MDMA DECREASES THE EFFECTS OF SIMULATED SOCIAL REJECTION (original) (raw)
. Author manuscript; available in PMC: 2015 Feb 1.
Published in final edited form as: Pharmacol Biochem Behav. 2013 Dec 3;117:1–6. doi: 10.1016/j.pbb.2013.11.030
Abstract
3-4-methylenedioxymethamphetamine (MDMA) increases self-reported positive social feelings and decreases the ability to detect social threat in faces, but its effects on experiences of social acceptance and rejection have not been determined. We examined how an acute dose of MDMA affects subjective and autonomic responses to simulated social acceptance and rejection. We predicted that MDMA would decrease subjective responses to rejection. On an exploratory basis, we also examined the effect of MDMA on respiratory sinus arrhythmia (RSA), a measure of parasympathetic cardiac control often thought to index social engagement and emotional regulation. Over three sessions, healthy adult volunteers with previous MDMA experience (N = 36) received capsules containing placebo, 0.75 or 1.5 mg/kg of MDMA under counter-balanced double-blind conditions. During expected peak drug effect, participants played two rounds of a virtual social simulation task called “Cyberball” during which they experienced acceptance in one round and rejection in the other. During the task we also obtained electrocardiograms (ECGs), from which we calculated RSA. After each round, participants answered questionnaires about their mood and self-esteem. As predicted, MDMA decreased the effect of simulated social rejection on self-reported mood and self-esteem and decreased perceived intensity of rejection, measured as the percent of ball tosses participants reported receiving. Consistent with its sympathomimetic properties, MDMA decreased RSA as compared to placebo. Our finding that MDMA decreases perceptions of rejection in simulated social situations extends previous results indicating that MDMA reduces perception of social threat in faces. Together these findings suggest a cognitive mechanism by which MDMA might produce pro-social behavior and feelings and how the drug might function as an adjunct to psychotherapy. These phenomena merit further study in non-simulated social environments.
Keywords: MDMA, social cognition, respiratory sinus arrhythmia, Cyberball
1. Introduction
±3,4-methylenedioxymethamphetamine (MDMA) is a popular recreational drug with a reputation as a potent enhancer of social engagement and feelings of empathy. Controlled studies verifying some of these effects have led to interest in the drug as an adjunct to psychotherapy, with the idea that it could aid in the formation of the patient-therapist alliance and encourage positive ideation (Oehen et al., 2013). However, it is unclear by which neurocognitive mechanisms MDMA produces these “pro-social” effects.
Our lab has previously reported (Bedi et al., 2010) that MDMA increases subjective reports of feelings of lovingness, insight, and sociability when given under controlled conditions, supporting anecdotal reports and findings elsewhere in the literature (for reviews see Baylen and Rosenberg, 2006, Parrott, 2007). We have further found that MDMA decreases amygdalar activation in response to images of angry faces (Bedi et al., 2009) and hampers accurate identification of fearful faces (Bedi et al., 2010), while others have reported that it biases emotional identification of faces towards positive emotions and away from negative ones (Hysek et al., 2012). Together, these findings suggest that one way that MDMA may work to induce social engagement and increase pro-social feelings is by decreasing perception of negative social cues indicating social rejection, such as angry or fearful facial expressions. Thus, for the present study, we had two goals: 1) to examine MDMA’s effects on responses to and perception of simulated social rejection and acceptance, and 2) to investigate one potential psychophysiological mechanism that could contribute to the effects of MDMA in social situations: changes in activity of the parasympathetic system, measured as respiratory sinus arrhythmia.
The aforementioned results indicating that MDMA reduces perception of negative social cues were all found in tasks involving cognitive judgments of social information, i.e. identification of facial expression of emotion. To determine whether the same phenomenon occurs in judgments of more complete social situations, we used the widely-implemented “Cyberball” task drawn from ostracism research (Williams and Jarvis, 2006). Cyberball is a virtual ball-toss game in which players are either ‘accepted’ or ‘rejected’ by others. Participants ‘play’ the game with two other, computer-controlled characters whose behavior can be modified by the experimenter to mimic acceptance (human player receives lots of throws) or rejection (human player receives very few throws). Individuals report lower levels of mood and self-worth after games involving rejection (Zadro et al., 2004), and fMRI scans from individuals excluded during Cyberball games show greater activation in the anterior cingulate cortex (Eisenberger et al., 2003), a brain area involved in emotional and nociceptive processing (Devinsky et al., 1995).
In addition to examining MDMA’s effects on subjective responses to social situations, we also explored its effect on the parasympathetic nervous system. MDMA is a sympathomimetic drug (De La Torre et al., 2000) and pharmacological stimulation of the sympathetic nervous system often decreases the activity of the parasympathetic nervous system (Davidson et al., 2005, Gaillard and Tbumbo, 1976), and on that basis it would be expected that MDMA would decrease parasympathetic nervous system activity. However, co-activation of both sympathetic and parasympathetic branches can be observed (Paton et al., 2005), and MDMA also increases levels of the hormone oxytocin (Dumont et al., 2009, Wolff, 2005), which increases activity in the vagal nerve of the parasympathetic nervous system (Hashimoto et al., 2012, Norman et al., 2011). Higher activity in the vagus nerve has been connected to the same phenomena observed under MDMA administration, i.e. positive social emotions, desire to socialize, and reduced responses to negative social stimuli (Porges, 2007). Thus we examined the effect of MDMA on parasympathetic nervous system activity on an exploratory basis using a measure of high-frequency heart rate variability, or respiratory sinus arrhythmia (RSA), which tracks vagal activity (Eckberg, 1983). Previous studies have found that experiencing rejection during Cyberball decreases RSA (Murray-Close, 2011), so we set out to determine how self-esteem, mood, and RSA were related during Cyberball under placebo and MDMA administration conditions.
We hypothesized that rejection during Cyberball would decrease positive mood and self-esteem and that MDMA would mitigate the effects of rejection, consistent with its effects on facial expression cues of social rejection. Further, we examined whether these effects were related to MDMA-induced changes in RSA.
2. Materials and methods
2.1 Study design
Participants received placebo, 0.75, and 1.5 mg/kg MDMA across three sessions in counterbalanced order under double-blind conditions. Sessions were separated by at least 96 h. At regular intervals during each session, they filled out subjective effects questionnaires. During peak drug effect, they played the Cyberball game twice, once under acceptance and once under rejection conditions, while an ECG was recorded. After each game they answered questions about their mood and self-esteem and about what percentage of throws they believed that they had received. They also performed other tasks as part of a larger study, the results of which will be reported elsewhere.
2.2 Participants
Healthy participants (N = 36, 18 female) ages 18 – 35 were recruited through flyers and online advertisements. Participants completed a 2-h screening that included a physical examination, ECG, modified Structured Clinical Interview for DSM-IV (SCID; First et al., 1996) and self-reported health and drug use history. We only accepted participants who had used MDMA at least 4 but less than 40 times with no adverse reactions. Other exclusion criteria were: pregnancy, BMI <19 or >30, less than high school education, non-fluency in English, hypertension, abnormal ECG, any medical condition requiring regular medication (except birth control), any medical contraindication determined by our study physician, a DSM-IV Axis I diagnosis in the past year (apart from non-treatment-seeking drug abuse), and history of stimulant drug dependence. We furthermore excluded persons smoking more than 25 cigarettes per week due to concern that nicotine withdrawal due to abstinence during the study would interfere with the effects of drug. Participants were primarily Caucasian (n=23, 65.7%), in their 20s (M=24.6, SD=4.7), with some college education (M=15.1 yrs education, SD =1.5), and light to moderate drug use (see Table 1).
Table 1.
Demographic and substance use characteristics
| n(%) or M(SD) | |
|---|---|
| Demographic variables | |
| Sex | 18 (50 %) female |
| Ethnicity | 28 (80 %) Non-Hispanic |
| Race | 23 (65.7 %) Caucasian |
| 4 (11.4 %) African-American | |
| 1 (2.9 %)Asian | |
| 7 (20 %)Other/mixed race | |
| Age | 24.6 (4.7) years |
| Education | 15.1 (1.5) years |
| Current substance use | |
| Typical alcoholic drinks/week | 9.8 (10.7) drinks |
| Smoking regularly in the past month | 8 (22.2 %) |
| Lifetime occasions recreational use | |
| Cannabis | 0 (0 %) never |
| 5 (13.9 %) 1 – 10 x | |
| 3 (8.3 %) 11 – 50 x | |
| 5 (13.9 %) 51 – 100 x | |
| 23 (63.9 %) >100 x | |
| Tranquilizers | 21 (58.3 %) never |
| 11 (30.6 %) 1 – 10 x | |
| 4 (11.1 %) 11 – 50 x | |
| Stimulants | 7 (19.4 %) never |
| 14 (38.9 %) 1 – 10 x | |
| 11 (30.6 %) 11 – 50 x | |
| 2 (5.6 %) 51 – 100 x | |
| 2 (5.6 %) >100 x | |
| Opiates | 16 (44.4 %) never |
| 14 (38.9 %) 1 – 10 x | |
| 4 (11.1 %) 11 – 50 x | |
| 0 (0 %) 51 – 100 x | |
| 1 (2.8 %) >100 x | |
| Hallucinogens | 2 (5.6 %) never |
| 24 (66.7 %) 1 – 10 x | |
| 9 (25.0 %) 11 – 50 x | |
| 1 (2.8 %) 51 – 100 x | |
| MDMA | 25 (69.4 %) 1 – 10 x |
| 11 (30.6 %) 11 – 50 x | |
| Other drugs | 26 (72.2%) never |
| 6 (16.7 %) 1 – 10 x | |
| 3 (8.3 %) 11 – 50 x | |
| 1 (2.8 %) 51 – 100 x |
Participants were required to refrain from recreational drugs for 48 h before sessions and from alcohol and over-the-counter drugs for 24h before sessions, with compliance verified using breath alcohol (Alcosensor III, Intoximeters, St. Louis, MO) and urine tests (ToxCup, Branan Medical, Irvine, CA). Participants were instructed to fast for two hours and maintain normal caffeine and nicotine intake before the beginning of the session. Before each session, urine pregnancy tests were performed for female participants. Women not on hormonal birth control were scheduled only during the follicular phase of their menstrual cycle, as menstrual cycle may affect responses to stimulant drugs (White et al., 2002). Participants were informed that they might receive any of the following kinds of drugs: a stimulant, a tranquilizer, a marijuana-like drug, or a placebo. All participants provided informed consent. All procedures were approved by the University of Chicago Institutional Review Board and were carried out in accordance with the Declaration of Helsinki.
2.3 Procedure
Participants first attended a 1-h orientation during which they were familiarized with the study task and the ECG equipment. They then completed three 5-h individual study sessions. Participants arrived at 9:00 am, completed the compliance checks described above, applied the ECG sensors under the direction of a trained research assistant, had their baseline blood pressure and heart rate measured, and filled out subjective effects questionnaires. At 9:30 a.m., they took two opaque size-00 gelatin capsules containing 0.75 or 1.5 mg/kg of body weight MDMA with D-glucose filler, or placebo (D-glucose only). Between 9:30 a.m. and 10:30 a.m., participants were allowed to relax and watch a movie if they wished. Subjective drug effects, blood pressure and heart rate were re-assessed at 10:00am. At 10:30 a.m., they completed a series of behavioral tasks that are not presented here. At 11:30 a.m., subjective effects, blood pressure and heart rate were reassessed, and participants’ ECGs were recorded to obtain RSA during peak drug effect in the absence of a task. At approximately 12:15 p.m., they played two Cyberball games while their ECG was recorded. Immediately after each game they answered mood and self-esteem questionnaires. Upon completion of the games (approximately 12:30 p.m.), subjective drug effects, blood pressure, and heart rate were reassessed immediately and again at 1:00 and 1:30 p.m, after which the ECG sensors were removed. At 2:00 p.m., participants were allowed to leave after completing an end of session questionnaire, provided they reported no remaining drug effects and their cardiovascular measures were back within normal range.
2.4 Measures
2.4.1 Subjective drug effects
Subjective drug effects on social emotions were measured using single-item Visual Analog Scales (VAS) that asked participants to rate how strongly they felt the following: ‘Insightful’, ‘Sociable’, ‘Confident’, ‘Lonely’, ‘Playful’, ‘Loving’, and ‘Friendly’ on a scale of 0–100. For this study, we used the ‘Loving’ measure, which has previously been shown to be sensitive to the pro-social subjective effects of MDMA (Bedi et al., 2010). We also administered the Drug Effects Questionnaire (DEQ; Fischman and Foltin, 1991) which comprises five visual analog scales from 0–100 on which participants indicate how much they like, feel, dislike and want more of the drug, as well as how ‘high’ they feel. We focused on DEQ ‘High’ scores. Participants completed these scales at baseline (−15) and at 30, 60, 120, 180, 210, and 240 min after capsule administration.
2.4.2 Cardiovascular drug effects
Cardiovascular effects were measured using disposable self-adhesive electrodes arranged in the standard Lead II configuration. These signals were amplified and processed by an integrated Mindware Bionex system (Mindware Technologies, Gahanna, OH). We analyzed the ECG waveform with Heart Rate Variability Analysis Software 2.51, also by Mindware Technologies. The software prepared the interbeat interval (IBI) series for spectral analysis as follows: each IBI series was interpolated and sampled at 4 Hz to ensure adequate resolution of the appropriate frequencies and equal intervals between samples, and then de-trended with a quadratic function to ensure stationarity (full details of this procedure in Berntson et al., 1997). This signal was brought into the frequency domain using a fast Fourier transform, and integrating the power over the respiratory frequency band (0.12 to 0.40 Hz) gave us the measurement we report as RSA. Values were obtained at baseline and at peak drug effect for each sixty-second segment of a five-minute recording period, and for each sixty-second segment of a three-minute recording period during the Cyberball games and then ensemble averaged. To ensure that participants were aware of their social condition before we measured RSA, we allowed ninety seconds of game time to elapse before taking three minutes of cardiovascular data.
2.5 Cyberball task
Social rejection and acceptance were simulated using the widely-implemented “Cyberball” virtual ball-toss game (Williams and Jarvis, 2006). Participants played games of “catch” with two computer players whose behavior was manipulated by the experimenters. Games lasted between three and four minutes. Participants played two games per session: one, to simulate social acceptance, in which they were thrown the ball 63 ± 3% of the time, the other, to simulate rejection, in which they were thrown the ball 30 ± 3% of the time (in a “fair” game in which all players are included exactly equally, participants would receive 50% of tosses from the other players). In each session, participants played one game of each kind in a counter-balanced fashion, with questionnaires administered in between the two games and after the second. Participants were asked to mentally visualize the experience and then answer questions about how they felt during the game. These questions were separated into two measures: positive mood and self-esteem. Both scales asked participants to rate their agreement on a seven-point scale with statements like “I felt sad”, “I felt somewhat inadequate during the game”, and “I felt like an outsider during the game”. The mood scale was composed of six questions (α = 0.77) and the self-esteem scale was composed of 12 questions (α = 0.89). We also asked the participants to estimate what percentage of throws they received during the game from 0–100% in order to ensure participants perceived the manipulation correctly. We did not attempt to blind the participants to the fact that they were playing against a computer, but the deleterious effect of the rejection games on self-esteem and mood has been demonstrated to be robust whether the participants are told that the other players are computers or that they are other humans (Zadro et al., 2004).
2.6 Statistical analyses
We analyzed our data using a planned contrast approach to repeated measures analysis of variance (RMANOVA; Judd and Kenny, 2010). Because the distributions of the raw subjective measures at individual time points violated assumptions of normality, we analyzed the subjective drug effects (DEQ Feel High and VAS Loving) by computing area-under-the-curve scores relative to the participant’s baseline for each session. These were distributed normally, and we compared them using a one-way (Drug) RMANOVA. We then conducted post-hoc tests on individual time points using the non-parametric Wilcoxon signed-rank test to determine if they were significantly different across doses. Similarly, relative area-under-the-curve scores were calculated for heart rate and compared using one-way (Drug) RMANOVA, but differences at individual time points were analyzed using Student’s t-test. Peak drug effect on RSA in the absence of a task was analyzed using a 3 (Drug) by 2 (Time) RMANOVA. RSA during Cyberball was first averaged across segments and then, as with the responses to the game-specific questionnaire, analyzed using a 3 (Drug) by 2 (Social Condition) RMANOVA. Due to equipment malfunction, sample sizes for the cardiovascular data are smaller than for the subjective measures (n = 24/36). All of our analyses were also performed with session order as a covariate, but since this did not alter the pattern of results, we omit the session covariate here for simplicity’s sake.
3 Results
3.1 Typical MDMA effects
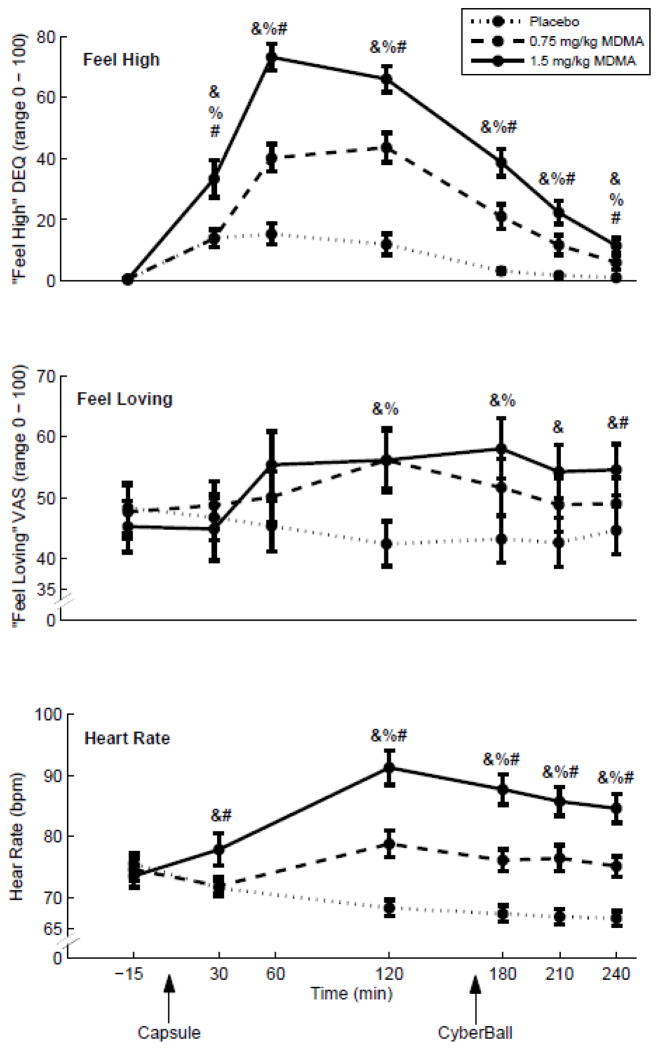
MDMA increased both DEQ ‘Feel High’ and VAS ‘Loving’ area-under-the-curve scores (linear dose, F[1, 34] = 130.5, p<.001 and F[1, 34] = 8.231, p<.01, respectively). Follow-up RMANOVA tests showed that the effects were significant at both doses, and Wilcoxon signed-rank tests of the differences across dose at individual time points showed that these effects were present at the time points before and after the Cyberball task (Fig. 1, top and middle panels). Both doses of MDMA increased area-under-the-curve scores for heart rate (linear dose, F[1, 26]=71.815, p<.001, Fig. 1, bottom panel).
Figure 1.
Replication of typical MDMA effects: MDMA dose-dependently increases subjective feelings of “high-ness” and “loving-ness” and increases heart rate. Solid traces indicate the high dose, dashed the low, and dotted the placebo. Symbols indicate significant differences from 0 (p<.05 using the Wilcoxon Signed-Rank test) for the medians of the following change scores: &, high – placebo; %, low – placebo; #, high – low. Error bars represent standard error of the mean. Arrows indicate the timing of capsule administration and Cyberball, the social simulation game. Top, responses to “are you high right now?”; Center, responses to “do you feel loving?”; Bottom, mean heart rates in beats per minute.
3.2 Cyberball results
Rejection during Cyberball robustly decreased self-reported mood and self-esteem when compared to the same values after acceptance (social condition, F[1, 34] = 29.247, p<.001 and F[1, 34] = 76.154, p<.001, respectively, see Fig. 2, left and middle panel). MDMA reduced the effect of rejection on mood and self-esteem (linear dose × social condition, F[1, 34] = 4.206, p<.05 and F[1, 34] = 5.626, p<.05, respectively, see Fig. 2, left and middle panel). Post-hoc follow up t-tests confirmed that an effect of MDMA was present only in the rejection condition. As expected, there was a strong effect of social condition on the perceived percentage of throws received because this percentage was in fact reduced from 63% to 30% (social condition, F[1, 34]=139.00, p<.001, Fig. 2 right panel). MDMA also increased the perceived percentage of throws selectively under the rejection condition (linear dose × social condition, F[1,34]=4.206, p<.05, Fig. 2, right-most panel).
Figure 2.
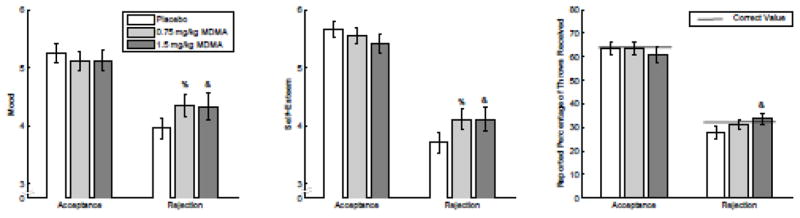
The high dose of MDMA increases self-reported mood, self-esteem, and reported percentage of throws received after rejection during a ball-tossing social simulation game without increasing them after acceptance in the same. Color indicates dose as follows: dark gray, high; light gray, low; white, placebo. Symbols indicate significant differences from 0 (p<.05 using Student’s _T_-test) for the following change scores: &, high – placebo; %, low – placebo; #, high – low. Error bars represent standard error of the mean. The thick black line in the right panel indicates the correct value for percentage throws received in each condition.
3.3 RSA results
The high dose of MDMA decreased RSA area-under-the-curve scores, though RSA still increased over the course of the session (linear dose, F[1,26]=9.573 p<.05, Fig. 3). Simulated rejection in Cyberball had no significant effect on RSA (social condition, F[1, 23]=.013, p=.910). MDMA resulted in decreased RSA power during Cyberball compared to placebo (linear dose, F[1,23]=29.483, p<.001), and there was no significant interaction between MDMA and social condition on RSA (linear dose × social condition, F[1,23]=1.602, p=.218, Fig. 3).
Figure 3.
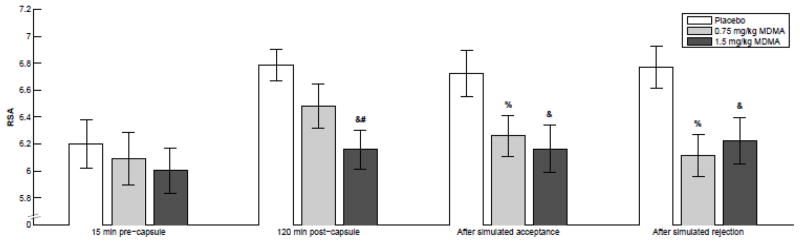
MDMA dose-dependently decreases respiratory sinus arrhythmia (RSA), and there was no detectable effect of Cyberball on RSA. Color indicates dose as follows: dark gray, high; light gray, low; white, placebo. Symbols indicate significant differences from 0 (p<.05 using Student’s _T_-test) for the following change scores: &, high – placebo; %, low – placebo; #, high – low. Error bars represent standard error of the mean. Bar groupings indicate, from left to right, RSA power calculated from EKGs taken: before capsule administration, 120 minutes after capsule administration, after simulated social acceptance, and after simulated social rejection.
4 Discussion
MDMA increased subjective pro-social feelings of lovingness, consistent with previous reports. MDMA also reduced the impact of simulated social rejection on mood and self-esteem without changing responses to simulated acceptance. The high dose of MDMA also decreased the perceived objective level of rejection, i.e. it increased the percentage of throws that participants reported receiving. Because, contrary to previous results, we found no effect of simulated rejection on RSA, we could not evaluate MDMA’s effect on autonomic responses to rejection. MDMA did, however, decrease RSA relative to placebo over the course of the whole session, consistent with its sympathomimetic profile. In summary, MDMA reduced both subjective perceptions of and responses to social rejection, increased positive social feelings, and decreased a cardiac index of vagal activity.
Our finding that MDMA decreased perceived intensity of rejection extends previous work (Bedi et al., 2010, Hysek et al., 2012), which showed that MDMA impairs perception of negative social information in faces, to a simulated social situation. Previous findings indicated that the facilitation of social motivation and engagement under MDMA administration may result from impaired perception of rejection. However, we report here that MDMA reduces the effect of rejection on mood and self-esteem at lower doses and to a greater degree than it affects perception of rejection (defined here as participants’ self-reported perception of how many times they were thrown the ball). This suggests that MDMA may affect social processing and behavior in more ways than just impairing perception. These findings merit further investigation into the effects of MDMA on social perception in more naturalistic social settings.
We argue that this suggests the most prominent effect of MDMA on processing of social information is impairment of perception of rejection and responses to rejection, rather than an introduction of positivity bias, because we found no effect of MDMA on any of these subjective measures during simulated social acceptance (though our subjective measures were close to their maximum value after social acceptance, neither distribution showed evidence of the truncation that would be expected if a ceiling effect was masking an effect of MDMA). This is in contrast to the effects of the closely related drug amphetamine, which has been found to generally increase sensitivity to subtle emotional content in facial expressions, an important form of social information, regardless of the valence of the emotion (Wardle et al., 2012), and to specifically increase emotional reactivity to positive images without affecting responses to negative images. (Wardle and de Wit, 2011). However, fMRI studies have shown that MDMA increases activation in the ventral striatum (a component of the reward pathway) during presentation of happy faces, in addition to a larger decrease in activity in the amygdala (which is associated with the identification of noxious stimuli) during presentation of angry faces (Bedi et al., 2009), indicating that perhaps MDMA has a more subtle effect on processing of positive social information that we were unable to detect here. Examining this possibility would be another goal of studying MDMA in more natural social settings.
These findings also have implications for putative pharmacological mechanisms for MDMA’s pro-social effects. Following the demonstration in (Thompson et al., 2007) that oxytocin blockade in rats results in the elimination of MDMA’s pro-social effects, there has been substantial research directed at establishing the extent of that nonapeptide neurohormone’s role in MDMA’s action. Intranasally-administered oxytocin has been shown to have a variety of effects on social cognition, including decreasing amygdalar activation and subjective anxiety in response to social stress (Lee et al., 2009), increasing attentiveness to social cues, and facilitating social memory processes (Guastella and MacLeod, 2012). However, though oxytocin, like MDMA, biases perception of facial expressions towards positive emotions (Bartz et al., 2011), it does not alter subjective responses to Cyberball on the same set of measures used here (Alvares et al., 2010). This may indicate an important role for other neurohormones, like norepinephrine and serotonin, in MDMA’s pro-social effects.
These same neurohormones also likely play a role in our finding that RSA was lower during MDMA administration, consistent with lower activity in the vagus nerve, as selective serotonin and norepinephrine reuptake-inhibiting compounds have been found to reduce RSA (Davidson et al., 2005, Licht et al., 2010), and MDMA has been found to increase release of both hormones (Steele et al., 1994, White et al., 1996). Lowered levels of vagal activity are typically associated with decreased social engagement behavior and less effective emotional regulation. However, we observed that MDMA increased participants’ self-reported feelings of lovingness (Fig. 1) and reduced responses to social rejection. It is therefore possible that under conditions of MDMA administration, vagal activity is not a good psychophysical indicator of psychological orientation towards social engagement or emotional regulation.
We further found no effect of the Cyberball simulated social situations on RSA, in contrast to the effect noted in (Murray-Close, 2011). That study was, however, with an exclusively female subject pool, and gender differences have been reported in RSA response to social stress (Grossman et al., 2001). Further, in that study participants played the game only once and were deceived into believing that were playing with other human beings, rather than with a computer, unlike in this study, two differences that might increase emotional responses to the experience of exclusion. Our findings here, then, indicate that, in a non-deceptive, repeated-measures context, social exclusion in Cyberball does not affect RSA in a mixed-gender population.
This study has a number of limitations. As noted previously, our simulated rejection manipulation did not elicit any autonomic response, precluding any analysis of the effects of MDMA on autonomic responses to rejection. Additionally, Cyberball is only a simulation of rejection and does not reflect all aspects of social rejection in the real world. Further studies could use more complex simulations, like the “O-Cam” technique reported in (Goodacre and Zadro, 2010). Further, anecdotal reports of MDMA’s effects on social feelings and behavior generally come from settings in which the drug is taken with others, while in this and all other laboratory studies so far, participants have taken the drug alone. Our lab has shown (Kirkpatrick and de Wit, 2013) that participants report different subjective effects of alcohol when they consume it in a social group than when they consume it alone. This phenomenon may extend to MDMA, such that laboratory studies like this one in which the drug is consumed alone may miss effects that are an important part of its use and abuse. Thus, a natural extension of this work would be to administer the drug to dyads and examine subjective and autonomic responses. Lastly, since the results presented here were gathered as part of a larger study, the Cyberball task occurred after peak drug effect, which is between 90 and 120 min (Dumont et al., 2009). Thus, it is possible that this effect could be even stronger if the task were performed during peak drug effect. As is clear from Figure 1, however, drug effects were still significantly present both before and after the task.
Despite these limitations, the findings reported here, taken together, indicate that MDMA induces a drug state that is pro-social in terms of self-report measures and responses to social stimuli, and that one manner in which MDMA induces this state appears to be by impairing perception of negative social information. Some have expressed concern that MDMA might increase negative cognitions (Parrott 2007), but we did not have findings supporting that effect here. The impairment of the processing of and concomitant decrease in deleterious effects from negative social information could have a role in MDMA-assisted psychotherapy, where the drug could be reducing patients’ perception of the risk associated with speaking openly about their issues, encouraging them to perceive that their psychotherapist is accepting of them, and reducing any negative effects of difficult psychotherapy sessions on their mood and self-esteem. It may also contribute to abuse of the drug, as pro-social effects are commonly cited by users as part of the motivation to take MDMA (Morgan et al., 2013, Soellner, 2005). We hope that this study informs research into these mechanisms and guides the development of improved strategies for both MDMA’s use as a psychotherapeutic adjunct and for prevention of the drug’s abuse.
Highlights.
- 3-4-methylenedioxymethamphetamine (MDMA) has “pro-social” effects
- MDMA’s pro-social effects could be related to decreased responses to social threat
- We measured subjective and autonomic responses to simulated social situations
- MDMA decreased the effect of social rejection on mood and self-esteem
- MDMA decreased respiratory sinus arrhythmia, a measure of autonomic function
Acknowledgments
The authors wish to thank Celina Joos, Lindsey Davis, and Michael Helzer for aiding in data collection and scoring. The research was supported by a grant from the National Institute on Drug Abuse (R01 DA002812) to HdW, and MCW was supported during the execution of this study by a National Institute on Drug Abuse training grant (T32 DA007255).
Role of funding source
NIDA had no involvement in the design, data collection, analysis, interpretation, writing of the report or the decision to submit this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvares GA, Hickie IB, Guastella AJ. Acute effects of intranasal oxytocin on subjective and behavioral responses to social rejection. Experimental and Clinical Psychopharmacology. 2010;18:316–21. doi: 10.1037/a0019719. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baylen CA, Rosenberg H. A review of the acute subjective effects of MDMA/ecstasy. Addiction. 2006;101:933–47. doi: 10.1111/j.1360-0443.2006.01423.x. [DOI] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of ±3, 4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biological psychiatry. 2010;68:1134–40. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology. 2009;207:73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger T, Eckberg D, Grossman P, Kaufmann P, Malik M, et al. Heart rate variability: Origins, Methods, and Interpretive Caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Davidson J, Watkins L, Owens M, Krulewicz S, Connor K, Carpenter D, et al. Effects of paroxetine and venlafaxine XR on heart rate variability in depression. Journal of clinical psychopharmacology. 2005;25:480–4. doi: 10.1097/01.jcp.0000177547.28961.03. [DOI] [PubMed] [Google Scholar]
- De La Torre R, Farré M, Roset PN, López CH, Mas M, Ortuño J, et al. Pharmacology of MDMA in Humans. Annals of the New York Academy of Sciences. 2000;914:225–37. doi: 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. REVIEW ARTICLE: Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dumont GJH, Sweep FCGJ, van der Steen R, Hermsen R, Donders ART, Touw DJ, et al. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Social Neuroscience. 2009;4:359–66. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Human sinus arrhythmia as an index of vagal cardiac outflow. Journal of Applied Physiology. 1983;54:961–6. doi: 10.1152/jappl.1983.54.4.961. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does Rejection Hurt? An fMRI Study of Social Exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders‚ Research Version. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. British Journal of Addiction. 1991;86:1563–70. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Gaillard AWK, Tbumbo DA. Drug Effects on Heart Rate and Heart Rate Variability during a Prolonged Reaction Task‚ Ć. Ergonomics. 1976;19:611–22. doi: 10.1080/00140137608931572. [DOI] [PubMed] [Google Scholar]
- Goodacre R, Zadro L. O-Cam: A new paradigm for investigating the effects of ostracism. Behavior Research Methods. 2010;42:768–74. doi: 10.3758/BRM.42.3.768. [DOI] [PubMed] [Google Scholar]
- Grossman P, Wilhelm FH, Kawachi I, Sparrow D. Gender Differences in Psychophysiological Responses to Speech Stress Among Older Social Phobics: Congruence and Incongruence Between Self-Evaluative and Cardiovascular Reactions. Psychosomatic Medicine. 2001;63:765–77. doi: 10.1097/00006842-200109000-00010. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Hormones and Behavior. 2012;61:410–8. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kemp AH, Quintana DS, Kuhnert R-L, Griffiths K, Hickie IB, et al. Oxytocin Increases Heart Rate Variability in Humans at Rest: Implications for Social Approach-Related Motivation and Capacity for Social Engagement. PLoS ONE. 2012;7:e44014. doi: 10.1371/journal.pone.0044014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology. 2012;222:293–302. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Judd C, Kenny D. Data Analysis in Social Psychology - Recent and Recurring Issues. In: Fiske S, Gilbert D, Lindzet G, editors. Handbook of Social Psychology. 5. 2010. pp. 115–43. [Google Scholar]
- Kirkpatrick M, de Wit H. In the company of others: social factors alter acute alcohol effects. Psychopharmacology. 2013:1–12. doi: 10.1007/s00213-013-3147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Macbeth AH, Pagani J, Young WS. Oxytocin: The Great Facilitator of Life. Progress in Neurobiology. 2009 doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht CMM, de Geus EJC, van Dyck R, Penninx BWJH. Longitudinal Evidence for Unfavorable Effects of Antidepressants on Heart Rate Variability. Biological psychiatry. 2010;68:861–8. doi: 10.1016/j.biopsych.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Morgan CJA, Noronha LA, Muetzelfeldt M, Fielding A, Curran HV. Harms and benefits associated with psychoactive drugs: findings of an international survey of active drug users. Journal of Psychopharmacology. 2013;27:497–506. doi: 10.1177/0269881113477744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Close D. Autonomic reactivity and romantic relational aggression among female emerging adults: Moderating roles of social and cognitive risk. International Journal of Psychophysiology. 2011;80:28–35. doi: 10.1016/j.ijpsycho.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, DeVries AC. Oxytocin increases autonomic cardiac control: Moderation by loneliness. Biological Psychology. 2011;86:174–80. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, Schnyder U. A randomized, controlled pilot study of MDMA (±3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD) Journal of Psychopharmacology. 2013;27:40–52. doi: 10.1177/0269881112464827. [DOI] [PubMed] [Google Scholar]
- Parrott AC. The psychotherapeutic potential of MDMA (3,4-methylenedioxymethamphetamine): an evidence-based review. Psychopharmacology. 2007;191:181–93. doi: 10.1007/s00213-007-0703-5. [DOI] [PubMed] [Google Scholar]
- Paton J, Boscan P, Pickering A, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Brain Res Rev. 2005:49. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Porges S. The Polyvagal Perspective. Biological Psychology. 2007;74:116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soellner R. Club Drug Use in Germany. Substance Use & Misuse. 2005;40:1279–93. doi: 10.1081/JA-200066791. [DOI] [PubMed] [Google Scholar]
- Steele T, McCann U, Ricaurte G. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”): pharmacology and toxicology in animals and humans. Addicition. 1994:89. doi: 10.1111/j.1360-0443.1994.tb03330.x. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT1A receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146:509–14. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Wardle MC, de Wit H. Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacology. 2011;220:143–53. doi: 10.1007/s00213-011-2498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Garner MJ, Munafò MR, de Wit H. Amphetamine as a social drug: effects of d-amphetamine on social processing and behavior. Psychopharmacology. 2012;223:199–210. doi: 10.1007/s00213-012-2708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. THE EFFECTS OF METHYLENEDIOXYMETHAMPHETAMINE (MDMA, “ECSTASY”) ON MONOAMINERGIC NEUROTRANSMISSION IN THE CENTRAL NERVOUS SYSTEM. Progress in Neurobiology. 1996;49:455–79. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- White T, Justice A, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacology, Biochemistry, and Behavior. 2002;73:729–41. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Williams, Jarvis Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods. 2006;38:174–80. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- Wolff K. Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. Journal of Psychopharmacology. 2005;20:400–10. doi: 10.1177/0269881106061514. [DOI] [PubMed] [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. Journal of Experimental Social Psychology. 2004;40:560–7. [Google Scholar]