Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib (original) (raw)
Key Points
- Imatinib achieves deep and durable remissions in patients with myeloid neoplasms bearing PDGFRB.
- Allogeneic stem cell transplantation is no longer indicated for patients with chronic myeloproliferative neoplasm bearing PDGFRB who respond to imatinib.
Abstract
Myeloid neoplasms and eosinophilia with rearrangements of PDGFRB are uncommon Philadelphia-negative myeloproliferative neoplasms. Patients are typically male, with morphologic features of a Philadelphia-negative chronic myeloproliferative syndrome or chronic myelomonocytic leukemia with eosinophilia. Reciprocal translocations involving PDGFRB result in fusion genes with constitutively activated receptor tyrosine kinase sensitive to inhibition with imatinib. We present an updated and expanded analysis of a cohort of 26 such patients treated with imatinib. After a median follow-up of 10.2 years (range, 1.8-17 years), the 10-year overall survival rate was 90% (95% confidence interval, 64%-97%); after median imatinib duration of 6.6 years (range, 0.1-12 years), the 6-year progression-free survival rate was 88% (95% confidence interval, 65%-96%). Of the patients, 96% responded; no patients who achieved a complete cytogenetic (n = 13) or molecular (n = 8) remission lost their response or progressed to blast crisis. Imatinib is well-tolerated and achieves excellent long-term responses in patients with PDGFRB rearrangements.
Introduction
Myeloid neoplasms with PDGFRB rearrangements are genotypically and phenotypically diverse, typically presenting as myeloproliferative neoplasm (MPN) with eosinophilia.1 More than 20 fusion partners have been reported,2 with a spectrum of morphologic presentations, including atypical chronic myeloid leukemia (CML), primary myelofibrosis, and acute leukemia.3,4 A recent study found evidence of PDGFRB rearrangements in 10 (1.8%) of 556 consecutive patients with MPN.2 The most common morphologic diagnosis is chronic myelomonocytic leukemia with eosinophilia, associated with t(5;12)(q33;p13), resulting an ETV6-PDGFRB fusion gene (formerly TEL-PDGFRB).5 Previous publications have detailed the successful use of imatinib, but long-term outcomes remain inadequately described.6-10 In our previous publication, the median duration of imatinib therapy was 3.9 years7; in this report, we present an expanded cohort of 26 such patients treated with imatinib for a median duration of 6.6 years.
Patients and methods
We contacted physicians contributing patients to the original reports6,7 and searched PubMed, Medline, and Google Scholar for the terms PDGFRB and imatinib or STI571. Corresponding authors of published cases before 2010 (to allow a minimum of 2 years’ follow-up) were invited to contribute updated follow-up data.8-17 Baseline patient demographics, disease characteristics, treatment, response, and follow-up data were collected in a study-specific case report form. Data collection was compliant with institutional review board requirements. Cytogenetic analysis, fluorescence in situ hybridization, and nested reverse transcriptase polymerase chain reaction for quantification of fusion transcripts were performed as previously described.7
Statistical analysis
Continuous variables are expressed as median and range, and categorical variables as numbers and percentages. Overall survival (OS) was determined from date of diagnosis; progression was defined as failure to achieve hematologic response (HR), loss of complete cytogenetic response (CCR) or molecular remission (MolR), or death and progression-free survival, and was calculated from initiation of imatinib; both were calculated using the method of Kaplan and Meier. All statistical analyses were performed using STATA, version 12.1 (StataCorp).
Results
We identified 26 patients with MPN and PDGFRB rearrangements: 12 from our prior report,7 7 initially reported elsewhere,8-10,13 and 7 unreported. Three authors declined to provide follow-up data; all others responded, and the baseline and treatment characteristics of these 26 patients are summarized in Table 1. The morphologic diagnoses were either chronic MPN or chronic myelomonocytic leukemia with eosinophilia with 2 exceptions: the first was a 19-year-old man who had acute myeloid leukemia with minimal differentiation and complex cytogenetics, with eosinophilia and PDGFRB gene rearrangement demonstrated by fluorescence in situ hybridization at postinduction reassessment, and the second was a 45-year-old man with systemic mastocytosis and t(4;5)(q21;q33) rearrangement.13
Table 1.
Baseline patient characteristics, details of imatinib therapy and response
| Characteristic | Data available | Result |
|---|---|---|
| Median age at diagnosis, range | 26 | 50 (0.9-78) years |
| Male, % | 26 | 21/26 (81%) |
| Median white cell count at diagnosis, range, ×109/L | 23 | 51 (4-138) |
| Median eosinophils at diagnosis, range, ×109/L | 21 | 3.5 (0.7-12) |
| Median platelets at diagnosis, range | 24 | 119 (60-506) |
| Cytogenetics at diagnosis | 26 | |
| t(5;12)(q33;p13) | 14 (54%) | |
| t(5;12) no further details available | 4 (12%) | |
| t(5;12)(q23;p23) | 2 (8%) | |
| t(5;12)(q33;p12.2) | 1 (4%) | |
| ins (2;12)(p21;q?13q?22),del(5)(q33q35) | 1 (4%) | |
| t(5;17)(q33;p13.3) | 1 (4%) | |
| t(1;5)(q23;q35) | 1 (4%) | |
| t(4;5)(q21;q33) | 1 (4%) | |
| t(2;5)(p21;q33) | 1 (4%) | |
| Fusion partners of PDGFRB | 26 | |
| ETV6 | 18 (69%) | |
| WDR48 | 1 (4%) | |
| PDE4DIP | 1 (4%) | |
| RAB5EP | 1 (4%) | |
| PRKG2 | 1 (4%) | |
| SPTBN1 | 1 (4%) | |
| BIN2 | 1 (4%) | |
| TP53BP1-PDGFRB | 1 (4%) | |
| Partner unknown | 1 (4%) | |
| Number of prior lines of therapy | 24 | |
| 0 | 8 (33%) | |
| 1 | 8 (33%) | |
| 2+ | 8 (33%) | |
| Median time from diagnosis to imatinib, range | 23 | 8.6 (0–123) months |
| Starting imatinib dose, milligrams daily | 26 | |
| 100 | 3 (12%) | |
| 300 | 1 (4%) | |
| 400 | 22 (84%) | |
| Best response to imatinib | 25 | |
| Stable disease | 1 (4%) | |
| HR | 1 (4%) | |
| Partial cytogenetic response | 1 (4%) | |
| CCR | 13 (52%) | |
| MolR | 9 (36%) | |
| Disease status at time of reporting | 25 | |
| Dead | 2 (8%) | |
| Stable disease | 1 (4%) | |
| HR | 0 (0%) | |
| Partial cytogenetic response | 1 (4%) | |
| CCR | 12 (40%) | |
| MolR | 9 (36%) | |
| Time to HR after imatinib | 22 | 1 (0.5–4*) months |
| Time best response after imatinib | 17 | 5 (1.5–36) months |
Eight patients had received no therapy before imatinib. The patient with acute myeloid leukemia received induction chemotherapy with daunorubicin and cytarabine, followed by imatinib. Hydroxyurea had been used before imatinib in 11 patients (44%), interferon-α in 5 (20%), busulphan in 3 (12%), prednisolone in 2 (8%), and cladribine in 1 (4%). Most patients were initially treated with imatinib 400 mg daily, and imatinib response was achieved in 25 (96%) of 26 patients. CCR was achieved in 13 of 25 and MolR in 8 of all patients.
Dose reductions were implemented in 7 patients in either CCR (n = 4) or MolR (n = 3). The baseline dose of 400 mg was reduced to 100 (n = 2), 200 (n = 4), or 300 (n = 1) because of toxicity (grade 4 hematologic toxicity, n = 1), physician preference (n = 3), patient request (n = 1), or reason unknown (n = 2). All patients remain in CCR or MolR, respectively. Four patients began receiving lower doses of 300 mg daily (n = 1) or 100 mg daily (n = 3). All 4 rapidly achieved and remain in CCR (n = 1) or MolR (n = 3), with a median time to best response of 10.0 months (range, 6.0-15.0 months).
In 3 patients, imatinib was discontinued. The first case was a 50-year-old woman with ETV6-PDGFRB fusion who commenced imatinib 8 years after diagnosis. She was the only patient not to achieve HR; imatinib was stopped after 5 months because of futility. The second patient was a 29-year-old man with chronic myelomonocytic leukemia and _RAB5EP_-PDGFRB who was experiencing molecular relapse 15 months after sibling-donor allogeneic stem cell transplantation. Imatinib 400 mg daily resulted in MolR by 6 weeks, sustained until deliberate drug withdrawal after 29 months.8 This patient was alive in clinical and hematologic remission at last follow-up, 6.2 years after discontinuation of imatinib. In the third patient, imatinib was ceased after prolonged grade 4 hematologic toxicity. This patient subsequently died of intracranial bleeding (described in “Safety and toxicity”).
Imatinib was temporarily interrupted in 2 patients. One patient (with acute myeloid leukemia) had erratic compliance, with multiple short treatment interruptions, but remained in CCR after 38 months on imatinib at last follow-up. The other was a female infant (diagnosed at 18 months of age) with a variant PDE4DIP-PDGFRB fusion.10 She achieved only partial cytogenetic response, and imatinib was discontinued after 4.5 years of treatment (reason unavailable). Within 3 months of cessation, hematologic relapse occurred and imatinib was restarted, with restoration of disease control. The last marrow assessment after 7 years of treatment showed sustained partial cytogenetic response. At last follow-up (age 13 years), she was continuing to receive imatinib.
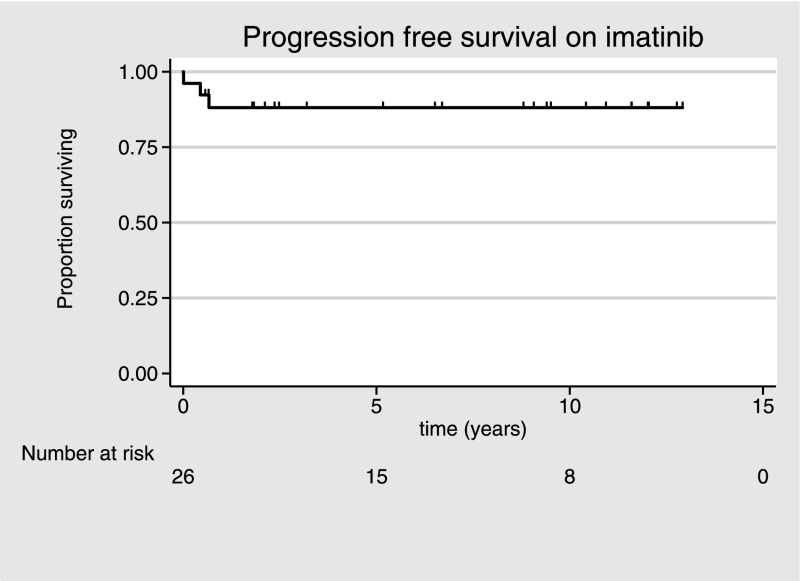
After a median follow-up of 10.2 years (range, 0-17 years) from diagnosis, 2 patients have died with an actuarial 10-year OS rate of 90% (95% confidence interval, 64%-97%). After a median duration of imatinib therapy of 6.5 years (range, 0.1-12 years), the actuarial 6-year progression-free survival rate is 88% (95% confidence interval, 65%-97%), with the 21 patients achieving CCR or better maintaining this response for a median of 62 months (range, 8-139 months; Figure 1). All 8 patients achieving MolR have sustained it, with a median of 112 months (range, 7-125 months) receiving imatinib. No patients developed secondary resistance or required treatment with second-generation tyrosine kinase inhibitors.
Figure 1.
Progression-free survival of patients with PDGFRB gene rearrangements receiving imatinib.
Safety and toxicity
As previously reported, the adverse effect profile was similar to that seen in patients with CML.7 These include mild nausea, fatigue, fluid retention, and myalgia not necessitating cessation of therapy. Both deaths were previously reported in detail.7 Briefly, 1 patient died of invasive fungal infection after transformation to blast crisis, and the other died of intracranial hemorrhage during a period of prolonged pancytopenia (grade 5 thrombocytopenia) after achievement of CCR. No other grade 3 to 4 toxicities were reported. The youngest patient (aged 18 months at diagnosis) is now aged 14 years; although there was no acute toxicity, she has mild marrow fibrosis and short stature after 11.6 years receiving imatinib, although causality is uncertain. Other than the patient previously reported, no leukemic transformations occurred, with a crude 10-year transformation rate of 4%.
Discussion
This report highlights the excellent efficacy and durable long-term remissions achieved in patients with _PDGFRB_-rearranged MPN treated with imatinib. Recognition of a platelet-derived growth factor receptor translocation is important, given that this exquisite sensitivity has altered the natural history from an aggressive (2-year OS 55%)18 to indolent (10-year OS 90%) neoplasm paralleling CML. Importantly, the variant PDGFRB fusion partners appear responsive. All patients initially treated with 400 mg daily who achieved HR did so within 2 months. Thus, if patients at this dose fail to achieve HR after 3 months of therapy, our data suggest ongoing treatment may be futile.
Published data regarding MPN with PDGFRA rearrangement suggest patients can be maintained successfully on doses of imatinib as low as 100 mg weekly, once remission is achieved.19-21 The lower doses required in patients with PDGFRA/B rearrangements relative to CML likely stem from the lower half maximal inhibitory concentration (IC50) for imatinib: 3.2 nM for FIP1L1-PDGFRA22 and 150 nM for ETV6-PDGFRB6 compared with a median 600 nM for BCR-ABL.23 The rapid and durable achievement of CCR/MolR in 4 patients commenced on doses lower than 400 mg, and lack of progression among 7 patients in whom doses were reduced suggests that patients with PDGFRB rearrangements may be more sensitive to imatinib than patients with CML. The durability of responses to imatinib suggests chronic MPN with PDGFRB rearrangement is no longer an indication for allogeneic stem cell transplantation. Given that only 3 patients stopped imatinib, we currently have insufficient data to comment on the safety of deliberate withdrawal of tyrosine kinase inhibitor in sustained MolR; however, enrolling such patients in a prospective study with close monitoring (as in the Stop Imatinib24 and trial of withdrawing imatinib in stable remission25 studies in CML) is the next rational step in determining whether imatinib can achieve durable treatment-free remission.
The study is limited by its retrospective nature and potential for publication bias overestimating response rate. However, in this rare disease, prospective studies are difficult to conduct. Nonetheless, these data confirm imatinib achieves deep and durable responses in patients with MPN bearing PDGFRB rearrangements.
Acknowledgments
The authors thank Dr N. Carvalho and Dr E. Velloso (São Paulo, Brazil) for clinical updates and Professor Francois Xavier Mahon for providing molecular analysis for French patients. This work was funded in part by the Victorian Cancer Agency (grant CTCB11_18) and the Haematology Society of Australia and New Zealand (New Investigator Scholarship).
Footnotes
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.Y.C. and K.B. designed the case report form, coordinated data collection, analyzed data and wrote the first draft of the manuscript; J.F.A. coordinated data collection and provided patients; J.F.S. designed the study and provided patients; F.H., V.P., M.G., N.P., O.O., A.G., P.G., C.E.D., S.I., G.S., P.R., R.C.T.A., D.M.R., and D.F. provided data and treated patients; N.C.P.C. performed molecular analysis; and all authors wrote and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.F.A. has received honoraria from and serves on advisory boards for Novartis. N.C.P.C. has received honoraria and research funding from Novartis. The remaining authors declare no competing financial interests.
Correspondence: John F. Seymour, Department of Haematology, Peter MacCallum Cancer Centre, East Melbourne, VIC, 8006 Australia; e-mail: john.seymour@petermac.org.
References
- 1.Havelange V, Demoulin JB. Review of current classification, molecular alterations, and tyrosine kinase inhibitor therapies in myeloproliferative disorders with hypereosinophilia. J Blood Med. 2013;4:111–121. doi: 10.2147/JBM.S33142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arefi M, García JL, Peñarrubia MJ, et al. Incidence and clinical characteristics of myeloproliferative neoplasms displaying a PDGFRB rearrangement. Eur J Haematol. 2012;89(1):37–41. doi: 10.1111/j.1600-0609.2012.01799.x. [DOI] [PubMed] [Google Scholar]
- 3.Lengline E, Beldjord K, Dombret H, Soulier J, Boissel N, Clappier E. Successful tyrosine kinase inhibitor therapy in a refractory B-cell precursor acute lymphoblastic leukemia with EBF1-PDGFRB fusion. Haematologica. 2013;98(11):e146–e148. doi: 10.3324/haematol.2013.095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tokita K, Maki K, Tadokoro J, et al. Chronic idiopathic myelofibrosis expressing a novel type of TEL-PDGFRB chimaera responded to imatinib mesylate therapy. Leukemia. 2007;21(1):190–192. doi: 10.1038/sj.leu.2404397. [DOI] [PubMed] [Google Scholar]
- 5.Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77(2):307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 6.Apperley JF, Gardembas M, Melo JV, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med. 2002;347(7):481–487. doi: 10.1056/NEJMoa020150. [DOI] [PubMed] [Google Scholar]
- 7.David M, Cross NC, Burgstaller S, et al. Durable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood. 2007;109(1):61–64. doi: 10.1182/blood-2006-05-024828. [DOI] [PubMed] [Google Scholar]
- 8.Magnusson MK, Meade KE, Nakamura R, Barrett J, Dunbar CE. Activity of STI571 in chronic myelomonocytic leukemia with a platelet-derived growth factor beta receptor fusion oncogene. Blood. 2002;100(3):1088–1091. doi: 10.1182/blood-2002-01-0165. [DOI] [PubMed] [Google Scholar]
- 9.Pitini V, Arrigo C, Teti D, Barresi G, Righi M, Alo G. Response to STI571 in chronic myelomonocytic leukemia with platelet derived growth factor beta receptor involvement: a new case report. Haematologica. 2003;88(5):ECR18. [PubMed] [Google Scholar]
- 10.Wilkinson K, Velloso ER, Lopes LF, et al. Cloning of the t(1;5)(q23;q33) in a myeloproliferative disorder associated with eosinophilia: involvement of PDGFRB and response to imatinib. Blood. 2003;102(12):4187–4190. doi: 10.1182/blood-2003-04-1150. [DOI] [PubMed] [Google Scholar]
- 11.Bastie JN, Garcia I, Terré C, Cross NC, Mahon FX, Castaigne S. Lack of response to imatinib mesylate in a patient with accelerated phase myeloproliferative disorder with rearrangement of the platelet-derived growth factor receptor beta-gene. Haematologica. 2004;89(10):1263–1264. [PubMed] [Google Scholar]
- 12.Curtis CE, Grand FH, Waghorn K, Sahoo TP, George J, Cross NC. A novel ETV6-PDGFRB fusion transcript missed by standard screening in a patient with an imatinib responsive chronic myeloproliferative disease. Leukemia. 2007;21(8):1839–1841. doi: 10.1038/sj.leu.2404728. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher G, Horsman DE, Tsang P, Forrest DL. Fusion of PRKG2 and SPTBN1 to the platelet-derived growth factor receptor beta gene (PDGFRB) in imatinib-responsive atypical myeloproliferative disorders. Cancer Genet Cytogenet. 2008;181(1):46–51. doi: 10.1016/j.cancergencyto.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JL, Font de Mora J, Hernandez JM, et al. Imatinib mesylate elicits positive clinical response in atypical chronic myeloid leukemia involving the platelet-derived growth factor receptor beta. Blood. 2003;102(7):2699–2700. doi: 10.1182/blood-2003-05-1579. [DOI] [PubMed] [Google Scholar]
- 15.Levine RL, Wadleigh M, Sternberg DW, et al. KIAA1509 is a novel PDGFRB fusion partner in imatinib-responsive myeloproliferative disease associated with a t(5;14)(q33;q32). Leukemia. 2005;19(1):27–30. doi: 10.1038/sj.leu.2403548. [DOI] [PubMed] [Google Scholar]
- 16.Vizmanos JL, Novo FJ, Román JP, et al. NIN, a gene encoding a CEP110-like centrosomal protein, is fused to PDGFRB in a patient with a t(5;14)(q33;q24) and an imatinib-responsive myeloproliferative disorder. Cancer Res. 2004;64(8):2673–2676. doi: 10.1158/0008-5472.can-04-0144. [DOI] [PubMed] [Google Scholar]
- 17.Walz C, Haferlach C, Hänel A, et al. Identification of a MYO18A-PDGFRB fusion gene in an eosinophilia-associated atypical myeloproliferative neoplasm with a t(5;17)(q33-34;q11.2). Genes Chromosomes Cancer. 2009;48(2):179–183. doi: 10.1002/gcc.20629. [DOI] [PubMed] [Google Scholar]
- 18.Steer EJ, Cross NC. Myeloproliferative disorders with translocations of chromosome 5q31-35: role of the platelet-derived growth factor receptor Beta. Acta Haematol. 2002;107(2):113–122. doi: 10.1159/000046641. [DOI] [PubMed] [Google Scholar]
- 19.Helbig G, Stella-Hołowiecka B, Majewski M, et al. A single weekly dose of imatinib is sufficient to induce and maintain remission of chronic eosinophilic leukaemia in FIP1L1-PDGFRA-expressing patients. Br J Haematol. 2008;141(2):200–204. doi: 10.1111/j.1365-2141.2008.07033.x. [DOI] [PubMed] [Google Scholar]
- 20.Baccarani M, Cilloni D, Rondoni M, et al. The efficacy of imatinib mesylate in patients with FIP1L1-PDGFRalpha-positive hypereosinophilic syndrome. Results of a multicenter prospective study. Haematologica. 2007;92(9):1173–1179. doi: 10.3324/haematol.11420. [DOI] [PubMed] [Google Scholar]
- 21.Pardanani A, D’Souza A, Knudson RA, Hanson CA, Ketterling RP, Tefferi A. Long-term follow-up of FIP1L1-PDGFRA-mutated patients with eosinophilia: survival and clinical outcome. Leukemia. 2012;26(11):2439–2441. doi: 10.1038/leu.2012.162. [DOI] [PubMed] [Google Scholar]
- 22.Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 23.White D, Saunders V, Lyons AB, et al. In vitro sensitivity to imatinib-induced inhibition of ABL kinase activity is predictive of molecular response in patients with de novo CML. Blood. 2005;106(7):2520–2526. doi: 10.1182/blood-2005-03-1103. [DOI] [PubMed] [Google Scholar]
- 24.Mahon FX, Réa D, Guilhot J, et al. Intergroupe Français des Leucémies Myéloïdes Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 25.Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–522. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]