Comparison of Chronic Kidney Disease Prevalence Examined by the Chronic Kidney Disease Epidemiology Collaboration Equation With That by the Modification of Diet in Renal Disease Equation in Korean Adult Population (original) (raw)
Abstract
Background
The new estimated glomerular filtration (eGFR) equation, Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation, was recently introduced. We compared the prevalence of CKD examined by the CKD‐EPI equation with that by the Modification of Diet in Renal Disease (MDRD) equation.
Methods
We analyzed the data from a total of 14,605 Korean adults (age ≥20 years), who were enrolled in the Korean National Health and Nutrition Examination Survey in 2007, 2009, and 2010. CKD stages 1 and 2 were defined as eGFR ≥60 mL/min/1.73 m2 with proteinuria measured by dipstick. CKD stages 3–5 were defined as eGFR <60 mL/min/1.73 m2.
Results
The eGFRs calculated by the CKD‐EPI equation were higher than those calculated by the MDRD equation (P < 0.001), especially in women and young people. The prevalence of CKD stages 3–5 calculated by the MDRD equation was 6.8%, 3.0%, and 3.0% in 2007, 2009, and 2010, respectively. The prevalence of CKD stages 3–5 calculated by CKD‐EPI equation was 7.7%, 2.7%, and 2.6% in 2007, 2009, and 2010, respectively. When defining the CKD using the CKD‐EPI equation, 55 (32.7%) of 350 cases were reclassified into more advanced stages and 295 cases (67.3%) were reclassified into less‐advanced stages.
Conclusion
The CKD‐EPI equation caused an overall low prevalence of CKD compared to the MDRD. Therefore, CKD‐EPI equation might be helpful to prevent an overestimation of CKD.
Keywords: CKD, MDRD, CKD‐EPI
INTRODUCTION
Chronic kidney disease (CKD) is a global health problem owing to its relative high prevalence and adverse complications 1. The prevalence of CKD is widely increasing around the world. In the United States, the prevalence of CKD stage 3 increased from 5.4 to 7.7% over a period of 5 years 2. In Japan, the prevalence of CKD stage 3–5 increased from 4.8 to 15.7% in men, and from 5.8 to 11.7% in women over three decades 3. Moreover, the risk factors of CKD such as obesity, hypertension (HTN), and diabetes mellitus (DM) have also increased worldwide 4, 5. Therefore, investigation of the trends of CKD prevalence and early detection of CKD patients are important in the establishment of strategies for CKD management.
The glomerular filtration rate (GFR), evidence kidney damage (albuminuria, proteinuria, and hematuria), and duration (≥3 months) are used to define and classify the CKD 6. In general, GFRs are calculated using an equation, most commonly the MDRD (Modification of Diet in Renal Disease) equation. It is well known that the major limitation of the MDRD equation is underestimation of estimated GFR (eGFR) in young people and low accuracy in measured GFR >60 mL/min/1.73 m2. In order to overcome these problems, a new equation (CKD epidemiology collaboration, CKD‐EPI) was recently introduced and the US National Kidney Foundation recommended the use of CKD‐EPI instead of the MDRD equation 7. Comparison studies of the two equations are being conducted in many countries. However, there has been no study comparing CKD‐EPI to the MDRD equation in the calculation of GFR among the general healthy population of Korea until now. We compared CKD‐EPI and the MDRD equation using the data from the Korean National Health and Nutrition Examination Survey (KNHANES). We also investigated the trends of CKD prevalence.
MATERIALS AND METHODS
Study Population and Data Selection
KNHANES is a cross‐sectional and nationwide survey on health and nutrition status. We analyzed the data from the first and third year of fourth KNHANES (2007, 2009), and from the first year of the fifth KNHANES (2010). We excluded the data from the second year of the fourth KNHANES (2008) due to heterogeneity of creatinine assay methods and control materials resulting from the change of laboratories. After excluding the cases of missing data on laboratory findings or clinical information, a total of 14,605 cases were enrolled in this study (Table 1).
Table 1.
Characteristics of KNHANES Participants
| 2007 | 2009 | 2010 | _P‐_value | |
|---|---|---|---|---|
| n | 2,692 | 6,627 | 5,286 | |
| Age group (%) | ||||
| 20–39 years | 30.8 | 31.0 | 30.2 | 0.6 |
| 40–59 years | 36.8 | 38.3 | 39.5 | 0.06 |
| ≥60 years | 32.4 | 30.7 | 30.4 | 0.2 |
| Male (%) | 43.5 | 45.9 | 45.4 | 0.1 |
| Medical history | ||||
| ACS history (%) | 3.1 | 2.0 | 3.0 | 0.001 |
| CVA history (%) | 2.6 | 2.0 | 1.8 | 0.04 |
| DM history (%) | 7.6 | 8.0 | 8.4 | 0.5 |
| DM medicationa (%) | 90.4 | 95.0 | 94.5 | 0.06 |
| Hypercholesterolemia history (%) | 6.1 | 8.6 | 10.9 | <0.001 |
| HTN history (%) | 19.2 | 21.5 | 22.9 | 0.001 |
| HTN medicationb (%) | 99.2 | 99.0 | 97.2 | 0.001 |
| Systolic BP (mmHg, mean ± SD) | 118.1 ± 17.5 | 121.0 ± 17.6 | 121.5 ± 17.7 | <0.001 |
| Diastolic BP (mmHg, mean ± SD) | 75.9 ± 10.0 | 78.4 ± 10.6 | 77.6 ± 10.6 | <0.001 |
| Fasting glucose (mg/dL, mean ± SD) | 96.3 ± 22.5 | 98.2 ± 24.3 | 97.5 ± 21.3 | 0.002 |
| Serum urea nitrogen (mg/dL, mean ± SD) | 15.0 ± 4.8 | 14.6 ± 4.4 | 14.1 ± 4.4 | <0.001 |
| Serum creatinine (mg/dL, mean ± SD) | 0.97 ± 0.26 | 0.83 ± 0.23 | 0.82 ± 0.20 | <0.001 |
| Total cholesterol (mg/dL, mean ± SD) | 189.1 ± 36.1 | 187.6 ± 35.6 | 189.4 ± 36.7 | 0.02 |
| Triglycerides (mg/dL, mean ± SD) | 132.3 ± 78.2 | 136.6 ± 110.2 | 133.7 ± 109.0 | 0.1 |
| LDL cholesterol (mg/dL, mean ± SD) | 115.0 ± 32.2 | 112.8 ± 33.6 | 114.4 ± 33.8 | 0.003 |
| HDL cholesterol (mg/dL, mean ± SD) | 47.6 ± 10.0 | 47.5 ± 10.8 | 48.3 ± 11.0 | 0.001 |
| Proteinuria (≥1+, %) | 9.0 | 7.0 | 7.9 | 0.005 |
| Present smoking (%) | 21.1 | 23.4 | 21.8 | 0.02 |
| Height (cm, mean ± SD) | 161.0 ± 9.4 | 162.3 ± 9.4 | 162.5 ± 9.2 | <0.001 |
| Weight (kg, mean ± SD) | 61.7 ± 11.1 | 62.7 ± 11.6 | 62.5 ± 11.5 | <0.001 |
| BMI (kg/m2, mean ± SD) | 23.7 ± 3.2 | 23.7 ± 3.3 | 23.7 ± 3.3 | 0.4 |
| Waist circumference (cm, mean ± SD) | 82.5 ± 9.6 | 81.4 ± 9.9 | 81.2 ± 9.9 | <0.001 |
| Obesity (BMI ≥25 kg/m2, %) | 32.3 | 32.7 | 32.1 | 0.8 |
| CKD stage 3 ≤ MDRD (%) | 6.8 | 3.0 | 3.0 | <0.001 |
| CKD stage 3 ≤ CKD‐EPI (%) | 7.7 | 2.7 | 2.6 | <0.001 |
| eGFR (mL/min/1.73 m2, MDRD (mean ± SD)) | 77.2 ± 12.5 | 89.6 ± 17.1 | 89.6 ± 17.1 | <0.001 |
| eGFR (mL/min/1.73 m2, CKD‐EPI (mean ± SD)) | 80.3 ± 14.7 | 96.0 ± 17.0 | 95.9 ± 16.7 | <0.001 |
Methods of Health Examination
The characteristics analyzed were as follows: age, sex, medical history (HTN, DM, hypercholesterolemia, cerebrovascular accident (CVA), and acute coronary syndrome (ACS), smoking history, height, waist circumference, and obesity (body mass index, BMI, ≥25 kg/m2). The percent of medication was defined as the percent of patients taking medicines among patients with DM or HTN.
Urine protein was semiquantitatively measured by urine dipstick. We defined proteinuria as ≥1+. Serum creatinine was measured by the Jaffe method. In 2007, serum creatinine was measured by ADVIA 1650 (Siemens, Deerfield, IL) that was traceable to the HPLC (high‐performance liquid chromatography) method. In 2009 and 2010, serum creatinine was measured by a Hitachi Automatic Analyzer (Hitachi, Tokyo, Japan) using Roche reagent, CREA (Roche, Basel, Switzerland) that was traceable to the ID‐MS (isotope dilution mass spectrometry) reference method. Serum low‐density lipoprotein (LDL) cholesterol was calculated by the equation, LDL cholesterol = total cholesterol – high‐density lipoprotein (HDL) cholesterol – (triglyceride/5). Blood pressure (BP) was measured by a sphygmomanometer with standard protocols. The mean of the second and third results was used as the final diastolic and systolic BP.
eGFR and CKD Staging
The four‐variable MDRD equation is eGFR = 186 × serum creatinine−1.154 × age−0.203 × 1.212 (if black) × 0.742 (if female), where serum creatinine is expressed in milligrams per deciliters. The CKD‐EPI equation is eGFR = 141 × min(Scr/κ, 1)α × max(Scr/κ, 1)−1.209 × 0.993Age × 1.018 (if female) − 1.159 (if black), where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1 7. The eGFR is expressed as milliliters (mL) per minute per 1.73 m2. When calculating using the MDRD equation with creatinine values measured by ID‐MS calibrated assay, final eGFR was obtained by multiplying by 175/186 8.
We classified the CKD stage according to the Kidney Diseases Outcomes Quality Initiative (KDOQI) guidelines 6. The CKD stages were defined as follows: no CKD, eGFR ≥60 mL/min/1.73 m2 without proteinuria; stage 1, eGFR ≥90 mL/min/1.73 m2 with proteinuria; stage 2, 60 mL/min/1.73 m2 ≤ eGFR < 90 mL/min/1.73 m2 with proteinuria; stage 3, 30 mL/min/1.73 m2 ≤ eGFR < 60 mL/min/1.73 m2; stage 4, 15 mL/min/1.73 m2 ≤ eGFR < 30 mL/min/1.73 m2; and stage 5, eGFR <15 mL/min/1.73 m2.
Statistics
Continuous data of the three groups were compared using the Kruskal–Wallis test or one‐way analysis of variance. Categorical data were compared using the chi‐square test or Fisher's exact test. The linear‐by‐linear association was used to evaluate the trends of CKD prevalence according to age and survey year. A kappa value was used to evaluate the degree of agreement. A _P_‐value <0.05 was considered statistically significant. Statistical analysis was performed by PASW Statistics 20.0 (IBM, Armonk, NY).
RESULTS
A total of 14,605 participants were enrolled in this study. The proportion of males to females and age groups were similar between survey years (P = 0.1). The prevalence of DM patients was 7.6, 8.0, and 8.4% in 2007, 2008, and 2010, respectively (P = 0.5). The prevalence of HTN patients was 19.2, 21.5, and 22.9% in 2007, 2008, and 2010, respectively (P = 0.001). The prevalence of hypercholesterolemia was 6.1, 8.6, and 10.9% in 2007, 2008, and 2010, respectively (P < 0.001). However, the prevalence of CVA patients decreased (2.6, 2.0, and 1.8% in 2007, 2008, and 2010, respectively). The serum creatinine levels significantly decreased, and the levels were 0.97 ± 0.26, 0.83 ± 0.23, and 0.82 ± 0.20 mg/dL in 2007, 2009, and 2010, respectively (P < 0.001). The prevalence of proteinuria was 9.0, 7.0, and 7.9% in 2007, 2009, and 2010, respectively (P = 0.005). The characteristics of participants are summarized in Table 1.
The eGFRs calculated by the MDRD equation were 77.2 ± 12.5, 89.6 ± 17.1, and 89.6 ± 17.1 mL/min/1.73 m2 in 2007, 2009, and 2010, respectively (P < 0.001). The eGFRs calculated by the CKD‐EPI equation were 80.3 ± 14.7, 96.0 ± 17.0, and 95.9 ± 16.7 mL/min/1.73 m2 in 2007, 2009, and 2010, respectively (P < 0.001). The eGFRs calculated by the CKD‐EPI equation were higher than those calculated by the MDRD equation especially in younger patients (Fig. 1). However, in the old population (men ≥70 years, women ≥80 years), eGFRs calculated by the MDRD equation were higher than those calculated by the CKD‐EPI equation. The Bland–Altman plots of difference between eGFRs calculated by the MDRD and CKD‐EPI equations are shown in Figure 2.
Figure 1.
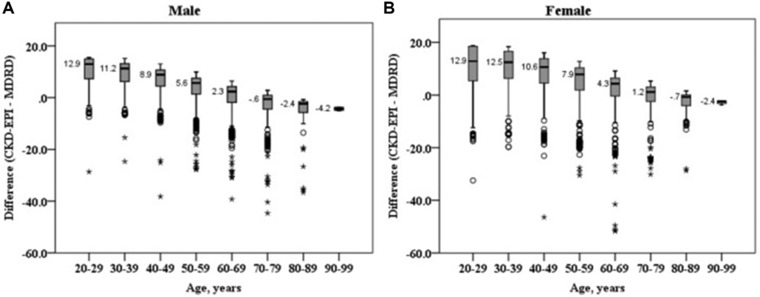
Box and whisker plot of the difference of eGFR (mL/min/1.73 m2) calculated by the MDRD and CKD‐EPI equations in male (A) and female (B). Boxes show interquartile ranges and median values. The difference of eGFR decreased with age (_P_‐value for linear by linear association was <0.001).
Figure 2.
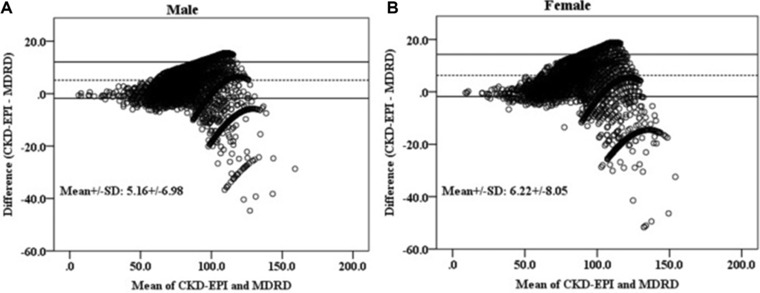
Bland–Altman plots of eGFR (mL/min/1.73 m2) calculated by the MDRD and CKD‐EPI equations in male (A) and female (B).
The prevalence of CKD stages 3–5 (calculated by the MDRD equation) was 6.8, 3.0, and 3.0% in 2007, 2009, and 2010, respectively (P < 0.001, Table 2). The prevalence of CKD stages 3–5 (calculated by CKD‐EPI equation) was 7.7, 2.7, and 2.6% in 2007, 2009, and 2010, respectively (P < 0.001). The prevalence of CKD stages 3–5 increased with age (P < 0.001).
Table 2.
Trends of CKD Prevalence in Korea Over 4 Years (2007–2010)
| CKD stage 3–5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDRD | CKD‐EPI | ||||||||||
| Year | Age | Male (%) | Female (%) | Total (%) | _P_‐value | Male (%) | Female (%) | Total (%) | _P_‐value | ||
| 2007 | 20–39 | 1/368 (0.3) | 2/461 (0.4) | 3/829 (0.4) | <0.001a | <0.001b | 0/368 (0) | 0/461(0) | 0/829(0) | <0.001a | <0.001b |
| 40–59 | 7/430 (1.6) | 20/560 (3.6) | 27/990 (2.7) | 5/430 (1.2) | 14/560 (2.5) | 19/990 (1.9) | |||||
| ≥60 | 44/374 (11.8) | 108/499 (21.6) | 152/873 (17.4) | 67/374 (17.9) | 122/499 (24.4) | 189/873 (21.6) | |||||
| Total | 52/1,172 (4.4) | 130/1,520 (8.6) | 182/2,692 (6.8) | 72/1,172 (6.1) | 136/1,520 (8.9) | 208/2,692 (7.7) | |||||
| 2009 | 20–39 | 1/1,003 (0) | 0/1,050 (0) | 1/2,053 (0) | <0.001b | 1/1,003 (0) | 0/1,050 (0) | 1/2,053 (0) | <0.001b | ||
| 40–59 | 25/1,145 (2.2) | 8/1,392 (0.6) | 33/2,537 (1.3) | 12/1,145 (1.0) | 5/1,392 (0.4) | 17/2,053 (0.8) | |||||
| ≥60 | 82/896 (9.2) | 85/1,141 (7.4) | 167/2,037 (8.2) | 79/896 (8.8) | 79/1,141 (6.9) | 158/2,037 (7.8) | |||||
| Total | 108/3,044 (3.5) | 93/3,583 (2.6) | 201/6,627 (3.0) | 92/3,044 (3.0) | 84/3,583 (2.3) | 176/6,627 (2.7) | |||||
| 2010 | 20–39 | 0/715 (0) | 0/879 (0) | 0/1,594 (0) | <0.001b | 0/715 (0) | 0/879 (0) | 0/1,594 (0) | <0.001b | ||
| 40–59 | 19/942 (2.0) | 12/1,144 (1.0) | 31/2,086 (1.5) | 12/942 (1.3) | 2/1,144 (0.2) | 14/2,086 (0.7) | |||||
| ≥60 | 66/742 (8.9) | 64/864 (7.4) | 130/1,606 (8.1) | 64/742 (8.6) | 57/864 (6.6) | 121/1,606 (7.5) | |||||
| Total | 85/2,399 (3.5) | 76/2,887 (2.6) | 161/5,286 (3.0) | 76/2,399 (3.2) | 59/2,887 (2.0) | 135/5,286 (2.6) |
When defining the CKD using the CKD‐EPI equation, 350 cases (24.0%) were reclassified compared to the results by the MDRD equation (Table 3). Fifty‐five cases (32.7%, up‐stage group) of three hundred fifty cases were reclassified into more advanced stages and 295 cases (67.3%, down‐stage group) were reclassified into less‐advanced stages. Thirty‐seven cases of fifty‐five cases (67.3%) were reclassified from no‐CKD stage to CKD stage 3. Two hundred twenty‐six cases (76.6%) of two hundred ninety‐five cases were reclassified from CKD stage 2 to CKD stage 1. Fifty‐nine cases of two hundred ninety‐five cases (16.9%) were reclassified from CKD 3 and 4 to no‐CKD. The proportion of the old‐age (≥60 years) population was higher in the up‐stage group than that in the down‐stage group (100 vs. 20%, Table 4). The proportion of patients with HTN history was significantly higher in the up‐stage group than in the down‐stage group (40.0 vs. 25.1%). The proportions of DM, CVA, and ACS history were also higher in the up‐stage group than in the down‐stage group.
Table 3.
Comparison of CKD Stages Calculated by the CKD‐EPI Equation with Those by the MDRD Equation
| CKD‐EPI | Reclassification (MDRD → CKD‐EPI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | |||||||||
| MDRD | No CKD | 1 | 2 | 3 | 4 | 5 | Total | Up stage | Down stage |
| No CKD | 5,750 | 0 | 0 | 19 | 0 | 0 | 5,769 | 19 | 0 |
| 1 | 0 | 202 | 4 | 0 | 0 | 0 | 206 | 4 | 0 |
| 2 | 0 | 143 | 246 | 6 | 0 | 0 | 395 | 6 | 143 |
| 3 | 25 | 0 | 5 | 200 | 0 | 0 | 230 | 0 | 30 |
| 4 | 0 | 0 | 0 | 0 | 9 | 0 | 9 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 6 | 6 | 0 | 0 |
| 5,775 | 345 | 255 | 255 | 9 | 6 | 6,615 | 29 | 173 | |
| Female | |||||||||
| No CKD | 7,257 | 0 | 0 | 18 | 0 | 0 | 7,275 | 18 | 0 |
| 1 | 0 | 182 | 8 | 0 | 0 | 0 | 190 | 8 | 0 |
| 2 | 0 | 83 | 143 | 0 | 0 | 0 | 226 | 0 | 83 |
| 3 | 0 | 0 | 4 | 249 | 0 | 0 | 287 | 0 | 4 |
| 4 | 34 | 0 | 0 | 1 | 8 | 0 | 9 | 0 | 35 |
| 5 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 |
| 7,291 | 265 | 155 | 268 | 8 | 3 | 7,990 | 26 | 122 | |
| Total | 13,066 | 610 | 410 | 523 | 17 | 9 | 14,605 | 55 | 295 |
Table 4.
Characteristics of the Subgroups Divided by the Reclassification Status
| Not reclassified | Up stage | Down stage | _P_‐value | |
|---|---|---|---|---|
| n | 14,255 | 55 | 295 | |
| Age group (%) | ||||
| 20–39 years | 30.6 | 0 | 39.7 | <0.001 |
| 40–59 years | 38.5 | 0 | 40.3 | <0.001 |
| ≥60 years | 30.9 | 100 | 20.0 | <0.001 |
| Male (%) | 45.0 | 52.7 | 58.6 | <0.001 |
| HTN history (%) | 21.4 | 40.0 | 25.1 | <0.001 |
| DM history (%) | 7.9 | 20.0 | 12.5 | <0.001 |
| Hypercholesterolemia history (%) | 8.9 | 5.5 | 13.2 | 0.02 |
| CVA history (%) | 2.0 | 7.3 | 2.0 | 0.02 |
| ACS history (%) | 2.5 | 9.0 | 3.4 | 0.006 |
| Present smoking (%) | 35.1 | 36.3 | 43.7 | 0.009 |
| Obesity (%, BMI ≥25kg/m2) | 32.2 | 40.0 | 42.0 | 0.001 |
| Serum creatinine (mg/dl, mean ± SD) | 0.85 ± 0.23 | 1.01 ± 0.22 | 0.96 ± 0.17 | <0.001 |
| eGFR (mL/min/1.73 m2, mean ± SD, MDRD) | 88 ± 17 | 69 ± 14 | 79 ± 12 | <0.001 |
| eGFR (mL/min/1.73 m2, mean ± SD, CKD‐EPI) | 93 ± 17 | 64 ± 12 | 87 ± 15 | <0.001 |
DISCUSSION
The MDRD equation was made based on the data of patients with mildly decreased renal function with a mean measured GFR of 40 mL/1.73 m2 9. Therefore, MDRD has a limitation of underestimating GFR in individuals with GFR >60 mL/min/1.73 m2. This limitation results in an overestimation of CKD prevalence. To overcome this limitation, the CKD‐EPI equation was developed based on the data of individuals with normal or mildly decreased renal function (measured mean GFR of 68 mL/min/1.73 m2) 7. In comparison with the MDRD equation, the CKD‐EPI equation shows less bias (median difference between measured and estimated GFR of 2.5 vs. 5.5 mL/min/1.73 m2, respectively), improved precision (interquartile range of the differences of 16.6 vs. 18.3 mL/min/1.73 m2), and improved accuracy (percent of the difference of GFR within 30% of measured GFR of 84.1 vs. 80.6% respectively) especially in cases with higher GFR (≥60 mL/min/1.73 m2) 7. Several studies have shown the effect of changing the equation from MDRD to CKD‐EPI. A comparison study using a Caucasian population showed that younger people were more often classified to a higher GFR and older people, especially males, to a lower GFR 10. An AusDiab (Australian diabetes, obesity and lifestyle) study showed that 266 patients with CKD according to the MDRD equation were reclassified into no‐CKD stage by the CKD‐EPI equation 11. All of the reclassified 266 cases corresponded to CKD stage 3 (30–59 mL/min/1.73 m2) according to the MDRD equation. Two hundred and five of two hundred sixty‐six were female. The atherosclerosis risk in communities (ARIC) study showed that participants with eGFR 30–89 mL/min/1.73 m2 by the MDRD equation tended to be reclassified into upward eGFR by the CKD‐EPI equation 12. Participants who were reclassified upward showed lower mortality, had coronary heart diseases and stroke compared with those who were not reclassified. Korhonen et al. 13 also reported that 44.8% of CKD stage 3 cases according to the MDRD equation were reclassified as "no CKD," and most of them were women (87.7%).
In our study, the CKD‐EPI equation also yielded higher eGFR compared to the MDRD equation, especially in women and young people. As a result, the CKD‐EPI equation caused an overall low prevalence of CKD compared to the MDRD. The patients with DM, HTN, CVA, and ACS tended to be reclassified into CKD of more advanced stages. Cases of CKD stage 2 and 3 (30–89 mL/min/1.73 m2) by MDRD were most commonly reclassified after applying the CKD‐EPI equation. These results are consistent with other previous studies 11, 12, 13. Previous comparison studies and our results indicate that the CKD‐EPI equation might be more useful in the diagnosis and classification of CKD than the MDRD equation due to less false‐positive results and accurate classification of CKD according to the risk factors of CKD 11, 12, 13, 14.
Lee et al. 3 reported that the prevalence of CKD stages 3–5 in Korea was 7.2% in 2007. Lee et al. 3 used the data from KNHANES and calculated CKD by the MDRD equation. Chin et al. 15 also reported that the prevalence of CKD stages 3–5 was 6.24% in 2005, 7.99% in 2006, and 6.45% in 2008. Chin et al. 15 used the data from subjects who had undergone routine health checkups and defined CKD by the MDRD equation. In the present study, CKD prevalence in 2007 (of CKD stages 3–5 was 6.8% by the MDRD equation) was slightly less (0.4%) than that of Lee et al.'s study (3). This difference may be due to the different total number of enrolled cases (2,692 in our study vs. 2,960 in Lee et al.'s study 3). We excluded the cases with missing values. In our study, the prevalence of CKD in 2009 and 2010 (3.0 and 3.0% by the MDRD equation, 2.7 and 2.6% by the CKD‐EPI equation) was significantly lower than that in 2007 (P = 0.001). The age distribution and male/female ratio were not different among the survey years (Table 1). Although the number of individuals with DM and HTN history increased over the survey periods and the BPs were also higher in 2009 and 2010 than those in 2007, the serum creatinine level was significantly lower in 2009 and 2010 (0.83 ± 0.23 and 0.82 ± 0.20 mg/dL, respectively) compared to that in 2007 (0.97 ± 0.26 mg/dL). The major difference among KNHANES 2007, 2009, and 2010 was the assay instrument used to measure the serum creatinine level. In 2007, serum creatinine was measured by ADVIA 1650 that was traceable to HPLC, while in 2009 and 2010, serum creatinine was measured by an Automatic Analyzer using CREA reagent that was traceable to ID‐MS. Considering the unchanged or increased risk factors of CKD from 2007 to 2010 (Table 1), the cause of decreased CKD prevalence from 2007 to 2009 and 2010 might be interlaboratory variations of serum creatinine measurement.
Standardization of serum creatinine measurement is an ongoing problem. Jaffe methods have fundamental limitations related to calibration and noncreatinine chromogens. To overcome these two analytical problems, calibrations traceable to the ID‐MS reference method and correction factors for noncreatinine chromogens were recommended. The original MDRD equation was developed using a Jaffe method on a Beckman Astra CX3 analyzer (Beckman Coulter, Fullerton, CA) that was not traceable to the ID‐MS reference method 9. Later, repressed four‐variable MDRD equation standardization with ID‐MS was introduced 8. The repressed MDRD equation is as follows: eGFR = 175 × serum creatinine−1.154 × age−0.203 × 1.212 (if black) × 0.742 (if female); serum creatinine standardized with ID‐MS is expressed in milligrams per deciliters. The creatinine calibration to be traceable to the ID‐MS and repressed MDRD equation showed improved performance 16, 17. To overcome the bias effect of noncreatinine chromogens, subtraction of the constant correction factor is used. Unfortunately, this "compensated Jaffe method" has limitations. Subtraction of the constant correction factor for noncreatinine chromogens could cause both overestimation and underestimation of serum creatinine according to the different levels of noncreatinine chromogens among patients 18, 19. Moreover, the effect of calibration and correction for noncreatinine chromogens on eGFR is great in eGFR of near 60 mL/min/1.73 m2 16, 20, 21, 22. Therefore, despite the effort for standardization, inter‐ and intralaboratory variations still remain. For example, Miller et al. 23 reported that the mean bias for 50 instrument‐method peer groups varied from −0.06 to 0.31 mg/dL at a concentration of 0.902 mg/dL measured by ID‐MS, and 63% of Jaffe method peer groups showed significant bias compared to ID‐MS methods. According to the analysis of variance, they concluded that the bias was primarily associated with the difference of instrument manufacturer (P < 0.001) rather than the difference of method type (P = 0.02). Moreover, the Roche peer group showed negative bias compared to ID‐MS. To accurately investigate CKD prevalence and clinical usefulness, studies involving long‐term follow‐ups and standardization of measurement may be needed.
Our study has several limitations. First, a single measurement of serum creatinine was used to calculate the eGFR. Also, the instrument for measuring serum creatinine was changed during the survey. It is well known that a single measurement of serum creatinine is less appropriate than repeated measurements in the evaluation of renal function. In general, repeated measurements are difficult in studies with a large population such as nationwide survey. Second, serum creatinine measurements were not recalibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory. Third, causality of CKD and risk factors were limited in use, due to the cross‐sectional nature of this study. Moreover, history of CKD risk factors was obtained exclusively through a questionnaire. This is a common limitation, which can be observed in other similar studies using data from nationwide surveys.
In conclusion, the CKD‐EPI equation yields higher GFR than the MDRD equation, especially in women and young people. CKD prevalence was lowered by the CKD‐EPI equation compared to that by the MDRD equation. Therefore, CKD‐EPI equation might be helpful to prevent an overestimation of CKD, which is the limitation of MDRD equation.
REFERENCES
- 1.Obrador GT, Pereira BJ, Kausz AT. Chronic kidney disease in the United States: An underrecognized problem. Semin Nephrol 2002;22(6):441–448. [DOI] [PubMed] [Google Scholar]
- 2.Castro AF, Coresh J. CKD surveillance using laboratory data from the population‐based National Health and Nutrition Examination Survey (NHANES). Am J Kidney Dis 2009;53(3 Suppl 3):S46–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SW, Kim YC, Oh SW, et al. Trends in the prevalence of chronic kidney disease, other chronic diseases and health‐related behaviors in an adult Korean population: Data from the Korean National Health and Nutrition Examination Survey (KNHANES). Nephrol Dial Transplant 2011;26(12):3975–3980. [DOI] [PubMed] [Google Scholar]
- 4.Kawamoto R, Kohara K, Tabara Y, Miki T. An association between metabolic syndrome and the estimated glomerular filtration rate. Intern Med 2008;47(15):1399–1406. [DOI] [PubMed] [Google Scholar]
- 5.Ruan X, Guan Y. Metabolic syndrome and chronic kidney disease. J Diabetes 2009;1(4):236–245. [DOI] [PubMed] [Google Scholar]
- 6.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53(4):766–772. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 10.van den Brand JA, van Boekel GA, Willems HL, Kiemeney LA, den Heijer M, Wetzels JF. Introduction of the CKD‐EPI equation to estimate glomerular filtration rate in a Caucasian population. Nephrol Dial Transplant 2011;26(10):3176–3181. [DOI] [PubMed] [Google Scholar]
- 11.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD‐EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: The AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis 2010;55(4):660–670. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD‐EPI) equation compared with the MDRD Study equation for estimated GFR: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2010;55(4):648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korhonen PE, Kivela SL, Aarnio PT, Kautiainen H, Jarvenpaa S, Kantola IM. Estimating glomerular filtration rate in hypertensive subjects: Comparison of the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) and Modification of Diet in Renal Disease (MDRD) Study equations. Ann Med 2012;44(5):487–493. [DOI] [PubMed] [Google Scholar]
- 14.McFarlane SI, McCullough PA, Sowers JR, et al. Comparison of the CKD Epidemiology Collaboration (CKD‐EPI) and Modification of Diet in Renal Disease (MDRD) study equations: Prevalence of and risk factors for diabetes mellitus in CKD in the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2011;57(3 Suppl 2):S24–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chin HJ, Ahn JM, Na KY, et al. The effect of the World Kidney Day campaign on the awareness of chronic kidney disease and the status of risk factors for cardiovascular disease and renal progression. Nephrol Dial Transplant 2010;25(2):413–419. [DOI] [PubMed] [Google Scholar]
- 16.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis 2007;50(1):21–35. [DOI] [PubMed] [Google Scholar]
- 17.Vickery S, Stevens PE, Dalton RN, van Lente F, Lamb EJ. Does the ID‐MS traceable MDRD equation work and is it suitable for use with compensated Jaffe and enzymatic creatinine assays? Nephrol Dial Transplant 2006;21(9):2439–2445. [DOI] [PubMed] [Google Scholar]
- 18.Parry DM. Use of single‐value protein compensation of the Jaffe creatinine assay contributes to clinically significant inaccuracy in results. Clin Chem 2008;54(1):215–216. [DOI] [PubMed] [Google Scholar]
- 19.Kuster N, Bargnoux AS, Pageaux GP, Cristol JP. Limitations of compensated Jaffe creatinine assays in cirrhotic patients. Clin Biochem 2012;45(4–5):320–325. [DOI] [PubMed] [Google Scholar]
- 20.Murthy K, Stevens LA, Stark PC, Levey AS. Variation in the serum creatinine assay calibration: A practical application to glomerular filtration rate estimation. Kidney Int 2005;68(4):1884–1887. [DOI] [PubMed] [Google Scholar]
- 21.Panteghini M. Enzymatic assays for creatinine: Time for action. Scand J Clin Lab Invest Suppl 2008;241:84–88. [DOI] [PubMed] [Google Scholar]
- 22.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 2002;39(5):920–929. [DOI] [PubMed] [Google Scholar]
- 23.Miller WG, Myers GL, Ashwood ER, et al. Creatinine measurement: State of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med 2005;129(3):297–304. [DOI] [PubMed] [Google Scholar]