, we analyzed the effect of the insertion of pG1 downstream of the repE and repA genes. Strain GMP209, carrying pG1 inserted downstream of the repA homolog, shows a similar copy number as the wild-type strain GMP201. In BM110 transconjugants with pG1 inserted downstream of the repE homolog (GMP211), the replication of Tn_GBS1_ was not affected, as it was stably maintained as a circular form (Fig. 5B). These results demonstrate the involvement of the RepE and RepA proteins in the replication of Tn_GBS1_ and Tn_GBS2_, respectively. Furthermore, it shows that both ICEs replicate not only in the recipient strains but also in the donor strains.
The conjugation modules show a broader host range than the ICEs Tn_GBS1_ and Tn_GBS2_.
All except one ICE related to Tn_GBS1_ and Tn_GBS2_ are inserted in streptococcal genomes. To further analyze their host range, we tested whether Tn_GBS1_ and Tn_GBS2_ could be transferred under laboratory conditions to other streptococci and to closely related Firmicutes. We performed conjugation assays using GMP205 and GMP201 as donor strains for Tn_GBS1_ and Tn_GBS2_, respectively, and S. gallolyticus, S. pyogenes, E. faecalis, and Bacillus subtilis strains as recipients (Table 1). However, TCs were obtained with S. gallolyticus, with a 1,000-fold reduced frequency for Tn_GBS1_ and Tn_GBS2_. When S. pyogenes was used as the recipient, TCs were only obtained with Tn_GBS2_, and no TCs were obtained with E. faecalis and B. subtilis in three independent experiments for both ICEs (Table 1). Our results obtained under laboratory conditions substantiate the observation that Tn_GBS1_ and Tn_GBS2_ show a restricted host range for streptococci, with a high preference for its natural host S. agalactiae.
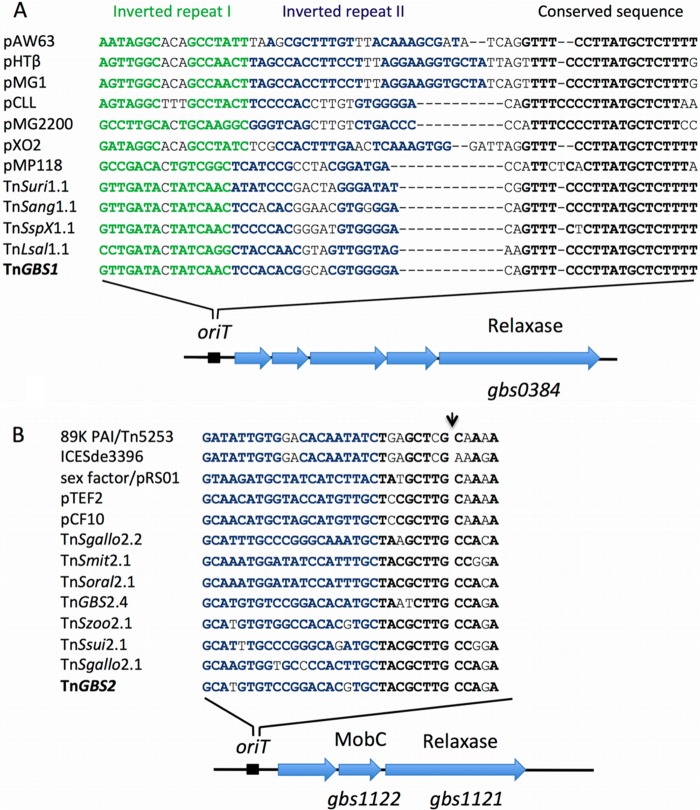
To test whether the limited host range of Tn_GBS_s is due to the incompatibility of their respective conjugation machineries, we analyzed their capacity to mobilize plasmids carrying the respective origin of transfer by conjugation. In Tn_GBS1_, gbs0384 encodes a protein distantly related to a family of relaxases responsible for the mobility of the vancomycin resistance conjugative plasmids pHTβ and pMG2200 of Enterococcus (29). As in pMG2200, the functional oriT sequence has been identified (30), and we searched for sequences showing a similar organization in Tn_GBS1_-like ICEs. Indeed, we identified a conserved sequence sharing similar features: two inverted repeats followed by a 20-bp conserved sequence (Fig. 6A). This sequence is located upstream of gbs0380, the first gene of the putative relaxase-encoding operon. To determine the functionality of this putative oriT_Tn_GBS1 site, we cloned a 731-bp-long fragment encompassing this region into the nonmobilizable vector pTCV_erm_ (15) and tested for the conjugative mobilization of the resulting plasmid (pTCV_erm-oriT_Tn_GBS1_) from strain NEM316 to strain BM110. Indeed, pTCV_erm-oriT_Tn_GBS1_ was efficiently transferred to strain BM110, demonstrating that this region encompassed the origin of transfer of Tn_GBS1_ (Table 3).
Fig 6.
Tn_GBS oriT_ regions. (A) Tn_GBS1 oriT_ regions. The predicted origin of transfer of Tn_GBS1_ located upstream of the gbs0384 putative relaxase gene in Tn_GBS1_ was aligned with validated or hypothetical oriT sites of Gram-positive plasmids. The two inverted repeats are shown in green and blue, and the conserved nucleotides are in black and bold. (B) Tn_GBS2 oriT_ region. The conserved parts of the predicted origin of transfer of selected Tn_GBS2_-like ICEs located upstream of the gbs1121 relaxase gene in Tn_GBS2_ were aligned with the validated or hypothetical oriT sites of Gram-positive plasmids and ICEs. The inverted repeat is shown in blue, and the conserved sequence that includes the nick site is in bold. The demonstrated nick sites in pRS01 and pCF10 are indicated by an arrow.
Table 3.
Tn_GBS1_ and Tn_GBS2 oriT_ sites: mobilization efficiency of pTCV-erm derivatives
| Donor strain | Mobilization efficiency of indicated recipient strain_a_ | ||
|---|---|---|---|
| S. agalactiae BM110 | S. gallolyticus UCN34 | E. faecalis JH2-2 | |
| NEM316 pTCV-erm_Ω_oriT_Tn_GBS1 | 1.7 · 10−5 (0.8) | 4.6 · 10−6 (1.9) | 1.4 · 10−5 (0.3) |
| NEM316 pTCV-erm_Ω_oriT_2Tn_GBS2 | 2.2 · 10−6 (0.3) | 1.1 · 10−7 (0.2) | 1.2 · 10−6 (0.2) |
| NEM316 pTCV-erm | 7.2 · 10−9 (2.6) | <10−9_b_ | <10−9 |
In Tn_GBS2_-related ICEs, the relaxase (encoded by gbs1121 in Tn_GBS2_) is highly similar to the clade of MOBP7 relaxases of the pheromone-responsive plasmid pCF10 from E. faecalis and of an ICE-called sex factor from L. lactis (31). In pCF10 and in the sex factor, the recruitment of the relaxase on the double-stranded oriT requires a small accessory protein, PcfF and LtrF, respectively. Interestingly, all Tn_GBS2_-related ICEs encode a homolog of this protein (Gbs1122 in Tn_GBS2_). In Tn_GBS2_, an oriT region was previously identified between the IR-right and the transposase gene (12). However, this oriT is unrelated to the one described in pCF10 and in the sex factor. Comparison of the DNA sequence upstream of gbs1123 revealed an approximately 100-bp sequence conserved among Tn_GBS2_-like ICEs, reminiscent of the MOBP7 oriT (31). These oriT loci show a conserved organization with an inverted repeat upstream of a short conserved sequence (Fig. 6B). The prediction of this putative second origin of transfer prompted us to test its functionality. The corresponding DNA region from Tn_GBS2_ was cloned into pTCV_erm_, and the resulting plasmid (pTCV_erm-oriT2_Tn_GBS2_) was assayed for conjugative transfer from strain NEM316 to strain BM110. Indeed, pTCV_erm-oriT_2Tn_GBS2_ was efficiently transferred (Table 3), showing that this region carries a genuine origin of transfer that promotes the efficient transfer of pTCV_erm_ among S. agalactiae strains.
Plasmid pTCV_erm_ is able to replicate in diverse Firmicutes (15). In order to test whether the conjugative machineries of Tn_GBS1_ and Tn_GBS2_ are responsible for their restricted host range, we tested for the transfer of pTCV_erm-oriT_Tn_GBS1_ and pTCV_erm-oriT_2Tn_GBS2_ to S. gallolyticus and E. faecalis, respectively. For both plasmids, we observed similar efficiencies of conjugation from NEM316 to E. faecalis and from NEM316 to the S. agalactiae strain BM110 (Table 3) and a 10-fold reduction from NEM316 to S. gallolyticus. The intergenus plasmid mobilization by Tn_GBS1_ and Tn_GBS2_ to E. faecalis demonstrates that the narrow host specificity of Tn_GBS_ ICEs is not due to the specificity of their conjugation machineries.
DISCUSSION
The identification and characterization of 58 ICEs related to Tn_GBS1_ and Tn_GBS2_ revealed that they represent a broad family of conjugative elements that use a DDE transposase and not a phage-like integrase for their mobility. Among the experimentally described ICEs (32), Tn_GBS_s are as diverse as the Tn_916_/Tn_1545_ family in Firmicutes, which express the tetracycline resistance protein TetM and other antibiotic resistance genes like erm(B) and aphA-3 (33) or the SXT/R391 family of Gram-negative bacteria that express diverse antibiotic resistance genes and/or other specific functions (34). The absence of typical cargo genes (e.g., antibiotic or heavy metal resistance genes) explains why, despite the expansion of this family among streptococcal species, they were discovered only through systematic genome sequencing (35). Nevertheless, some functions encoded by these ICEs might affect the phenotype of their host, providing a selective advantage or disadvantage under certain circumstances. In particular, the putative aggregation proteins (Gbs0393 and Gbs1143 expressed by Tn_GBS1_ and Tn_GBS2_, respectively) are related to the streptococcal antigen I/II family of polypeptide adhesins. In streptococci, members of this family have been shown to be involved in host colonization, biofilm formation, and immune modulation (36, 37). Interestingly, Tn_GBS_ ICEs are underrepresented among pathogenic isolates of human origin. Among the eight published human S. agalactiae genomes, only NEM316 carries Tn_GBS_ elements. However, the human origin of NEM316 has been questioned (38). Furthermore, the recently released sequences of two bovine isolates, ATCC 13813 (NZ_AEQQ00000000) and FSL S3-026 (39) contain two and three Tn_GBS_ ICEs, respectively. Tn_GBS_s are frequent among the human commensal species Streptococcus oralis and S. mitis, whereas they are virtually absent from S. pneumoniae and completely absent from all S. pyogenes genomes available. It seems therefore that Tn_GBS_s are counterselected in human-pathogenic streptococci. Furthermore, in S. agalactiae, Tn_GBS2_ promotes the conjugative transfer of chromosomal DNA (7). This may also hold true for other streptococci in which they would contribute to chromosomal recombination and to the evolution of these species.
Conjugative elements, plasmids, and ICEs have been shown to share numerous features (32). In particular, they often code for closely related conjugation machineries (5). Tn_GBS1_- and Tn_GBS2_-related ICEs express conjugative modules related to different conjugative plasmids. The global congruence of the phylogenetic trees of the VirD4, VirB4, and relaxase proteins indicates the en bloc exchange of these modules. Interestingly, the closest conjugation modules of the Tn_GBS1_ and Tn_GBS2_ subfamilies are harbored by conjugative plasmids of different origin, suggesting that streptococcal Tn_GBS_ evolved by two successive conversions from conjugative plasmids to ICEs by the recruitment of a transposition module of IS origin.
Ser/Tyr site-specific recombinase-dependent ICEs represent a broad family of mobile genetic elements combining diverse integrases and conjugative modules (32). Despite the diversity of transposases encoded by ISs (40), the association of a conjugative module and a DDE transposase was reported for only one other ICE: ICE_6013_ of S. aureus. ICE_6013_ expresses an IS_30_-like transposase and carries a conjugative module similar to that of ICE_Bs1_ (41, 42). A shared feature of the transposase of Tn_GBS_ elements and of the IS_30_ family is the formation of a circular intermediate that is required for the conjugation process (43). However, different transposases like those from the broad IS_3_/IS_911_ family generate such circular intermediates (44) but have not been shown to be associated with a conjugative module to form an ICE. One could speculate that the conversion of a plasmid to an ICE could have been frequent, resulting from a Campbell-type recombination (45) of a plasmid at a conserved IS sequence in the chromosome to generate as a first step a composite conjugative transposon. However, as such combinations were, until now, rarely found, it is likely that this association imposes additional constraints on both the transposition and the conjugative processes.
Similar mobilization/conjugation modules may be stabilized either by replicating as a plasmid or by integrating into the host chromosome (5, 32). Tn_GBS_ ICEs are stabilized by integration in the genome. In addition, we show here that for their propagation, Tn_GBS1_ and Tn_GBS2_ exquisitely combine replication and transposition. Indeed, both ICEs behave transiently as a plasmid in the recipient cell, further underscoring the proximity of ICEs and conjugative plasmids. Although the replication modules belong to the same class as those of related conjugative plasmids (Fig. 1 and 2), the evolutionary trees of their Rep proteins are not congruent with the trees of the conjugation machineries (data not shown). This suggests that they have probably been acquired independently from the conjugation module. We also observed that accessory proteins involved in replication control and plasmid stability are missing. Therefore, the capacity to replicate is probably not selected for the long-term stabilization of these ICEs, but this replication is transient before integration. Similarly, it has recently been shown that ICE_B_s_1_ replicates in the donor strain. The replication of ICE_B_s_1_ is not required for its conjugative transfer but contributes to its stability after excision (46). However, it was suggested to contribute to the spreading of the ICE to many cells in chains after the initial conjugative transfer to one of the cells in the chain (47), similar to the increased donor efficiency of Tn_GBS_ transconjugants we have observed. Nevertheless, replication of Tn_GBS_ ICEs is different from that of ICE_B_s_1_, as it uses typical θ-replication machineries of Firmicutes plasmids and not a rolling circle replication catalyzed by the relaxase NickA (46).
Our results allow us to propose a model for the propagation and stabilization of Tn_GBS_ ICEs. As an integrated form, the ICE is inactive; thus, it is not leading to a burden for the host and it is stably transmitted to its descendants. After stochastic or regulated formation of the circular form, the ICE might replicate with a low efficiency, may express conjugative functions, and may be transferred to a recipient cell, which would be highly proficient in replicating and retransferring the ICE to secondary recipient bacteria. After integration into safe sites upstream of σA promoters, the ICE again becomes silent and is stably maintained. We identified repA genes within several typical ICEs expressing a phage-like integrase (e.g., Tn_5253_) (see Fig. S7 in the supplemental material). Therefore, the combination of insertion and replication might be more common than previously anticipated, further blurring the distinction between ICEs and plasmids (48).
We have identified Tn_GBS_ ICEs in about one-half of the streptococcal species and ISs encoding related transposases in more than 38 Firmicutes species (data not shown). Strikingly, despite the number of available genomes, we identified only one distantly related element in a Lactobacillus species. This raises the question for the reasons of this narrow host range. Under laboratory conditions, Tn_GBS_s show similar restricted host specificities. Indeed, whereas Tn_GBS1_ and Tn_GBS2_ could be efficiently transferred among S. agalactiae isolates, the efficiency of transfer dropped in other streptococcal species and no transfer was detected in closely related Firmicutes, such as Enterococcus or Bacillus species. This host specificity is not due to a limitation of the transfer, as the conjugative module of both Tn_GBS1_ and Tn_GBS2_ promotes the efficient mobilization to E. faecalis of a broad-host-range plasmid carrying their putative oriT sites. The host range of a plasmid seems to be mainly determined by its capacity to replicate in different hosts (49). It has been shown that the host specificity of pAD1 and pIP1017 for E. faecalis results from the specificity of the RepA_N protein (50). The species specificity of replication may also contribute to the host specificity of Tn_GBS_ ICEs. Supporting this hypothesis, in the few S. gallolyticus TCs obtained, unlike in S. agalactiae TCs, Tn_GBS1_ was readily inserted into the chromosome. Tn_GBS1_ is probably unable to efficiently replicate in this streptococcal species, supporting the hypothesis that the efficient transfer process requires replication. Together, our results suggest that the fine adaptation of Tn_GBS_s to their host for replication and insertion has ensured their evolutionary success within the Streptococcus genus but led also to their narrow host range.
Supplementary Material
Supplemental material
ACKNOWLEDGMENTS
We thank Mathieu Brochet, Isabelle Rosinski-Chupin, Patrick Trieu-Cuot, Maria-José Lopez-Sanchez, and Laura Gomez Valero for their interest and critical comments. We are most grateful to Carmen Buchrieser for discussions and critical reading of the manuscript. We also thank Marie-France Lartigue for the gift of plasmid pJIM2246-Ptet.
This work was supported by the French National Research Agency (grants ANR-08-GENM-027-001 and 2010-PATH-004-02), the LabEx project IBEID, and the Fondation pour la Recherche Médicale (FRM).
Footnotes
Published ahead of print 22 February 2013
REFERENCES
- 1.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. 2011. PHAST: a fast phage search tool. Nucleic Acids Res. 39:W347–W352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama K, Yamashita A, Kurokawa K, Morimoto T, Ogawa M, Fukuhara M, Urakami H, Ohnishi M, Uchiyama I, Ogura Y, Ooka T, Oshima K, Tamura A, Hattori M, Hayashi T. 2008. The whole-genome sequencing of the obligate intracellular bacterium Orientia tsutsugamushi revealed massive gene amplification during reductive genome evolution. DNA Res. 15:185–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies J, Davies D. 2010. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74:417–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burrus V, Pavlovic G, Decaris B, Guedon G. 2002. Conjugative transposons: the tip of the iceberg. Mol. Microbiol. 46:601–610 [DOI] [PubMed] [Google Scholar]
- 5.Guglielmini J, Quintais L, Garcillan-Barcia MP, de la Cruz F, Rocha EP. 2011. The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. PLoS Genet. 7:e1002222 doi:10.1371/journal.pgen.1002222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lederberg J, Tatum EL. 1946. Gene recombination in Escherichia coli. Nature 158:558. [DOI] [PubMed] [Google Scholar]
- 7.Brochet M, Rusniok C, Couve E, Dramsi S, Poyart C, Trieu-Cuot P, Kunst F, Glaser P. 2008. Shaping a bacterial genome by large chromosomal replacements, the evolutionary history of Streptococcus agalactiae. Proc. Natl. Acad. Sci. U. S. A. 105:15961–15966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manson JM, Hancock LE, Gilmore MS. 2010. Mechanism of chromosomal transfer of Enterococcus faecalis pathogenicity island, capsule, antimicrobial resistance, and other traits. Proc. Natl. Acad. Sci. U. S. A. 107:12269–12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schubert S, Darlu P, Clermont O, Wieser A, Magistro G, Hoffmann C, Weinert K, Tenaillon O, Matic I, Denamur E. 2009. Role of intraspecies recombination in the spread of pathogenicity islands within the Escherichia coli species. PLoS Pathog. 5:e1000257 doi:10.1371/journal.ppat.1000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llosa M, Gomis-Ruth FX, Coll M, de la Cruz Fd F. 2002. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 45:1–8 [DOI] [PubMed] [Google Scholar]
- 11.Wallden K, Rivera-Calzada A, Waksman G. 2010. Type IV secretion systems: versatility and diversity in function. Cell. Microbiol. 12:1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brochet M, Da Cunha V, Couve E, Rusniok C, Trieu-Cuot P, Glaser P. 2009. Atypical association of DDE transposition with conjugation specifies a new family of mobile elements. Mol. Microbiol. 71:948–959 [DOI] [PubMed] [Google Scholar]
- 13.Da Cunha V, Guerillot R, Brochet M, Glaser P. 2011. Integrative and conjugative elements encoding DDE transposases. _In_Roberts AP, Mullany P. (ed), Bacterial integrative mobile genetic elements. Landes Bioscience, Austin, TX [Google Scholar]
- 14.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poyart C, Trieu-Cuot P. 1997. A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to beta-galactosidase in gram-positive bacteria. FEMS Microbiol. Lett. 156:193–198 [DOI] [PubMed] [Google Scholar]
- 16.Trieu-Cuot P, Poyart-Salmeron C, Carlier C, Courvalin P. 1990. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn_1545_. Nucleic Acids Res. 18:3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez E, Bartolome B, de la Cruz F. 1988. pACYC184-derived cloning vectors containing the multiple cloning site and lacZ alpha reporter gene of pUC8/9 and pUC18/19 plasmids. Gene 68:159–162 [DOI] [PubMed] [Google Scholar]
- 18.Cruz-Rodz AL, Gilmore MS. 1990. High efficiency introduction of plasmid DNA into glycine treated Enterococcus faecalis by electroporation. Mol. Gen. Genet. 224:152–154 [DOI] [PubMed] [Google Scholar]
- 18b.Biswas I, Gruss A, Ehrlich SD, Maguin E. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36:W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abascal F, Zardoya R, Posada D. 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21:2104–2105 [DOI] [PubMed] [Google Scholar]
- 22.Jensen LB, Garcia-Migura L, Valenzuela AJ, Lohr M, Hasman H, Aarestrup FM. 2010. A classification system for plasmids from enterococci and other Gram-positive bacteria. J. Microbiol. Methods 80:25–43 [DOI] [PubMed] [Google Scholar]
- 23.Le Chatelier E, Janniere L, Ehrlich SD, Canceill D. 2001. The RepE initiator is a double-stranded and single-stranded DNA-binding protein that forms an atypical open complex at the onset of replication of plasmid pAMbeta 1 from Gram-positive bacteria. J. Biol. Chem. 276:10234–10246 [DOI] [PubMed] [Google Scholar]
- 24.Tinsley E, Naqvi A, Bourgogne A, Koehler TM, Khan SA. 2004. Isolation of a minireplicon of the virulence plasmid pXO2 of Bacillus anthracis and characterization of the plasmid-encoded RepS replication protein. J. Bacteriol. 186:2717–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lioy VS, Pratto F, de la Hoz AB, Ayora S, Alonso JC. 2010. Plasmid pSM19035, a model to study stable maintenance in Firmicutes. Plasmid 64:1–17 [DOI] [PubMed] [Google Scholar]
- 26.Weaver KE, Kwong SM, Firth N, Francia MV. 2009. The RepA_N replicons of Gram-positive bacteria: a family of broadly distributed but narrow host range plasmids. Plasmid 61:94–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwong SM, Skurray RA, Firth N. 2004. Staphylococcus aureus multiresistance plasmid pSK41: analysis of the replication region, initiator protein binding and antisense RNA regulation. Mol. Microbiol. 51:497–509 [DOI] [PubMed] [Google Scholar]
- 28.Francia MV, Weaver KE, Goicoechea P, Tille P, Clewell DB. 2007. Characterization of an active partition system for the Enterococcus faecalis pheromone-responding plasmid pAD1. J. Bacteriol. 189:8546–8555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomita H, Ike Y. 2005. Genetic analysis of transfer-related regions of the vancomycin resistance Enterococcus conjugative plasmid pHTbeta: identification of oriT and a putative relaxase gene. J. Bacteriol. 187:7727–7737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng B, Tomita H, Inoue T, Ike Y. 2009. Isolation of VanB-type Enterococcus faecalis strains from nosocomial infections: first report of the isolation and identification of the pheromone-responsive plasmids pMG2200, encoding VanB-type vancomycin resistance and a Bac41-type bacteriocin, and pMG2201, encoding erythromycin resistance and cytolysin (Hly/Bac). Antimicrob. Agents Chemother. 53:735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Staddon JH, Dunny GM. 2007. Specificity determinants of conjugative DNA processing in the Enterococcus faecalis plasmid pCF10 and the Lactococcus lactis plasmid pRS01. Mol. Microbiol. 63:1549–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wozniak RA, Waldor MK. 2010. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat. Rev. Microbiol. 8:552–563 [DOI] [PubMed] [Google Scholar]
- 33.Roberts AP, Mullany P. 2011. Tn916-like genetic elements: a diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol. Rev. 35:856–871 [DOI] [PubMed] [Google Scholar]
- 34.Wozniak RA, Fouts DE, Spagnoletti M, Colombo MM, Ceccarelli D, Garriss G, Dery C, Burrus V, Waldor MK. 2009. Comparative ICE genomics: insights into the evolution of the SXT/R391 family of ICEs. PLoS Genet. 5:e1000786 doi:10.1371/journal.pgen.1000786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaser P, Rusniok C, Buchrieser C, Chevalier F, Frangeul L, Msadek T, Zouine M, Couve E, Lalioui L, Poyart C, Trieu-Cuot P, Kunst F. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 45:1499–1513 [DOI] [PubMed] [Google Scholar]
- 36.Brady LJ, Maddocks SE, Larson MR, Forsgren N, Persson K, Deivanayagam CC, Jenkinson HF. 2010. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol. Microbiol. 77:276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maddocks SE, Wright CJ, Nobbs AH, Brittan JL, Franklin L, Stromberg N, Kadioglu A, Jepson MA, Jenkinson HF. 2011. Streptococcus pyogenes antigen I/II-family polypeptide AspA shows differential ligand-binding properties and mediates biofilm formation. Mol. Microbiol. 81:1034–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorensen UB, Poulsen K, Ghezzo C, Margarit I, Kilian M. 2010. Emergence and global dissemination of host-specific Streptococcus agalactiae clones. mBio 1:e00178–10 doi:10.1128/mBio.00178–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards VP, Lang P, Bitar PD, Lefebure T, Schukken YH, Zadoks RN, Stanhope MJ. 2011. Comparative genomics and the role of lateral gene transfer in the evolution of bovine adapted Streptococcus agalactiae. Infect. Genet. Evol. 11:1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34:D32–D36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smyth DS, Robinson DA. 2009. Integrative and sequence characteristics of a novel genetic element, ICE6013, in Staphylococcus aureus. J. Bacteriol. 191:5964–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han X, Ito T, Takeuchi F, Ma XX, Takasu M, Uehara Y, Oliveira DC, de Lencastre H, Hiramatsu K. 2009. Identification of a novel variant of staphylococcal cassette chromosome mec, type II.5, and its truncated form by insertion of putative conjugative transposon Tn_6012_. Antimicrob. Agents Chemother. 53:2616–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiss J, Olasz F. 1999. Formation and transposition of the covalently closed IS30 circle: the relation between tandem dimers and monomeric circles. Mol. Microbiol. 34:37–52 [DOI] [PubMed] [Google Scholar]
- 44.Ton-Hoang B, Betermier M, Polard P, Chandler M. 1997. Assembly of a strong promoter following IS911 circularization and the role of circles in transposition. EMBO J. 16:3357–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell A. 1981. Evolutionary significance of accessory DNA elements in bacteria. Annu. Rev. Microbiol. 35:55–83 [DOI] [PubMed] [Google Scholar]
- 46.Lee CA, Babic A, Grossman AD. 2010. Autonomous plasmid-like replication of a conjugative transposon. Mol. Microbiol. 75:268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babic A, Berkmen MB, Lee CA, Grossman AD. 2011. Efficient gene transfer in bacterial cell chains. mBio 2:e00027–11 doi:10.1128/mBio.00027–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grohmann E. 2010. Autonomous plasmid-like replication of Bacillus ICE_Bs_1: a general feature of integrative conjugative elements? Mol. Microbiol. 75:261–263 [DOI] [PubMed] [Google Scholar]
- 49.Kues U, Stahl U. 1989. Replication of plasmids in gram-negative bacteria. Microbiol. Rev. 53:491–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcinek H, Wirth R, Muscholl-Silberhorn A, Gauer M. 1998. Enterococcus faecalis gene transfer under natural conditions in municipal sewage water treatment plants. Appl. Environ. Microbiol. 64:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material