The Association Between Dietary Flavonoid and Lignan Intakes and Incident Type 2 Diabetes in European Populations: The EPIC-InterAct study (original) (raw)
Abstract
OBJECTIVE
To study the association between dietary flavonoid and lignan intakes, and the risk of development of type 2 diabetes among European populations.
RESEARCH DESIGN AND METHODS
The European Prospective Investigation into Cancer and Nutrition-InterAct case-cohort study included 12,403 incident type 2 diabetes cases and a stratified subcohort of 16,154 participants from among 340,234 participants with 3.99 million person-years of follow-up in eight European countries. At baseline, country-specific validated dietary questionnaires were used. A flavonoid and lignan food composition database was developed from the Phenol-Explorer, the U.K. Food Standards Agency, and the U.S. Department of Agriculture databases. Hazard ratios (HRs) from country-specific Prentice-weighted Cox regression models were pooled using random-effects meta-analysis.
RESULTS
In multivariable models, a trend for an inverse association between total flavonoid intake and type 2 diabetes was observed (HR for the highest vs. the lowest quintile, 0.90 [95% CI 0.77–1.04]; P valuetrend = 0.040), but not with lignans (HR 0.88 [95% CI 0.72–1.07]; P valuetrend = 0.119). Among flavonoid subclasses, flavonols (HR 0.81 [95% CI 0.69–0.95]; P valuetrend = 0.020) and flavanols (HR 0.82 [95% CI 0.68–0.99]; P valuetrend = 0.012), including flavan-3-ol monomers (HR 0.73 [95% CI 0.57–0.93]; P valuetrend = 0.029), were associated with a significantly reduced hazard of diabetes.
CONCLUSIONS
Prospective findings in this large European cohort demonstrate inverse associations between flavonoids, particularly flavanols and flavonols, and incident type 2 diabetes. This suggests a potential protective role of eating a diet rich in flavonoids, a dietary pattern based on plant-based foods, in the prevention of type 2 diabetes.
The prevalence of diabetes is markedly increasing worldwide, with the number of people with diabetes projected to rise from 366 million in 2011 to 552 million in 2030 (1). Dietary patterns characterized by higher consumption of fruit and vegetables (2), such as within a Mediterranean diet (3), are associated with a reduced risk of type 2 diabetes. Flavonoids and lignans are bioactive polyphenols that are contained in plant-based foods such as fruits, vegetables, nuts, legumes, cocoa, and cereals, and in beverages such as tea, wine, and juices (4), and have been proposed to have a potential role in the prevention of type 2 diabetes through diverse biological effects, including antioxidant and anti-inflammatory properties and insulin sensitivity–enhancing effects (5–7).
Epidemiological evidence for an association between dietary intake of flavonoids and the risk of type 2 diabetes is inconsistent (8–13). For the six flavonoid subclasses, flavanols (including flavan-3-ol monomers, proanthocyanidins, and theaflavins), anthocyanidins, flavonols, flavanones, flavones, and isoflavones (Supplementary Table 1), a range of associations with diabetes has been reported in six prospective studies (8–13). An inverse significant association with type 2 diabetes was observed with anthocyanidins (15% risk reduction in a comparison of extreme quintiles), and significant inverse trends were observed with some flavonols (quercetin and myricetin) in a pooled analysis of Nurses’ Health Study I and II and the Health Professionals Follow-Up Study (8) and the Finnish Mobile Clinic Health Examination Survey (10), respectively. However, no associations were reported in the other two U.S.-based studies (Women’s Health Study and Iowa Women’s Health Study) (9,11) and for any other flavonoid subclasses (8–11). Among two Asian studies, the Singapore Chinese Health Study reported an inverse association of diabetes with soy intake and an inverse borderline significant association with isoflavone intake (12), whereas the Japan Public Health Centre-Based Prospective Study observed no significant association between soy or isoflavone intakes and type 2 diabetes in the whole population; however, among overweight Japanese women there was an inverse association (13). To our knowledge, there are no studies evaluating the association of dietary lignan intake with type 2 diabetes, although some experimental studies have shown promising antidiabetic properties (14,15).
In light of the inconsistent current evidence, and in particular the paucity of information in European populations with considerable variability in flavonoid and lignan intakes, the aim of this study was to investigate the association between dietary flavonoid and lignan intakes, and the risk of developing type 2 diabetes in Europe. In particular, the use of the European Prospective Investigation into Cancer and Nutrition (EPIC)-InterAct study, which was conducted across eight countries in Europe with substantial variation in the intake of flavonoids, enabled us to examine these associations comprehensively in a European population.
RESEARCH DESIGN AND METHODS
Study design and population
The EPIC-InterAct is a large prospective type 2 diabetes case-cohort study (16) nested within the EPIC study (17) with more than half a million adult participants recruited in the 1990s from the following 10 European countries: Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom. With the exception of Greece and Norway, all EPIC countries participated in the EPIC-InterAct study (n = 455,680). After the exclusion of individuals without stored blood (n = 109,680) or with prevalent diabetes at baseline (5,821), 340,234 participants with 3.99 million person-years of follow-up were included in this study. All participants gave written informed consent, and the study was approved by the local ethics committee in the participating countries and the Internal Review Board of the International Agency for Research on Cancer.
Type 2 diabetes case ascertainment and verification
A pragmatic, high-sensitivity approach for case ascertainment was used in order to identify all potential incident type 2 diabetes cases and to exclude all individuals with prevalent diabetes (16), using at least two multiple sources of evidence including self-report and linkage to primary care registers, secondary care registers, medication registers, and hospital admissions and mortality data. Cases in Germany were additionally validated by diagnostic records. Cases in Denmark and Sweden were not ascertained by self-report, but were identified via local and national diabetes and pharmaceutical registers, and hence were considered as verified. Follow-up was censored either on 31 December 2007, the date of type 2 diabetes diagnosis, or the date of death, whichever occurred first. In total, 12,403 verified incident type 2 diabetic cases were identified.
Subcohort selection and population for current analysis
A random subcohort of 16,835 individuals was selected from the 340,234 participants with available stored blood samples, stratified by center. After the exclusion of 681 individuals without information on diabetes status, 16,154 subcohort individuals were included, of whom 778 individuals developed incident type 2 diabetes during follow-up.
Of the 27,779 participants (12,403 case subjects, of whom 778 were within the subcohort of 16,154 participants) in the EPIC-InterAct study, we excluded 619 participants within the lowest and the highest 1% of the distribution of the ratio of reported energy intake (determined from the questionnaire) to estimate energy requirements (calculated from age, sex, body weight, and height). In addition, we excluded 1,072 participants with missing information on nutritional intake or other covariates used in the statistical analysis. This resulted in a final sample of 26,088 participants for inclusion in the current analysis with 11,559 case subjects and a subcohort of 15,258 participants, including 729 case subjects in the subcohort.
Flavonoid and lignan intake and other dietary variables
Habitual diet during the 12 months prior to recruitment was recorded using country-specific validated food frequency questionnaires or diet histories (17,18). Most centers adopted a self-administered questionnaire of 98 to 266 food items. In Spain and Ragusa (Italy), the questionnaire was administered at a personal interview using a computerized dietary program. Questionnaires in France, Italy, Spain, the Netherlands, and Germany were quantitative, estimating individual average portion size systematically. Those in Denmark, Naples (Italy), and Umeå (Sweden) were semiquantitative, with the same standard portion assigned to all subjects. In Malmö (Sweden) and the U.K., a questionnaire method combined with a food record was used. Total energy and nutrient intakes were estimated using the standardized EPIC Nutrient Database (19).
Estimated flavonoid and lignan intake was derived from foods included in the dietary questionnaires through a comprehensive food composition database on flavonoids and lignans, as we have previously described (20,21). Our database on flavonoids was based on U.S. Department of Agriculture databases (22), Phenol-Explorer (23) and the U.K. Food Standards Agency database (24). This database compiles composition data on lignans and the six flavonoid subclasses (Supplementary Table 1). Furthermore, our flavonoid food composition database was expanded by using retention factors when no analytical data were provided by cooked food. The retention factors applied to all flavonoid classes, except isoflavones, were 0.70, 0.35, and 0.25, respectively, after frying, cooking in a microwave oven, and boiling (25).
These retention factors were not applied to isoflavones and lignans because their cooking losses are usually minimal. Our database was also expanded by calculating the flavonoid content of recipes, estimating missing values based on similar foods (by botanical family and plant part), obtaining consumption data for food group items, and using botanical data for logical zeros (when negligible amounts of flavonoids or lignans would be present in a food type, e.g., anthocyanidins in plant foods without red, blue or purple color). In nature, flavonoids and lignans are usually found as glycosides, mainly with glucose or rhamnose moieties, but other sugars may also be involved. Therefore, data on flavonoids and lignans are expressed as aglycone equivalents, after conversion of the flavonoid glycosides into aglycone contents using their respective molecular weights. The final database contains 1,877 food items, including raw foods, cooked foods, and recipes, and 10% of values for these food items are missing.
Other variables
A lifestyle questionnaire was used to collect information about sociodemographic characteristics, smoking status, and medical history (17). Occupational and leisure-time physical activity was assessed by questionnaire and classified according to the Cambridge Physical Activity Index (26). A history of previous illness included hypertension, hyperlipidemia, previous cancers, and/or cardiovascular diseases (angina, stroke, and myocardial infarction). Information on family history of type 2 diabetes in a first-degree relative was collected for all participants except for individuals in Italy, Spain, Germany, and Oxford (U.K.). Height, weight, and waist circumference were measured by trained health professionals using standardized protocols, except in Oxford (U.K.) and France, where self-reported measurements were obtained, and Umeå (Sweden), where waist circumference was not recorded (16). BMI was calculated as weight in kilograms divided by height in square meters. Blood samples were collected at baseline, and hemoglobin A1c (HbA1c) was measured using high-performance liquid chromatography (Diamat Automated Glycated Hemoglobin Analyzer; Bio-Rad Laboratories Ltd., Hemel Hempstead, U.K.).
Statistical analysis
Dietary questionnaire-derived means, SDs, medians, and 5th and 95th percentiles of total intake and intakes of subclasses of flavonoids and lignans were calculated. Total flavonoid intake by country was also visualized in a box-and-whisker plot. Baseline characteristics and dietary intakes in the subcohort were summarized by quintiles of total flavonoid intake using means and SDs or frequencies. Prentice-weighted Cox regression models accounting for the case-cohort design (27) were used to estimate the associations between flavonoid and lignan intakes and type 2 diabetes of each EPIC country. Total intake and intakes of subclasses of flavonoids and lignans were categorized using sub-cohort-wide quintiles. Tests for linear trend were performed by assigning the medians of each quintile as scores. Intakes were also analyzed continuously, after a log2 transformation that indicates a doubling in flavonoid and lignan intakes. Hazard ratios (HRs) were calculated using the following modeling strategy. Age was used as the underlying time scale, with entry time defined as the participant’s age at baseline, and exit time as the participant’s age at diagnosis of diabetes, censoring, or death (whichever came first). All analyses were stratified by center to control for center effects such as follow-up procedures and questionnaire design. Model 1 included age (as underlying time scale), sex, and total energy intake (kilocalories per day). Model 2 was additionally adjusted for the following potential lifestyle confounders: educational level (none, primary school, technical/professional, secondary school, higher education); physical activity (inactive, moderately inactive, moderately active, and active); smoking status (never, former, and current); BMI (kilograms per square meter); and alcohol intake (grams per day). Model 3 was additionally adjusted for the following potential dietary confounders: intakes of red meat, processed meat, sugar-sweetened soft drinks, and coffee (grams per day). Model 4 was additionally adjusted for the following potential mediators: intakes of fiber (grams per day), vitamin C (milligrams per day), and magnesium (milligrams per day). HRs and 95% CIs were estimated within each country and then combined by using random-effects meta-analysis. Between-country heterogeneity was assessed using the _I_2 statistic.
Effect modification by sex, baseline BMI category (BMI <25, 25 to <30, and ≥30 kg/m2), and smoking status (never, current, former smokers) was assessed by modeling interaction terms, in model 4, between these variables and total flavonoid intake, and conducting stratified analyses. Moreover, the proportional hazards assumption was assessed by testing the interaction between flavonoid intake and age (<60 and ≥60 years of age), and for all exposures there was no evidence against the assumption.
Sensitivity analyses were conducted excluding 975 diabetes case subjects in whom type 2 diabetes had been diagnosed within the first 2 years of recruitment. In a second sensitivity analysis, model 4 was additionally adjusted for hypertension and hyperlipidemia, after the exclusion of 1,971 participants with cancer and/or cardiovascular diseases at recruitment, because participants in these subgroups may have modified their diets. In a third sensitivity analysis, model 4 was additionally adjusted for history of diabetes in a first-degree relative (with the exclusion of 12,977 participants with missing data), an important risk factor of type 2 diabetes (28); finally, model 4 was additionally adjusted for waist circumference (exclusion of 1,824 participants without this data), another independent risk factor strongly associated with type 2 diabetes (29). In a further sensitivity analysis, non-case subjects from the subcohort were excluded if they had an HbA1c level ≥6.5% (48 mmol/mol), as this cutoff can be used as a diagnostic criterion for type 2 diabetes (as per the American Diabetes Association and the World Health Organization).
All statistical analyses were performed using Stata/SE 12.0 (StataCorp, College Station, TX). All P values were based on two-sided tests, and statistical significance was set at P < 0.05.
RESULTS
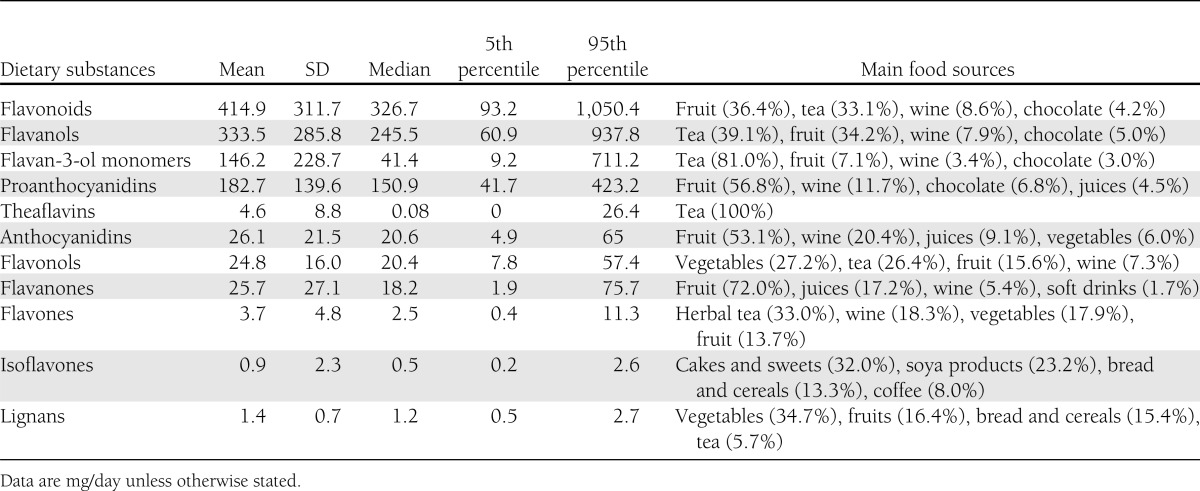
Table 1 shows the mean (SD) and median and percentiles (5th and 95th) of both total intake and intakes of subclasses of dietary flavonoids and lignans. As indicated by the large differences between means and medians, the distributions were skewed to higher values. Flavanols were the most important contributor (80%) to total flavonoid intake (proanthocyanidins 44%, flavan-3-ols monomers 35%, theaflavins 1%), followed by anthocyanidins (6.3%), flavanones (6.2%), and flavonols (6.0%). Total flavonoid intake varied markedly across countries, with median intakes ranging from 201.7 mg/day in Sweden to 850.6 mg/day in the U.K. (Supplementary Fig. 1). Total flavonoid intake and intake of some flavonoid subclasses (flavanols and flavonols) were highly correlated (R > 0.8), whereas other flavonoid subclasses (such as anthocyanidins, flavanones, flavones, and isoflavones) had low to moderate correlation (R = between 0.1 and 0.4). The main food sources of total flavonoid intake were fruits (36.4%), tea (33.1%), wine (8.6%), chocolate products (4.2%), fruit juices (3.9%), beer (2.5%), vegetables (2.3%), and legumes (2.3%) (Table 1).
Table 1.
Dietary intake of flavonoids and lignans in the EPIC-InterAct subcohort (n = 15,258)
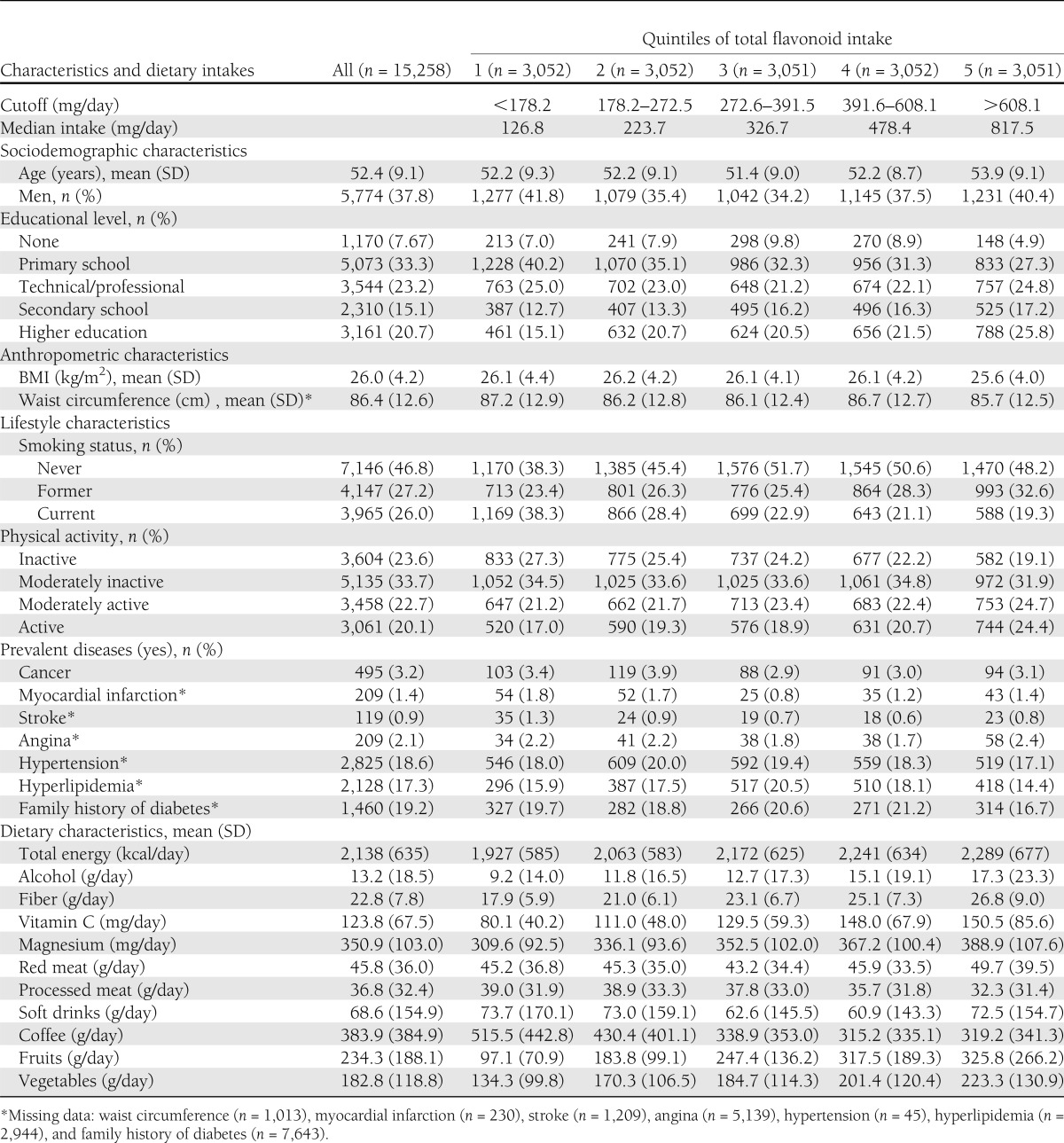
Baseline characteristics of the subcohort according to quintiles of total flavonoid intake are shown in Table 2. Participants in the highest quintile of total flavonoid intakes were likely to be older and to have the lowest BMI and waist circumference compared with those participants in the lowest quintile. With increasing total intake of flavonoids, participants tended to have a more health-conscious lifestyle pattern with greater educational level and physical activity; lower tobacco consumption; a higher intake of fruits, vegetables, fiber, vitamin C, and magnesium; and a lower consumption of processed meat. However, participants in the top quintile reported greater alcohol and red meat intake and lower coffee intake. Participants across the quintiles had similar frequencies of prevalent diseases.
Table 2.
Baseline characteristics and dietary intakes of the EPIC-InterAct subcohort according to quintiles of total flavonoid intake
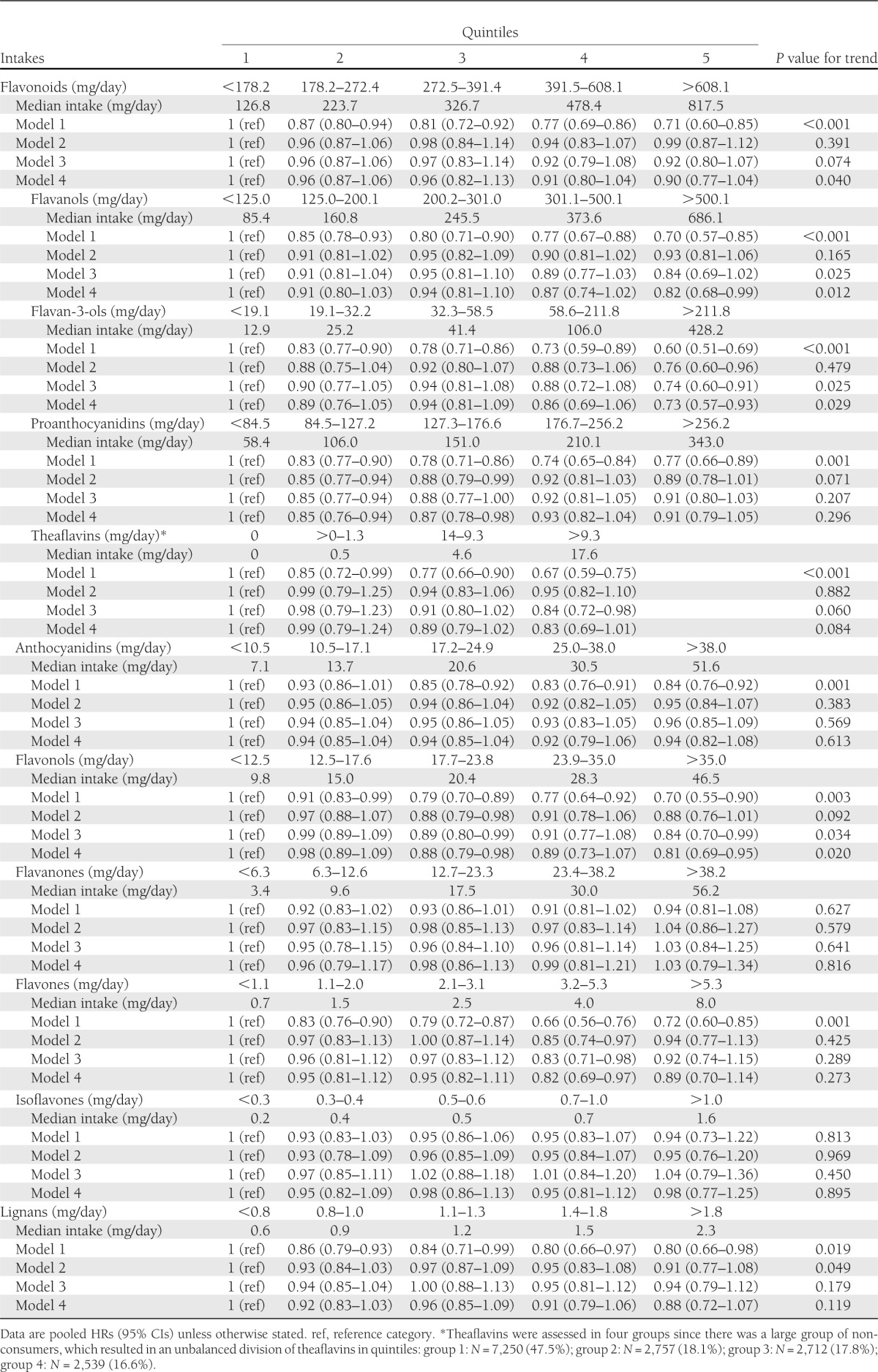
The pooled HRs (95% CIs) for type 2 diabetes by quintiles of total intake and intakes of subclasses of flavonoids and lignans are shown in Table 3. Significant inverse associations were observed in model 1 (stratified by center and adjusted for age [as underlying time-scale], sex, and total energy) for total intakes of flavonoids, flavanols (including flavan-3-ol monomers, proanthocyanidins, and theaflavins), anthocyanidins, flavonols, flavones, and lignans. After further adjustment for potential confounders (models 2 and 3), all associations were attenuated but were still statistically significant for flavan-3-ol monomers and flavonols. When fiber, vitamin C, and magnesium intakes were additionally included in the multivariable models (model 4), similar risk estimates were observed between the intake of all flavonoid subclasses and lignans, and the incidence of type 2 diabetes as in model 3, showing significant inverse associations with intakes of flavanols (HR for highest vs. lowest quintile 0.82 [95% CI 0.68–0.99]; P for trend 0.012); flavan-3-ol monomers (HR 0.73 [95% CI 0.57–0.93]; P for trend 0.029); and flavonols (HR 0.81 [95% CI 0.69–0.95]; P for trend 0.020). A significant trend was also detected for total flavonoids (HR 0.90 [95% CI 0.77–1.04]; P for trend 0.040). A borderline significant trend was seen for theaflavins (HR 0.83 [95% CI 0.69–1.01]; P for trend 0.084). No significant association was observed with lignans (HR 0.88 [95% CI 0.72–1.07]; P for trend 0.119).
Table 3.
Association between flavonoid and lignan intakes and type 2 diabetes: EPIC-InterAct study
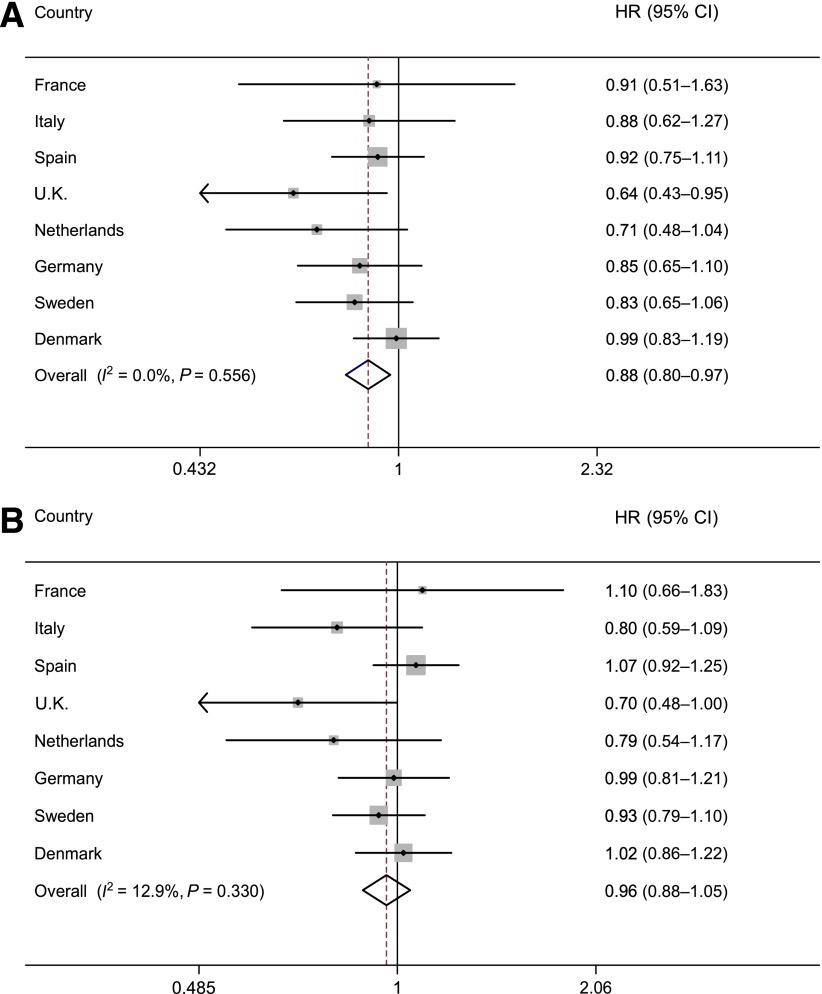
In multivariable analyses (model 4), similar associations of type 2 diabetes were observed when dietary flavonoid and lignan exposures were assessed as continuous variables after a log2 transformation (Fig. 1 and Supplementary Fig. 2). No statistically significant heterogeneity between countries was detected for the associations of total intake and intakes of subclasses of flavonoids and lignans with type 2 diabetes, except for flavanones (_I_2 = 52.8%, P = 0.038) and flavones (_I_2 = 53.3%, P = 0.036) (Supplementary Fig. 2). No interactions were found with sex (P for interaction = 0.609), BMI (P = 0.680), or smoking status (P = 0.526) for total flavonoid intake.
Figure 1.
HRs (and 95% CIs) for incident type 2 diabetes for a doubling of total flavonoid (A) and lignan (B) intakes across countries in the InterAct study. The pooled HR is based on a random-effects meta-analysis using Prentice-weighted Cox regression analysis with age as the underlying time scale (model 4; see Statistical analysis section); stratified by center; and adjusted for sex, educational level, smoking status, physical activity levels, BMI, total energy, and intakes of alcohol, red meat, processed meat, sugar-sweetened soft drinks, coffee, fiber, vitamin C, and magnesium.
In sensitivity analyses (Supplementary Table 2), similar results were observed after the exclusion of diabetes case subjects in whom type 2 diabetes had been diagnosed within the first 2 years of follow-up or participants with prevalent cardiovascular diseases. When family history of diabetes was added in model 4, associations were strengthened. After further adjustment for waist circumference, the findings were almost identical. After the exclusion of 84 non-case subjects from the subcohort with an HbA1c level ≥6.5% (48 mmol/mol) at baseline, the results were almost identical.
CONCLUSIONS
In this large European case-cohort study, an inverse trend between dietary total flavonoid intake and incidence of type 2 diabetes was observed. Flavanols, including flavan-3-ol monomers and flavonols, were the flavonoid subclasses significantly related to a lower hazard of type 2 diabetes.
To date, there are only two large U.S. cohort studies that have evaluated the association between the total flavonoid intake and incident type 2 diabetes, each using a different update of the U.S. Department of Agriculture database on flavonoids (22). Only the study using the database release 2.1 (year 2007) observed a consistent inverse association between intake of anthocyanidins and type 2 diabetes risk (8,11). This is in line with the crude, but not the multivariable adjusted, findings in our study, based on the database version from 2007. This inconsistency could be due to the different dietary intakes between studies; in our study, the median anthocyanidin intake in the first quintile (7.1 mg/day) was similar to that in the third quintile (8.1 mg/day) in the U.S. study (8). Moreover in the U.S. study, the HRs were almost identical for the third (HR 0.87 [95% CI 0.80–0.94]), fourth (HR 0.88 [95% CI 0.83–0.94]), and fifth quintiles (HR 0.85 [95% CI 0.80–0.91]) compared with the first quintile (8). This suggests that the lower risk of type 2 diabetes due to intake of anthocyanidins might reach a plateau at a certain intake level. Two other prospective studies have assessed the relationships between the intake of some flavonoid subclasses and the risk of the development of type 2 diabetes (9,10). The U.S. study reported no association with intake of either flavonols or flavones (9); however, the Finnish study reported inverse significant trends for two individual flavonols (10), as in our study. These differences in the results for intakes of flavonols and flavanols between European and U.S. studies could be a result of European countries having approximately twice the intake compared with the U.S. (8,21). In both Asian studies, inverse associations with isoflavone intakes were reported (12,13), but not in Western studies (8,11). Asian countries still have the highest isoflavone intakes worldwide (∼10-fold higher than in European countries) (20,30), which may explain the differences observed in association with type 2 diabetes between Asian and Western countries. In our study, there was no association between lignan intake and risk of type 2 diabetes, although in a U.S. study, lignan levels were significantly associated with a lower fasting insulin level (31). Indeed, in two recent experimental studies lignans have been associated with an improvement of glucose homeostasis by increasing glucose disposal rates and enhancing hepatic insulin sensitivity (14) and an inhibition of α-amylase activity (15).
The main food sources of flavonoids were fruits and vegetables, tea, and wine. These foods (2,32,33), as well as the Mediterranean diet, a dietary pattern based on flavonoid-rich foods (e.g., fruits and vegetables, olive oil, and moderate wine consumption) (3) were associated with a reduced risk of type 2 diabetes in the EPIC-InterAct study. Similar results were observed in previous U.S. studies, where anthocyanidin-rich foods (blueberries and apples/pears) (8) and wine consumption (11), a rich source of anthocyanidins and flavanols, were inversely associated with type 2 diabetes risk. Notably, after adjustment for potential compounds co-occurring in flavonoid-rich foods, such as fiber, vitamin C, magnesium, and alcohol, associations between flavonoids and the risk of type 2 diabetes were still statistically significant in the current study, suggesting that it is unlikely that these compounds confound or mediate the association between intake of flavonoids and type 2 diabetes risk.
The potential mechanisms underlying these inverse associations between flavonoids and type 2 diabetes risk may include the modulation of the postprandial glucose levels by reducing the activity of digestive enzymes (e.g., α-amylase and α-glucosidase) (34) and decreasing the active transport of glucose across intestinal brush border membrane, inhibiting sodium GLUT2 (35). Furthermore, some flavonoid-rich extracts improved hyperglycemia and insulin sensitivity in type 2 diabetic mice via activation of AMP-activated protein kinase and accompanied by an upregulation of GLUT4 (36). In vitro, flavonoids also had a protective effect on pancreatic β-cells by reducing the inducible form of nitric oxide synthase gene expression mediated through the suppression of nuclear factor-κB and c-Jun NH2-terminal kinase signaling pathways (37,38). Other antioxidant, anti-inflammatory, and antiangiogenic activities of flavonoids may also contribute to their potential protective effect against type 2 diabetes (5).
Strengths of the current study include the multicenter design and the large sample size at recruitment, from which a large number of verified incident cases of type 2 diabetes accrued during 3.99 million person-years of follow-up. This study also includes a wide variation in flavonoid and lignan intakes among participants in eight European countries. Furthermore, we were able to control for a number of plausible confounders and factors that may mask the etiological pathway of the association between flavonoid and lignan intake and type 2 diabetes. In all sensitivity analyses, the associations were almost identical, denoting the robustness of our results. Limitations of the current study included the use of a single baseline assessment of diet and other lifestyle variables. Therefore, changes in lifestyle could not be taken into account in these analyses. In addition, our results may be influenced by measurement errors of the dietary questionnaires that may have attenuated our findings, although country-specific validated questionnaires for some flavonoid-rich foods, such as fruits, vegetables, tea, and wine (17,18), were used. Furthermore, flavonoid and lignan intakes are likely to be underestimated since the flavonoid database was incomplete (although an extensive common database was used) (20,21) and herb/plant supplement intakes were omitted in these analyses (up to 5% in Denmark, the highest consumer country) (39). Nutritional biomarkers offer an alternative and objective method for estimating dietary intake and provide more accurate measures than self-reported questionnaires. To date, there are only a few validated biomarkers of flavonoid and lignan intakes, so further research in this field is warranted (40). However, we were unable able to evaluate the association between the intakes of other polyphenols, such as phenolic acids and stilbenes, and type 2 diabetes because data on these are not yet available in the EPIC cohort. Moreover, the association of dietary intakes of flavonoids and lignans with type 2 diabetes risk might be susceptible to confounding since high flavonoid and lignan intake reflects a healthier lifestyle. In our models, we have adjusted for other determinants of healthy lifestyle; however, possible residual confounding cannot be excluded.
In conclusion, this large case-cohort study conducted in eight European countries supports a role for dietary intake of flavonoids in the prevention of type 2 diabetes in men and women. High total intakes of flavonoids, flavanols, flavan-3-ol monomers, and flavonols were associated with a 10, 18, 27, and 19% lower risk, respectively, of type 2 diabetes. These results highlight the potential protective effect of eating a diet rich in flavonoids (a dietary pattern based on plant-based foods) on type 2 diabetes risk.
Acknowledgments
The EPIC-InterAct study was supported by the European Union (Integrated Project LSHM-CT-2006-037197 in the Framework Programme 6 of the European Community). In addition, InterAct investigators received the following funding: R.Z.-R. was supported by a postdoctoral programme Fondo de Investigación Sanitaria (no. CD09/00133) from the Spanish Ministry of Science; R.Z.-R. and C.A.G. were supported by the Health Research Fund (Fondo de Investigación Sanitaria) of the Spanish Ministry of Health (RTICC DR06/0020/0091); N.G.F. was supported by the Medical Research Council Epidemiology Unit (MC_UP_A100_1003); M.G., P.A., E.M.-M., and M.J.T. were supported by the Health Research Fund of the Spanish Ministry of Health, CIBER en Epidemiología y Salud Pública (Spain); Y.T.v.d.S. was supported by NL Agency grant IGE05012 and an Incentive Grant from the Board of the Universitair Medisch Centrum Utrecht (the Netherlands); L.B., K.O., and A.T. were supported by the Danish Cancer Society; P.W.F. was supported by the Swedish Research Council, Novo Nordisk, the Swedish Heart Lung Foundation, and the Swedish Diabetes Association; V.K. and T.K. were supported by Deutsche Krebshilfe; A.M.W.S. and D.L.v.d.A. were supported by the Dutch Ministry of Public Health, Welfare and Sports, the Netherlands Cancer Registry, LK Research Funds, Dutch Prevention Funds, Dutch ZON, World Cancer Research Fund, and Statistics Netherlands; G.M. was supported by Ministero della Saluteregione Toscana Progetto Integrato Oncologia; T.J.K. and K.-T.K. were supported by Cancer Research U.K.; F.C.-C., G.F., and F.P. were supported by Ligue contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, and INSERM; A.M. was supported by Associazione Italiana per la Ricerca sul Cancro; J.R.Q. was supported by the Asturias Regional Government; M.J.T. was supported by the Murcia Regional Government; and R.T. was supported by AIRE-ONLUS Ragusa, AVIS-Ragusa, the Sicilian Regional Government. No other potential conflicts of interest relevant to this article were reported.
R.Z.-R. designed the research, analyzed the data, and wrote the manuscript. N.G.F. designed the research and wrote the manuscript. S.J.S. analyzed the data and reviewed and edited the manuscript. C.A.G. designed the research and reviewed and edited the manuscript. B.B. contributed to the discussion and reviewed and edited the manuscript. M.G. contributed to the discussion. Y.Y.v.d.S. contributed to the discussion and reviewed and edited the manuscript. P.A., H.B., L.B., F.C.-C., G.F., E.J.F., P.W.F., S.G., V.K., T.J.K., K.-T.K., T.K., G.M., A.M., E.M.-M., P.M.N., K.O., F.P., J.R.Q., I.R., C.S., A.S., M.S., N.S., A.M.W.S., A.T., M.J.T., R.T., D.L.v.d.A., and C.L. reviewed and edited the manuscript. E.R. is the coordinator of the EPIC study, and reviewed and edited the manuscript. N.J.W. is the coordinator of EPIC-InterAct, and reviewed and edited the manuscript. N.G.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all EPIC participants and staff for their contribution to the study. The authors also thank Nicola Kerrison (Medical Research Council Epidemiology Unit, Cambridge) for managing the data for EPIC-InterAct.
Footnotes
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–321 [DOI] [PubMed] [Google Scholar]
- 2.Cooper AJ, Forouhi NG, Ye Z, et al. InterAct Consortium Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr 2012;66:1082–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romaguera D, Guevara M, Norat T, et al. InterAct Consortium Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: the InterAct project. Diabetes Care 2011;34:1913–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez-Jiménez J, Fezeu L, Touvier M, et al. Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr 2011;93:1220–1228 [DOI] [PubMed] [Google Scholar]
- 5.Dembinska-Kiec A, Mykkanen O, Kiec-Wilk B, Mykkanen H. Antioxidant phytochemicals against type 2 diabetes. Br J Nutr 2008;99(E Suppl. 1):ES109–ES117 [DOI] [PubMed] [Google Scholar]
- 6.Hanhineva K, Törrönen R, Bondia-Pons I, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci 2010;11:1365–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr Opin Lipidol 2013;24:25–33 [DOI] [PubMed] [Google Scholar]
- 8.Wedick NM, Pan A, Cassidy A, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012;95:925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr 2005;24:376–384 [DOI] [PubMed] [Google Scholar]
- 10.Knekt P, Kumpulainen J, Järvinen R, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002;76:560–568 [DOI] [PubMed] [Google Scholar]
- 11.Nettleton JA, Harnack LJ, Scrafford CG, Mink PJ, Barraj LM, Jacobs DR., Jr Dietary flavonoids and flavonoid-rich foods are not associated with risk of type 2 diabetes in postmenopausal women. J Nutr 2006;136:3039–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller NT, Odegaard AO, Gross MD, et al. Soy intake and risk of type 2 diabetes mellitus in Chinese Singaporeans [corrected]. Eur J Nutr 2012;51:1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanri A, Mizoue T, Takahashi Y, et al. Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J Nutr 2010;140:580–586 [DOI] [PubMed] [Google Scholar]
- 14.Kwon DY, Kim da S, Yang HJ, Park S. The lignan-rich fractions of Fructus Schisandrae improve insulin sensitivity via the PPAR-γ pathways in in vitro and in vivo studies. J Ethnopharmacol 2011;135:455–462 [DOI] [PubMed] [Google Scholar]
- 15.Hano C, Renouard S, Molinié R, et al. Flaxseed (Linum usitatissimum L.) extract as well as (+)-secoisolariciresinol diglucoside and its mammalian derivatives are potent inhibitors of α-amylase activity. Bioorg Med Chem Lett 2013;23:3007–3012 [DOI] [PubMed] [Google Scholar]
- 16.Langenberg C, Sharp S, Forouhi NG, et al. InterAct Consortium Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia 2011;54:2272–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 2002;5(6B):1113–1124 [DOI] [PubMed] [Google Scholar]
- 18.Margetts BM, Pietinen P. European Prospective Investigation into Cancer and Nutrition: validity studies on dietary assessment methods. Int J Epidemiol 1997;26(Suppl. 1):S1–S5 [DOI] [PubMed] [Google Scholar]
- 19.Slimani N, Deharveng G, Unwin I, et al. The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr 2007;61:1037–1056 [DOI] [PubMed] [Google Scholar]
- 20.Zamora-Ros R, Knaze V, Luján-Barroso L, et al. Dietary intakes and food sources of phytoestrogens in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-hour dietary recall cohort. Eur J Clin Nutr 2012;66:932–941 [DOI] [PubMed] [Google Scholar]
- 21.Zamora-Ros R, Knaze V, Luján-Barroso L, et al. Differences in dietary intakes, food sources and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr 2013;109:1498–1507 [DOI] [PubMed] [Google Scholar]
- 22.U.S. Departament of Agriculture USDA Database for the Flavonoid Content of Selected Foods. Beltsville, MD, U.S. Department of Agriculture, 2007 [Google Scholar]
- 23.Neveu V, Perez-Jiménez J, Vos F, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward HA, Kuhnle GG, Mulligan AA, Lentjes MA, Luben RN, Khaw KT. Breast, colorectal, and prostate cancer risk in the European Prospective Investigation into Cancer and Nutrition-Norfolk in relation to phytoestrogen intake derived from an improved database. Am J Clin Nutr 2010;91:440–448 [DOI] [PubMed] [Google Scholar]
- 25.Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuces, and celery. J Agric Food Chem 1997;45:590–595 [Google Scholar]
- 26.Wareham NJ, Jakes RW, Rennie KL, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 2003;6:407–413 [DOI] [PubMed] [Google Scholar]
- 27.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–1172 [DOI] [PubMed] [Google Scholar]
- 28.InterAct Consortium The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia 2013;56:60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langenberg C, Sharp SJ, Schulze MB, et al. InterAct Consortium Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: the EPIC-InterAct case-cohort study. PLoS Med 2012;9:e1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SA, Wen W, Xiang YB, et al. Assessment of dietary isoflavone intake among middle-aged Chinese men. J Nutr 2007;137:1011–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Schouw YT, Sampson L, Willett WC, Rimm EB. The usual intake of lignans but not that of isoflavones may be related to cardiovascular risk factors in U.S. men. J Nutr 2005;135:260–266 [DOI] [PubMed] [Google Scholar]
- 32.Beulens JW, van der Schouw YT, Bergmann MM, et al. InterAct Consortium Alcohol consumption and risk of type 2 diabetes in European men and women: influence of beverage type and body size The EPIC-InterAct study. J Intern Med 2012;272:358–370 [DOI] [PubMed] [Google Scholar]
- 33.InterAct Consortium Tea consumption and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. PLoS One 2012;7:e36910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubilar M, Jara C, Poo Y, et al. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): sources of antioxidant compounds and α-Glucosidase/α-Amylase inhibitors. J Agric Food Chem 2011;59:1630–1637 [DOI] [PubMed] [Google Scholar]
- 35.Kwon O, Eck P, Chen S, et al. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J 2007;21:366–377 [DOI] [PubMed] [Google Scholar]
- 36.Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr 2010;140:527–533 [DOI] [PubMed] [Google Scholar]
- 37.Han MK. Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic beta-cell damage. Exp Mol Med 2003;35:136–139 [DOI] [PubMed] [Google Scholar]
- 38.Matsuda T, Ferreri K, Todorov I, et al. Silymarin protects pancreatic beta-cells against cytokine-mediated toxicity: implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology 2005;146:175–185 [DOI] [PubMed] [Google Scholar]
- 39.Skeie G, Braaten T, Hjartåker A, et al. Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study. Eur J Clin Nutr 2009;63(Suppl. 4):S226–S238 [DOI] [PubMed] [Google Scholar]
- 40.Zamora-Ros R, Rabassa M, Llorach R, González CA, Andres-Lacueva C. Application of dietary phenolic biomarkers in epidemiology: past, present, and future. J Agric Food Chem. 22 February 2012 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]