FUS/TLS assembles into stress granules and is a prosurvival factor during hyperosmolar stress (original) (raw)
. Author manuscript; available in PMC: 2014 Apr 25.
Published in final edited form as: J Cell Physiol. 2013 Nov;228(11):2222–2231. doi: 10.1002/jcp.24395
Abstract
FUsed in Sarcoma/Translocated in LipoSarcoma (FUS/TLS or FUS) has been linked to several biological processes involving DNA and RNA processing, and has been associated with multiple diseases, including myxoid liposarcoma and amyotrophic lateral sclerosis (ALS). ALS-associated mutations cause FUS to associate with stalled translational complexes called stress granules under conditions of stress. However, little is known regarding the normal role of endogenous (non-disease linked) FUS in cellular stress response. Here, we demonstrate that endogenous FUS exerts a robust response to hyperosmolar stress induced by sorbitol. Hyperosmolar stress causes an immediate re-distribution of nuclear FUS to the cytoplasm, where it incorporates into stress granules. The redistribution of FUS to the cytoplasm is modulated by methyltransferase activity, whereas the inhibition of methyltransferase activity does not affect the incorporation of FUS into stress granules. The response to hyperosmolar stress is specific, since endogenous FUS does not redistribute to the cytoplasm in response to sodium arsenite, hydrogen peroxide, thapsigargin, or heat shock, all of which induce stress granule assembly. Intriguingly, cells with reduced expression of FUS exhibit a loss of cell viability in response to sorbitol, indicating a prosurvival role for endogenous FUS in the cellular response to hyperosmolar stress.
Keywords: FUS/TLS, Hyperosmolar stress, Stress granules
Introduction
Fused in sarcoma/translocated in liposarcoma (FUS/TLS or FUS) is an RNA/DNA-binding protein that is implicated in a diverse array of cellular processes. FUS, also known as heterogeneous ribonuclear protein hnRNP P2 (Calvio et al., 1995), is a member of the TET family of proteins that also includes EWS (Ewing's sarcoma) and TAF15 (TATA-binding protein-associated factor 15) (Tan and Manley, 2009). FUS was originally discovered in the context of a fusion oncoprotein in myxoid liposarcoma cells (Crozat et al., 1993). Since then, this multifunctional protein has been linked to various aspects of RNA and DNA-processing, including mRNA splicing (Ishigaki et al., 2012), transcription (Wang et al., 2008), and DNA repair (Kuroda et al., 2000). Recently, mutations in FUS have been linked to the fatal neurodegenerative disease amyotrophic lateral sclerosis (ALS) (Kwiatkowski et al., 2009; Vance et al., 2009).
FUS is predominately expressed in the nucleus of most cells (Andersson et al., 2008), although it shuttles between the nucleus and cytoplasm during mRNA transport (Fujii and Takumi, 2005; Zinszner et al., 1997). Several reports have shown that ALS-linked FUS mutants associate with cytoplasmic stress granules under conditions of oxidative stress and heat shock (Bosco et al., 2010; Dormann et al., 2010; Gal et al., 2010). Stress granules are stalled translational complexes comprised of mRNA, ribosomes, and RNA-binding proteins that form in response to induced stress, such as hyperosmolar stress, oxidative stress, heat shock, ultraviolet irradiation and viral infection (Anderson and Kedersha, 2009). These dynamic complexes are thought to play a role in sorting mRNAs for expression, storage or degradation (Kedersha and Anderson, 2002). More recently, stress granules have also been shown to directly regulate protein activity in the context of cellular signaling (Wippich et al., 2013). In contrast to the aforementioned mutant FUS, much less is known about the association of endogenous FUS with stress granules and the role of endogenous FUS in stress response.
Herein, we sought to examine the response of endogenous FUS to various cellular stressors. We found that inducers of stress granule assembly shown to direct mutant-FUS to stress granules, such as sodium arsenite, thapsigargin, hydrogen peroxide, and heat shock, had no effect on the subcellular distribution of endogenous FUS. In striking contrast, endogenous FUS exhibited a robust redistribution from the nucleus to the cytoplasm and assembled into stress granules under conditions of hyperosmolar stress induced by sorbitol and sucrose. Not only was the response of FUS stress-specific, it was also regulated by methyltransferase activity. Cells with reduced FUS expression were more susceptible to sorbitol-induced toxicity, suggesting that FUS plays protective role with regard to cellular homeostasis. These data establish a novel role for the multifunctional FUS protein in cellular stress response.
Materials and Methods
Cell culture and induced stress
HeLa cells and HEK293 cells were cultured in minimal essential medium (MEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA) and 1% penicillin and streptomycin (P/S, Gibco, Grand Island, NY, USA) under standard culture conditions (37°C, 5% CO2/95% air). NSC-34 cells and mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco's MEM (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% FBS and 1% P/S under standard culture conditions. FlpIn HEK293 cells with stably integrated GFP-FUS G515X were cultured as described previously (Bosco et al., 2010). Sorbitol (Sigma, St. Louis, MO, USA) was dissolved directly into the media to obtain a concentration of 0.4 M and added to the cells (Kedersha and Anderson, 2007). Sucrose (Electron Microscopy Sciences, Fort Washington, PA, USA) was dissolved into media to obtain a final concentration of 600 osmol/L and added to the cells (Bevilacqua et al., 2010). Stock solutions of 100 mM sodium arsenite (Sigma, St. Louis, MO, USA) in DMSO (Sigma, St. Louis, MO, USA), 10 mM thapsigargin (Sigma, St. Louis, MO, USA) in DMSO, 1 M hydrogen peroxide (Sigma, St. Louis, MO, USA) in media, 30 mM emetine (Sigma, St. Louis, MO, USA) in water and 20 mM Adenosine-2′,3′-dialdehyde (AdOx, Sigma, St. Louis, MO, USA) in water were prepared and added to the media to obtain the final concentrations of 0.5 mM, 50 μM, 1.5 mM, 50 μg/ml and 50 μM respectively. Doxycycline (Sigma, St. Louis, MO, USA) was used at a final concentration of 1 μg/ml from a stock of 50 mg/ml prepared in water. Cells were exposed to heat shock by adding media, pre-warmed to 43°C, followed by immediate transfer to an incubator set to 43°C. ON-TARGETplus SMARTpool (Dharmacon, Waltham, MA, USA ) consisting of a pool of siRNAs against FUS (Cat # L-009497-00-0005,) and ON-TARGETplus Non-targeting pool siRNA (Cat # D-001810-10-05) as control were transfected using Lipofectamine-2000 (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions.
NSC-34 cell lines were a kind gift from Dr. Neil Cashman (University of British Columbia). Stable NSC-34 cell lines expressing short hairpin (sh) RNA against mouse FUS (shFUS) or non-targeting scrambled RNA (shSC) were prepared by first transducing with the Tet repressor. A single clone that demonstrated good induction without any leaky expression was then selected. NSC34-TetR cells were then transduced with inducible lentivirus-Tet-on/shFUS or Tet-on/shSC(Ishigaki et al., 2012). Cells were treated with 1 μg/ml doxycycline to induce the expression of the shRNAs.
Immunofluorescence
Immunofluorescence was performed as described in (Bosco et al., 2010). Primary antibody incubation conditions were as follows: 1:500-1000 rabbit anti-FUS (A300-293A, Bethyl Labs, Montgomery, TX, USA); 1:2500 mouse anti-TIAR (610352, BD Transduction Labs, San Jose, CA, USA); 1:1500 rabbit ant-ASYM24 (07-414, Millipore, Billerica, MA, USA); 1:2500 mouse anti-G3BP (611126, BDTransduction Labs, San Jose, CA, USA) for 1 hr at room temperature; and 1:250 mouse anti-GE-1/hedls/p70 S6 kinase (sc-8418, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 12 hrs at 4°C. Secondary anti-mouse IgG antibody conjugated to Dylight 549 (715-505-151, Jackson ImmunoResearch Labs, West Grove, PA, USA) was used at 1:1500–1:3000. Secondary anti-rabbit IgG antibody conjugated to Dylight 488 (711-485-152, Jackson ImmunoResearch Labs, West Grove, PA, USA) and secondary anti-rabbit IgG antibody conjugated to Cy5 (711-175-152, Jackson ImmunoResearch Labs, West Grove, PA, USA) were used at 1:1500–1:3000. GFP signal was enhanced using 1:2000 Alexa Fluor 488-conjugated rabbit anti-GFP (A21311, Invitrogen, Grand Island, NY, USA). Nuclei were stained with 50 nM 4’,6’-diamidine-2-phenylindole dihydrochloride (DAPI; D1306, Invitrogen, Grand Island, NY, USA) for 5 min at room temperature. Coverslips were mounted with ProLong Gold Antifade Reagent (P36930, Invitrogen, Grand Island, NY, USA).
Image acquisition and quantification
Fixed cell images were acquired using a Solamere Technology Group CSU10B (Salt Lake City, UT, USA) spinning disk confocal system as described (Bosco et al., 2010) or using a Leica DMI6000B microscope (Leica Microsystems, Buffalo grove, IL, USA). For images acquired with the Leica microscope, a 100x objective was used with LAS AF One Software (Leica Microsystems, Buffalo grove, IL, USA) and the Leica DFC365FX camera. Maximum projection images were created from acquired image stacks (z=0.2-0.25μm, n=6-44 planes) and analyzed using NIH Image J software.
For quantifying the percentage (%) of nuclear FUS, image stacks (z=0.2μm, n=13 planes) of 60 cells were collected from n=3 experiments with the spinning disk confocal system above. Images were analyzed using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). Sum projections of each image stack were created after subtracting the background signal as described (Bosco et al., 2010). The integrated morphometry analysis tool was used to calculate the percent (%) nuclear FUS. Statistical significance between conditions was determined by an ANOVA and Tukey's post-hoc pairwise test.
Western blots
Western blots were performed essentially as described in (Bosco et al., 2010). Briefly, blots were incubated at 4°C with shaking overnight in the presence of primary antibodies as per the following dilutions: 1:500 anti-tubulin (Sigma. St. Louis, MO, USA), 1:500 anti-FUS (in house antibody created against 264-284 peptide sequence of FUS, Genscript, Piscataway, NJ, USA), 1:500 anti-FUS (47711, Santacruz, Santa Cruz, CA, USA) and 1:1000 anti-ASYM24 (07-414, Millipore, Billerica, MA, USA). Densitometry was performed using the Odyssey infrared imaging systems software (Licor Biosciences, Lincoln, NE, USA).
Immunoprecipitation
Cells resuspended in 50 mM Tris HCl (pH 7.5) supplemented with 1% NP-40, 150 mM NaCl, 5 mM EDTA, 10% glycerol and complete protease inhibitor (lysis buffer) were briefly sonicated and incubated at 4°C with shaking for 30 min. The lysates were centrifuged for 15 min at 13000 rpm and 4°C. Pre-clearing of the supernatants was achieved by incubation with 100 μl of Biomag Protein G beads (Invitrogen, Grand Island, NY, USA) at 4°C with shaking for 2 hrs. The beads were removed with a magnet and the protein concentration of the supernatant was determined using a bicinchoninic assay (ThermoScientific, Billerica, MA, USA). Anti-FUS (Genscript, Piscataway, NJ, USA) or anti-GFP antibody (ab290, Abcam, Cambridge, MA, USA) was bound to fresh beads with shaking for 2 hrs at 4°C. A total of 1 mg of the pre-cleared supernatant was then added to 100μl of antibody-bound beads and incubated overnight with shaking at 4°C. The lysate was removed and beads were washed three times with lysis buffer. Proteins bound to the beads were eluted with 1X SDS sample buffer at 95°C for 5 min, and probed by western as described above.
Cell toxicity assays
NSC-34 cell lines shSC and shFUS were plated in 24 well dishes. 48 hrs after induction with doxycyline, cells were treated with 0.4 M sorbitol or 0.25 mM sodium arsenite for 8 hrs. For the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay 100 μl of 5 mg/ml MTT (Invitrogen, Grand Island, NY, USA) was added to the wells for 35 min followed by cell lysis overnight with 300 μl lysis buffer (10% SDS in 1:1 N,_N_-dimethylformamide:water/2% acetic acid/2.5% HCl 1 M ) and absorbance measurement at 550 nm using the VICTOR V plate reader (Perkin Elmer, Waltham, MA, USA). Cell viability for each sample was calculated using the formula: % viability = 100 × (ODSample – ODBlank)/(ODUntreated – ODBlank). LDH (Lactate dehyrodenase) assay was performed as per manufacturer's protocol (CytoTox 96® Non-radioactive Cytotoxicity Assay, Promega, Madison, WI, USA). After the 8 hr treatment, 50 μl of media from each well was transferred to wells of 96 well plate. 50 l of substrate mix was then added to each well and the plates were covered and incubated at ambient temperature, protected from light for 30 min. After the incubation, 50 l stop solution was added to each well and absorbance was recorded at 490 nm using the above plate reader. Percentage (%) cytotoxicity was determined for each experimental condition (Expt) using the formula: % cytotoxicity = 100 × (ODExpt – ODUntreated)/(ODMax – ODUntreated), where ODMax represents the absorbance of the media from a well with complete lysis of cells releasing maximum LDH. All assays were performed at least three independent times. Statistical significance was determined by a two-tailed Student's t-test.
Results
Endogenous FUS redistributes to the cytoplasm and assembles into stress granules in response to hyperosmolar stress
In order to investigate the role of FUS in stress response, we examined the nucleo-cytoplasmic distribution of FUS in response to various cellular stressors. Hyperosmolar stress induced by the administration of 0.4 M sorbitol to HeLa cells for 1 hr resulted in a striking redistribution of FUS from the nucleus to the cytoplasm, where FUS assembled into numerous puncta. A majority of FUS-positive puncta co-localized with the stress granule marker proteins, G3BP (Fig. 1A; supplementary material Movie M1) and TIAR (Fig. 1B; supplementary material Movie M2) (Kedersha and Anderson, 2007). The redistribution and incorporation of FUS into stress granules in response to sorbitol is reminiscent of other nuclear hnRNPs, such as hnRNP A1 (Guil et al., 2006) and TDP-43 (Dewey et al., 2011). However, not all hnRNP proteins redistribute to stress granules in response to sorbitol (van der Houven van Oordt et al., 2000), suggesting a functional role in stress response for those hnRNPs that do localize to these structures. In addition to sorbitol, hyperosmolar stress induced by sucrose also caused FUS to redistribute to the cytoplasm and incorporate into stress granules (supplementary Fig. S1).
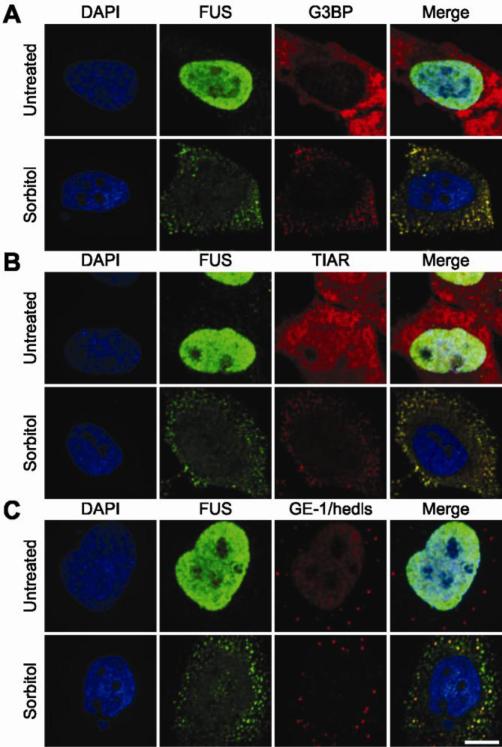
Figure 1. Endogenous FUS redistributes to the cytoplasm and localizes to cytoplasmic stress granules in response to sorbitol.
Confocal images of untreated HeLa cells (top row in each panel) as compared to cells treated with 0.4 M sorbitol for 1 hr (bottom row in each panel; A-C) are shown. Cells probed with an anti-FUS antibody (green) and either the stress granule marker anti-G3BP (A) or anti-TIAR (B) revealed that FUS co-localizes with stress granules in response to sorbitol (see also supplementary material Movies M1 and M2). (C) P-bodies were detected by anti-GE-1/hedls antibody in both untreated and treated conditions; however, the majority of P-bodies did not exhibit co-localization with FUS (see also Supplementary Material Movie M3). Cells were counter stained with the nuclear marker DAPI (blue; A-C). Images are representative of at least n=3 experiments. Scale bar represents 10 μm.
Since stress granules are functionally related to processing bodies (P-bodies), which are cellular sites of mRNA degradation (Moore, 2005), we also probed for the co-localization of endogenous FUS with GE-1/hedls, a constituent of P-bodies but not stress granules (Kedersha and Anderson, 2007). The majority of P-bodies did not co-localize with FUS-positive granules. However, some P-bodies appeared to associate with and/or dock onto FUS-positive granules (Fig. 1C; supplementary material Movie M3), consistent with the physical association between P-bodies and stress granules that has been previously described (Bosco et al., 2010; Kedersha et al., 2005).
Next, we investigated the effect of sorbitol in other cell lines. Administration of sorbitol to HEK (human embryonic kidney)-293T, MEFs (mouse embryonic fibroblasts) and NSC-34 (neuroblastoma × spinal cord hybrid) (Cashman et al., 1992) cell lines recapitulated the results from HeLa cells; FUS redistributed from the nucleus to the cytoplasm, where it assembled into G3BP- and TIAR-positive stress granules (supplementary material Fig. S2). Therefore, the response of FUS to hyperosmolar stress is not a cell type-specific phenomenon, but rather is detected in several different mammalian cell lines.
In contrast to sorbitol and sucrose, FUS did not redistribute to the cytoplasm when HeLa cells were exposed to inducers of oxidative stress (e.g., sodium arsenite and hydrogen peroxide), endoplasmic reticulum (ER) stress (e.g., thapsigargin) or heat shock, all of which induce the formation of stress granules in a majority of cells (Fig. 2) (Emara et al., 2012; Kedersha and Anderson, 2007). Endogenous FUS was not detected in any of the G3BP-positive stress granules that formed under these conditions; we did not detect any cells with elevated cytoplasmic FUS or FUS-positive stress granules under these conditions. Similarly, exogenously expressed wild-type FUS did not redistribute nor assemble into stress granules under the aforementioned conditions (Bentmann et al., 2012; Bosco et al., 2010; Daigle et al., 2013; Dormann et al., 2010), although the effect of sorbitol on endogenous or exogenous FUS has not been reported. Thus our results demonstrate that the formation of stress granules by various stressors is not sufficient to cause a redistribution of FUS to the cytoplasm, indicating that there are specific factors associated with hyperosmolar stress that elicit this response for endogenous FUS.
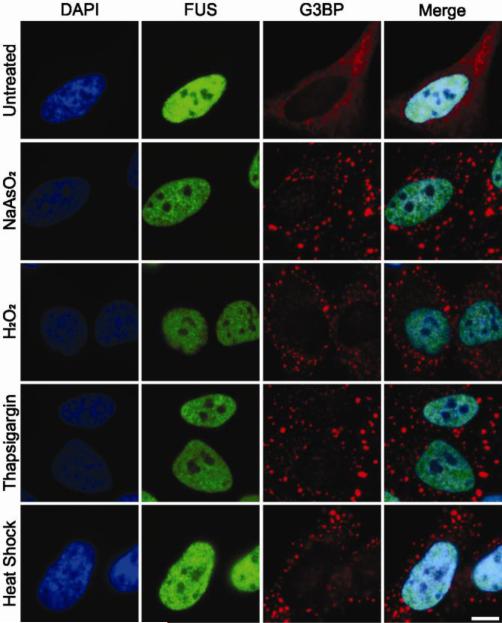
Figure 2. The recruitment of FUS to cytoplasmic stress granules is stress-specific.
HeLa cells were treated with either 0.5 mM sodium arsenite (NaAsO2) for 1 hr, 1.5 mM hydrogen peroxide (H2O2) for 2 hrs, 50 μM thapsigargin for 30 min, or heat shock at 43 °C for 30 min. Immunofluorescence revealed that G3BP-positive stress granules (red) formed under all stress conditions. FUS (green) remained nuclear and absent from stress granules under these stress conditions, similar to the unstressed condition (top panel). Nuclei were stained with DAPI (blue). All images are representative of n=3 independent experiments. Scale bar represents 10 μm.
The assembly of FUS into stress granules is rapid and reversible
The formation of stress granules represents a fast, almost immediate, response of cells to induced stress. To determine the time frame in which FUS responds to sorbitol, we monitored the cellular redistribution of FUS by immunofluorescence microscopy over a one-hour time course of sorbitol exposure. G3BP is an effector of stress granule assembly (Aulas et al., 2012; Tourriere et al., 2003) and was therefore used as a marker to monitor the assembly process. The cytoplasmic redistribution of FUS was detected within 10 min of sorbitol treatment, a time point that preceded the appearance of discreet G3BP-positive cytoplasmic foci, demonstrating that FUS starts to accumulate in the cytoplasm before stress granules are fully formed (Fig. 3A). Within 20 min of sorbitol treatment, discreet G3BP-positive stress granules containing FUS were detected. Therefore FUS appears to incorporate into stress granules on the same time scale that these foci are being formed. G3BP- and FUS-positive stress granules appear fully formed by 60 min, at which time a substantial fraction of FUS was redistributed to the cytoplasm.
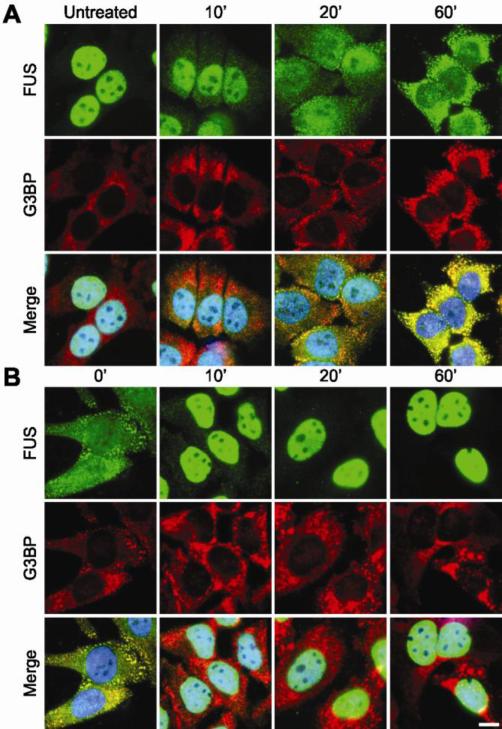
Figure 3. The response of FUS to sorbitol is rapid and reversible.
(A) A representative time-course for the cytoplasmic redistribution of FUS into stress granules upon exposure to hyperosmolar stress. HeLa cells were treated with 0.4 M sorbitol for the indicated time points, fixed, and assessed by immunofluorescence with anti-FUS (green) and anti-G3BP (red) antibodies, and the nuclear marker DAPI (blue). Elevated levels of cytoplasmic FUS were detected as early as 10 min. FUS accumulated into discreet stress granules by 20 min. The nucleo-cytoplasmic distribution of FUS continued to shift towards the cytoplasm over the remaining time course. (B) A representative time-course for the return of FUS to the nucleus and the concomitant disassembly of stress granules upon withdrawal of sorbitol. HeLa cells were treated with 0.4 M sorbitol for 1 hr, after which the sorbitol was replaced with fresh media and the cells were processed as described in (A). A majority of FUS re-localized to the nucleus within 10 min. Some G3BP positive stress granules persisted for up to 1 hr. Images are representative of at least n=3 experiments. Scale bar represents 10 μm.
The formation of stress granules is a reversible process (Anderson and Kedersha, 2008). After the induced stress is removed, stress granules disassemble as the cell re-establishes homeostasis. We monitored the disassembly of stress granules in HeLa cells pre-treated with sorbitol to determine the subcellular fate of FUS as cells re-established homeostasis. The disassembly of stress granules was initiated by replacing media containing sorbitol with fresh media lacking sorbitol, and cells were monitored for 60 min by immunofluorescence microscopy as described above. Within 10 min of removing sorbitol from the media, FUS dissociated from stress granules and re-distributed to the nucleus in virtually all (~90%) cells. However, G3BP-positive, FUS-negative foci persisted in approximately one third of cells at this time point (Fig. 3B). For the remainder of the time course, FUS was localized to the nucleus while G3BP-positive stress granules gradually continued to disassemble until the 60 min time point, when ~20% of cells contained G3BP-positive stress granules. These data show that FUS exhibits a rapid response not only to the administration of sorbitol (Fig. 3A), but also to the removal of this stressor (Fig. 3B).
Stress granule assembly is required for robust cytoplasmic redistribution of FUS
FUS is a nucleo-cytoplasmic shuttling protein. Therefore, the accumulation of FUS in the cytoplasm can result from increased export of the protein from the nucleus and/or decreased import to the nucleus from the cytoplasm. The nucleo-cytoplasmic equilibrium of FUS may be shifted towards the cytoplasm through FUS binding interactions. For example, injection of anti-FUS antibodies into cells trapped the majority of FUS in the cytoplasm within 2 hrs (Zinszner et al., 1997). Since the timescale of FUS redistribution from the nucleus to the cytoplasm under conditions of hyperosmolar stress (1 hour, Fig. 3) is similar to that in aforementioned antibody study (2 hours, (Zinszner et al., 1997)), we asked whether or not stress granules serve as a “cytoplasmic sink” that effectively traps FUS in the cytoplasm through mass action. Stress granule assembly was inhibited by the addition of 50 g/ml emetine, which stabilizes polysomes and blocks translation elongation (Kedersha et al., 2000), for 1 hr prior to the administration of hyperosmolar stress. As expected, only diffuse G3BP signal (i.e., no G3BP-positive stress granules) was observed under these conditions (Fig 4A). Interestingly, emetine treatment also markedly attenuated the cytoplasmic redistribution of FUS (Fig. 4A) in the presence of sorbitol. These data implicate stress granule formation as a requisite for the cytoplasmic redistribution of FUS, and suggest that the full response of FUS to hyperosmolar stress includes its assembly into stress granules.
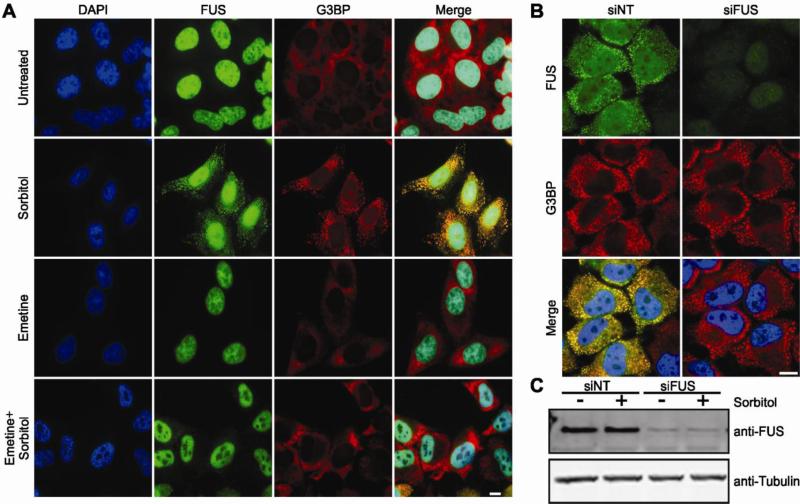
Figure 4. An inhibitor of stress granule assembly prevents the cytoplasmic redistribution of FUS, though stress granules still assemble in the absence of FUS.
(A) HeLa cells were treated with 0.4 M sorbitol for 1 hr, 50 μg/ml emetine for 1 hr or pre-treated with emetine followed by sorbitol treatment. Cells were then fixed and probed by immunofluorescence for DAPI (blue), FUS (green) and G3BP (red). Emetine pre-treatment inhibited both stress granule assembly, as evidenced by the diffuse G3BP signal, and the cytoplasmic redistribution of FUS in the presence of sorbitol. (B) HeLa cells were transfected with non-targeting siRNA (siNT) or siRNA against FUS (siFUS) for 48 hrs, subsequently treated with 0.4 M sorbitol for 1 hr, and then processed for immunofluorescence as described above. Cells treated with either siFUS or siNT exhibited normal stress granule formation (B, red) in response to sorbitol, despite a significant reduction in FUS protein levels in siFUS treated cells as evidenced by immunofluorescence (green; B) and western blot (C). All images are representative of at least n=3 independent experiments. Scale bar represents 10 μm.
Next we investigated the role of FUS in stress granule assembly under conditions of hyperosmolar stress. HeLa cells were first treated with either siRNA specific for FUS or non-targeting siRNA as a control for 48 hrs, and were then exposed to 0.4 M sorbitol for 30 min to induce the formation of stress granules (Fig. 4B). Although cells treated with FUS siRNA exhibited a ~90% reduction in FUS protein levels (Fig. 4C), these cells produced G3BP-positive stress granules in response to sorbitol that were indistinguishable from control cells (Fig. 4B). While the physical response of FUS to hyperosmolar stress depends on the stress granule assembly pathway (Fig. 4A), FUS does not appear to dictate the ability of stress granules to form.
Methylation regulates the nucleo-cytoplasmic distribution of FUS under hyperosmolar stress
Next we investigated the mechanisms by which FUS relocalizes to the cytoplasm and incorporates into stress granules in response to hyperosmolar stress. Methylation of arginine residues is a post-translational modification that modulates the nucleo-cytoplasmic distribution of hnRNP proteins, such as the cold-inducible RNA-binding protein (CIRP) (De Leeuw et al., 2007). Some reports implicate a link between the arginine methylation status of ALS-linked FUS and its subcellular localization (Tradewell et al., 2012; Yamaguchi and Kitajo, 2012). In fact, mass spectrometry analyses demonstrate that up to 20 arginine residues are asymmetrically dimethylated in FUS (Rappsilber et al., 2003). That protein arginine N-methyltransferase-1 (PRMT1), which accounts for ~85% of arginine methylation in the cell (Bedford and Clarke, 2009), and FUS interact suggests that the methylation of FUS is catalyzed by PRMT1(Du et al., 2011; Tradewell et al., 2012; Yamaguchi and Kitajo, 2012). Interestingly, stress granules contain arginine methylated hnRNP proteins, raising the possibility that this post-translational modification influences stress granule dynamics (Xie and Denman, 2011). This notion is supported by an attenuation of fragile X mental retardation protein (FMRP) in stress granules upon exposure to adenosine-2’, 3’-dialdehyde (AdOx) (Dolzhanskaya et al., 2006), a general inhibitor of methyltransferases (O'Dea et al., 1987).
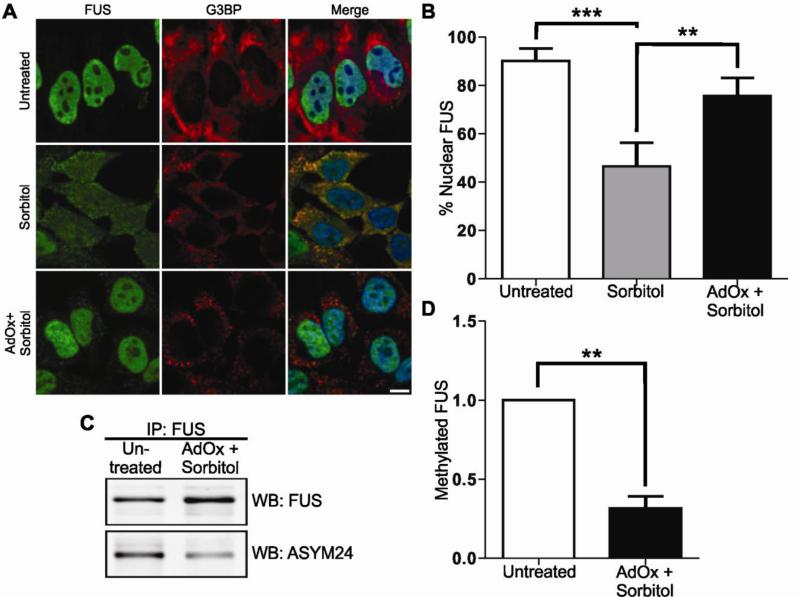
To determine whether or not the methylation status of FUS regulates its subcellular localization under conditions of hyperosmolar stress, we examined the nuclear-cytoplasmic distribution of FUS after treatment of HeLa cells with AdOx. FUS remained predominately nuclear in the presence of AdOx alone (data not shown). However, when cells were pre-treated with AdOx prior to sorbitol exposure, there was a significant effect on the nuclear-cytoplasmic partitioning of FUS compared to cells treated with sorbitol alone (Fig. 5A and B). While sorbitol treatment resulted in a ~50% reduction of nuclear FUS compared to control cells, pre-treatment with AdOx restored ~30% of FUS to the nucleus (Fig. 5B). To quantify the methylation status of the FUS protein itself, FUS was immunoprecipitated from untreated cells or from cells treated with AdOx in combination with sorbitol and probed for asymmetrically dimethylated arginine residues with the ASYM24 antibody (Tradewell et al., 2012). Arginine methylation of FUS in untreated cells was detected by ASYM24 (Fig. 5C), which is expected since FUS is reportedly arginine methylated under homeostatic conditions (Rappsilber et al., 2003). The level of methylated FUS was not significantly altered by the addition of sorbitol (data not shown). However, the arginine methylation status of FUS decreased by more than 50% in cells pre-treated with AdOx (data not shown) or AdOx in combination with sorbitol (Figs. 5C and D). Since AdOx is a general methyltransferase inhibitor, we cannot exclude the possibility that other methylation events influence the subcellular distribution of FUS in these experiments. Nonetheless, these data suggest that the methylation status of FUS must be maintained in order for it to redistribute to the cytoplasm under conditions of hyperosmolar stress, and are consistent with the notion that hypomethylated forms of FUS fail to shuttle out of the nucleus (Tradewell et al., 2012; Yamaguchi and Kitajo, 2012).
Figure 5. Methylation regulates the nucleo-cytoplasmic distribution of FUS.
(A,B) HeLa cells were treated with 0.4 M sorbitol for 1 hr, or pre-treated with 50 μM AdOx for 24 hrs prior to sorbitol treatment (AdOx + sorbitol) and subjected to confocal immunofluorescence imaging with anti-FUS (green) and anti-G3BP (red) antibodies. Sorbitol decreased the percentage of cellular FUS in the nucleus from 90±5.1% in untreated cells to 46.5±9.8%. Pre-treatment of cells with AdOx prior to sorbitol increased the percentage of cellular FUS in the nucleus to 75.6±7.4%. Data shown are the average of three independent experiments ± standard deviation. Statistical significance was determined by ANOVA and Tukey's post-hoc pairwise test (**p<0.005, ***p<0.0005). No other comparisons were statistically significant. Scale bar represents 10 μm. (C) FUS was immunoprecipitated from untreated HeLa cells or from AdOx + sorbitol cells and probed with the ASYM24 antibody by western blot. FUS was used as a loading control. (D) Densitometry analysis of (C) revealed a 68.6±7.8% decrease in the amount of FUS that is arginine dimethylated when cells were pre-treated with AdOx compared to untreated cells. Data shown are the average of three independent experiments ± standard deviation. Statistical significance was determined by Student's t-test (** p<0.005).
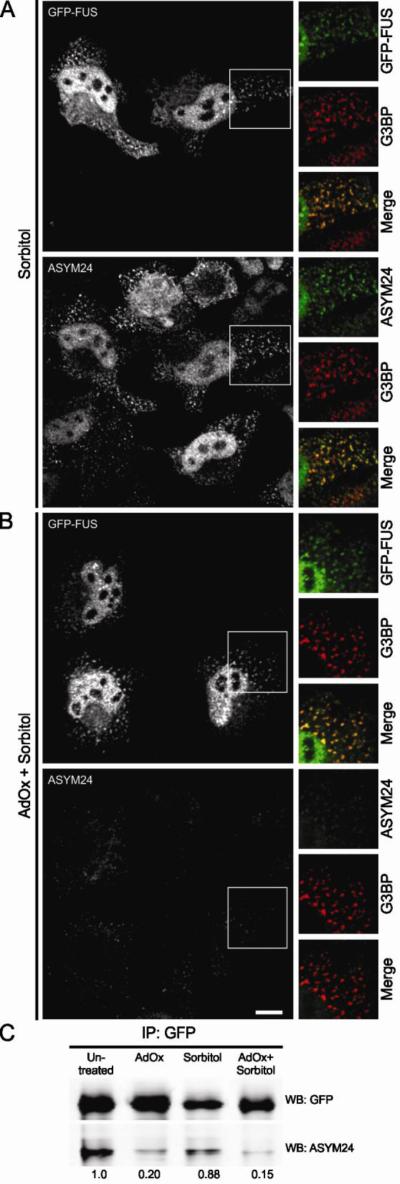
Next we sought to determine if hypomethylated FUS could still assemble into stress granules. Since the assembly of FUS into stress granules occurs concomitantly with cytoplasmic accumulation (Fig. 3), it was necessary to first dissect these two processes. To this end, we transiently transfected HeLa cells with the GFP-tagged FUS 515X truncation construct, which lacks the nuclear localization signal and is therefore retained in the cytoplasm under homeostatic conditions (Bosco et al., 2010). GFP-FUS 515X assembled into stress granules in response to 0.4 M sorbitol, and the extent of this association was the same whether cells were pre-treated with AdOx or not (Figs. 6A and B). The same outcome was observed in HEK-293 cells stably expressing GFP-FUS 515X (data not shown). In contrast to the GFP-FUS signal, there was a dramatic decrease in the ASYM24 signal in cells pre-treated with AdOx (Figs. 6A and B), indicating that pre-treatment with AdOx effectively inhibited methyltransferase activity within these cells. Immunoprecipitation with anti-GFP followed by western blot analysis with the ASYM24 antibody confirmed that GFP-FUS 515X was indeed hypomethylated due to AdOx pre-treatment (Fig. 6C). Thus, despite a large reduction in the methylation status of FUS in AdOx pre-treated cells (Figs. 5 and 6), FUS still robustly associated with stress granules. We note that a small fraction of FUS remained dimethylated in the AdOx condition (Fig. 6C), presumably FUS protein that was methylated prior to AdOx exposure but had not turned over during the course of the experiment (Xie and Denman, 2011). In the absence of commercially available antibodies that are specific for dimethylated FUS, we cannot exclude the possibility that stress granules contain some dimethylated FUS in these experiments. However, the dramatic decrease in ASYM24 signal is consistent with a reduced load of methylated proteins within stress granules, and therefore it is unlikely that all of the residual methylated FUS is sequestered into these structures. Together, these studies argue against a role for arginine methylation in regulating the incorporation of FUS in stress granules.
Figure 6. Methylation does not regulate the incorporation of FUS into stress granules.
HeLa cells were transiently transfected to express GFP-FUS G515X. Cells were exposed to 0.4 M sorbitol for 1 hr either (A) in the absence of AdOx or (B) after cells had been pre-treated with 25 μM AdOx for 8 hrs. (A) Confocal imaging showed that GFP-FUS G515X (green) assembles into G3BP-positive stress granules (red) upon sorbitol treatment (top panel). Co-staining with the ASYM24 antibody (a far-red fluorescence probe was employed; green is used in the images for clarity) revealed that these same stress granules contained asymmetrically dimethylated proteins (bottom panel). (B) While the ASYM24 signal is dramatically decreased within stress granules and cells pre-treated with AdOx (bottom panel), there is still a robust association of GFP-FUS with stress granules under the same conditions (top panel). Scale bar represents 10 m. (C) Immunoprecipitation of GFP-FUS G515X with an anti-GFP antibody and a subsequent western blot analysis with ASYM24 revealed that FUS is hypomethylated due to AdOx pretreatment. All data are representative of n=3 independent experiments.
Cells are susceptible to sorbitol toxicity and death when FUS expression is reduced
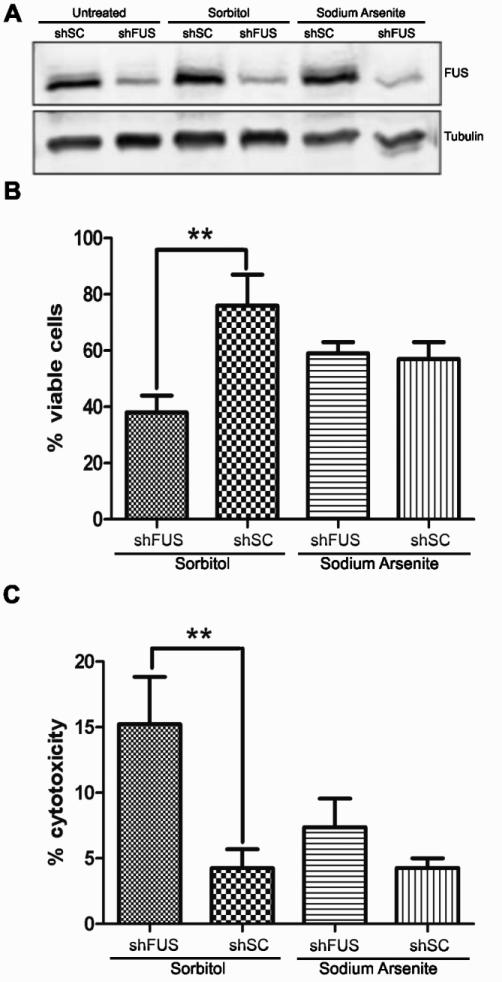
Given that the full response of FUS to hyperosmolar stress includes its assembly into stress granules (Fig. 4), and that the role of stress granules is to overcome stress and re-establish cellular homeostasis, we investigated whether the expression of FUS is important for cellular viability under conditions of hyperosmolar stress. The normal cellular response to hyperosmolar stress includes cell cycle arrest, during which time cells may adapt to stress and resume proliferation (Burg et al., 2007). However, severe hyperosmolar stress induces apoptosis and cell death (Bevilacqua et al., 2010; Burg et al., 2007). To address the susceptibility of cells to hyperosmolar toxicity in the absence of FUS, we employed inducible NSC-34 cell lines that stably express either shRNA specific for FUS (shFUS) or a scrambled control shRNA (shSC) sequence. These cell lines are advantageous for cell viability measurements since cell death resulting from chemical transfection protocols is eliminated. NSC-34 cells were induced with doxycycline for 48 hrs, resulting in ~70% knock down of FUS in the shFUS line (Fig. 7A) but not a loss of cell viability in either shFUS or shSC cells (data not shown). Cells were then treated for 8 hrs with either 0.4 M sorbitol or 0.25 mM sodium arsenite as a negative control. Sodium arsenite induces stress granule assembly, however endogenous FUS does not associate with stress granules under this condition (Fig. 2). Moreover, others have reported that mammalian cells with knocked-down FUS expression are not susceptible to sodium arsenite (Aulas et al., 2012). In agreement with this report, we did not detect a difference in percentage cell viability (Fig. 7B) or cell death (Fig. 7C) between shFUS and shSC cells in response to sodium arsenite using the MTT and LDH assays, respectively. In contrast, the percentage of viable cells was approximately two-fold lower in the shFUS cells compared to shSC cells after sorbitol treatment (Fig. 7B). That shFUS cells are more susceptible to sorbitol-induced toxicity was confirmed by the LDH cell death assay, which revealed 3-fold greater cell death in shFUS cells treated with sorbitol compared to shSC cells under the same conditions. Therefore, while the expression of FUS is not required for the assembly of stress granules (Fig. 4B), cellular homeostasis and survival during hyperosmolar stress is mediated by the expression of FUS.
Figure 7. Reduced FUS expression causes cells to become susceptible to sorbitol induced toxicity.
(A) Expression of either a non-targeting scrambled shRNA (shSC) or shRNA against FUS (shFUS) was induced by doxycycline for 48 hrs in NSC-34 cell lines, resulting in ~70% knock-down of the FUS protein as determined by western blot. Tubulin was used as a loading control. Cells were then treated with 0.4 M sorbitol or 0.25 mM sodium arsenite for 8 hrs and subjected to the (B) MTT cell viability assay or (C) LDH cell toxicity assay. (B) A significant decrease in cell viability was detected in shFUS cells (38±6%) compared to shSC cells (76±11%) when treated with sorbitol, whereas shFUS cells did not exhibit an analogous susceptibility to sodium arsenite (59±4% for shFUS versus 57±6% for shSC). (C) A higher percentage of cell death was detected in shFUS cells (15.2±3.6%) compared to shSC cells (4.2±1.4%) in response to sorbitol, whereas no difference in cell death was detected when these lines were stressed with sodium arsenite (7.4±2.2% for shFUS versus 4.3±0.7% for shSC). (B,C) Data shown are an average from n=3 independent experiments ± standard deviation. Statistical significance was determined by Student's t-test (** p<0.005).
Discussion
Although FUS is predominately expressed in the nucleus of most cell types (Andersson et al., 2008), it can shuttle between the nucleus and the cytoplasm during mRNA transport (Fujii and Takumi, 2005; Zinszner et al., 1997). The equilibrium of FUS expression can be shifted towards the cytoplasm using inhibitors against RNA polymerase II (Pol II) (Zinszner et al., 1994) or against the nuclear import receptor Transportin-1 (Trp), also known as Karyopherin β2 (Dormann et al., 2010). Genetic perturbations of its nuclear localization signal (NLS) also increase the cytoplasmic expression of FUS in the neurodegenerative disease ALS (Kwiatkowski et al., 2009; Vance et al., 2009). Herein we demonstrate a novel and robust response of endogenous FUS to hyperosmolar stress, whereby FUS redistributes from the nucleus to the cytoplasm within minutes of exposure to sorbitol (Figs. 1, 2 and 3) or sucrose (Fig. S1).
A role for FUS in hyperosmolar stress response is further supported by its association with stress granules under this condition. Stress granules are stalled translational complexes; as such, they are thought to regulate mRNAs processing during stress (Anderson and Kedersha, 2008). Recently, the activity of mTORC1 was shown to correlate with its sequestration inside stress granules, suggesting that these complexes can also regulate cell signaling at the protein level (Wippich et al., 2013). Importantly, no other chemical or environmental stressor has been shown to cause endogenous FUS to redistribute from the nucleus into the cytoplasm and enter into stress granules. While different stressors, such as oxidative stress and heat shock, have been shown to influence the association of ALS-linked mutant forms of FUS with stress granules, the nature of the NLS mutations causes FUS to accumulate in the cytoplasm a priori of stress (Bosco et al., 2010; Dormann et al., 2010). In contrast, hyperosmolar stress triggers both the cytoplasmic redistribution of FUS and its assembly into stress granules. Therefore, the response of endogenous FUS to hyperosmolar stress represents an altogether different mechanism compared to the previously described mutant forms of FUS. Further, our data support a normal and important role for endogenous FUS in stress response (discussed further below), whereas the association of ALS-linked FUS with stress granules is thought represent a pathogenic mechanism in disease (Wolozin, 2012).
In order to dissect the processes governing the cytoplasmic redistribution of FUS from its incorporation into stress granules, we employed the GFP-FUS G515X construct, which lacks the nuclear localization domain. This allowed us to investigate the role of methylation as a post-translational modification in both events. Inhibition of methyltransferases with AdOx significantly reduced the cytoplasmic redistribution of FUS during hyperosmolar stress (Fig. 5). Moreover, analysis with the ASYM24 antibody revealed that FUS is asymmetrically dimethylated at arginine residues under homeostatic conditions but is hypomethylated in the presence of AdOx (Figs. 5 and 6). These observations, together with a mass spectrometry study demonstrating that ~20 arginine residues within FUS are asymmetrically dimethylated (Rappsilber et al., 2003), supports the possibility that methylation of the FUS protein itself dictates its subcellular localization during hyperosmolar stress. Conversely, the methylation status of FUS, or other cellular factors for that matter, does not appear to regulate the association of FUS with stress granules (Fig. 6). A remaining possibility is that other post-translational modifications of FUS influence its association with stress granules.
What are the biological implications of FUS in hyperosmolar stress response? Hyperosmolar stress is implicated in a myriad of disease conditions in humans, including renal failure, diabetes, neurodegeneration and inflammation, as well as disorders of the eye, heart and liver (Brocker et al., 2012). Moreover, the cell shrinkage caused by hyperosmolar stress triggers many adverse subcellular events, such as mitochondrial depolarization, inhibition of DNA replication and transcription, damage to DNA and proteins, and cell cycle arrest, all of which can ultimately lead to cell death (Alfieri and Petronini, 2007; Brocker et al., 2012; Burg et al., 2007).
Our results are consistent with a prosurvival mechanism for endogenous FUS in human conditions that involve hyperosmolar stress. First, the response to hyperosmolar stress is specific, since alternative stressors that induce stress granule assembly such as oxidative stress and heat shock fail to elicit a similar response from endogenous FUS (Figs. 1-3). This data suggests a potentially distinct cellular response to hyperosmolar conditions compared to other stressors. Second, cells are more susceptible to hyperosmolar toxicity when FUS expression is reduced (Fig. 7), supporting a prosurvival role for FUS in this type of stress response.
Other nuclear hnRNPs, such as hnRNP A1, also respond to hyperosmolar stress by redistributing to the cytoplasm and assembling into stress granules. When localized to stress granules, hnRNP A1 is thought to specifically suppress the translation of anti-apoptotic factors and in turn initiates apoptosis under conditions of severe hyperosmolar stress (Bevilacqua et al., 2010). An intriguing possibility is that FUS sequesters specific mRNAs and proteins into stress granules, thereby altering their expression and/or function in response to the hyperosmolar stress. Indeed, recent PAR-CLIP (Hoell et al., 2011) and RIP-Chip (Colombrita et al., 2012) analyses have identified thousands and hundreds, respectively, of mRNA transcripts that are bound by FUS in the cell under homeostatic conditions. Interestingly, FUS binds mRNA that encodes genes involved in DNA damage repair and cell cycle regulation (Colombrita et al., 2012), two pathways that are altered during hyperosmolar stress (Burg et al., 2007).
In summary, our results support a prosurvival function for endogenous FUS during hyperosmolar stress. These findings have implications for human disorders with an etiology that involves hyperosmolar stress. Identifying the factors that regulate the response of FUS to hyperosmolar stress, as well as the pathways affected by FUS under this stress condition, will be critical to further our understanding of this prosurvival role of FUS.
Supplementary Material
Supp Movie 1
Supp Movie 2
Supp Movie 3
01
Acknowledgements
We acknowledge the assistance from Dr. Paul Furcinitti of the UMass Medical School Core Digital Imaging Facility. We would like thank Maeve Tischbein for her assistance with the immunofluorescence experiments, and Dr. Neil Cashman from University of British Columbia for naïve NSC-34 cell lines. We acknowledge financial support from the Worcester Foundation (DAB) and the US National Institutes of Health/ National Institute on Neurological Disorders and Stroke (R01NS078145-01 to DAB) for this work.
Contract grant sponsor: Worcester Foundation (DAB) and the US National Institutes of Health/ National Institute on Neurological Disorders and Stroke (R01NS078145-01 to DAB)
Footnotes
Author Contributions: RRKS, CLW, LJK, and DAB designed the experiments and analyzed the data; RRKS, CLW, LJK and NL performed the experiments; SI and FU constructed the inducible FUS knock-down NSC-34 cells; RRKS and DAB wrote the manuscript. All authors approved the manuscript.
References
- Alfieri RR, Petronini PG. Hyperosmotic stress response: comparison with other cellular stresses. Pflugers Arch. 2007;454(2):173–185. doi: 10.1007/s00424-006-0195-x. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33(3):141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. Stress granules. Curr Biol. 2009;19(10):R397–398. doi: 10.1016/j.cub.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Andersson MK, Stahlberg A, Arvidsson Y, Olofsson A, Semb H, Stenman G, Nilsson O, Aman P. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008;9:37. doi: 10.1186/1471-2121-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulas A, Stabile S, Vande Velde C. Endogenous TDP-43, but not FUS, contributes to stress granule assembly via G3BP. Mol Neurodegener. 2012;7:54. doi: 10.1186/1750-1326-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33(1):1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentmann E, Neumann M, Tahirovic S, Rodde R, Dormann D, Haass C. Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43). J Biol Chem. 2012;287(27):23079–23094. doi: 10.1074/jbc.M111.328757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua E, Wang X, Majumder M, Gaccioli F, Yuan CL, Wang C, Zhu X, Jordan LE, Scheuner D, Kaufman RJ, Koromilas AE, Snider MD, Holcik M, Hatzoglou M. eIF2alpha phosphorylation tips the balance to apoptosis during osmotic stress. J Biol Chem. 2010;285(22):17098–17111. doi: 10.1074/jbc.M110.109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ, Jr., Sapp P, McKenna-Yasek D, Brown RH, Jr., Hayward LJ. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2010;19(21):4160–4175. doi: 10.1093/hmg/ddq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker C, Thompson DC, Vasiliou V. The role of hyperosmotic stress in inflammation and disease. Biomol Concepts. 2012;3(4):345–364. doi: 10.1515/bmc-2012-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87(4):1441–1474. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- Calvio C, Neubauer G, Mann M, Lamond AI. Identification of hnRNP P2 as TLS/FUS using electrospray mass spectrometry. RNA. 1995;1(7):724–733. [PMC free article] [PubMed] [Google Scholar]
- Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194(3):209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- Colombrita C, Onesto E, Megiorni F, Pizzuti A, Baralle FE, Buratti E, Silani V, Ratti A. TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post- transcriptional fate in motoneuron-like cells. J Biol Chem. 2012;287(19):15635–15647. doi: 10.1074/jbc.M111.333450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993;363(6430):640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- Daigle JG, Lanson NA, Jr., Smith RB, Casci I, Maltare A, Monaghan J, Nichols CD, Kryndushkin D, Shewmaker F, Pandey UB. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum Mol Genet. 2013;22(6):1193–1205. doi: 10.1093/hmg/dds526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leeuw F, Zhang T, Wauquier C, Huez G, Kruys V, Gueydan C. The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor. Exp Cell Res. 2007;313(20):4130–4144. doi: 10.1016/j.yexcr.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Dewey CM, Cenik B, Sephton CF, Dries DR, Mayer P, 3rd, Good SK, Johnson BA, Herz J, Yu G. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol Cell Biol. 2011;31(5):1098–1108. doi: 10.1128/MCB.01279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzhanskaya N, Merz G, Aletta JM, Denman RB. Methylation regulates the intracellular protein-protein and protein-RNA interactions of FMRP. J Cell Sci. 2006;119(Pt 9):1933–1946. doi: 10.1242/jcs.02882. [DOI] [PubMed] [Google Scholar]
- Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IR, Capell A, Schmid B, Neumann M, Haass C. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. Embo J. 2010;29(16):2841–2857. doi: 10.1038/emboj.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Arai S, Kawamura T, Matsushita A, Kurokawa R. TLS and PRMT1 synergistically coactivate transcription at the survivin promoter through TLS arginine methylation. Biochem Biophys Res Commun. 2011;404(4):991–996. doi: 10.1016/j.bbrc.2010.12.097. [DOI] [PubMed] [Google Scholar]
- Emara MM, Fujimura K, Sciaranghella D, Ivanova V, Ivanov P, Anderson P. Hydrogen peroxide induces stress granule formation independent of eIF2alpha phosphorylation. Biochem Biophys Res Commun. 2012;423(4):763–769. doi: 10.1016/j.bbrc.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii R, Takumi T. TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. J Cell Sci. 2005;118(Pt 24):5755–5765. doi: 10.1242/jcs.02692. [DOI] [PubMed] [Google Scholar]
- Gal J, Zhang J, Kwinter DM, Zhai J, Jia H, Jia J, Zhu H. Nuclear localization sequence of FUS and induction of stress granules by ALS mutants. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil S, Long JC, Caceres JF. hnRNP A1 relocalization to the stress granules reflects a role in the stress response. Mol Cell Biol. 2006;26(15):5744–5758. doi: 10.1128/MCB.00224-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoell JI, Larsson E, Runge S, Nusbaum JD, Duggimpudi S, Farazi TA, Hafner M, Borkhardt A, Sander C, Tuschl T. RNA targets of wild-type and mutant FET family proteins. Nat Struct Mol Biol. 2011;18(12):1428–1431. doi: 10.1038/nsmb.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki S, Masuda A, Fujioka Y, Iguchi Y, Katsuno M, Shibata A, Urano F, Sobue G, Ohno K. Position-dependent FUS-RNA interactions regulate alternative splicing events and transcriptions. Scientific reports. 2012;2:529. doi: 10.1038/srep00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30(Pt 6):963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- Kedersha N, Anderson P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000;151(6):1257–1268. doi: 10.1083/jcb.151.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169(6):871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Sok J, Webb L, Baechtold H, Urano F, Yin Y, Chung P, de Rooij DG, Akhmedov A, Ashley T, Ron D. Male sterility and enhanced radiation sensitivity in TLS(−/−) mice. Embo J. 2000;19(3):453–462. doi: 10.1093/emboj/19.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH., Jr. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309(5740):1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- O'Dea RF, Mirkin BL, Hogenkamp HP, Barten DM. Effect of adenosine analogues on protein carboxylmethyltransferase, S-adenosylhomocysteine hydrolase, and ribonucleotide reductase activity in murine neuroblastoma cells. Cancer Res. 1987;47(14):3656–3661. [PubMed] [Google Scholar]
- Rappsilber J, Friesen WJ, Paushkin S, Dreyfuss G, Mann M. Detection of arginine dimethylated peptides by parallel precursor ion scanning mass spectrometry in positive ion mode. Anal Chem. 2003;75(13):3107–3114. doi: 10.1021/ac026283q. [DOI] [PubMed] [Google Scholar]
- Tan AY, Manley JL. The TET family of proteins: functions and roles in disease. J Mol Cell Biol. 2009;1(2):82–92. doi: 10.1093/jmcb/mjp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160(6):823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tradewell ML, Yu Z, Tibshirani M, Boulanger MC, Durham HD, Richard S. Arginine methylation by PRMT1 regulates nuclear-cytoplasmic localization and toxicity of FUS/TLS harbouring ALS-linked mutations. Hum Mol Genet. 2012;21(1):136–149. doi: 10.1093/hmg/ddr448. [DOI] [PubMed] [Google Scholar]
- van der Houven van Oordt W, Diaz-Meco MT, Lozano J, Krainer AR, Moscat J, Caceres JF. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J Cell Biol. 2000;149(2):307–316. doi: 10.1083/jcb.149.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454(7200):126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, Pelkmans L. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell. 2013;152(4):791–805. doi: 10.1016/j.cell.2013.01.033. [DOI] [PubMed] [Google Scholar]
- Wolozin B. Regulated protein aggregation: stress granules and neurodegeneration. Mol Neurodegener. 2012;7(1):56. doi: 10.1186/1750-1326-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Denman RB. Protein methylation and stress granules: posttranslational remodeler or innocent bystander? Mol Biol Int. 2011;2011:137459. doi: 10.4061/2011/137459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Kitajo K. The effect of PRMT1-mediated arginine methylation on the subcellular localization, stress granules, and detergent-insoluble aggregates of FUS/TLS. PLoS One. 2012;7(11):e49267. doi: 10.1371/journal.pone.0049267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Albalat R, Ron D. A novel effector domain from the RNA-binding protein TLS or EWS is required for oncogenic transformation by CHOP. Genes Dev. 1994;8(21):2513–2526. doi: 10.1101/gad.8.21.2513. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Sok J, Immanuel D, Yin Y, Ron D. TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J Cell Sci. 1997;110(Pt 15):1741–1750. doi: 10.1242/jcs.110.15.1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Movie 1
Supp Movie 2
Supp Movie 3
01