Cinnamon Treatment Upregulates Neuroprotective Proteins Parkin and DJ-1 and Protects Dopaminergic Neurons in a Mouse Model of Parkinson’s Disease (original) (raw)
. Author manuscript; available in PMC: 2015 Sep 1.
Published in final edited form as: J Neuroimmune Pharmacol. 2014 Jun 20;9(4):569–581. doi: 10.1007/s11481-014-9552-2
Abstract
Upregulation and/or maintenance of Parkinson’s disease (PD)-related beneficial proteins such as Parkin and DJ-1 in astrocytes during neurodegenerative insults may have therapeutic efficacy in PD. Cinnamon is a commonly used natural spice and flavoring material throughout the world. Here we have explored a novel use of cinnamon in upregulating Parkin and DJ-1 and protecting dopaminergic neurons in MPTP mouse model of PD. Recently we have delineated that oral feeding of cinnamon (Cinnamonum verum) powder produces sodium benzoate (NaB) in blood and brain of mice. Proinflammatory cytokine IL-1β decreased the level of Parkin/DJ-1 in mouse astrocytes. However, cinnamon metabolite NaB abrogated IL-1β-induced loss of these proteins. Inability of TNF-α to produce nitric oxide (NO) and decrease the level of Parkin/DJ-1 in wild type (WT) astrocytes, failure of IL-1β to reduce Parkin/DJ-1 in astrocytes isolated from iNOS (−/−) mice, and decrease in Parkin/DJ-1 in WT astrocytes by NO donor DETA-NONOate suggest that NO is a negative regulator of Parkin/DJ-1. Furthermore, suppression of IL-1β-induced expression of iNOS in astrocytes by NaB and reversal of NaB-mediated protection of Parkin/DJ-1 by DETA-NONOate in astrocytes indicate that NaB protects Parkin/DJ-1 in activated astrocytes via suppressing iNOS. Similarly MPTP intoxication also increased the level of iNOS and decreased the level of Parkin/DJ-1 in vivo in the nigra. However, oral treatment of MPTP-intoxicated mice with cinnamon powder and NaB reduced the expression of iNOS and protected Parkin/DJ-1 in the nigra. These findings paralleled dopaminergic neuronal protection, normalized striatal neurotransmitters, and improved motor functions by cinnamon in MPTP-intoxicated mice. These results suggest that cinnamon may be beneficial for PD patients.
Keywords: Cinnamon, Parkin, DJ-1, Nitric oxide, MPTP mouse model, Dopaminergic neurons, Dopamine
Introduction
Parkinson’s disease (PD), the most common neurodegenerative movement disorder in human, is caused due to the loss of dopaminergic neurons in the substancia nigra pars compacta (SNpc) (Dauer and Przedborski 2003; Fahn 2010). Parkin and DJ-1 are known to stimulate and support the survival of existing dopaminergic neurons. Consistently, in PD, Parkin and DJ-1 have been suggested as rescuers of these vulnerable cells (Zhou and Freed 2005; Ng et al. 2009; Bian et al. 2013). However, clinical application of these molecules in PD has been limited because of difficulties in delivery. These small proteins do not readily diffuse across the blood-brain barrier (BBB) or ventricular lining and have limited or unstable bioavailability. Gene delivery and/or protein delivery by stereotactic injection is definitely an option but it has several limitations. It seems from the therapeutic angle, the best option is to stimulate/induce the production of Parkin and DJ-1 within the SNpc of PD patients. Therefore, finding out drugs or small molecules to up-regulate Parkin and DJ-1 within the SNpc represents an important area of research.
Cinnamon, the brown bark of cinnamon tree, is a commonly used spice and flavoring material for deserts, candies, chocolate, etc. It has a long history as a medicine as well. Medieval physicians used cinnamon in medicines to treat a variety of disorders including arthritis, coughing, hoarseness, sore throats, etc. It was once so highly-prized that several wars were fought over it. In addition to containing manganese, dietary fiber, iron, and calcium, cinnamon contains a major compound, cinnamaldehyde, which is converted into cinnamic acid by oxidation. In the liver, this cinnamic acid is β-oxidized to benzoate (Abd El-Mawla et al. 2001) that exists as sodium salt (NaB) or benzoyl-CoA. Recently we have demonstrated that oral administration of ground cinnamon increases the level of NaB in serum and brain of mice (Jana et al. 2013). It has been also reported that minor amount of NaB is also excreted in the urine of human (Bridges et al. 1970; Kubota and Ishizaki 1991). NaB is of medical importance as it is a component of Ucephan, a FDA-approved drug used in the treatment for hepatic metabolic defects associated with hyperammonemia such as urea cycle disorder in children (Leonard and Morris 2002; Scaglia et al. 2004). It is also widely used as a preservative in broad range of foods and cosmetic products (Nair 2001). It is non-toxic and can be administered as a solution in drinking water. It has been reported that 2 % solution of NaB in drinking water is safe for lifelong treatment in mice without any noticeable side effects (Toth 1984).
Here we delineate that levels of Parkin and DJ-1 decrease in activated glial cells and that treatment of activated glial cells with cinnamon metabolite NaB blocks such loss. Interestingly, NaB protects Parkin and DJ-1 in activated astrocytes via suppressing the production of NO and expression of iNOS. Similarly in MPTP mouse model of PD, we find increased level of iNOS and decreased level of Parkin and DJ-1 in vivo in the nigra. Finally, we also demonstrate that oral treatment of MPTP-intoxicated mice with cinnamon powder and NaB reduces the nigral expression of iNOS, blocks nigral loss of Parkin and DJ-1, protects the nigrostriatal axis, and restores locomotor activities. These results suggest that cinnamon may be used to protect dopaminergic neurons in the nigra of PD patients.
Methods
Reagents
Sodium benzoate (NaB) and sodium formate (NaFO), were purchased from Sigma Aldrich (St. Louis, MO). Original Ceylon cinnamon (Cinnamonum verum) in ground form was obtained from Indus Organics (San Ramon, CA). Fetal bovine serum (FBS), Hank’s balanced salt solution (HBSS), trypsin, and DMEM/F-12 were from Mediatech (Manassas, VA).
Isolation of Primary Mouse Astroglia
Astroglia were isolated from 7–9 days old mouse pups as described earlier (Brahmachari et al. 2006; Khasnavis et al. 2012; Mondal et al. 2012a). Briefly, on day 9, the mixed glial cultures were subjected to shaking at 240 rpm for 2 h at 37 °C on a rotary shaker to remove microglia followed by another round of shaking on day 11 at 190 rpm for 18 h to remove oligodendroglia and residual microglia. The attached cells were washed and seeded onto new plates for further studies. About ninety-eight percent of this preparation was found to be positive for GFAP, a marker of astrocytes.
Animals and MPTP Intoxication
Six- to eight-week old C57BL/6 mice were purchased from Harlan, Indianapolis, IN. Animal maintenance and experiments were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use committee of the Rush University Medical Center, Chicago, IL. For acute MPTP intoxication, mice received four intraperitoneal (i.p.) injections of MPTP-HCl (18 mg/kg of free base; Sigma Chemical Co., St. Louis, MO) in saline at 2-h intervals (Ghosh et al. 2007, 2009; Roy et al. 2012). Control animals received only saline.
Cinnamon and NaB Treatment
Ground cinnamon (Cinnamonum verum) was solubilized in 0.5 % methylcellulose (MC) and MPTP-intoxicated mice were treated daily with 100 μL cinnamon-MC mixture from 3 h after the last injection of MPTP via gavage using a gavage needle. Therefore, control MPTP mice received only 100 μL 0.5 % MC. For NaB treatment, NaB was solubilized in water and mice were treated daily with NaB-solubilized water via gavage. Sodium formate (NaFO) was used as a control for NaB, which was also treated via gavage after solubilization in water.
Antibodies
Rabbit anti-mouse iNOS antibodies were obtained from Calbiochem, Gibbstown, NJ. Goat anti-glial fibrillary acidic protein (GFAP) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-mouse Parkin and goat anti-mouse DJ-1 were purchased from Abcam (Cambridge, MA). Cy2- and Cy5-conjugated antibodies were obtained from Jackson Immuno Research Laboratories (West Grove, PA).
Western Blot Analysis
Immunoblot analysis for DJ-1, Parkin, iNOS, and GFAP was carried out as described earlier (Saha et al. 2006, 2007). Briefly, cell homogenates were electrophoresed, proteins were transferred onto a nitrocellulose membrane, and bands were visualized with an Odyssey infrared scanner after immunolabeling with respective primary antibodies followed by infra-red fluorophore-tagged secondary antibody (Invitrogen).
Immunohistochemistry
Seven days after MPTP intoxication, mice were sacrificed and their brains fixed, embedded, and processed for tyrosine hydroxylase (TH) and thionin staining as previously described (Benner et al. 2004; Jana et al. 2007; Ghosh et al. 2009). Quantitation of striatal TH immunostaining was performed as described (Benner et al. 2004; Jana et al. 2007; Ghosh et al. 2009). Optical density measurements were obtained by digital image analysis (Scion, Frederick, MD). Striatal TH optical density reflected dopaminergic fiber innervation.
HPLC Analyses
Striatal level of dopamine was quantified in Complete Stand-Alone HPLC-ECD System EiCOMHTEC-500 (JM Science Inc., Grand Island, NY) as described earlier (Ghosh et al. 2007, 2009; Mondal et al. 2012b; Roy et al. 2012). Briefly, mice were sacrificed by cervical dislocation after 7 days of MPTP intoxication and their striata were collected and immediately frozen in dry ice and stored at −80C until analysis. On the day of the analysis, tissues were sonicated in 0.2 M perchloric acid containing isoproterenol and resulting homogenates were centrifuged at 20,000×g for 15 min at 4°C. After pH adjustment and filtration, 10 μl of supernatant was injected onto an Eicompak SC-3ODS column (Complete Stand-Alone HPLC-ECD System EiCOMHTEC-500 from JM Science Inc., Grand Island, NY) and analyzed following the manufacturer’s protocol.
Behavioral Analyses
Two types of behavioral experiments were conducted. These included an open field experiment for locomotor activity and a rotorod experiment for feet movement as described earlier (Roy and Pahan; Ghosh et al. 2007; Ghosh et al. 2009). Locomotor activity was measured after 7 days of the last dose of MPTP injection in a Digiscan Monitor (Omnitech Electronics, Inc., Columbus, OH). This Digiscan Monitor records stereotypy and rearing, behaviors that are directly controlled by striatum, as well as other basic locomotion parameters, such as horizontal activity, total distance traveled, number of movements, movement time, rest time, mean distance, mean time, and center time. Before any insult or treatment, mice were placed inside the Digiscan Infra-red Activity Monitor for 10 min daily and on rotorod for 10 min daily for 3 consecutive days to train them and record their baseline values. Briefly, animals were removed directly from their cages and gently placed nose first into a specified corner of the open-field apparatus and after release, data acquisition began at every 5 min interval. DIGISCAN software was used to analyze and store horizontal and vertical activity data, which were monitored automatically by infrared beams. In rotorod, the feet movement of the mice was observed at different speeds. To eliminate stress and fatigues, mice were given a 5-min rest interval. Then 7 d after the last dose of MPTP, open field assays and rotorod tests were carried out twice at 6 h intervals on each mouse separately. Locomotor activity measures were assessed after baseline value comparison.
Statistics
All values are expressed as means±SEM. One-way ANOVA was performed while analyzing time-dependent effect of TNF-α on the expression of DJ-1 and Parkin in astrocytes. In other cases, Student’s _t_-test was used to compare outcome between two groups (e.g. control vs MPTP, MPTP vs cinnamon or NaB etc.).
Results
Sodium Benzoate Inhibits the Loss of DJ-1 and Parkin in Activated Mouse Astrocytes
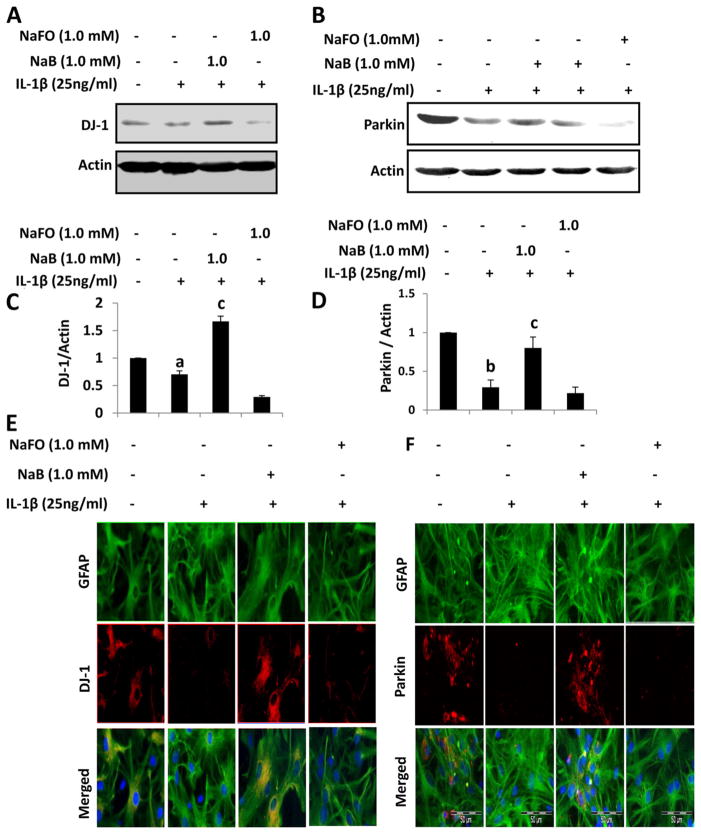
Although DJ-1 and Parkin are expressed in both neurons and astrocytes in culture, during the pathogenesis of PD, dopaminergic neurons remain under stress, do not produce trophic factors and eventually die. Under similar condition, however, astrocytes do not die and undergo activation. Therefore, repurposing activated astrocytes for supporting existing neurons is an important area of research. Because dysfunction of Parkin and DJ-1 in astrocytes may lead to nigrostriatal degeneration by loss of tropic support, free radical damage, and/or release of cytokines by astrocytes, it is important to delineate how Parkin and DJ-1 become dysfunctional in astrocytes and how such processes could be stopped. Astrocytes are known to be activated by proinflammatory cytokine IL-1β since only this proinflammatory cytokine is capable of activating C/EBPβ in astrocytes (Jana et al. 2005). Therefore, we examined the status of DJ-1 and Parkin in IL-1β-stimulated astrocytes. As evident from Western blot analysis, levels of both DJ-1 (Fig. 1a & c) and Parkin (Fig. 1b & d) decreased in IL-1β-activated astrocytes as compared to normal astrocytes. Similarly, immunofluorescence analyses also show decrease in DJ-1 (Fig. 1e) and Parkin (Fig. 1f) in activated astrocytes. Recently, we have demonstrated that cinnamon metabolite sodium benzoate (NaB) upregulates DJ-1 in normal astrocytes (Khasnavis and Pahan 2012). Here, we examined if NaB was capable of upregulating the levels of DJ-1 and Parkin in IL-1β-activated astrocytes. Treatment of activated astrocytes with NaB normalized and/or upregulated DJ-1 (Fig. 1a, c & e) and Parkin (Fig. 1b, d & f) as compared to IL-1β stimulation. These results were specific as sodium formate, a compound similar to NaB but without the benzene ring, had no such effect (Fig. 1). These findings demonstrate that NaB is capable of upregulating DJ-1 and Parkin in activated astrocytes.
Fig. 1.
Effect of sodium benjoate (NaB) on IL-1β-mediated down-regulation of DJ-1 and Parkin in mouse primary astrocytes. Mouse astrocytes pre-treated with NaB for 6 h were stimulated with IL-1β for 18 h under serum-free condition. Sodium formate (NaFO) was used as a negative control for NaB. Western blot was performed to analyze the levels of DJ-1 (a) and Parkin (b). Actin was used as a house keeping protein. Densitometric analysis was performed and results presented as protein expression relative to actin (DJ-1, c; Parkin, d). Results are mean±SD of three different experiments. a p<0.05 vs control; b p<0.001 versus control; c p<0.001 vs IL-1β. After similar treatments, astrocytes were _double_-labeled for GFAP & DJ-1 (e) and GFAP & Parkin (f)
NO Donor Abrogates the Inhibitory Effect of NaB on the Down-Regulation of DJ-1 and Parkin in Activated Mouse Astrocytes
Next, we investigated mechanisms by which NaB inhibited the down-regulation of DJ-1 and Parkin in activated astrocytes. Earlier we have demonstrated that NaB suppresses the production of nitric oxide (NO) in activated microglia and astroglia (Brahmachari et al. 2009). Therefore, we examined the involvement of NO in NaB-mediated protection of DJ-1 and Parkin in activated astrocytes. As reported earlier, NaB dose-dependently inhibited the production of NO (measured as nitrite) in IL-1β-stimulated astrocytes (Fig. 2a). Expectedly, addition of NO donor, DETA-NONOate, increased the level of NO in NaB-treated astrocytes (Fig. 2a). Abrogation of NaB-mediated protection of DJ-1 and Parkin in IL-1β-activated astrocytes by DETA-NONOate (Fig. 2b–c) suggests that NO scavenging is involved in NaB-mediated protection of these PD-related beneficial proteins.
Fig. 2.
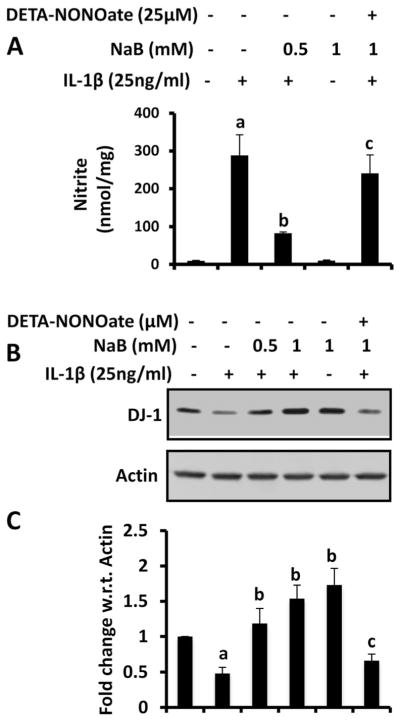
Effect of NaB on IL-1β-mediated production of nitric oxide in mouse primary astrocytes. Mouse astrocytes pre-treated with NaB for 6 h were stimulated with IL-1β for 18 h under serum-free condition in the presence or absence of DETA-NONOate (25 μM). Levels of nitrite (a) were measured in supernatants by Griess reagent. Expression of DJ-1 protein (b) was monitored in cells by Western blot. Densitometric analysis was performed and results presented as protein expression relative to actin (c). Results are mean±SD of three different experiments. a p<0.001 vs control; b p<0.001 vs IL-1β; c p<0.05 vs NaB
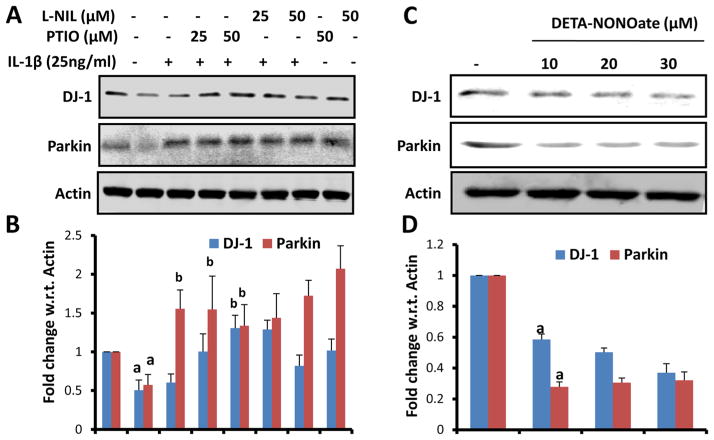
NO Alone is Sufficient for the Down-Regulation of DJ-1 and Parkin in Primary Mouse Astrocytes
Since NO removal is involved NaB-mediated protection of DJ-1 and Parkin in activated astrocytes, we examined if NO alone is sufficient to down-regulate these proteins in astrocytes. Therefore, we tested the effect of L-NIL, an inhibitor of iNOS, and PTIO, a scavenger of NO, on the status of DJ-1 and Parkin in IL-1β-activated astrocytes in the absence of NaB. Protection of DJ-1 and Parkin in IL-1β-activated astrocytes by L-NIL and PTIO (Fig. 3a & c) suggests that scavenging of NO alone is sufficient to protect these beneficial proteins in activated astrocytes. Next, we examined the effect of DETA-NONOate on the expression of DJ-1 and Parkin in normal astrocytes. Suppression of DJ-1 and Parkin protein expression by DETA-NONOate in the absence of IL-1β (Fig. 3b & d) suggests that production of NO alone is capable of down-regulating DJ-1 and Parkin in astrocytes.
Fig. 3.
Involvement of nitric oxide in IL-1β-mediated down-regulation of DJ-1 and Parkin in mouse primary astrocytes. Mouse astrocytes were stimulated with IL-1β in the presence of different concentrations of L-NIL and PTIO for 24 h followed by monitoring the expression of DJ-1 and Parkin by Western blot (a). Densitometric analysis was performed and results presented as relative to actin (b). Cells were also treated with different concentrations of DETA-NONOate for 24 h followed by monitoring levels of DJ-1 and Parkin by Western blot analysis (c). Densitometric analysis was performed and results presented as relative to actin (d). Results are mean±SD of three different experiments. a p<0.001 versus control; b p<0.001 vs IL-1β
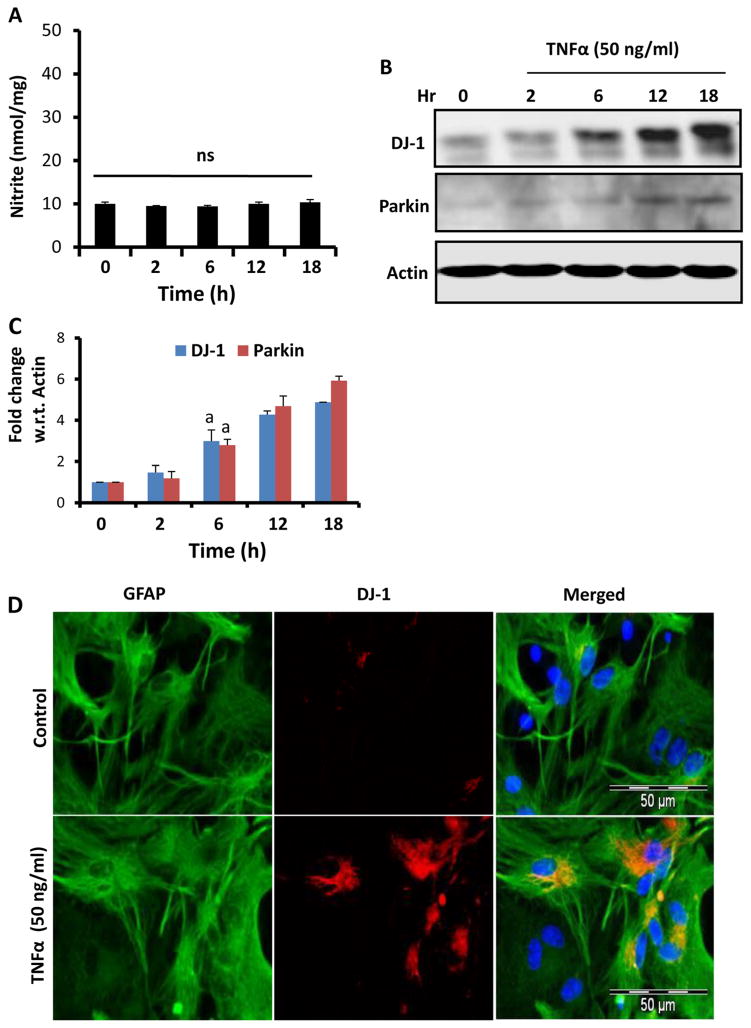
TNF-α Does not Induce the Production of NO and Increases the Level of DJ-1 and Parkin in Mouse Astrocytes
Since NO is critical for down-regulating DJ-1 and Parkin, we examined the effect of a proinflammatory cytokine that does not produce NO in astrocytes. As reported earlier (Jana et al. 2005), TNF-α alone did not induce the production of NO in astrocytes (Fig. 4a). When investigated the effect of TNF-α on DJ-1 and Parkin, we did not find any decrease in expression of these proteins in astrocytes (Fig. 4b–c). To our surprise, TNF-α alone increased the expression of DJ-1 and Parkin in astrocytes (Fig. 4b–c). This finding was also corroborated by immunofluorescence analysis (Fig. 4d). These results suggest that activation of astrocytes in the absence of NO in fact increases the expression of these beneficial proteins in astrocytes.
Fig. 4.
Effect of TNF-α on the expression of DJ-1 and Parkin in mouse primary astrocytes. Cells were stimulated with TNF-α for different time periods under serum-free condition followed by monitoring levels of nitrite in supernatants (a). Levels of DJ-1 and Parkin were monitored in cells by Western blot analysis (b). Densitometric analysis was performed and results presented as relative to actin (c). Results are mean±SD of three different experiments. a p<0.001 versus control. After 18 h of stimulation with TNF-α, cells were _double_-labeled for GFAP and DJ-1 (d). Results represent three independent analyses
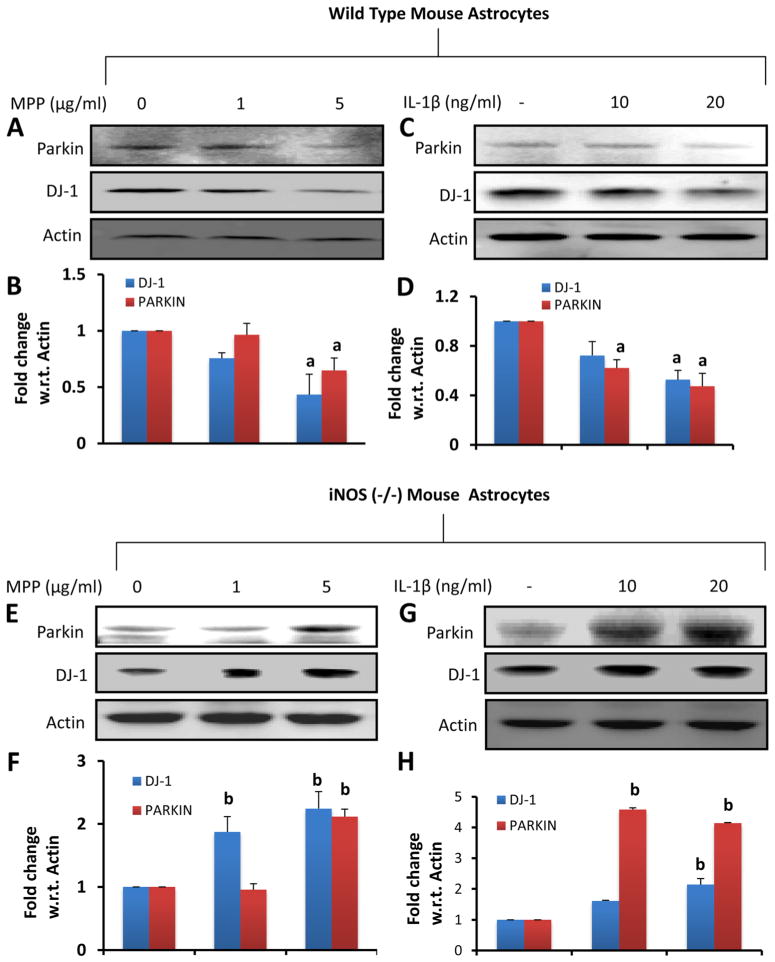
IL-1β and MPP+ Increase the Level of DJ-1 and Parkin in Astrocytes Isolated from iNOS (−/−) Mice
Since TNF-α increased the level of DJ-1 and Parkin in astrocytes, we examined the effect of NO-producing inducers in iNOS (−/−) astrocytes. Primary astrocytes isolated from wild type (WT) and iNOS (−/−) mice were stimulated with different doses of MPP+ (a Parkinsonian toxin) and IL-1β. As shown above, both MPP+ (Fig. 5a–B) and IL-1β (Fig. 5c–d) reduced the level of DJ-1 and Parkin in WT astrocytes. Although MPP+ was ineffective in decreasing the level of DJ-1 and Parkin at 1 μM, significant inhibition of these proteins was observed at 5 μM concentration (Fig. 5a–b). This is because MPP+ was not very effective in inducing the production of NO at 1 μM concentration but it produced NO in mouse astrocytes at 5 μM (data not shown). In contrast to WT astrocytes, both MPP+ (Fig. 5e–f) and IL-1β (Fig. 5g–h) stimulated the expression of DJ-1 and Parkin in iNOS (−/−) astrocytes, suggesting that proinflammatory molecules induce neuroprotective proteins like DJ-1 and Parkin in the absence of iNOS.
Fig. 5.
The role of inducible nitric oxide synthase (iNOS) in the regulation of DJ-1 and Parkin in mouse primary astrocytes. (a–d) Astrocytes isolated from wild-type mice were stimulated with MPP+ and IL-1β for 24 h followed by monitoring levels of DJ-1 and Parkin by Western blot analysis (a, MPP+; c, IL-1β). Densitometric analysis was performed and results presented as relative to actin (b, MPP+; d, IL-1β). (e–h) Astrocytes isolated from iNOS (−/−) mice were stimulated with MPP+ and IL-1β for 24 h followed by monitoring levels of DJ-1 and Parkin by Western blot analysis (E, MPP+; G, IL-1β). Densitometric analysis was performed and results presented as relative to actin (f, MPP+; h, IL-1β). Results are mean±SD of three different experiments. a p<0.001 vs WT-control; b p<0.001 vs iNOS (−/−)-control
Cinnamon Suppresses the Expression of iNOS and Increases the Level of DJ-1 and Parkin in the SNpc of MPTP-Intoxicated Mice
Since cinnamon metabolite NaB suppressed the production of NO and protected DJ-1 and Parkin in activated astrocytes, next, we examined whether cinnamon itself was also capable of inhibiting the expression of iNOS and upregulating the level of DJ-1 and Parkin in vivo in the SNpc of MPTP-intoxicated mice. Chinese cinnamon (Cinnamonum cassia) and original Ceylon cinnamon (Cinnamonum verum or Cinnamonum zylencum) are two major types of cinnamon that are available in the US. Recently by mass spectrometric analysis, we have found that Cinnamonum verum is much more pure than Cinnamonum cassia (Jana et al. 2013). Although cinnamaldehyde is present as the major peak in both Cinnamonum cassia and Cinnamonum verum, Cinnamonum cassia contains more styrene, benzene, 1,1′-(2-butene-1,4-diyl) bis-, benzene, 1,1′-(1,2-cyclobutanediyl) bis-, palmitic acid, stearic acid, 4-phenylbutyl chloride, and (2,3-diphenylcyclopropyl) methyl phenyl sulfoxide than Cinnamonum verum (Jana et al. 2013). Furthermore, Cinnamonum cassia, but not Cinnamonum verum, contains some hepatotoxic 1-benzopyran-2-one or coumarin (Jana et al. 2013). We have also found that oral treatment of mice with ground Cinnamonum verum via gavage markedly increases the level of NaB in serum and midbrain (Jana et al. 2013), suggesting that cinnamon is metabolized into NaB and that cinnamon-derived NaB is capable of entering into the CNS.
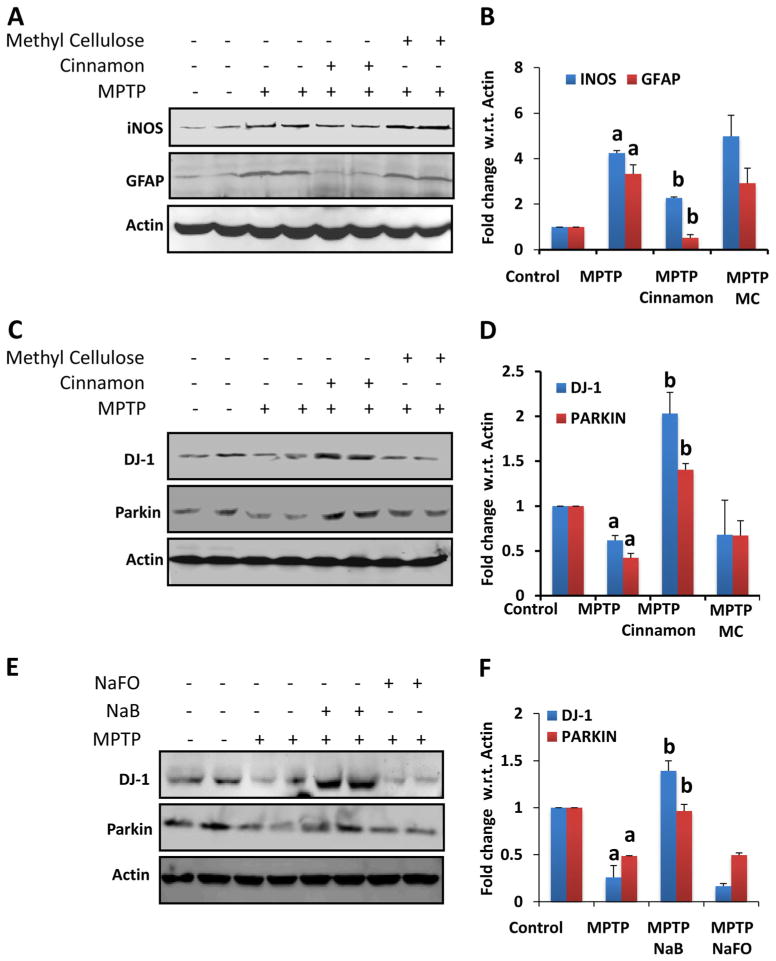
Therefore, we tested the neuroprotective efficacy of Cinnamonum verum in acute MPTP mouse model. From 3 h after the last injection of MPTP, mice received Cinnamonum verum daily via gavage and after 7 days of the last injection of MPTP, levels of iNOS and GFAP were monitored in the SNpc by Western blot. As expected, after MPTP intoxication, levels of both iNOS and GFAP increased in the SNpc as compared to controls (Fig. 6a–b). However, cinnamon treatment reduced nigral expression of iNOS and GFAP in MPTP-intoxicated mice (Fig. 6a–b). This effect was specific as vehicle (methyl cellulose) had no such inhibitory effect (Fig. 6a–b). These results suggest that oral treatment of cinnamon powder is capable of reducing inflammation in vivo in the SNpc of MPTP-intoxicated mice.
Fig. 6.
Effect of cinnamon and its metabolite NaB on the expression of DJ-1 and Parkin in vivo in the nigra of MPTP-intoxicated mice. Mice (_n_=4) receiving cinnamon powder (100 mg/kg body wt/d) or vehicle (methyl cellulose) from 3 h after the last injection of MPTP were sacrificed 7 days after the last injection of MPTP followed by monitoring levels of iNOS & GFAP (a) and DJ-1 & Parkin (c) by Western blot. Densitometric analysis was performed and results presented as relative to actin (b, iNOS & GFAP; d, DJ-1 & Parkin). Mice (_n_=4) receiving NaB (50 mg/kg body wt/d) or NaFO (negative control) from 3 h after the last injection of MPTP were sacrificed 7 days after the last injection of MPTP followed by monitoring levels of DJ-1 & Parkin (E) by Western blot. Densitometric analysis was performed and results presented as relative to actin (f). Results are mean±SEM of four mice per group. a p<0.001 vs control; b p<0.001 vs MPTP
Since cinnamon treatment decreased nigral level of iNOS, we examined if cinnamon treatment was capable of protecting and/or increasing DJ-1 and Parkin in the nigra. Decrease in DJ-1 and Parkin in the nigra of MPTP-intoxicated mice as compared to normal mice suggests that MPTP insult reduces the expression of these beneficial proteins in the nigra (Fig. 6c–d). However, oral treatment of MPTP-intoxicated mice with cinnamon, but not vehicle, led to the protection and/or increase of DJ-1 and Parkin in the nigra (Fig. 6c–d), suggesting that cinnamon treatment protects DJ-1 and Parkin in the nigra from MPTP insult. Since cinnamon is metabolized to NaB (Jana et al. 2013) and NaB protects DJ-1 and Parkin in activated astrocytes (Fig. 1), we examined if NaB treatment also rescued DJ-1 and Parkin in the nigra of MPTP-intoxicated mice. Similar to cinnamon, treatment of MPTP-intoxicated mice with NaB also protected the level of DJ-1 and Parkin in the nigra (Fig. 6e–f).
Cinnamon and NaB Protect the Nigrostriatum in MPTP-Intoxicated Mice
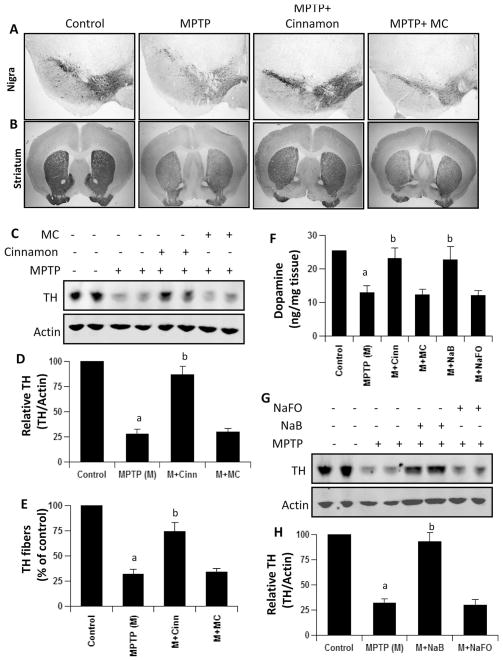
Since cinnamon and NaB protected DJ-1 and Parkin in the nigra of MPTP-intoxicated mice, next, we investigated if cinnamon and NaB protected the nigrostriatum from MPTP insult. MPTP-intoxication led to approximately 75 % loss of nigral TH (Fig. 7a, c & d) and 70 % reduction of striatal TH ODs (Fig. 7b & e) compared with saline-injected controls. However, in MPTP-injected mice treated with cinnamon, less reduction in nigral TH and striatal TH ODs was observed (Fig. 7a–e). Next, to determine whether cinnamon protects against biochemical deficits caused by MPTP, we quantified levels of dopamine (DA) in the striata 7 days after the MPTP treatment. As evident from Fig. 7f, MPTP intoxication led to about 50 % decrease in striatal DA compared to striata of saline-injected mice. In contrast, MPTP-intoxicated animals that received cinnamon showed only 15 % decrease in striatal dopamine (Fig. 7f). Similarly, NaB treatment also protected nigral TH (Fig. 7g–h) and striatal DA (Fig. 7f) from MPTP insult.
Fig. 7.
Cinnamon and its metabolite NaB protect dopaminergic neurons in MPTP-intoxicated mice. Mice receiving cinnamon powder (100 mg/kg body wt/d) or vehicle from 3 h after the last injection of MPTP were sacrificed 7 days after the last injection of MPTP followed by TH immunostaining of SNpc (a) and striatum (b), quantification of nigral TH (c, Western blot; d, densitometric analysis), quantification of TH-positive fibers in striatum (e), and assay of neurotransmitters in striatum (f). Similarly, mice receiving NaB (50 mg/kg body wt/d) or NaFO from 3 h after the last injection of MPTP were sacrificed 7 days after the last injection of MPTP followed by quantification of nigral TH (g, Western blot; h, densitometric analysis) and assay of neurotransmitters in striatum (f). Data are means±SEM of six mice per group. ap<0.001 vs control; bp<0.001 vs MPTP
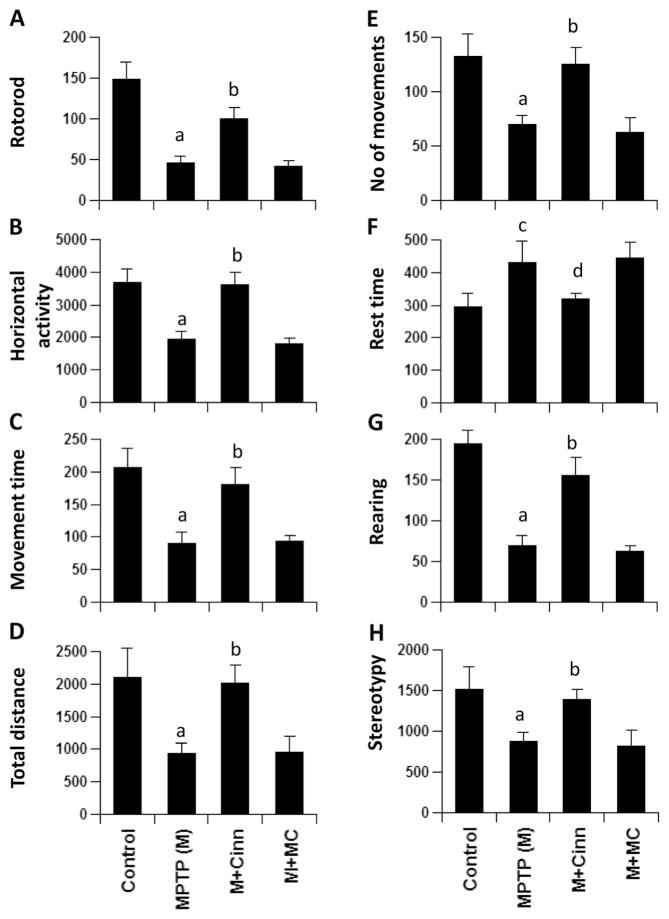
Cinnamon and NaB Improve Locomotor Functions in MPTP-Intoxicated Mice
The ultimate therapeutic goal of neuroprotection is to decrease functional impairment. Therefore, to examine whether cinnamon and NaB protect not only against structural and neurotransmitter damage but also against functional deficits caused by MPTP, we monitored locomotor and open-field activities. As reported earlier (Ghosh et al. 2007; Ghosh et al. 2009; Khasnavis et al. 2014), MPTP injection caused marked decrease in rotorod performance (Fig. 8a), horizontal activity (Fig. 8b), movement time (Fig. 8c), total distance (Fig. 8d), number of movements (Fig. 8e), rearing (Fig. 8g), and stereotypy (Fig. 8h). On the other hand, MPTP increased the rest time (Fig. 8f). Interestingly, cinnamon significantly improved MPTP-induced hypolocomotion (Fig. 8).
Fig. 8.
Cinnamon treatment improves motor functions in MPTP-intoxicated mice. Mice receiving cinnamon powder (100 mg/kg body wt/d) or vehicle from 3 h after the last injection of MPTP were tested for motor functions (a, rotorod; b, horizontal activity; c, movement time; d, total distance; e, number of movements; f, rest time; g, rearing; h, stereotypy) 7 days after the last injection of MPTP. Data are means±SEM of six mice per group. a_p_<0.001 vs control; c_p_<0.05 vs control; b_p_<0.001 vs MPTP; d_p_<0.05 vs MPTP
Discussion
PD is second only to Alzheimer’s disease as the most common devastating human neurodegenerative disorder. Despite intense investigation, no interdictive therapy is available for PD. Administration of a dopamine agonist or levodopa has been the standard treatment for PD. However, it is often associated with a number of side effects and unsatisfactory outcomes. Therefore, understanding the mechanism of the disease process of PD and development of effective neuroprotective therapeutic approach to halt the disease progression are of paramount importance. Parkin and DJ-1 are PD-related beneficial proteins and upregulation of these proteins may have therapeutic efficacy for PD. Here, we describe a novel, but natural, way to protect these proteins from Parkinsonian insult.
Cinnamon, the brown bark of cinnamon tree, is a commonly used spice and flavoring material for deserts, candies, chocolate, etc. It has a long history as a medicine as well. Medieval physicians used cinnamon in medicines to treat a variety of disorders including arthritis, coughing, hoarseness, sore throats, etc. In addition to containing manganese, dietary fiber, iron, and calcium, cinnamon contains a major compound, cinnamaldehyde, which is converted into cinnamic acid by oxidation. In the liver, this cinnamic acid is β-oxidized to benzoate (Abd El-Mawla et al. 2001) that exists as sodium salt (NaB) or benzoyl-CoA. Recently we have also demonstrated that oral administration of original Ceylon cinnamon (Cinnamonum verum) markedly increases the level of NaB in serum and brain of mice (Jana et al. 2013). NaB is of medical importance as it is a component of Ucephan, a FDA-approved drug used in the treatment for hepatic metabolic defects associated with hyperammonemia such as urea cycle disorder in children (Leonard and Morris 2002; Scaglia et al. 2004). It is also widely used as a preservative in broad range of foods and cosmetic products (Nair 2001). Earlier we have demonstrated that NaB modifies T cells at multiple steps and protects experimental allergic encephalomyelitis, an animal model of MS (Brahmachari and Pahan 2007). We have also found that NaB upregulates DJ-1 in normal astrocytes (Khasnavis and Pahan 2012). Here, we demonstrate that DJ-1 and Parkin are down-regulated in activated astrocytes and that NaB treatment of activated cells led to the protection of these beneficial proteins. Similarly, cinnamon and NaB also protected DJ-1 and Parkin in vivo in the SNpc of MPTP-insulted mice. We also tested the hypothesis that cinnamon may protect the nigrostriatum in MPTP-insulted mice. While nigral TH disappeared in MPTP-intoxicated mice, treatment with cinnamon protected TH from MPTP toxicity. Cinnamon treatment also protected striatal TH fibers from MPTP toxicity and restored the level of neurotransmitters. Most importantly cinnamon ameliorated functional impairment in MPTP-intoxicated mice. We did not notice any drug related side effect (e.g. hair loss, weight loss, untoward infection etc.) in any of the mice used during the course of the study, suggesting that cinnamon may not exhibit any side effects. Because cinnamon is a natural, non-toxic, widely-used, and an economical compound, these results may have therapeutic importance in PD.
Signaling mechanisms by which the expression of DJ-1 and Parkin is regulated are poorly understood. Several lines of evidence presented in this manuscript clearly demonstrate that NO plays a key role in NaB-mediated upregulation of DJ-1 and Parkin. First, NaB suppressed the production of NO and stopped the loss of DJ-1 and Parkin in IL-1β-stimulated astrocytes. Second, IL-1β was unable to inhibit the expression of DJ-1 and Parkin in astrocytes where either NO was scavenged by PTIO or NO production was inhibited by L-NIL. Third, DETA-NONOate, a NO donor, abrogated NaB-mediated protection of DJ-1 and Parkin in IL-1β-stimulated astrocytes. Fourth, DETA-NONOate alone was capable of suppressing the expression of DJ-1 and Parkin in normal astrocytes. Fifth, TNF-α did not induce the production of NO and remained unable to suppress the expression of DJ-1 and Parkin in astrocytes. Surprisingly, TNF-α stimulated the expression of DJ-1 and Parkin. Sixth, IL-1β and MPP+ remained unable to suppress DJ-1 and Parkin in iNOS (−/−) astrocytes. Interestingly, these proinflammatory molecules increased the level of DJ-1 and Parkin in iNOS (−/−) astrocytes. Together these results identify an important role of NO in suppressing the expression of DJ-1 and Parkin in astrocytes and suggest that proinflammatory molecules are capable of increasing these beneficial proteins in the absence of iNOS.
NO, a short-lived and diffusible free radical, plays many roles as a signaling and effector molecule in diverse biological systems; it is a neuronal messenger and is involved in vasodilation as well as in antimicrobial and antitumor activities (Nathan 1992; Saha and Pahan 2006). On the other hand, NO has also been implicated in several CNS disorders, including inflammatory, infectious, traumatic, and degenerative diseases (Merrill et al. 1993; Brosnan et al. 1994; Akama et al. 1998). There are considerable evidences for the transcriptional induction of iNOS (the high-output isoform of NOS) in the CNS that is associated with autoimmune reactions, acute infection, and degenerative brain injury (Merrill et al. 1993; Brosnan et al. 1994; Akama et al. 1998). NO is potentially toxic to neurons and oligodendrocytes that may mediate toxicity through the formation of iron-NO complexes of iron-containing enzyme systems (Drapier and Hibbs 1988), oxidation of protein sulf-hydryl groups (Radi et al. 1991), nitration of proteins, and nitrosylation of nucleic acids and DNA strand breaks (Wink et al. 1991). Earlier we have shown that NO is responsible for astrogliosis and that NO increases the expression of GFAP in astrocytes (Brahmachari et al. 2006). Here we demonstrate that NO is a key player in the regulation of PD-related beneficial proteins DJ-1 and Parkin, in which NO decreases the expression of DJ-1 and Parkin in astroglia. Therefore, specific targeting of NO either by iNOS inhibitors or NO scavengers may be an important step for suppressing astrogliosis and the upregulation and/or protection of DJ-1 and Parkin.
In summary, we have demonstrated that cinnamon and its metabolite NaB protect DJ-1 and Parkin from inflammatory insult and that oral administration of cinnamon saves the nigrostriatum from Parkinsonian insult. These results highlight a novel neuroprotective role of cinnamon and suggest that this widely-used spice may be explored for therapeutic intervention in PD.
Acknowledgments
This study was supported by grants from NIH (AT6681 and NS83054).
Contributor Information
Saurabh Khasnavis, Department of Neurological Sciences, Rush University Medical Center, 1735 West Harrison St, Suite 320, Chicago, IL 60612, USA.
Kalipada Pahan, Email: kalipada_pahan@rush.edu, Department of Neurological Sciences, Rush University Medical Center, 1735 West Harrison St, Suite 320, Chicago, IL 60612, USA.
References
- Abd El-Mawla AM, Schmidt W, Beerhues L. Cinnamic acid is a precursor of benzoic acids in cell cultures of Hypericum androsaemum L. but not in cell cultures of Centaurium erythraea RAFN. Planta. 2001;212:288–293. doi: 10.1007/s004250000394. [DOI] [PubMed] [Google Scholar]
- Akama KT, Albanese C, Pestell RG, Van Eldik LJ. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc Natl Acad Sci U S A. 1998;95:5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, Nemachek C, Green SR, Przedborski S, Gendelman HE. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian M, Liu J, Hong X, Yu M, Huang Y, Sheng Z, Fei J, Huang F. Overexpression of parkin ameliorates dopaminergic neurodegeneration induced by 1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. PLoS ONE. 2013;7:e39953. doi: 10.1371/journal.pone.0039953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2007;179:275–283. doi: 10.4049/jimmunol.179.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Jana A, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol. 2009;183:5917–5927. doi: 10.4049/jimmunol.0803336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JW, French MR, Smith RL, Williams RT. The fate of benzoic acid in various species. Biochem J. 1970;118:47–51. doi: 10.1042/bj1180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan CF, Battistini L, Raine CS, Dickson DW, Casadevall A, Lee SC. Reactive nitrogen intermediates in human neuropathology: an overview. Dev Neurosci. 1994;16:152–161. doi: 10.1159/000112102. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Drapier JC, Hibbs JB., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988;140:2829–2838. [PubMed] [Google Scholar]
- Fahn S. Parkinson’s disease: 10 years of progress, 1997–2007. Mov Disord. 2010;25(Suppl 1):S2–S14. doi: 10.1002/mds.22796. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, Pahan K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci. 2009;29:13543–13556. doi: 10.1523/JNEUROSCI.4144-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic Biol Med. 2005;38:655–664. doi: 10.1016/j.freeradbiomed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Jana M, Jana A, Liu X, Ghosh S, Pahan K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of I kappa B alpha in anti-inflammatory effect of gemfibrozil in microglia. J Immunol. 2007;179:4142–4152. doi: 10.4049/jimmunol.179.6.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K. Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J Neuroimmune Pharmacol. 2013;8:739–755. doi: 10.1007/s11481-013-9447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasnavis S, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, upregulates neuroprotective Parkinson disease protein DJ-1 in astrocytes and neurons. J Neuroimmune Pharmacol. 2012;7:424–435. doi: 10.1007/s11481-011-9286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasnavis S, Jana A, Roy A, Mazumder M, Bhushan B, Wood T, Ghosh S, Watson R, Pahan K. Suppression of nuclear factor-kappaB activation and inflammation in microglia by physically modified saline. J Biol Chem. 2012;287:29529–29542. doi: 10.1074/jbc.M111.338012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasnavis S, Roy A, Ghosh S, Watson R, Pahan K. Protection of dopaminergic neurons in a mouse model of parkinson’s disease by a physically-modified saline containing charge-stabilized nanobubbles. J Neuroimmune Pharmacol. 2014 doi: 10.1007/s11481-013-9503-3. [DOI] [PubMed] [Google Scholar]
- Kubota K, Ishizaki T. Dose-dependent pharmacokinetics of benzoic acid following oral administration of sodium benzoate to humans. Eur J Clin Pharmacol. 1991;41:363–368. doi: 10.1007/BF00314969. [DOI] [PubMed] [Google Scholar]
- Leonard JV, Morris AA. Urea cycle disorders. Semin Neonatol. 2002;7:27–35. doi: 10.1053/siny.2001.0085. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- Mondal S, Martinson JA, Ghosh S, Watson R, Pahan K. Protection of Tregs, suppression of Th1 and Th17 cells, and amelioration of experimental allergic encephalomyelitis by a physically-modified saline. PLoS ONE. 2012a;7:e51869. doi: 10.1371/journal.pone.0051869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, Pahan K. Testing NF-kappaB-based therapy in hemiparkinsonian monkeys. J Neuroimmune Pharmacol. 2012b;7:544–556. doi: 10.1007/s11481-012-9377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int J Toxicol. 2001;20(Suppl 3):23–50. doi: 10.1080/10915810152630729. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- Ng CH, Mok SZ, Koh C, Ouyang X, Fivaz ML, Tan EK, Dawson VL, Dawson TM, Yu F, Lim KL. Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci. 2009;29:11257–11262. doi: 10.1523/JNEUROSCI.2375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- Roy A, Ghosh A, Jana A, Liu X, Brahmachari S, Gendelman HE, Pahan K. Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson’s disease. PLoS ONE. 2012;7:e38113. doi: 10.1371/journal.pone.0038113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal. 2006;8:929–947. doi: 10.1089/ars.2006.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglia F, Carter S, O’Brien WE, Lee B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab. 2004;81(Suppl 1):S79–S85. doi: 10.1016/j.ymgme.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Toth B. Lack of tumorigenicity of sodium benzoate in mice. Fundam Appl Toxicol. 1984;4:494–496. doi: 10.1016/0272-0590(84)90208-2. [DOI] [PubMed] [Google Scholar]
- Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254:1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]