Quantifying the effects of the division of labor in metabolic pathways (original) (raw)
. Author manuscript; available in PMC: 2015 Nov 7.
Published in final edited form as: J Theor Biol. 2014 Jul 17;360:222–242. doi: 10.1016/j.jtbi.2014.07.011
Abstract
Division of labor is commonly observed in nature. There are several theories that suggest diversification in a microbial community may enhance stability and robustness, decrease concentration of inhibitory intermediates, and increase efficiency. Theoretical studies to date have focused on proving when the stable co-existence of multiple strains occurs, but have not investigated the productivity or biomass production of these systems when compared to a single ‘super microbe’ which has the same metabolic capacity. In this work we prove that if there is no change in the growth kinetics or yield of the metabolic pathways when the metabolism is specialized into two separate microbes, the biomass (and productivity) of a binary consortia system is always less than that of the equivalent monoculture. Using a specific example of Escherichia coli growing on a glucose substrate, we find that increasing the growth rates or substrate affinities of the pathways is not sufficient to explain the experimentally observed productivity increase in a community. An increase in pathway efficiency (yield) in specialized organisms provides the best explanation of the observed increase in productivity.
Keywords: Microbial ecology, Mathematical modeling, Syntrophic consortia, Cross-feeding, Chemostat
1. Introduction
From the earliest observations of microbial organisms, it has been apparent that microbial consortia are ubiquitous in nature. In fact, naturally occurring ecosystems are almost exclusively organized as consortia. Recent metagenomic studies from the soil (Fierer and Jackson, 2006), to the ocean (Venter et al., 2004), to the human gut (Gill et al., 2006), have found that microbial communities are incredibly diverse, often consisting of thousands of interacting species. Subsets of these communities form consortia that act together to enhance their capabilities and survival (Eiteman et al., 2008).
Early theoretical ecology studies led to the development of the competitive exclusion principle (CEP), which states that the maximum number of species that can coexist in a system is equal to the total number of limiting (essential) resources (Hardin, 1960; MacArthur and Levins, 1964; Rescigno and Richardson, 1965). However, in nature we see many examples of multiple microbial species stably coexisting. Frequent explanations for coexistence in a natural population can be categorized into three main types: the development of multiple niches due to spatial heterogeneity or self-organized segregation, the system not being in equilibrium due to environmental fluctuations or external forcing, and the presence of inter- and intra-species interactions. Despite the limited direct applicability of CEP to natural systems, the clear mathematical formulation of CEP allows for significant insight by identifying which conditions of the CEP have been violated to lead to the observed coexistence of species. For example, by applying the CEP to the dynamics in a chemostat, we see that due to the consistent flow of nutrients and continuous mixing, the environment is kept constant and the development of different niches due to spatial heterogeneity is not possible; therefore, it must be some form of inter- or intra-species interactions such as crowding, chemical signaling, cooperativity, or mutual inhibition that lead to any observed coexistence. Studying these interactions in simple chemostat systems gives us a better understanding of the factors that maintain the diversity in naturally occurring microbial consortia.
Natural consortia are often found to form syntrophic systems, where the microbes depend on each other for survival, either by the production of required metabolic substrates or by the maintenance of chemically advantageous conditions (Schink, 2002). It is often observed that this syntrophic cooperation within microbial consortia increases their productivity and can allow the consortia to perform advanced functions that the microbial species are not capable of individually. These microbial interactions are known to be important in diverse areas including chronic medical infections (e.g. diabetic ulcers, Gardner et al., 2013; James et al., 2008), biofuel synthesis (e.g. biodiesel production, Peralta-Yahya et al., 2012; Zuroff and Curtis, 2012), environmental nutrient cycling (e.g. CO2 sequestering, nitrification, Costa et al., 2006), bioprocessing (Shong et al., 2012), and wastewater treatment (Schink, 1997; Seitz et al., 1990a, 1990).
A frequently observed syntrophic system is a cross-feeding chain where microbes work together to perform the sequential degradation of complex compounds like lingocellulosic material (Schink, 2002). In these syntrophic cross-feeding systems a single substrate must be broken down in many steps, with one species catabolizing the available substrate and oxidizing it to produce a byproduct that the next species in the chain can consume. The intermediate byproducts in these systems are often found to be inhibitory. In this work we will consider the case where the intermediate byproduct inhibits growth. However, this more complicated system can be reduced to the case where there is no inhibition by taking the appropriate limit.
It has been observed experimentally that these syntrophic chain systems where the metabolic pathways are split among separate organisms (known as ‘_microbial specialization_’ or a ‘_division of labor_’) are more productive than a single organism with the equivalent metabolic capabilities (Bernstein et al., 2012; Wintermute and Silver, 2010). For example, if we compare a single organism that metabolizes A → B → C to a pair of organisms that metabolize A → B (organism 1) and B → C (organism 2), experimental observation has found the pair of organisms to be more productive than the single organism. Productivity is defined here as total biomass production per unit of input A.
An example of this division of labor that has been found to evolve repeatedly in different experiments occurs when E. coli is grown on a glucose substrate. The original population of E. coli can fully metabolize glucose (glucose → acetate → CO2 (TCA cycle)), but when grown on glucose for many generations the population splits into two main subpopulations: microbes that preferentially consume glucose and produce acetate (glucose → acetate) and microbes that preferentially consume acetate (acetate → CO2) (Rosenzweig et al., 1994; Rozen and Lenski, 2000; Treves et al., 1998).
In this paper we use standard chemostat modeling techniques to investigate whether the division of labor (splitting the pathways into two separate organisms) alone is sufficient to explain the observed increase in biomass, and if not, what other changes may be required. It is important to note that in contrast to the past work which has investigated the evolution of such cross-feeding systems, we will not consider the two systems in direct competition, instead comparing the maximum biomass (productivity) of the systems in isolation. This is motivated by industrial applications where the total productivity of the consortia, which is usually proportional to the biomass, is of primary interest.
This type of system has been studied mathematically, and it can be shown that for n species in a simple syntrophic cross-feeding chain, there is a stable coexistence steady state (Kreikenbohm and Bohl, 1986; Powell, 1985, 1986; Reilly, 1974). This simple system has been modified to include other forms of inhibition, external toxins, multiple substrates, and other forms of mutualism; in all cases, a stable, stationary, coexistence steady state is found (Aota and Nakajima, 2001; Burchard, 1994; Elkhader, 1991; Katsuyama et al., 2009; Sari et al., 2012). Previous research has focused on proving the existence and stability of coexistence steady-states. There has been no investigation of the productivity of these syntrophic chain systems. Some recent work (Doebeli, 2002; Estrela and Gudejl, 2010; Pfeiffer and Bonhoeffer, 2004) investigated the evolution of cross-feeding in microbial populations and found that there are a wide range of parameter values for which cross-feeding is seen to evolve. The aim of these evolution studies was to identify conditions for stable coexisting syntrophic chain systems to evolve and outcompete equivalent monocultures. They did not explicitly investigate the productivity of the systems that are found to evolve.
In our initial model, we assume that the metabolic dynamics of the pathways do not change when being split into separate microbes. In addition, we initially assume that the growth rate for the monoculture is a linear combination of the growth rates of the two pathways. This formulation allows us to obtain theoretical results and is effectively an upper bound on the growth rate of the monoculture microbe. Realistically, there are costs to utilizing both pathways at once for the monoculture, and there are changes that are known to occur to the metabolic pathway dynamics when the microbes specialize to a single substrate. For example, Pfeiffer and Bonhoeffer (2004) theorize that the evolution of cross-feeding could be due to the system minimizing the concentration of inhibitory intermediates and minimizing the concentration of enzymes it must produce, while maximizing the rate of ATP-production. Another explanation comes from Johnson et al. (2012) who find, by considering the biochemical conflicts which constrain the relationships between two metabolic processes that the division of metabolic pathways could be advantageous as it allows microbes to focus on producing a smaller number of enzymes and optimizing a smaller subset of pathways.
By starting with the assumption of no adaptation for the specialists and the maximal growth rate for the monoculture, we get a strong theoretical result. Then, by varying the growth kinetics (growth rates and substrate affinities) and yields between the monoculture and the binary culture systems, we are able to use our model to test possible explanations for the observed increase in productivity and to quantitatively investigate what changes are required for the division of labor to be advantageous (increased biomass). Considering the specific example of E. coli grown on a glucose substrate (Rosenzweig et al., 1994; Rozen and Lenski, 2000; Treves et al., 1998), we use standard Monod kinetics and measured experimental parameters to determine the conditions under which the model results match the experimentally observed increase in biomass.
The main result of this work is a comparison of the biomass production of a single microbe with full metabolic capacity to a syntrophic consortium of two specialized microbes each with a unique subset of the full metabolic chain, where the intermediate byproduct may be inhibitory. We prove that the monoculture system will always have higher biomass production (i.e., higher productivity) if there are no changes to the growth kinetics or yield of the pathways between the two systems. In a specific example, we show that increasing the growth rates or substrate affinities of the consortial pathways, a change that might be expected due to specialization, is not sufficient to generate the observed higher biomass production in the binary consortia. However, by varying the yields for the consortial pathways, higher biomass production can be achieved for the binary consortia relative to the monoculture. Yield changes are an expected result of microbial specialization because utilizing a smaller number of specialized pathways leads to reduced energy requirements. This result provides evidence for the argument that an increase in efficiency (yields) is a key factor underlying the observed productivity benefit of microbial consortia. Additionally, the finding that changing yields can lead to higher biomass production through specialization has important implications for industrial settings where higher biomass production is often highly desirable.
2. Model construction
To investigate the effects of separating metabolic pathways, we will analyze the simplest case of a syntrophic chain where there are only two microbial species. For clarity we will use a specific example in developing our notation, in this case the metabolism of glucose by E. coli. To construct the model we assume that the metabolism of a single provided substrate (glucose, G) can be split into two pathways. The first step (pathway) takes substrate G and produces an intermediate by-product A (acetate), creating new biomass at a growth rate _μ_1(G, A). The intermediate by-product acetate A serves as a substrate for the second step (pathway), which has a growth rate _μ_2(A). As well as being a substrate, acetate lowers the pH and is detrimental to both metabolism pathways in the cell, which slows the growth rates μi and thus slows the uptake of substrate. The lower pH caused by acetate production could also increase the energy required for cell homeostasis, which would affect the yield parameters. We do not consider this effect here, as if yield did decrease at higher A values, this would further disadvantage the binary consortia, and thus would not qualitatively affect our results but would greatly complicate the analysis.
We will consider the situation where the microbes are grown in continuous culture (chemostat). We compare the total biomass produced in two different systems: a binary culture system where the two pathways are in two separate microbes, and a monoculture system where the pathways are combined into a single microbe. These two systems are shown in Fig. 1. For the monoculture system, acetate that is produced through the first pathway is then available to be consumed through the second pathway. If the acetate consumption rate is lower than the acetate production rate, acetate will accumulate in the system, see Eqs. (4) and (7). In our model of the monoculture, we consider the case where acetate is consumed (metabolized) by the individual microbial cell that produced it, and the case where acetate is released from that microbe into the environment and then consumed by distinct microbes, to be equivalent. This is possible because this model does not include any cost involved in transporting acetate into or out of the cell. Including a cost for transporting acetate would disadvantage the binary consortia compared to the monoculture, and thus it would not qualitatively affect our results.
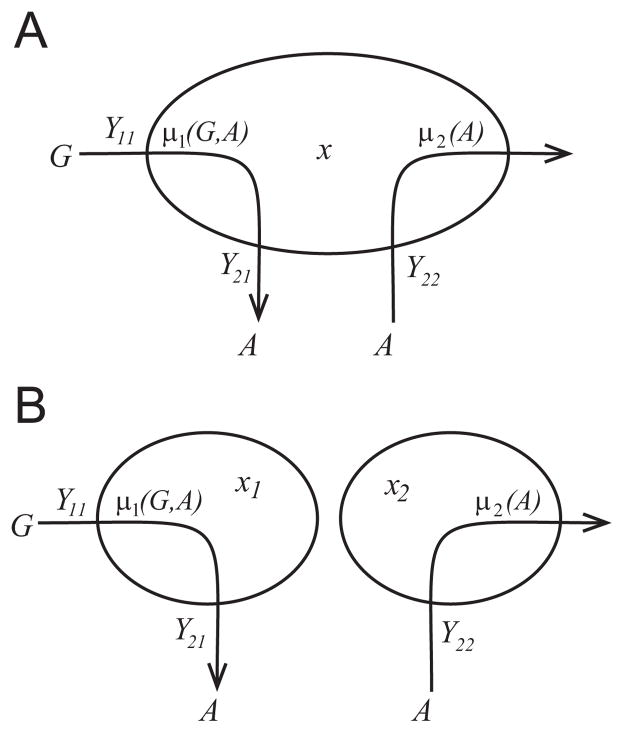
Fig. 1.
Diagram showing the metabolic pathways in (a) the monoculture system with the microbes x and (b) the binary culture system with the primary (glucose) consumer _x_1 and the scavenger (acetate consumer) _x_2, where G is the primary substrate (glucose) and A is the inhibitory intermediate substrate (acetate). The yields _Y_11 and _Y_22 fix the rates of biomass production per gram of substrate consumed, the yield _Y_21 is the ratio of biomass production to byproduct production, and the growth rates are given by the functions _μ_1(G, A) and _μ_2(A).
2.1. Model formulation
To model the dynamics in a chemostat we construct a system of ordinary differential equations (ODEs), following standard techniques (Smith and Waltman, 1995), that describe the evolution over time of the microbial biomass and substrate concentrations. In both systems we will track the concentration of primary substrate, G and the concentration of the intermediate by-product, A.
In binary culture, the biomass of a microbe that consumes G and produces A to grow at rate _μ_1(G, A) will be denoted by _x_1, and the biomass of the second microbe that consumes A and grows at rate _μ_2(A) will be _x_2. For the binary chemostat this situation can be described by the following system of ordinary differential equations:
| dGdt=(Gin-G)D-μ1(G,A)Y11x1, | (3) |
|---|
| dAdt=-AD+μ1(G,A)Y21x1-μ2(A)Y22x2, | (4) |
|---|
where D > 0 is the dilution rate (with units 1/h, and D = V/Q where V is the volume and Q is the volumetric flow rate) and Gin > 0 is the input concentration of primary substrate. The parameters D and Gin are set by experimental conditions. The yields, constants Yij, are determined by the stoichiometry of the metabolic pathways involved, which dictates the energy produced per unit of substrate consumed, and the proportion of energy produced that goes towards biomass production, which depends on additional factors including the energy required for cell homeostasis and for the production of enzymes. In this work we have chosen to define the yield terms _Y_11 and _Y_22 as the amount of biomass made by one unit of substrate, G and A respectively, and _Y_21 is the amount of biomass produced when one unit of intermediate substrate A is produced through the _G_-metabolism pathway. The parameter, r = _Y_11/_Y_21 defines the grams of the inhibitory intermediate substrate A produced per gram of primary substrate A consumed, and is fixed for a specific metabolic pathway.
To model the monoculture system, we assume that the two pathways are combined in a single microbe, whose biomass is represented by the variable x. The growth rate is assumed to be the sum of the metabolic pathways of the two microbes (μWT = _μ_1(G, A) + _μ_2(A)). This provides the maximal possible growth rate for the monoculture, without making further assumptions on whether monoculture can actually use this potential. In reality, using both pathways concurrently comes at a cost, as microbes have finite resources for the transport of nutrients into the cell, and the production of enzymes. We will later (Section 4.3) consider modeling the monoculture growth rate as a convex combination of the pathways (μ̄WT = βf(G)I(A) + (1 − β)m(A)). However, we start the linear combination (μWT = _μ_1(G, A) + _μ_2(A)), which is the upper bound for the wildtype growth rate, in order to be able to obtain theoretical results. This gives the system of rate equations for the monoculture system:
| x′=[μ1(G,A)+μ2(A)-D]x, | (5) |
|---|
| G′=(Gin-G)D-μ1(G,A)Y11x, | (6) |
|---|
| A′=-AD+[μ1(G,A)Y21-μ2(A)Y22]x. | (7) |
|---|
In this formulation we assume that there is no change in pathway efficiency (yield), growth rate, substrate affinity, or inhibition when the pathways are split into two separate microbes, thus the functions and parameters for the monoculture system (5)–(7) are identical to those in the binary culture system (1)–(4).
In this work we will assume that the inhibition by A on the first pathway is non-competitive, so that the growth rate of pathway 1 can be described by _μ_1(G, A) = f(G)I(A), where the function f(G) describes the growth of _x_1 on G and the function I(A) describes the inhibition of _x_1 by A. The growth of _x_2 due to the consumption of A we describe by the function _μ_2(A) = m(A), where m(A) increases at low A values but decreases when A gets too high.
2.2. Model non-dimensionalization
In order to simplify the calculations, we non-dimensionalize the original system using the scalings:
| x^=xGinY11,x1^=x1GinY11,x2^=x2GinY11,G^=GGin,A^=AY21GinY11,t^=tD,γ=Y21Y22. | (8) |
|---|
This gives us the dimensionless variables (x1^,x2^, Ĝ, Â) for the binary culture and (x̂, Ĝ, Â) for the monoculture. Both systems of equations depend upon the new dimensionless time variable t̂, and the only remaining parameter in the system is the dimensionless parameter γ.
Removing the hats for convenience and making the substitution _μ_1(G, A) = f(G)I(A), _μ_2(A) = m(A), gives us a dimensionless version of the binary culture system (Eqs. (1)–(4))
| x1′=(f(G)I(A)-1)x1,x2′=(m(A)-1)x2,G′=1-G-f(G)I(A)x1,A′=-A+f(G)I(A)x1-γm(A)x2, | (9) |
|---|
and a dimensionless version monoculture system (Eqs. (5)–(7))
| x′=[f(G)I(A)+m(A)-1]x,G′=1-G-f(G)I(A)x,A′=-A+[f(G)I(A)-γm(A)]x. | (10) |
|---|
2.3. Model assumptions
Before we describe our main result, it is important to specify our assumptions on the functions and parameters in the model (in the dimensionless setting).
- A1
The functions f(G), I(A) and m(A) are the same in both models, i.e. that the separation of the pathways does not modify the metabolic dynamics. - A2
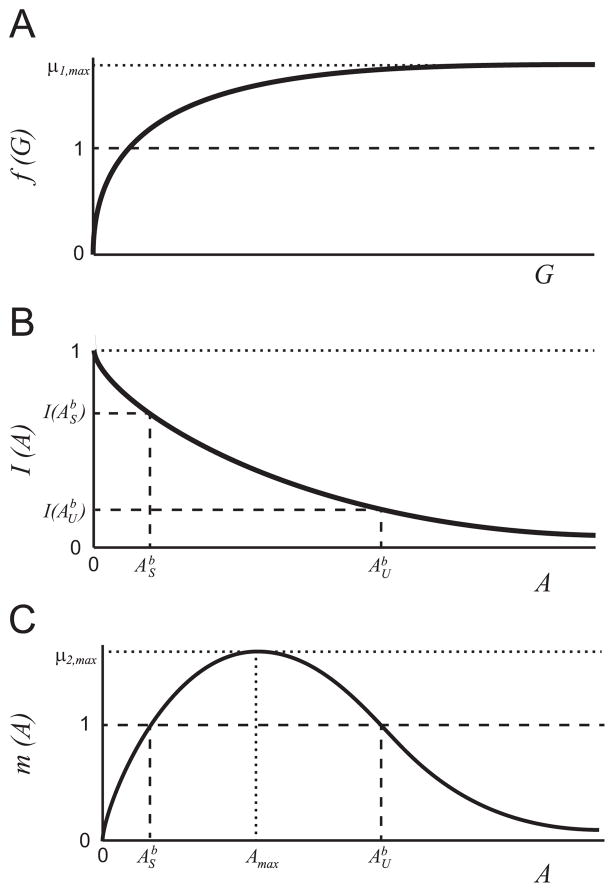
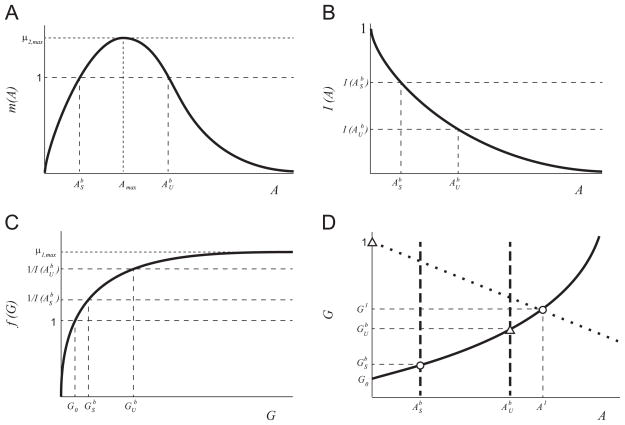
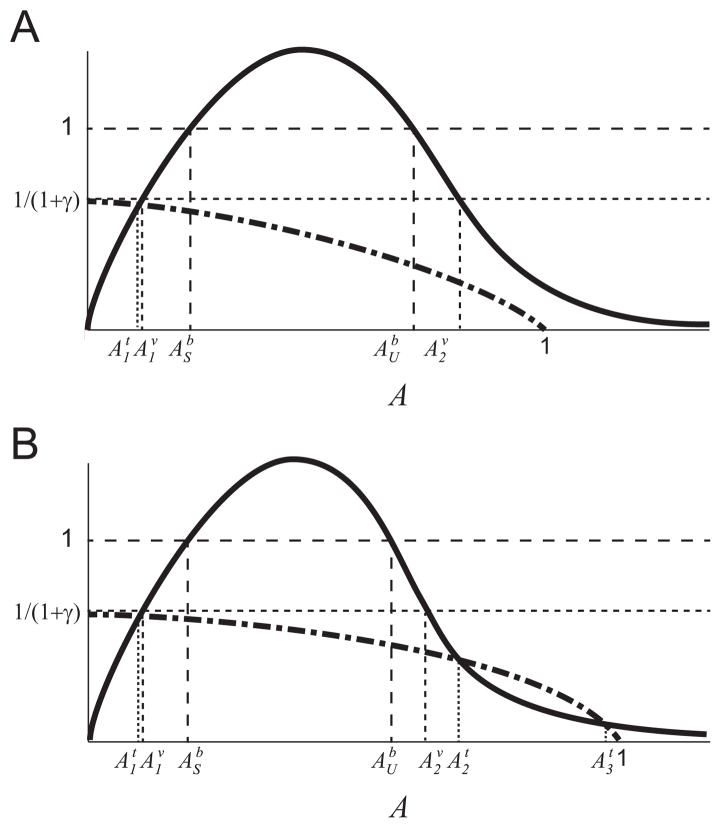
The function f(G) is a monotonically increasing function of substrate concentration, G, with limiting rate, _μ_1,max (see Fig. 2(a)). The properties of f(G) aref(0)=0,limG→∞f(G)=μ1,max,df(G)dG>0forG≥0,limG→∞df(G)dG=0. (11) These conditions on f(G) are readily satisfied by an increasing Hill function _μ_1,maxGn/(kn + Gn) for any n. - A3
The function I describes the inhibition of the _G_-metabolism pathway by the intermediate substrate A (see Fig. 2(b)). The properties of I(A) are:I(0)=1,I(A)>0forA≥0,limA→∞I(A)=0,∂I(A)∂A<0forA>0. (12) These conditions on I(A) are satisfied by a decreasing Hill function kn/(kn + An) for any n. - A4
The function m(A) describes the growth rate on the inhibitory substrate (A) (see Fig. 2(c)). m(A) has the following properties:m(0)=0,limA→∞m(A)=0,max(m(A))=m(Amax)=μ2,max,dm(A)dA>0for0≤A≤Amax,dm(A)dA=0atA=Amax,dm(A)dA≤0forAmax≤A,limA→∞dm(A)dA=0. (13) These conditions on m(A) are satisfied by commonly used substrate-inhibition functions (Han and Levenspiel, 1988). - A5
We consider the case where the growth rates of each microbe are sufficiently large that they can survive at the chosen dilution rate; i.e.,
For _μ_2,max > 1 the equation m(A) = 1 has two solutions, which we define as ASb and AUb, where ASb<AUb. - A6
We consider the case where the substrate inflow is in excess, by which we mean f(Gin) ≈ _μ_1,max and consequently, f(Gin) > D (in the original variables). These conditions correspond to f(1) ≈ _μ_1,max and f(1) > 1 in the dimensionless setting.
Fig. 2.
Qualitative sketch of the growth and inhibition functions: (a) f(G), (b) I(A), and (c) m(A) in nondimensional variables. The solutions to the equation m(A) = 1, ASb and AUb, are shown in panels (b) and (c).
We use the product form of the function μ_1(G, A) = f(G)I(A) since it captures the growth and non-competitive inhibition of pathway 1 and simplifies the analysis. However, a more general function μ_1(G, A) with the properties (∂_μ_1(G, A)/∂_G > 0) for all G > 0 and (∂_μ_1(G, A)/∂_A) < 0 for all _A_ > 0 may be used if inhibition is known to be competitive.
3. Main results
In this section we present our main result, the proof of which can be found in the Appendix. The implications of this technical result are then demonstrated with an example from a realistic situation in the following sections.
3.1. Binary culture system equilibria and stability
For the binary culture (9) we differentiate between three different types of equilibrium points: trivial equilibria where _x_1 = 0, _x_2 = 0, boundary equilibria where _x_1 ≠ 0, _x_2 = 0 (note there are no _x_1 = 0, _x_2 ≠ 0 equilibria, as G is the only substrate provided to the chemostat system), and co-existence equilibria where _x_1 ≠ 0, _x_2 ≠ 0. We consider only equilibria in the positive cone (x1,x2,G,A)∈ℝ+4.
Theorem 1
Given Assumptions [A 1]–[A 6] in Section 2.3, the binary culture system (9) has the following equilibria:
- There exists a trivial equilibrium when there are no microbes in the system at (_x_1, _x_2, G, A) = (0, 0, 1, 0), for all functions satisfying [A 2]–[A 4]. This trivial equilibrium point is an unstable saddle for all parameter values satisfying [A 5]–[A 6].
- There exists a boundary equilibrium point, where only _x_1 survives, at (x11,x21,G1,A1)=(1-G1,0,G1,1-G1), where _G_1 is implicitly defined by f(_G_1)I(1 − _G_1) = 1. The stability depends on the specific functions and parameters.
- We define a value A=Acritb, where Acritb is implicitly defined by I(Acritb)=1/μ1,max.
- If Acritb<ASb, or if ASb<Acritb but GSb+ASb>1, there are no co-existence equilibria. The boundary equilibrium point is a stable node.
- If ASb<Acritb<AUb, and GSb+ASb<1, or if AUb<Acritb, and GSb+ASb<1, but 1<GUb+AUb, there is a single, stable, co-existence equilibrium point S:=(x1,Sb,x2,Sb,GSb,ASb). The boundary equilibrium point is an unstable saddle.
- If AUb<Acritb, and GUb+AUb<1, there are two co-existence (_x_1 ≠ 0, _x_2 ≠ 0) equilibria, S:=(x1,Sb,x2,Sb,GSb,ASb) and U:=(x1,Ub,x2,Ub,GUb,AUb). The point U is an unstable saddle, and the point S is a stable node. The boundary equilibrium point is a stable node.
Proof
Remark 1
The conditions related to Acritb ensure that the equation f(G)I(A) = 1 has a solution for the given Ab value. The conditions Gb + Ab < 1 ensure that the equilibrium biomass values (_x_1 and _x_2) are strictly positive; if _Gb_ + _Ab_ > 1 then x2,Ub<0 and the equilibrium point is non-physical.
Remark 2
Note that in case (iii) of Theorem 1c the system is bistable, with the stable manifold of the saddle point U forming a separatrix that separates the basins of attraction of the two stable equilibria, S and the boundary equilibrium point.
3.2. Monoculture system equilibria and stability
For the monoculture system (10), there are two types of equilibria: trivial equilibria where x = 0 and non-trivial equilibria where x ≠ 0.
Theorem 2
Given Assumptions [A 1]–[A 6] in Section 2.3, the monoculture system (10) has the following equilibria:
- There exists a trivial equilibrium point at (x, G, A) = (0, 1, 0), which is an unstable saddle.
- There are 2_n_ + 1 non-trivial (x ≠ 0) equilibria Ei:=(xim,Gim,Aim) in the region A<ASb, where n ≥ 0, ordered by increasing Aim. If there is a unique internal equilibrium (n = 0) this equilibrium point is a stable node. If there are multiple internal equilibria, the odd equilibria (i = 1, 3, …, 2_n_ + 1) are stable nodes, and the even equilibria (i = 2, 4, …, 2_n_) are unstable saddles.
- If the curve G = 1 − A/(1 + γ − γ/(1 − m(A))) intersects the curve f(G)I(A) + m(A) = 1, in the region A>AUb, then there is an even number of equilibria Kj, j = 1, …, 2_m_. If we order these equilibria by increasing value of A, then the odd equilibria are saddles and even equilibria are nodes.
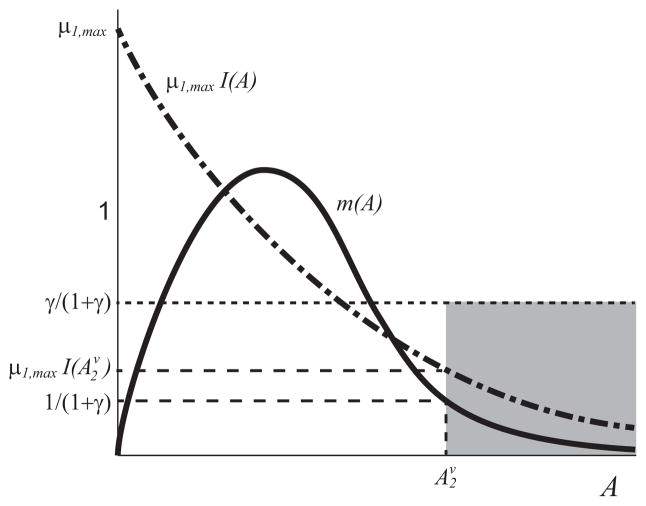
These curves can only intersect if m(A) = 1 − γ/(1 + γ − A) has more than one solution. This condition is illustrated in Fig. B3. If
these equilibria do not exist, where A2v is the upper solution of m(A) = 1/(1 + γ). This condition is illustrated in Fig. 3.
Fig. 3.
Qualitative sketch of the curves m(A) (solid black curve) and _μ_1,maxI(A) (dot-dash black curve) that satisfy the sufficient condition in Theorem 2c ruling out the upper monoculture equilibria. The curve _μ_1,maxI(A) lies in the shaded region defined by the value m(A2v), where A2v is the upper solution of m(A) = 1/(1 + γ).
Proof
Remark 3
The equilibria Kj only exist in specific circumstances when the monoculture growth is less inhibited by acetate than the acetate consuming specialist (see (14) for exact statement), while at least one lower equilibrium Ei exists for all parameter values satisfying [A1]–[A6]. The high A (low biomass) stable equilibria, when they exist, have a small basin of attraction, which is characterized by high levels of acetate and low levels of glucose. Only initial conditions with high initial acetate and low glucose can lead to a monoculture that evolves to such stable equilibrium. For this reason, we do not consider these equilibria in the results below.
3.3. Biomass comparison
We wish to compare the productivity of the binary culture and monoculture systems, using their total biomass as a measure of productivity.
The biomass for the binary system is largest at the stable coexistence equilibrium point S, since it can be easily shown that x1,Sb+x2,Sb>x11 (see Appendix C). The monoculture system has (at least) one stable non-trivial equilibrium point Ei, with high biomass. Since we do not consider the possible upper equilibria Kj with low biomass to be physical, we will compare the biomass at the binary system equilibrium S with an arbitrary monoculture equilibrium Ei.
Theorem 3
Given Assumptions [A 1]–[A 6] in Section 2.3, and the existence of at least one (stable) co-existence equilibrium point, S, in the binary system (conditions in Theorem 1c). Then the biomass of the monoculture, xim, at any of the stable non-trivial equilibrium points Ei for i = 1, 3, …2_n_ + 1 is always higher than the total biomass of the binary culture system x1,Sb+x2,Sb at its stable co-existence equilibrium point S:
Proof
Outline of the proof
We show in Appendix C that the monoculture biomass is given by
| xm=(1+1γ)(1-Gm)-Amγ, | (15) |
|---|
and the combined biomass of the binary culture is
| xtotb=x1,Sb+x2,Sb=(1+1γ)(1-GSb)-ASbγ. | (16) |
|---|
We can also show that for all Ei monoculture equilibria:
Thus,
(1+1γ)(1-GSb)-ASbγ<(1+1γ)(1-Gm)-Amγ
and the biomass of the binary culture is always less than the biomass of the monoculture:
Remark 4
If there are no upper equilibria in the monoculture, Ei for i = 1, 3, …2_n_ + 1 are the only stable equilibrium points of the system, and the monoculture biomass will always be higher than the total biomass of the binary culture, for all initial conditions (G,A)∈ℝ+2.
4. Effect of modifications to the model on relative biomass
In chemostat experiments, a binary culture of the type analyzed here shows a biomass increase of around 20% over a wild-type monoculture system (Bernstein et al., 2012). As we have shown in Theorem 3, under our assumptions the monoculture system always has a higher biomass. Clearly this means that one or more of the assumptions that we make in Section 2 are incorrect. This is useful, as by examining the assumptions we made, we can investigate which of the effects that we did not include are most relevant.
One of the assumptions we made in formulating the initial model is that there is no change in the growth dynamics or yields of the metabolic pathways when the microbes specialize to a single substrate. However, as discussed already, there is evidence that the growth dynamics and pathway efficiencies will change, and we will investigate the effects of incorporating these changes in this section.
4.1. Increasing the growth rates in the binary culture system
Experiments have observed that growing E. coli on a single nutrient source can increase its growth rate ~15–20% (Edwards et al., 2001; Ibarra et al., 2002; Bernstein et al., 2012), as the microbe adapts to the single nutrient and can up regulate the transport for that substrate (Oh et al., 2002) and down regulate the transport for the pathways it is not using (catabolite repression, Egli et al., 1993; Lendenmann and Egli, 1922). We can incorporate the effect of increasing the growth rate of the species in the binary culture system by introducing multiplicative parameters _α_1 and _α_2 in front of growth rates f(G) and m(A), thus making the replacements:
| f(G)→α1f(G),m(A)→α2m(A). | (18) |
|---|
Note that this increases the growth rates at all concentrations of G and A, not just for high growth regions of G or A. For the original systems (with no change in growth rate) we have _α_1 = 1, _α_2 = 1. To simulate an increase in growth rate when the metabolic pathways are split up we simply choose _α_1, _α_2 > 1 in the binary culture system.
Increasing _α_1 decreases GSb→0, while increasing _α_2 in the binary culture moves ASb→0, which in turn decreases GSb (bounded below by _G_0). We find that it is always possible to get higher biomass for the binary culture than the monoculture (GSb<Gm,ASb<Am) if there are no bounds on the increases for both _α_1 and _α_2. If we can only vary one pathway’s growth rate, i.e., keeping _α_1 = 1 or _α_2 = 1, then it is not always possible to get higher biomass in the binary culture. Whether or not it is possible to surpass the monoculture biomass, and the increases in growth rate required, will depend on the specific functions and parameters for the system of interest.
4.2. Increasing the substrate affinity in the binary culture system
When growing on a single carbon source, it has been found that some microbes, including E. coli, can increase their affinity when the concentration of the substrate remains low for an extended period of time (Helling et al., 1987; Senn et al., 1994). This is thought to be achieved through up-regulating higher affinity transporters (Ferenci, 1996). Decreasing KG, moves GSb→0, while decreasing KA moves ASb→0, which in turn decreases GSb (bounded below by _G_0). This matches the effects of increasing the growth rates by a factor _α_1 or _α_2 in Section 4.1. We can explain this mathematically as follows: in the region where G (or A) is low, increasing the substrate affinity acts to increase the growth rate for that G (or A) value. By considering f(G) at low G, we find that f(G) ≈ (_μ_1,max/KG)G for small G, so increasing _μ_1,max or decreasing KG by the same factor should have the same effect.
4.3. Varying the form of the monoculture growth rate
It could be argued that the monoculture out-performs the binary culture in our formulation due to the way we have described the monoculture growth rate (μWT(G, A) = f(G)I(A) + m(A)). In this formulation we are assuming that the microbe can use both pathways concurrently, with both functioning at their maximal rates for the specific G and A. This acts as an upper bound on the maximal possible growth rate for the monoculture. In reality, there are limitations due to the transport of nutrients into the cell and the energy required for the synthesis and maintenance of metabolic enzymes. It has been observed experimentally that often a microbe preferentially consumes the substrate sustaining the higher growth rate (catabolite repression). In this process, the synthesis of enzymes required for alternate pathway is down-regulated, which would decrease the maximal growth rate of these other pathways. If we assume that the cell must choose to allocate resources between the two pathways, we can incorporate this into our model by making the monoculture’s growth rate a convex combination of the two pathways’ growth rates:
| μ¯WT=βf(G)I(A)+(1-β)m(A). | (19) |
|---|
where β could be some function of the relative growth rates of the two pathways at the given G and A concentrations, following (Abrams, 1987; Egli et al., 1993; Lendenmann and Egli, 1922).
According to catabolite repression the microbe will only consume the preferred substrate. However, if the intermediate substrate is inhibitory there is often a ‘switch’ that overrides this effect at high A to consume the excess intermediate substrate and decrease the inhibitory effects (Wolfe, 2005). In our model, we could incorporate this by using (19), with β being a function of A, switching to low β (increased A consumption) when A reached a critical value, even in the presence of high G.
Using μ̄WT, we cannot get theoretical results for the relative productivity of the monoculture and binary culture systems in general, as the biomass will depend on the specific situation and the β values (or functions) chosen. However, we can easily show that for sufficiently high or low β the binary culture system would now out-perform the monoculture system:
- At β = 1, the monoculture growth rate is given solely by f(G)I(A), which is the equivalent of the binary culture system with _x_2 = 0. In Appendix C we showed that this _x_1-only equilibrium point has a lower biomass than the binary culture system coexistence equilibrium point, thus the binary culture outperforms the monoculture.
- As β → 0, the monoculture growth rate is increasingly dependent on the second pathway, m(A), which consumes exclusively acetate A. Since glucose G is the only substrate provided to the chemostat, we expect that the monoculture will not survive.
Based on optimal foraging theory and experiments, it is reasonable to assume that E. coli feeding on multiple substrates would allocate resources to pathways such that the growth rate is maximized (Abrams, 1987; Egli et al., 1993; Lendenmann and Egli, 1922), and this is indeed what is found with catabolite repression. In a chemostat at equilibrium, the growth rate is always fixed to the dilution rate D, so we cannot determine the optimum β value at equilibrium. One option could be to first find the β values required for the monoculture system to have lower biomass (for a given system), then to use the optimal foraging arguments to determine whether these β values are realistic by looking at the ratio of _μ_1–_μ_2 for the given G and A values. Another option would be to look at what β value maximizes biomass, and consider whether this leads to higher biomass in the monoculture than the binary culture, and whether this is a reasonable β value. We will consider both in Section 5.2.3 for the specific example of E. coli growing on glucose.
4.4. Increasing the efficiencies (yields) in the binary culture system
The yield terms _Y_11 and _Y_22 describe the amount of biomass produced per unit of substrate consumed, and are a measure of the microbe’s efficiency. The yields are determined by only two factors: how much energy the pathway can produce (given by the stoichiometry of the pathway), and how much of the energy produced is allocated to biomass production.
There are many ways that microbes can adapt to optimize their productivity, but only some of these will increase the yield terms in our formulation. For example, it is observed that when provided with multiple substrates, cells will consume the most productive substrate preferentially (also known as catabolite repression). In this way, the microbes are utilizing the most efficient pathways and thus increasing their efficiency. In our model the binary culture microbes only have one substrate, and thus catabolite repression will not affect their dynamics. In the monoculture, catabolite repression would lead to the most efficient pathways being prioritized. This increase in resources would increase the substrate uptake and growth rate of the preferred pathway, and could be incorporated into our model through changing the parameter β as described above, where β = 1 describes consumption of the primary substrate only. However, as the stoichiometry of the pathways does not change, this effect would not directly change _Y_11 or _Y_22 in our model.
In order to increase the yield, a microbe must reduce the energy that is required for functions other than biomass production, such as cell homeostasis and enzyme production. Microbes growing on a single source are thought to down-regulate the production of enzymes for the metabolism of other substrates (Johnson et al., 2012). Since the synthesis and maintenance of metabolic enzymes requires energy, if a microbe can reduce its net enzyme production in this way, this would reduce the energy required and its yield would increase. Thus it is possible that by specializing to a single substrate, the yields _Y_11 and _Y_22 would increase.
Another factor that could affect the energy requirements, is the cost of transporting substrates into the cell. So far we have not included this cost. If we were to include the cost of transporting the primary substrate G into the cell, this would decrease the term _Y_11 in both binary and monoculture systems equally. Including the cost of transporting the inhibitory intermediate substrate A into the cell, would decrease _Y_22. The binary culture needs to transport all of the consumed A into the scavenger specialist _x_2, whereas the monoculture can metabolize intracellular A as it is produced, as well as transporting A into the cell, thus deflecting some of this cost. Therefore, _Y_22 would decrease more in the binary culture than in the monoculture system.
Examining the equations for steady-state biomass we find that changing _Y_11 does not affect the nondimensionalized binary biomass, but from Eq. (8) it will affect the actual (dimensional) biomass. The total biomass of the binary culture increases when _Y_11 increases (the _G_-metabolism pathway becomes more efficient). When _Y_22 increases (the _A_-metabolism pathway becomes more efficient), or when _Y_21 decreases (the amount of inhibitory metabolite produced increases), γ decreases, which will increase the total biomass of the binary culture. As discussed in Section 2.1, the ratio r = _Y_11/_Y_21, which describes the grams of the inhibitory intermediate substrate produced per gram of primary substrate consumed, is fixed for a specific metabolic pathway. Therefore, varying _Y_21 or _Y_11 independently is not realistic, and in the latter analysis we will vary _Y_11 and _Y_21 together, keeping r constant. When making increases to _Y_11 or _Y_22 we must keep in mind that there is an upper bound on the yield terms that given by the stoichiometry of the utilized metabolic pathway. Whether the yield increase required to match the observed increase in biomass for the binary culture is above that bound will depend on the system of interest.
4.5. Varying Gin and D
Some earlier work has found that the binary culture is favored over a monoculture for certain substrate inflow concentrations Gin or dilution rates D (Doebeli, 2002; Pfeiffer and Bonhoeffer, 2004). However, we find that for all Gin and D, satisfying assumptions [A5] and [A6], the monoculture has higher biomass than the binary culture. That being said, the difference in total biomass between the two systems will vary as Gin and D change, so there may be some experimental conditions where the binary culture is less disadvantaged, and where the aforementioned modifications would be more effective.
5. Specific example using Monod kinetics and typical values for E. coli
In this section, the results of this paper will be applied to the specific example of E. coli growing on glucose as the sole carbon source. Experimentally observed parameters for E. coli growth on glucose or acetate are summarized in Table 1. In the majority of the literature, E. coli were grown in aerobic conditions in which both pathways would be utilized. This will lead to overestimation of the parameters: _μ_1,max, _Y_11, _Y_21, KIP, and underestimation of the parameter r.
Table 1.
Typical parameter values for E. coli grown on glucose or acetate as the carbon source. See Table D1 for further details of the strains used and the specific experimental conditions.
We can quantify the discrepancy between the actual yield of each pathway and the reported yield in wild-type aerobic conditions. If we assume that the growth rate from the first (anaerobic) pathway is given by _μ_1 and the growth from the aerobic metabolism of acetate (acetyl CoA) is given by _μ_2, then the discrepancy between the actual yield of each pathway and the reported yield in wild-type aerobic conditions will be
- The reported yield Y11WT (gBiomass/gGlucose consumed) will be overestimated by Y11WT=Y11(μ1+μ2)/μ1.
- The reported acetate production yield Y21WT (gBiomass/gAcetate produced) will be overestimated by Y21WT=Y21(μ1+μ2)/(μ1-γμ2).
- The observed ratio rWT (gAcetate produced/gGlucose consumed) will be underestimated by rWT = r(1 − _γμ_2/_μ_1).
From the equation for Y11WT it can be seen that if _μ_2 = 0 (i.e. there is no metabolism of acetate, which occurs in anaerobic conditions), then Y11WT=Y11. Thus, in order to estimate the parameters of just the first pathway (max f(G) and _Y_11), we use the yield and growth parameters from anaerobic experiments (Chen et al., 1997; Varma and Palsson, 1994; Xu et al., 1999). To estimate _Y_21 we use the ratio r = 0.667 determined by the reaction stoichiometry and calculate _Y_21 = _Y_11/r. Substituting reasonable values for growth rates, we find that our choice of parameters leads to observed Y21WTrWT that match experimental data (Table 1). The observed growth and yield parameters for E. coli grown on acetate as the sole carbon source provide good estimates for max (m(A)) and _Y_22. The chosen yields are given in Table 2.
Table 2.
Selected yield values for monoculture and binary culture systems.
| Parameter | Selected value | Notes |
|---|---|---|
| _Y_11 | 0.152 | from anaerobic growth experiments reaction stoichiometry calculated, _Y_21 = _Y_11/r |
| r = _Y_11/_Y_21 | 0.667 | |
| _Y_21 | 0.227 | |
| _Y_22 | 0.400 |
It is important to note that for the yield terms _Y_11 and _Y_22, which are the grams of biomass produced when one gram of substrate is consumed, only some of the energy from the substrate metabolism will go towards biomass synthesis. Depending on the efficiency of the pathway, the energy produced per gram of substrate will vary, and the energy required for cell homeostasis (i.e. not available for biomass production) also varies. Estimates for the ratio of energy produced that goes towards biomass production and the efficiencies of the pathways used vary depending on the E. coli strain and the environmental conditions (Carlson and Srienc, 2004; Han et al., 1992). We choose to use measured values for the total yield from the literature. These yields could vary with strain and condition, and we will consider this in Section 5.2.3. In all cases the yields are bounded above by the stoichiometry of the most efficient biomass production pathways assuming zero energy required for cell homeostasis, and our choices for yield parameters are below this bound and consistent with experimental findings.
To fit growth parameters, we must choose specific functions for f(G), I(A), and m(A). Here we select Monod type kinetics for the growth and inhibition functions:
| m(A)=μ2,maxAA+KA+A2KIS. | (22) |
|---|
which satisfy assumptions [A2]–[A4] and are found to be appropriate for describing growth and inhibition rates of E. coli in chemostats or batch growth. With the exception of the half-saturation of glucose, KG, experimental findings (Table 1) are reasonably consistent and the chosen parameters for these functions are given in Table 3.
Table 3.
Selected parameter values for growth and inhibition functions given in Eqs. (20), (21), (22), dilution rates, and substrate inflow concentrations.
| Parameter | Selected value | Notes |
|---|---|---|
| _μ_1,max | 0.6 h−1 | max (f(G)) = 0.6 h−1 |
| _K_G | 0.05 g/L | |
| _K_IP | 0.5 g/L | |
| _μ_2,max | 0.8 h−1 | max (m(A)) = 0.2 h−1 |
| _K_A | 1.5 g/L | half-saturation at _A_=0.23 g/L |
| _K_IS | 0.7 g/L | inhibited to half maximum at _A_=4.6 g/L |
| D | 0.1 h−1 | chosen to prevent wash-out |
| Gin | 10 g/L | chosen to ensure excess substrate |
At low glucose concentrations it is known that glucose affinity increases (Ferenci, 1996; Helling et al., 1987), and the parameter KG varies from O(0.01) to O(100) mg/L (Table D1) through the upregulation of higher affinity (and often slower or more energy intensive) pathways. In our model, KG is a fixed parameter, and to demonstrate the results of this paper we choose an intermediate KG value (see Table 3). This choice does not affect our result, as for equal substrate affinities the monoculture will outperform the binary culture for any choice of KG (Theorem 3). We will investigate the effect of varying the glucose affinities between the monoculture and the binary culture in Section 5.2.2. However, as increases in glucose affinity are found to depend mostly on the growth conditions, we would expect any increase in glucose affinity (KG) to affect both the monoculture and the binary culture similarly.
In E. coli there are two pathways for acetate assimilation: the phosphotransacetylase-acetate kinase (PTA-ACKA) pathway and the acetyl-CoA synthetase (acs) pathway. The PTA-ACKA pathway has lower substrate affinity, with Km ≈ 0.4 – −0.6 g/L (Brown et al., 1977; Wolfe, 2005), but is more efficient requiring only one ATP for acetate assimilation. The PTA-ACKA pathway is found to be active until acetate gets to low concentrations (A « 0.15 g/L) (Kumari et al., 1995). In contrast, the acs pathway has higher substrate affinity, with Km ≈ 0.01 g/L (Brown et al., 1977; Wolfe, 2005), but is more energy intensive, requiring two ATP for acetate assimilation. Kumari et al. (1995) find that both pathways are required for optimal growth on acetate across a range of concentrations, with the acs pathway being required for A < 0.6 g/L and the _PTA-ACKA_ pathway being required for _A_ > 1.5 g/L.
In our model, we use a single function m(A) and single yield term _Y_22 to describe the growth kinetics and efficiency of growth on acetate; we do not include the two pathways separately. We choose growth parameters _μ_2,max, KA, and KIS that match the experimentally observed growth in chemostat cultures which are utilizing both pathways. For example, the combined growth kinetics has a substrate affinity of Km = 0.23 g/L which is midway between the Km values of each pathway. We will investigate the effect of varying the acetate affinities between the monoculture and the binary culture in Section 5.2.2.
The yield parameter _Y_22 is fixed, so we have chosen _Y_22 = 0.4 which is at the lower end of the yields observed across a range of growth conditions (Kleman and Strohl, 1994). This is because we find that in our model acetate concentrations stay low and the yield will be determined predominantly by the lower efficiency, higher affinity acs pathway.
The dilution rate D and the glucose inflow concentration Gin are set by experimental conditions. We must select a dilution rate that satisfies non-wash-out conditions (from assumption [A6]: max(m(A)) > D, _μ_1,max > D). This leads to a low dilution rate (D = 0.1) due to the low value of max(m(A)) > D seen experimentally. We also choose a high substrate inflow Gin » _G_0, so that the system is in glucose-excess conditions (assumption [A5]).
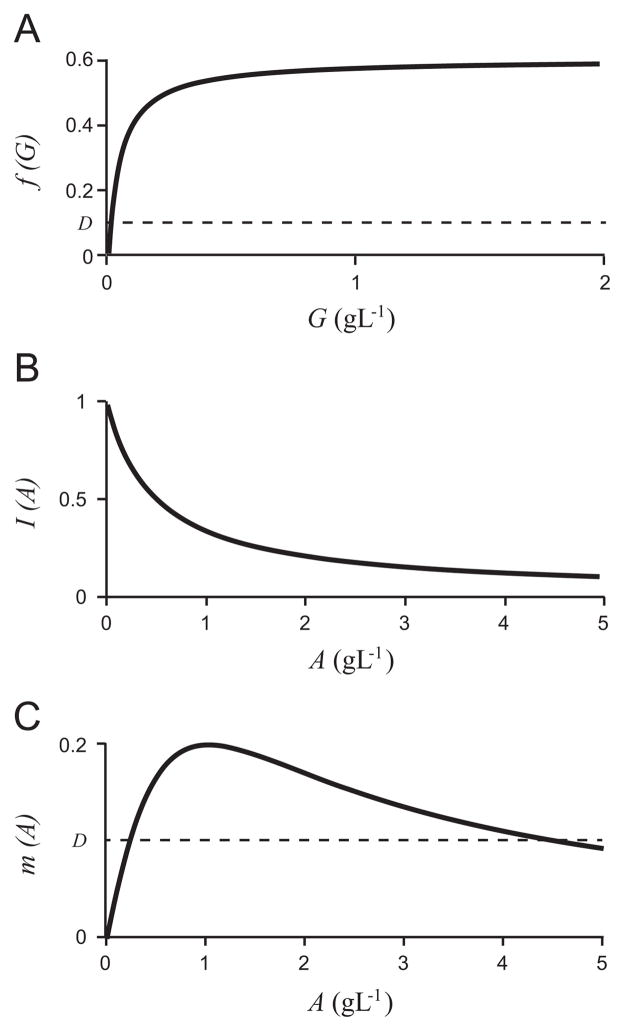
For the selected functions and parameter values, the growth and inhibition curves are depicted in Fig. 4. We graph them in terms of the original variables, glucose concentration, G (g/L), and acetate concentration, A (g/L), for ease of comparison to the experimental literature (Table 1). However, for the remainder of the analysis we will use the dimensionless variables and parameter values unless otherwise stated. We use the nondimensionalization in Eqs. (8) and the parameter scalings:
| μ^1,max=μ1,maxD,μ^2,max=μ2,maxD,KG^=KGGin,KA^=KArGin,KIS^=KISrGin,KIP^=KIPrGin. | (23) |
|---|
where r = _Y_11/_Y_21.
Fig. 4.
The functions f(G), I(A), and m(A) (20, 21, and (22), respectively), in the original (dimensional) variables, for the chosen parameter values.
5.1. Monoculture and binary culture with no changes
In the monoculture system (10), for growth and inhibition functions (20)–(22) and parameter values in Tables 2 and 3, we find a unique non-trivial equilibrium at
(x1m,G1m,A1m)=(2.72,0.000418,0.0197).
In the binary culture system (9), there is one stable coexistence equilibrium point
(x1,Sb,x2,Sb,GSb,ASb)=(0.998,1.70,0.00159,0.0337),
with x̄S = 2.70. There is also a stable equilibrium point where only _x_1 survives at
(x1b,x2b,Gb,Ab)=(0.371,0,0.629,0.371).
The biomass of the monoculture, x = 2.72, is greater than the total biomass in the binary culture at the stable co-existence equilibrium, x̄ = _x_1 + _x_2 = 2.70, as given by Theorem 3.
5.2. Quantitative investigation of how to increase the binary culture’s relative productivity
In chemostat experiments, a binary culture of this type shows a biomass increase of around 20% over the wild-type monoculture system (Bernstein et al., 2012). In Fig. 5 we plot the curves that determine the equilibrium points of the systems. The biomass is determined by the values of G and A, given in Eqs. (15) and (16), thus in the (A,G)-plane, the curves G = c − A/(1 + γ), for a constant c = 1 − γxc/(γ + 1), are the lines of constant biomass, xc. In order for the binary culture biomass to exceed that of the monoculture, the binary culture system must be modified such that the point (Ab, Gb) crosses the dashed line in Fig. 5, or, alternatively, the monoculture system must be modified such that the point (Am, Gm) crossed the dotted line in Fig. 5. (The only exception to this is if _Y_11 or Gin are varied between the two systems, as these parameters appear in the non-dimensionalization of the biomass variables.)
Fig. 5.
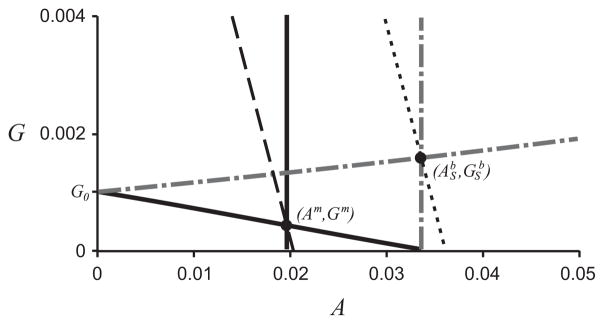
The curves _G_1 and _G_2, which determine the position of the monoculture equilibrium point(s) in the nondimensionalized system are shown in black solid lines, with the intersection labeled (Am, Gm). The curves f(G)I(A) = 1 and m(A) = 1, which determine the position of the binary culture equilibrium point(s) are shown in gray dot-dash lines, with the intersection labeled (ASb,GSb). The lines of constant biomass for the monoculture and binary culture are black dashed and dotted lines, respectively.
5.2.1. Varying growth rates independently
When microbes specialize to grow on a single substrate they are found to increase their growth rate. One explanation for this is that they are able to allocate more resources to synthesizing transporters that increase the substrate uptake rate. By making the substitutions in Eq. (18), we can use parameters, _α_1 and _α_2 to modify the growth rates independently and perform a more general investigation.
To increase the growth rate of the microbes in the binary culture compared to monoculture we set _α_1 > 1, or _α_2 > 1, or both. It is important to note that increasing αi will increase the growth rate of the pathway i by the factor αi across all substrate concentrations.
- If we only increase the maximal growth rate of the glucose metabolism, _α_1, we cannot increase the total biomass of the binary culture to match that of the monoculture, even if _α_1 → ∞.
- If we only increase the maximal growth rate of the acetate metabolism, _α_2, the binary culture has the same biomass of the monoculture at _α_2 = 1.69, i.e., the growth rate has to increase 69%.
- Finally, we allow both _α_1 and _α_2 to increase then we find multiple combinations of _α_1, _α_2 where the biomass has the same or larger mass than the monoculture. The combination of multipliers with minimal value of the sum _α_1 + _α_2 for which the total biomass of the binary culture matches that of monoculture is when we only increase the second pathway, so _α_1 = 1 and _α_2 = 1.69.
Increases of around 15–20% have been seen experimentally when microbes have been grown on a single substrate. The increases of 70% that are required here are not realistic. Moreover, Bernstein et al. (2012) observed a biomass increase of 20% in chemostat experiments. Even increasing _α_2 → ∞ the binary culture biomass never reaches 20% more than the monoculture
5.2.2. Increasing the substrate affinity in the binary culture
In E. coli the experimentally observed glucose affinity varies by orders of magnitude in different experimental conditions (Km = 0.0053–99 g/L Senn et al., 1994). This is because the bacteria have many different systems to transport and metabolize glucose as a substrate, that all have differing speeds and affinities. If we predict that the glucose specialist (_x_1) in the binary system has an increasing substrate (glucose) affinity, we can investigate the effect this would have on biomass production by decreasing KG in our model.
- Varying KG we find that there is no decrease in KG that will lead to the binary system having a higher biomass than the monoculture system. This is expected from our results in Section 5.2.1, since f(G) ≈ _μ_1,maxG/KG at low G, and there is no _α_1 that leads to the binary system biomass exceeding that of the monoculture.
Additionally, the phenomenon whereby E. coli in low glucose conditions uses higher affinity transporters would be predicted to occur in both the monoculture and primary microbe in the binary culture, as this depends mostly on growth conditions. If glucose affinities increase but remain equal, the monoculture will still outperform the binary culture by Theorem 3.
If the acetate scavenger specialist (_x_2) in the binary culture has an increased acetate substrate affinity, relative to the monoculture, we can investigate the effect this would have on biomass production by decreasing KA and KIS in our model.
- Varying KA (and KIS) we find that the binary culture has higher biomass when KA and KIS decrease to 54% of the monoculture values i.e. an increase in substrate affinity of 85%. This is to be expected from our results in Section 5.2.1, as increasing m(A) by 69% gave a higher biomass for the binary culture, and thus decreasing KA (which increases the growth rate at low A values) should have a similar effect.
Note: we decrease KIS concurrently with KA in order to keep max (m(A)) constant, however, if we only decrease KA and allow max(m(A)) to increase we see similar results (55% decrease cf. 54% decrease).
A twofold increase in the acetate affinity (halving the Km value) is within realistic realms, and brings the Km value of acetate metabolism to 0.12 g/L. However, the higher-affinity acs pathway, that would be required for an increased acetate affinity, is more energy intensive (lower yield); this means that although the increased substrate affinity would act to increase the biomass, the consequent decrease in yield would counteract this increase. In our model formulation, we cannot directly account for the use of different acetate assimilation pathways between the binary culture system and the monoculture in the comparison, as we do not include the two pathways separately. Furthermore, up-regulation of the higher affinity acs pathway has been observed in vivo in wild-type E. coli (Wolfe, 2005) growing on acetate as well, so we consider it difficult to justify a change that increases the binary culture acetate affinity by 85% more than that of the monoculture.
If we take our analysis one step further and attempt to match the observed biomass increase of 20% in the binary culture (Bernstein et al., 2012), we find that there is no increase in substrate affinity (Km → 0) which can produce the required increase in biomass.
5.2.3. Varying form of the monoculture growth rate
When there is glucose available, E. coli will preferentially consume glucose due to catabolite repression until the acetate concentration gets too high, at which point the E. coli will switch to consuming acetate (Nicolaou et al., 2010; Wolfe, 2005). This effect is incorporated implicitly into our model, as for high G, low A, f(G)I(A) > m(A) and μWT ≈ _μ_1. Whereas, for high A we find m(A) > f(G)I(A), and μWT → _μ_2. However, there may be some additional trade-off in the ability of the cell to produce the enzymes required for each pathway, so the increased utilization of one pathway may decrease the growth rate of the other (Oh et al., 2002). We can incorporate this into the model by using the modified growth function for the monoculture system in Eq. (19).
In experiments it is observed that the utilization of the two pathways, and thus the value of the parameter β, depends on the concentration of acetate A (Wolfe, 2005). We will not assume a form for the dependence of β on A, however, by considering the existing experimental evidence we can determine whether the equilibrium acetate concentration and pathway utilization for a given β value is a realistic scenario.
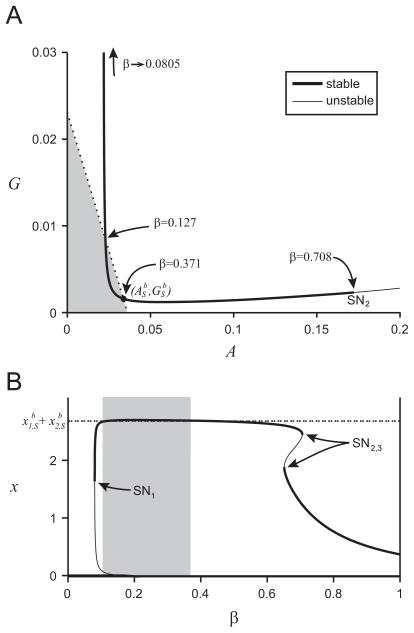
The position in the (A,G)-plane of the equilibrium point for the modified monoculture system as β varies is shown in Fig. 6(a). For β values (0.127 < β < 0.371) the monoculture equilibrium point is to the left of the dotted line (binary culture biomass equivalence), and thus has a higher biomass. As β decreases below β = 0.127 the monoculture equilibrium G value increases, until the monoculture does metabolize enough glucose to survive and the equilibrium point disappears in a saddle-node bifurcation at β = 0.0805 (_SN_1). As β increases from β = 0.371, the monoculture equilibrium point disappears in a saddle-node bifurcation at β = 0.708 (_SN_2). The system becomes bi-stable at β = 0.653 (_SN_3), shown in Fig. 6(b). The lower biomass equilibrium point persists and reaches the _x_1-only equilibrium point of the binary culture (_A_1, _G_1) at β = 1.
Fig. 6.
Panel (a) shows the position of the monoculture equilibrium point in the (A, G)-plane as β varies as a solid black line. The coexistence equilibrium point of the binary culture (ASb,GSb) is shown as a filled black circle. The dotted line shows the line of constant biomass for the binary culture at the coexistence equilibrium point, equilibrium points to the left of the curve (the shaded area) have a higher biomass than the binary culture. Important β values are labeled in the figure. Saddle-node bifurcations are labeled _SN_1,2,3, but only _SN_2 is within the range of this figure. Panel (b) shows the biomass (x value) of the monoculture equilibrium point as β varies which is shown as a solid black line; thick where it is stable, thin where it is unstable. The dashed line shows the biomass of the comparative binary culture system, x1,Sb+x2,Sb, and the shaded region represents the β values where the monoculture has higher biomass than the binary culture system.
At β = 1 the monoculture is the equivalent of the binary culture system with _x_2 = 0, which means it has a lower biomass than the binary culture system coexistence equilibrium point (not shown in Fig. 6). As β decreases from 1, the advantage of being able to consume the acetate increases the monoculture’s biomass. We find that the binary culture only has higher biomass than the monoculture for β > 0.371. However, for β > 0.371 the monoculture reaches an equilibrium point with high acetate concentration, but E. coli are observed to switch to consuming more acetate when the acetate concentration increases (Wolfe, 2005), which means that these high β values (a higher utilization of the glucose consumption pathway f(G)I(A) over the acetate consumption pathway m(A)) are unrealistic.
As _β_→ 0, the monoculture growth rate is increasingly dependent on the second pathway, m(A), which consumes exclusively acetate A. Since glucose G is the only substrate provided to the chemostat, we expect that the monoculture will not survive. Indeed, for β<0.0805 the monoculture washes out and the biomass of monoculture (_x_=0) will be less than the biomass of the binary culture.
The optimal β value (to maximize growth rate Abrams, 1987; Egli et al., 1993; Lendenmann and Egli, 1922) will depend on the concentration of G and A. However, for monoculture equilibrium values as β varies the growth rate is set to D by the chemostat dynamics. If we instead predict that the optimal β value would lead to a higher biomass, we find peak monoculture biomass at β = 0.208 (_xm_=2.71), and we reach the conclusion that the monoculture would be expected to have a higher biomass than the binary culture.
5.2.4. Varying yields
In this section we examine the effect of varying yields in our model of E. coli growing on glucose. If the microbes in the binary culture are able to reduce their energy requirements when they specialize to a single substrate, the yields _Y_11 and _Y_22 would increase. If we include the effect of the cost of transporting acetate into the cell, this would decrease _Y_22. Here we will assume that the metabolic pathway stoichiometry is fixed, i.e., the ratio r = Y 11/_Y_21 (grams of acetate produced per gram of glucose consumed) is fixed. The effects of varying the yields are as follows:
- By increasing _Y_11 (and _Y_21 as r is fixed) in the binary culture system, that is, reducing the amount of glucose G the primary consumer microbe requires to make one g of biomass _x_1, the total biomass of the binary culture can exceed the monoculture biomass. In this example we find that the two systems have equal biomass when _Ȳ_11 = 1.03 _Y_11 (and _Ȳ_21 = 1.03 _Y_21), which represents an increase in glucose pathway efficiency of 3%.
- By increasing _Y_22 in the binary culture system, which corresponds to a reduction in the amount of acetate A the secondary scavenger microbe requires to make one gram of biomass _x_2, the total biomass of the binary culture can exceed the monoculture biomass. In this example we find that the two systems have equal biomass with _Ȳ_22 = 1.02 _Y_22. This represents an increase in acetate pathway efficiency of 2%.
If the ratio r = _Y_21/_Y_11 is not fixed, we find that increasing _Y_11 independently of _Y_21, we can get the same total biomass with _Ȳ_11 = 1.01 _Y_11, and decreasing _Y_21 independently of _Y_11 we find that _Ȳ_21 = 0.98 _Y_21 gives the same total biomass in both systems. However, in order for _Y_21 to decrease without _Y_11 decreasing (or _Y_11 to increase without _Y_21 increasing), the glucose consuming microbe would have to utilize different metabolic pathways, with different stoichiometry, that produce more A per G consumed. In experiments, it is observed that wild-type E. coli can modify their metabolic strategies depending on the environmental conditions, specifically, in excess acetate the monoculture is seen to increase the flux through the acetate-metabolism pathway m(A) (Wolfe, 2005). This would be equivalent to varying β in our modified monoculture system (Eq. (19)); the stoichiometry of the pathways would not change. In this application, we argue that it is unrealistic to modify _Y_21 independently of _Y_11.
Finally, we can generate the 20% increase in biomass observed in Bernstein et al. (2012) if we increase by the efficiency of the glucose-metabolism pathway (_Y_11 and _Y_21) by 57%, or increase the efficiency of acetate-metabolism pathway, _Y_22, by 34%.
6. Discussion
In this paper we describe a minimal model of a syntrophic chain system in which the metabolism of a substrate is divided into two distinct organisms. We compare this to a monoculture, which is able to completely metabolize the substrate. Under very general assumptions we show that the monoculture will always produce larger biomass than the equivalent binary syntrophic chain system. This finding does not agree with experimental findings from microbial consortial systems, which show higher biomass for consortial systems (Bernstein et al., 2012). Two key assumptions in the creation of our minimal model are that there are no metabolic advantages to dividing the metabolic steps into separate organisms, and that there is no cost to the monoculture for using multiple substrates concurrently. Since the model under these assumptions does not reproduce the increased biomass in the consortium that is observed experimentally, this leads us to conclude that one (or both) of these assumptions is incorrect and allows us to investigate the effect of making these assumptions more realistic. By modifying the minimal model we are able to test hypotheses and investigate the effects of adaptations that can develop in a syntrophic chain system, clarifying the mechanisms that contribute to the experimentally observed advantage. We find that the division of metabolic pathways must have an effect on both the growth and yield of the system.
If we assume that in the binary culture the pathways are able to metabolize the substrate faster (increasing the growth rate), we find that it is sometimes possible to have a higher biomass in the binary culture system. However, considering a specific example from E. coli using typical parameters, the growth rate increases required are much larger than those observed experimentally when an organism adapts to a single food source. Considering instead the possibility of increases in substrate affinity, we find that, for the specific example of E. coli, a twofold decrease in the acetate Km value for the binary culture relative to the monoculture would lead to higher biomass. However, in similar low substrate conditions acetate affinity increases would be seen in both the monoculture and binary culture, and even if the acetate specialist in the binary culture could increase its substrate affinity, this would not necessarily increase the productivity, as the higher affinity acetate assimilation pathway has a lower yield.
We also test a hypothesis that the efficiencies of the metabolic pathways in the binary culture are increased, as the specialists do not have to produce the enzymes related to the other pathways, reducing the amount of energy required for enzyme synthesis and maintenance, and thereby increasing the proportion of energy that can go towards biomass production (Oh et al., 2002). By varying the yield, it is possible to increase the binary culture’s biomass to match that of the monoculture with an increase of only 2–3% in the parameters _Y_11 or _Y_22. This magnitude of yield change is within the range of what is observed experimentally, and we consider this to be the most likely cause of the observed productivity increase in the binary culture.
Comparing our results to past work on the question of whether organisms maximize growth rates or efficiency, we find that two main theories are the Maximum Power Principle (MPP) (DeLong, 2008; Lotka, 1922; Sciubba, 2011) and the Resource Ratio Theory (RRT) (de Mazancourt and Schwartz, 2010; Miller et al., 2005; Tilman, 1982, 1988). The MPP states that systems strive to increase ‘power’ within the system constraints. This has been used to successfully predict the outcome of two species competition experiments (DeLong, 2008). The RRT states that the system/species that can use as much as possible of the resources available (have the lowest amount of ‘wasted’ substrate) will win in a competition experiment (or will evolve) (de Mazancourt and Schwartz, 2010). In summary, MPP argues for maximizing substrate uptake rate, whereas RRT argues for optimizing the growth at low substrate concentrations. In a chemostat with fixed yield, the highest substrate uptake will correspond to the highest growth rate, and these two will be consistent. Thus, in evolution (or competition) in a chemostat, maximizing growth rate or maximizing substrate uptake rate may be advantageous strategies in order to survive.
In this work we are motivated by the observation of increased biomass in a division of labor system (Bernstein et al., 2012), which has important implications for industrial settings. We find that yield maximization should be the advantageous strategy for increasing the biomass production of a chemostat system. In all our work we consider the binary and monoculture systems in isolation, and investigate and compare the optimal productivity (biomass production) of these systems. We are not considering the evolution of these division of labor systems, as there is much work already investigating this and it is not the focus of our work. We are also not considering competition of these two systems, thus the arguments from RRT and MPP are not directly applicable to this situation. Some work has found that evolutionary advantage depends on the stress conditions, with some papers even finding that the ‘optimal’ robust state has lower biomass (Pfeiffer and Bonhoeffer, 2004). This is not unexpected as up-regulating pathways with higher substrate affinities is often more costly (decreased yield), but this enables survival at lower substrate concentrations and thus resistance to invasion.
For E. coli communities, the cross-feeding syntrophic chain system studied in Section 5 has been seen to evolve repeatedly (Rosenzweig et al., 1994; Rozen and Lenski, 2000; Treves et al., 1998). This suggests that there must be some increase in growth rate or substrate affinity following specialization in order for the system to out-compete the original monoculture. When synthetically engineered, this cross-feeding system has been found to have higher productivity (Bernstein et al., 2012). Our model shows that increases in growth rate or substrate affinity are not sufficient to explain this observed increase in productivity, and that an increase in the efficiency (yield) of the syntrophic system must have occurred.
An interesting contradiction that remains is that in the unmodified model ASb>Am for all parameters and growth functions, but in experiments the concentration of the inhibitory intermediate A is observed to be lower in a syntrophic chain system than in a monoculture. If we assume that an increase in efficiency when organisms specialize to a single substrate is sufficient to explain the observed increase in biomass, we find that there is no decrease in ASb in the binary culture when we increase the yield _Y_22 and only a small decrease when we increase the yield _Y_11. However, increasing the growth rates in the binary system does decrease ASb. This suggests that increases in both yield (increased efficiency) and growth kinetics play a role in metabolic specialization. By applying our model to experimental data, we will be able to investigate these factors in future work.
HIGHLIGHTS.
- We investigate the division of labor (metabolic function) in microbial systems.
- We compare a syntrophic consortia to a monoculture with equivalent metabolic capability.
- Consortia biomass is always lower than a monoculture with the same metabolic dynamics.
- Increasing the growth rate or substrate affinity does not explain the observed consortial advantage.
- Increased metabolic pathway efficiency (yield) provides the observed increase in productivity.
Appendix A. Binary culture equilibrium points existence and stability
In this appendix we prove Theorem 1.
A.1. Existence of equilibrium points
Equilibrium points in the binary system (9) must satisfy
| -A+f(G)I(A)x1-γm(A)x2=0, | (27) |
|---|
- A trivial equilibrium point, where _x_1 = 0, _x_2 = 0, must satisfy 1− G = 0 from Eq. (26) and − A = 0 from equation (27). This exists for all functions and parameter values at (_x_1, _x_2, G, A) = (0, 0, 1, 0).
- A boundary equilibrium point where only _x_1 survives (_x_1 ≠ 0) must satisfy:
f(G)I(A)-1=0fromEq.(24),1-G-f(G)I(A)x1=0fromEq.(26),-A+f(G)I(A)x1=0fromEq.(27).
A single boundary equilibrium point exists at
(x11,x21,G1,A1)=(1-G1,0,G1,1-G1),
where _G_1 is the solution to f (_G_1)I(1− _G_1) = 1. - Co-existence equilibrium points, where both _x_1 and _x_2 survive, are positive solutions (x1,x2,G,A)∈ℝ+4 that satisfy
f(G)I(A)-1=0fromEq.(24), (28) m(A)-1=0fromEq.(25), (29) ---------------------- ---- and Eqs. (26) and (27). The co-existence equilibria take the form 1-Gib,(1-Gib-Aib)/γ,Gib,Aib for i = S, U, where Aib are the solutions of m(A) = 1 (see Fig. A1(a)), and Gib are the solutions to f(G)I(Aib)=1 (see Fig. A1(b) and (c)).
Given Assumption [A5] in Section 2.3, Eq. (29) has two positive solutions ASb and AUb, where ASb<AUb (Fig. A1(a)).
For each of these Ab values, Eq. (28) has a unique positive solution for Gb, if and only if 1/I(Ab)<_μ_1,max (as shown in Fig. A1 (b) and (c)). Thus, we define the critical A value by I(Acritb)=1/μ1,max. For Ab<Acritb there is a unique positive Gb.
From Eqs. (26) and (27) the biomass at equilibrium is given by x1b=1-Gb and x2b=(1-Gb-Ab)/γ. Thus, the biomass of _x_2 is only positive if 1− Gb − Ab >0.
The co-existence equilibrium points are the intersections of the curves implicitly defined by f(G)I(A) = 1 and m(A) = 1. The equation m(A) = 1 defines two vertical lines A=ASb and A=AUb in the (A, G) plane. The slope of the curve h:={(A,G)∈ℝ+2∣f(G)I(A)=1} is given by
From assumptions [A2] and [A3] this gives dh/_dA_>0 for all (A, G). The curve has a vertical asymptote at Acritb. As A→Acritb, we find that f(G)→ μ_1,max, I(A)→1/μ_1,max, G_→∞, ∂_f/∂_G_→0, and ∂_I/∂_A<0. Combining this information, the numerator of dh/dA is positive and bounded away from zero, while the denominator of dh/dA goes to zero, thus dh/_dA_→∞ as A→Acritb from below. The assumptions [A1]–[A6] give six possible cases for the intersections of these curves (shown in Fig. A2). Their intersection is the projection of the co-existence equilibrium points to (A, G) plane.
- There are no co-existence equilibria in the following three cases:
- If Acritb<ASb, then 1/I(ASb)>μ1,max and there are no solutions to Eq. (29) for A=ASb or A=AUb. Thus, there are no co-existence equilibria.
- If ASb<Acritb<AUb and GSb+ASb>1. Then 1/I(ASb)<μ1,max and Eq. (29) with A=ASb has solution G=GSb, but 1/I(AUb)>μ1,max and there are no solutions to Eq. (29) with A=AUb. However, as 1-GSb-ASb<0,x2,Sb<0 and there are no (positive) co-existence equilibria.
- If AUb<Acritb and GSb+ASb>1. Then 1/I(ASb)<μ1,max and Eq. (29) with A=ASb has solution G=GSb, and 1/I(AUb)<μ1,max Eq. (29) with A=AUb has solution G=GUb. However, as 1-GSb-ASb<0,x2,Sb<0 and since GUb>GSb and AUb>ASb1-GUb-AUb<1-GSb-ASb<0 so x2,Ub<0, and there are no (positive) co-existence equilibria.
- There is a single co-existence equilibrium point in the following two cases:
- If ASb<Acritb<AUb, then 1/I(ASb)<μ1,max and Eq. (29) with A=ASb has solution G=GSb, but 1/I(AUb)>μ1,max and there are no solutions to Eq. (29) with A=AUb. If 1-GSb-ASb>0, then x1,Sb>0 and x2,Sb>0 and there is one co-existence equilibrium point S:=(x1,Sb,x2,Sb,GSb,ASb).
- If AUb<Acritb, then Eq. (29) with A=ASb and A=AUb has corresponding solutions G=GSb and G=GUb, where GSb<GUb (see Fig. A1(d)). If 1-GUb-AUb<0<1-GSb-ASb, then co-existence equilibrium point S:=(x1,Sb,x2,Sb,GSb,ASb) exists, but x2,Ub<0 so S is the only co-existence equilibrium point.
- There are two co-existence equilibria in the following case:
- If AUb<Acritb, then Eq. (29) with A=ASb and A=AUb has corresponding solutions G=GSb and G=GUb, where GSb<GUb (see Fig. A1(d)). If 1-GUb-AUb>0, then 1-GSb-ASb>0 and x1,Sb>0,x2,Sb>0,x1,Ub>0, and x2,Ub>0. Therefore there are two co-existence equilibrium point S:=(x1,Sb,x2,Sb,GSb,ASb) and U:=(x1,Ub,x2,Ub,GUb,AUb).
A.2. Stability of equilibrium points
We introduce the following variables:
| w2(t)=G(t)+γx2(t)+A(t). | (32) |
|---|
Note that the system obeys a conservation law of material consumed to biomass produced. The rate equations for these new variables are
which means that the set Ωb:={(x1,x2,G,A)∈ℝ+4:x1=1-G,γx2=1-G-A} is an exponentially attracting two-dimensional invariant subspace of the full ℝ4 phase space. The asymptotic behavior of the system is determined by the dynamics on Ωb, given by
| G.=1-G-f(G)I(A)x1(G), | (35) |
|---|
| A.=-A+f(G)I(A)x1(G)-γm(A)x2(G,A), | (36) |
|---|
with x_1(G) = 1 −_G, x_2(A, G) = (1 −_G −A)/γ. In this reduced system, we consider the region where _x_1 ≥0, _x_2 ≥0, given by G ≤1 − A in the positive quadrant. The Jacobian of this reduced system is
| J(G,A)=[-1+fI-∂f∂GIx1-f∂I∂Ax1-fI+∂f∂GIx1+m-1+f∂I∂Ax1-γ∂m∂Ax2+m], | (37) |
|---|
where x_1 = 1−_G, x_2 = (1−_G_−_A)/γ. The trivial equilibrium point (_x_1, _x_2, G, A) = (0, 0, 1, 0), I(0) = 1 and m(0) = 0 (from Assumptions [A3] and [A4]) and the Jacobian becomes
| J(G,A)=[-1+f(1)0-f(1)-1], | (38) |
|---|
which has eigenvalues λ = , −1, f (1) −1. From Assumption [A2] in Section 2.3 we know that 0<_f_ (1)< _μ_1,_max_. When _f_ (1)>1 the trivial equilibrium will have three negative eigenvalues (two from the components normal to Ωb and one from the 2D reduced subsystem) and one positive eigenvalue, thus it is an unstable saddle. The condition f (1)>1, which corresponds to f (Gin) >D in the original dimensions, is ensured by assumption [A6]: the trivial equilibrium point will be unstable as long as the substrate inflow is in excess.
The boundary equilibrium point (_fI_=1, _x_2 = 0) has the Jacobian
| J(G1,A1)=[-∂f∂GIx1-f∂I∂Ax1-1+∂f∂GIx1+m-1+f∂I∂Ax1+m]. | (39) |
|---|
From this we find
Tr(J)=-[(∂f∂GI-f∂I∂A)x1+(1-m)]
and
Det(J)=(1-m)[∂f∂GI-f∂I∂A]x1(G).
The boundary equilibrium point undergoes transcritical bifurcations when m(A) = 1, i.e., when A1=ASb and A1=AUb. For A1<ASb, _m_(_A_1)<1, Det (_J_)>0, Tr (J)<0, and (Tr2 −4 · Det)>0 and the equilibrium point is a stable node. For ASb<A1<AUb, _m_(_A_1)>1, Det (J)<0 and the equilibrium point is an unstable saddle. For AUb<A1, _m_(_A_1)<1, Det (_J_)>0, Tr (J)<0, and (Tr2 −4 · Det)>0 and the equilibrium point is a stable node again. The vector (A, G) = (1, −1) is a stable eigenvector which lies on the x_2 = 0 invariant line: G = 1−_A.
For the co-existence equilibria (_fI_=1, _m_=1) the Jacobian becomes
| J(G,A)=[-∂f∂GIx1-f∂I∂Ax1-∂f∂GIx1f∂I∂Ax1-γ∂m∂Ax2], | (40) |
|---|
with
Tr(J)=-[(∂f∂GI-f∂I∂A)x1(G)+γ∂m∂Ax2(G,A)]
and
Det(J)=∂f∂GIx1(G)γ∂m∂Ax2(G,A).
For (ASb,GSb) in the region _x_2 >0, Tr (J)<0 and Det (_J_)>0, and (Tr2 −4 · Det)>0 so the equilibrium point is a stable node. For (AUb,GUb) in the region _x_2 >0, Det (J)<0 and the equilibrium point is an unstable saddle. These equilibrium points go through transcritical bifurcations at _x_2 = 0 when they pass through the boundary equilibrium point. Details are shown in Fig. A2.
Fig. A1.
Qualitative sketches of the growth and inhibition curves and positions of equilibria for the case where there are two co-existence equilibrium points. Panel (a) shows the function m(A) showing Amax and the relative positions of ASb and AUb. Panel (b) shows the function I(A) with the relative positions of ASb and AUb. Panel (c) shows function f(G) and the relative positions of GSb and GUb. The position of G_0 defined by f(G) = 1 is shown as well. Panel (d) shows the curves f(G)I(A) = 1 (solid black curve) and m(A) = 1 (dashed black lines). The co-existence equilibrium points projected to (A, G) plane are at the two intersections of these curves. The dotted black line G = 1−_A (together with the solid black curve f (G)I(A) = 1) determines the position of the boundary (_x_2 = 0) equilibrium point projected into the (A, G) plane. The position of _G_0 defined by f(G) = 1 is shown as well.
Fig. A2.
Qualitative sketches of the six possible cases based on assumptions [A1]–[A4] in Section 2.3. Equilibrium points are marked with a circle for stable equilibria and a triangle for unstable equilibria. In the shaded gray region (G_>1−_A) any solutions are physically unrealistic as _x_2 <0. The trivial (_x_1 = 0, _x_2 = 0) equilibrium point at (0,1) is shown. For all cases, there is a boundary (_x_2 = 0) equilibrium point (_A_1, G_1) which, when projected into the (A, G) plane, lies at the intersection of the dotted black line G = 1−_A together with the solid black curve f (G)I(A) = 1. The curves f (G)I(A) = 1 (solid black curve) and m(A) = 1 (dashed black lines) are shown, the position of co-existence equilibrium points projected to (A, G) plane is at the intersections of these curves. The vertical asymptote of the curve f (G)I(A) = 1 is labeled Acritb. The position of _G_0 defined by f (G) = 1 is shown as well.
Appendix B. Monoculture equilibrium points
In this appendix we prove Theorem 2.
B.1. Existence
Given assumptions [A1]–[A6]
- The monoculture system (10) has a trivial equilibrium point with x_=0, which must satisfy 1−_G = 0 and −A = 0. This equilibrium point lies at (x, G, A) = (0, 1, 0) for all functions and parameters.
- Any non-trivial, _x_≠0, equilibrium points must satisfy
-A+(f(G)I(A)-γm(A))x=0, (43)
Eliminating f(G), I(A), and m(A) from Eqs. (41)–(43) yields the equation for equilibrium biomass
where Gm and Am lie in the intersection of the curves
| G1:={(x,G,A)∈ℝ+3:f(G)I(A)+m(A)=1}, | (45) |
|---|
and
| G2:={(x,G,A)∈ℝ+3:G=1-A1+γ-γ1-m(A)}. | (46) |
|---|
To determine the number of non-trivial equilibrium points, we must consider the shape and position of the curves _G_1 and _G_2 to find intersection points.
B.1.1. Shape and position of G1
The curve _G_1 has the following properties:
- The intersection of the curve _G_1 with _G_-axis (A = 0) satisfies f (G) = 1. Note that this equation has a unique solution since f is monotonically increasing and has _μ_1,max >1. We label this intersection _G_0.
- The intersection of the curve _G_1 with _A_-axis (G = 0) satisfies m(A) = 1, which has two solutions, ASb and AUb. We note for future reference that these values correspond to the equilibrium acetate concentration solutions for the binary system.
- Since DG(f(G)I(A)+m(A) −1)≠ 0 for all _G_>0, _A_>0 by the implicit function theorem, there is a scalar function h such that
and thus the points on _G_1 satisfy G = h(A). - Implicitly differentiating equation (45) we get an expression for the slope of this curve in (G,A) space:
∂∂A(f(h(A))I(A)+m(A)-1)=0∂(fI)∂h·dhdA+∂(fI)∂A+∂m∂A=0,
which we can rearrange to give the expression for the derivative of the function G = h(A)dhdA=-(∂(fI)∂A+∂m∂A)∂(fI)∂G. (47)
We now try to determine the sign of this derivative from Assumptions [A2]–[A4] in Section 2.3. First we note that
∂(fI)∂G>0forall(G,A),∂(fI)∂A<0forall(G,A),∂m∂A>0forA<Amax,∂m∂A<0forA>Amax.
The discussion that follows is illustrated in Fig. B1 (a).
- For the lower branch of the curve (A<_Amax_), (∂(_fI_)/∂_A_)<0 but (∂_m_/∂_A_)>0, so the sign of dh/dA is not determined by our assumptions. However, since the lower branch intersects _G_-axis in a point (0, _G_0) and the _A_-axis at (ASb, 0), the derivative dh/dA must become negative for some interval (A,ASb).
- For the upper branch of the curve (_A_>Amax) we know that dh/_dA_>0 and that the curve intersects the _A_-axis at A=AUb. As A increases, f (G)I(A)→0 and m(A)→0. At a critical A value, implicitly defined by _μ_1,maxI(A)+m(A) = 1, the curve reaches a vertical asymptote, Acritm, and for Acritm<A there is no solution to f (G)I(A)+m(A) = 1.
B.1.2. Shape and position of G2
We rewrite Eq. (46) as G_2 = 1 −_H(A), where
We summarize our observations about curve _G_2 = _G_2(A).
- When _A_=0, H(A) = 0 and so the curve _G_2 intersects the _G_-axis at _G_=1.
- The intersection of the curve G_2 = 1 −_H(A) with A_-axis satisfies H(A) = 1. This solution, which we denote At satisfies 1+γ − γ/(1 −_m(At)) = At, or rewritten, m(At) = (1 − γ)/(1+γ_−_At). This equation always has one solution, in the region 0<At<ASb, and in some cases can have two more solutions for high AUb<At<1, as shown in Fig. B3. The intersections with the _A_-axis will be ordered by increasing A with subscripts A1t and A2t,A3t (if applicable).
- The curve G_2 = 1 −_H(A) has vertical asymptotes when 1+ γ − γ/(1 −m(A)) = 0, which correspond to the value A satisfying 1+ γ = γ/(1 −m(A)). There is one asymptote in the region A<ASb(m(A)<1), which follows from the fact that the left hand side is bounded and the right hand side grows without bound as A→ASb, since m(ASb)=1 by definition. In the region A>AUb(m(A)<1), there is one asymptote, as the left hand side is bounded and the right hand side grows without bound as A→AUb from above, since m(AUb)=1 by definition. We denote the asymptotic values of A by A1v and A2v, which satisfy m(Aiv)=1/(1+γ), shown in Fig. B3.
- For A1v and A1t, which are both in the region A<ASb we wish to determine their relative positions. Consider equations 1+ γ_ = γ/(1 −m(Av)) and 1+ γ = At + γ/(1 −m(At)). It follows that
and since 0<_At_ <1 this means that
In the region A<ASb the function _γ_/(1−_m_(_A_)) is increasing as _A_ increases, thus A1t<A1v. In the region A>AUb the function γ/(1 −_m(A)) is now decreasing as A increases, thus (if A2t and A3t exist) A2v<A2t<A3t. - The slope of the curve is given by
dG2dA=-dH(A)dA=-1·(1+γ-γ1-m(A))-AddA(-γ1-m(A))(1+γ-γ1-m(A))2=-1·(1+γ-γ1-m(A))+dm(A)dAγA(1-m(A))2(1+γ-γ1-m(A))2 (48) For 0<A<A1v, 1 + _γ_ − _γ_/(1 −_m_(_A_))>0 and dm(A)/_dA_>0, thus _dG_2/dA <0. - The function H(A) has a removable singularity at ASb and AUb, with the limit value at these points lim_A_→A S,A U H(A) = 0. Thus, _G_2 = 1 at ASb and AUb.
- For A1v<A<ASb the slope of G_2 is negative, and for AUb<A<A2v the slope of _G_2 is positive. As _A_ approaches ASb and AUb, 1−_m_(_A_)≪1 and the second term in the numerator of (48) dominates. Near ASb:dm(A)/dA>0 and 1+ γ − γ/(1−_m(A))<0, therefore dG_2/dA <0. Near AUb:dm(A)/dA<0, 1+γ − γ/(1−_m(A))<0, and thus _dG_2/dA <0. As A increases from A=AUb, _dG_2/dA remains positive, and m(A) decreases until the curve _G_2 reaches an asymptote at A2v.
We summarize this information in Fig. B1(b) which shows a qualitative sketch of the curve _G_2.
B.1.3. Intersections of _G_1 and _G_2
Equilibrium points are at intersections between the curves _G_1 (45) and _G_2 (46). The curve _G_1 is in the region G >0 for A<ASb and AUb<A<Acritm. We will call the region A<ASb the lower region and AUb<A the upper region.
For A1v<A<ASb and AUb<A<A2v, curve _G_2 is above _G_ = 1, thus there can be no intersections with curve _G_1 in physically relevant (_x>_0) regions of phase space.
Intersections between the curves can only occur in the region A<A1v and the region A2v<A<1.
B.1.3.1. Intersections in the lower region
The lower branch of _G_1 (see Eq. 45) is a continuous curve connecting the point (0, _G_0) and the point (AS, b, 0), while _G_2 is a continuous curve that connects (0, 1) and (At, 0). Since 1>G_0 to A1t<ASb, thus these curves must intersect at 2_n+1 points, where _n_≥0. These correspond to equilibria of the system and we can order them based on their A value by _E_1, E_2, …_E_2_n + 1. In particular, there is at least one non-trivial equilibrium point between 0<A<A1t and 0<G<max(G1(A∈(0,A1t))).
B.1.3.2. Intersections in the upper region
The upper branch of _G_1 is a smooth curve that increases from _G_=0 at AUb until it reaches an asymptote at A defined by μ_1,maxI(A)+m(A) = 1. If the curve 1− γ/(1+ γ −_A) intersects m(A) in the upper region, the curve _G_2 will cross the _A_-axis to lie in the _G_>0 region for A2t<A<A3t, where AUb<A2v<A2t<A3t<1. In this case it is possible for the upper branch of the curve _G_2 to intersect G_1, thus creating equilibrium points. If these curves do intersect in the upper region, these new upper equilibria, Kj for j = 1…2_m, would appear in pairs at saddle-node bifurcations.
Fig. B2 shows the situation when there is an upper branch of _G_2, but it does not intersect _G_1. For there to be equilibrium points in the upper region the upper branch of the curve _G_2 must cross the _A_-axis, and it also must reach high enough G values to intersect with the curve _G_1 before _G_1 reaches its asymptote at Acritm.
As was mentioned in the introduction we do not consider these equilibria to represent biologically plausible state of the monoculture. The high stable equilibria only exist in specific circumstances when the acetate growth curve m(A) decreases rapidly for high acetate levels, while the lower stable equilibrium exists for all parameter values. The high stable equilibria, when they exist, have a small basin of attraction, which is characterized by high levels of acetate and low levels of glucose. So only initial conditions with high initial acetate and low glucose can lead to the monoculture that is positioned at such equilibrium. Below we provide some sufficient conditions under which the curves _G_1 and _G_2 do not intersect in the upper region and thus there are no equilibria in this region.
- We expect that the acetate consumer in the binary culture is better adapted to growth on acetate that the monoculture for large value of A. Our fist condition states that the maximal growth rate of monoculture is not greater than a multiple of the growth rate of acetate consumer
μ1,maxI(A2v)<γm(A2v) (49) where the inequality is evaluated at A2v defined implicitly by m(A2v)=1/(1+γ). This assumption is summarized in Fig. 3. Monotonicity implies that the curve _μ_1,maxI(A) is in the shaded region for A>A2v. The condition (49) comes from the observation that if I(A2v)<γ/(μ1,max(1+γ)), then Acritm<A2v and the curve _G_1 reaches its asymptote before _G_2 can enter the region _G_ >0. Therefore there can be no upper equilibria. - The second condition which rules out the upper equilibria asks that the function m(A) does not decrease too rapidly for large values of A. Note that if the curve _G_2 does not cross the _A_-axis into the _G_>0 region for upper A values, there can be no upper equilibria. This is equivalent to situation a condition that m(A) = 1 − γ/(1+ γ − A) has no solutions A2t and A3t, see Fig. B3. Finally, observe if
then 1<A2v which implies that _m_(1 − _γ_/(1+_γ_ − _A_) has only one solution A1t. The condition (50) is equivalent to
m(GinY21Y11)D>Y21Y21+Y22.
in terms of the original parameters and variables.
B.2. Stability of equilibrium points
We define a new variable in the monoculture system
z(t)=γx(t)+(1+γ)G(t)+A(t).
It is each to show that
which means that the plane
is an attracting (exponentially at a rate of e_−_t) invariant subspace of the phase space ℝ+3.
The long term behavior of the system is determined by the dynamics on this attracting plane, which is given by
| dAdt=-A+[f(G)I(A)-γm(A)]x(A,G) | (51) |
|---|
| dGdt=1-G-f(G)I(A)x(A,G) | (52) |
|---|
| x(A,G)=(1+1γ)(1-G)-Aγ | (53) |
|---|
This system has the Jacobian
J(A,G)=[-1+(f∂I∂A-γ∂m∂A)x-1γ(fI-γm)∂f∂GIx-(1+1γ)(fI-γm)-f∂I∂Ax+1γfI-1-∂f∂GIx+(1+1γ)fI].
For any 2D system, Tr(J) = _λ_1 + _λ_2 and Det(J) = _λ_1 · _λ_2. The conditions Det(J)>0 and Tr(J)<0 are necessary and sufficient conditions to show that an equilibrium point is stable. The condition Det(J)<0 is necessary and sufficient to show that an equilibrium point is an unstable saddle.
At the trivial equilibrium point, (A, G) = (0, 1) where _x_=0, I(0) = 1, and m(0) = 0, the Jacobian becomes
J(0,1)=[-1-(1γ)f(1)-(1+1γ)f(1)(1γ)f(1)-1+(1+1γ)f(1)].
The characteristic equation: λ_2 + λ (2−_f (1))+(1 −f(1)) has roots _λ_1= −1<0, _λ_2 = _f_(1)−1. We know that _f_(1)≈_μ_1,max >1 from assumption [A6], so _λ_2 >0, which means that (0, 1) is a saddle, with two negative (stable) eigendirections (one from the reduction to the 2D reduced system and the other from the dynamics of reduced flow) and one unstable eigendirection.
The corresponding eigenvectors are
An important observation to make here is that the stable manifold of the trivial equilibrium point in reduced system is an invariant line G = 1 −A/(1+ γ) which corresponds to _x_=0.
Now we analyze the stability of the non-trivial equilibria Ei and Kj. At these equilibria we have fI + m = 1, (fI − γm)x = A, and fIx = 1 −G, with x(A, G) = (1+1/γ)(1 −G) −A/γ. The Jacobian becomes
J(Am,Gm)=[(f∂I∂A-γ∂m∂A)x-(1+1γ)fI∂f∂GIx+(1+γ)(1-(1+1γ)fI)-f∂I∂Ax+1γfI-1-∂f∂GIx+(1+1γ)fI].
The determinant of this Jacobian can (after lengthy rearrangement) be reduced to:
Det(J(Am,Gm))=c0=∂f∂GI(∂m∂Aγx2+fIx)-A(∂m∂A+f∂I∂A).
And the trace of the Jacobian is
Tr(J(Am,Gm))=-c1=-1-x(∂f∂GI-f∂I∂A+γ∂m∂A).
To determine the sign of _c_0 and thus the stability of any monoculture equilibrium point, we consider the relative slopes of the curves _G_1 and G_2 and their intersections in the region A<ASb. We will show that the stability is determined by the relative slope of the curves _G_1 and _G_2. Note that at any odd lower equilibrium point _Ei_, _i_ = 1, 3, 5, …, 2_n_+1 and at any even upper equilibrium point _Kj_, _j_ = 2, 4, …2_m_, we have ∂_G_2/∂_A_< ∂_G_1/∂_A_, whereas at any even lower equilibrium points _Ei_, _i_ = 2, 4, 6, …, 2_n_, and any odd upper equilibrium points _Kj_, _j_ = 1, 3, …2_m_−1, we have ∂_G_2/∂_A_> ∂_G_1/∂_A.
Taking the expressions for the slopes from Eqs. (47) and (48), with some rearranging we can show that at non-trivial equilibria
and
thus
| c0=∂f∂GI(∂m∂Aγx2+fIx+AdG1dA) | (54) |
|---|
| =A∂f∂GI(dG1dA-dG2dA) | (55) |
|---|
- At even lower equilibrium points and odd upper equilibrium points, _dG_2/dA > _dG_1/dA, _c_0 <0 and the equilibrium point is an unstable saddle.
- At points where the curves _G_1 and _G_2 are tangent, _c_0 = 0 and bifurcations occur.
- At any odd lower equilibrium point and at any even upper equilibrium point _dG_1/dA >_dG_2/dA and _c_0 >0. The equilibrium point in the 2D system is a stable or unstable node, depending on the sign of Tr(J(Am, Gm)), which would correspond to a stable node or an unstable saddle in the full system.
For lower even equilibrium points, Tr(J(Am, Gm))<0 and _c_0 >0, so the equilibrium point is a stable node. We can show that these stable equilibria do not undergo Hopf bifurcations (where Re(λ) = 0) since Tr(J(Am, Gm)) = _λ_1+ _λ_2 ≠0.
For upper odd equilibrium points, c_0 >0 but ∂_m/∂A<, so the stability depends on the sign of Tr(_J_(_Am_, _Gm_). If Tr(_J_(_Am_, _Gm_))<0, then the equilibrium point in the 2D system is a stable node, which corresponds to a stable node in the full system, however, if Tr(_J_(_Am_, _Gm_))>0, then the equilibrium point in the 2D system is an unstable node, which corresponds to an unstable saddle in the full system.
Fig. B1.
(a) Qualitative sketch of the curve _G_1 implicitly defined by (45). (b) Qualitative sketch of the curve _G_2 defined by (46).
Fig. B2.
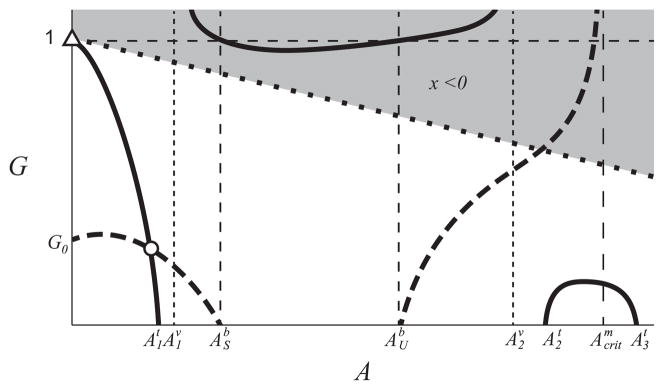
Qualitative sketch of the curves _G_1 (dashed black curve) and _G_2 (solid black curve). The position of non-trivial equilibrium points projected to (A, G) plane is at the intersections of these curves. Equilibria are marked with a circle for a stable equilibrium point and a triangle for an unstable saddle. The trivial (_x_=0) equilibrium point at (0,1) is shown. In the shaded gray region (G_>1−_A/(1+γ)) any solutions are physically unrealistic as x<0. The vertical asymptote of the curve f(G)I(A)+m(A) = 1 is labeled Acritm.
Fig. B3.
Qualitative sketch of the curves m(A) (solid black curve) and 1 − γ/(1+ γ −A) (dot-dashed black curve). The case with only one intersection A1t is shown in panel (a). In panel (b) the case with three intersections is shown. The A1v and A2v values which are solutions to m(A) = 1/(1+ γ) are also shown.
Appendix C. Biomass of monoculture and the binary system
In this section we will prove Theorem 3.
C.1. Comparison of binary culture and monoculture biomass
It can be shown that the biomass x¯1=x11+x21=x11+0 for the boundary equilibrium point in the binary system (Eqs. (9)) is always less than the total biomass of the stable co-existence equilibrium point, S: Since x_1 = 1 −_G at both equilibria for the binary system, and Gib<G1, where Gib is the equilibrium glucose concentration for the co-existence equilibria defined below. Thus x¯1<x1,ib<x¯ib. In order to compare the maximum biomass in the two systems, we must compare the stable co-existence equilibrium point (_x_1 ≠0, _x_2 ≠0) for the binary system with the nontrivial equilibria _E_1, …, E_2_n+1 with x ≠0 for the monoculture.
The biomass of the monoculture at equilibrium is given by
| xm=(1+1γ)(1-Gim)-Aimγ. | (C.1) |
|---|
The total biomass of the binary system at its stable equilibrium point x¯Sb=x1,Sb+x2,Sb:
| x¯Sb=(1+1γ)(1-GSb)-ASbγ. | (C.2) |
|---|
As shown in Appendix B, Aim<At<ASb for all monoculture equilibria in the region (on the lower A<AbS branch).
The slope of curve implicitly defined by f(G)I(A) = 1, on which GSb lies, is given by Eq. (30). The curve _G_1, on which Gim lies, has slope given by Eq. (47). Both curves intersect the _G_-axis at G = _G_0. The slope for the binary culture curve (Eq. (30)) is always larger than the slope of the monoculture curve (Eq. (47)) in the region A<_A_max, as ∂_m_/∂_A_>0. Thus, in the region A<At, the monoculture curve _G_1 lies below the binary culture curve implicitly defined by f(G)I(A) = 1 and Gim<GSb.
As Aim<ASb and Gim<GSb, from Eqs. (C.1) and (C.2) we know that xm>x¯Sb. In other words, given these assumptions, the monoculture will always have greater biomass than the binary culture (division of labor system).
Appendix D. Experimental values for E. coli growth
Table D1 details the experimental conditions and strains used for the parameters listed in Table 1. When the reported constants were obtained by fitting data to a specific model, we report the equivalent observable values. The maximum growth rates are the maximum values of the growth function on that substrate, assuming zero inhibitory products. The half-saturation values are the substrate concentration at which the growth has reached half its maximum value. The acetate product inhibition constant we define as the acetate concentration at which the growth rate has decreased to half its maximum value, in excess glucose (G » KG). The acetate substrate inhibition constant we define as the acetate concentration at which the growth rate has decreased to half its maximum value.
Table D1.
Typical parameter values for E. coli growth.
| Source | Strain | Experimental conditions | Parameter | Value |
|---|---|---|---|---|
| Bernstein et al. (2012) | E. coli MG1655 WT | Aerobic batch growth, 37 °C, M9 minimal media, acetate consumption deletion | Biomass yield (CDW[g]/g of glucose) | 0.23 |
| mutant 307 | Maximum growth rate on 4 g/L glucose (h−1) | 0.43–0.49a | ||
| mutant 403 | glucose consumption deletion | Maximum growth rate on 1.5 g/L acetate (h−1) | 0.11–0.15a | |
| Chen et al. (1997) | E. coli K-12 MG1655 | Aerobic batch growth, complex medium, 37 °C | Maximum growth rate on glucose (h−1) | 0.57 |
| Biomass yield (CDW[g]/g of glucose) | 0.255 | |||
| Chen et al. (1997) | E. coli K-12 MG1655 | Anaerobic batch growth, complex medium, 37 °C | Maximum growth rate on glucose (h−1) | 0.43 |
| Biomass yield (CDW[g]/g of glucose) | 0.175 | |||
| Dykhuizen (1978) | E. coli K-12 W3110 | Aerobic batch growth, Davis salts, 37 °C | Maximum growth rate on glucose (h−1) | 0.76 |
| Dykhuizen (1978) | E. coli K-12 W3110 | Chemostat growth, Davis salts, 37 °C | Glucose half-saturation constant (mg/L) | 7.2 |
| Edwards et al. (2001) | E. coli K-12 MG1655 | Aerobic batch growth, M9 minimal media 27.5–37 °C, 0.05–4 g/L acetate | Maximum growth rate on acetate (h−1) | 0.31 |
| Biomass yield (CDW[g]/g of acetate) | 0.38 | |||
| Farmer and Liao (1997) | E coli K-12 VJS632 | Aerobic batch growth, 37 °C, M9 minimal media, 0.5% glucose | Maximum growth rate on glucose(h−1) | 0.52 |
| Biomass yield (CDW[g]/g of glucose) | 0.30 | |||
| Acetate yield (g of acetate/g of glucose) | 0.25 | |||
| Review paper | E. coli various | various | Glucose half-saturation constant (mg/L) | 0.04–180 |
| Fuhrer et al. (2005) | E. coli K-12 MG1655 | Aerobic batch growth, 30 °C, M9 minimal media, 3 g/L glucose | Maximum growth rate on glucose (h−1) | 0.39 |
| Biomass yield (CDW[g]/g of glucose) | 0.30 | |||
| Guardia and Calvo (2001) | E. coli ATCC 8789 | Aerobic batch and chemostat growth, 37 °C, complex medium including 5 g/L yeast extract, varying 1–19 g/L glucose, parameter fits to model | Maximum growth rate on glucose (h−1) | 1.12 |
| Glucose half-saturation constant (mg/L) | 100 | |||
| Acetate product inhibition constant (g/L) | 4 | |||
| Maximum growth rate on acetate (h−1) | 0.19 | |||
| Acetate half-saturation constant (g/L) | 0.4 | |||
| Acetate substrate inhibition constant (g/L) | 3.8 | |||
| Acetate yield (g acetate produced/CDW[g]) | 0.4 | |||
| Kleman and Strohl (1994) | E. coli K-12 W3110 | Aerobic fed-batch with target 0.5 g/L glucose, mineral medium, 35 °C | Maximum growth rate on glucose (h−1) | 0.91 |
| Glucose half-saturation constant (mg/L) | 4 | |||
| Biomass yield (CDW[g]/g of glucose) | 0.43 | |||
| Acetate yield (g of acetate/g of glucose) | 0.38 | |||
| Acetate yield (g acetate produced/CDW[g]) | 0.3–0.9 | |||
| Natarajan and Srienc (2000) | E. coli BL21 | Chemostat, 30 °C, dilution rate 0.12–0.40 h−1 inflow: M9 minimal media, 0.25 g/L glucose | Maximum growth rate on glucose (h−1) | 0.768 |
| Glucose half-saturation constant (mg/L) | 0.68 | |||
| Oh et al. (2002) | E. coli MC4100 | Aerobic batch growth, M9 minimal media | Maximum growth rate on 0.25% acetate (h−1) | 0.33 |
| Paalme et al. (1997) | E. coli K-12 W3350 | A-stat, 37 °C, mineral medium, 10 g/L glucose | Maximum growth rate on glucose (h−1) | 0.57 |
| Biomass yield (CDW[g]/g of glucose) | 0.50 | |||
| Paalme et al. (1997) | E. coli K-12 W3350 | A-stat, 37 °C, mineral medium, 5 g/L acetate | Maximum growth rate on acetate (h−1) | 0.22 |
| Biomass yield (CDW[g]/g of acetate) max/min | 0.30/0.21 | |||
| Senn et al. (1994) | E. coli ML30 | Chemostat growth, mineral medium, 37 °C | Maximum growth rate on glucose (h−1) | 0.92 |
| Glucose half-saturation constant (mg/L) | 0.0072 | |||
| Literature review Senn et al. (1994) | E. coli various | various | Maximum growth rate on glucose (h−1) | 0.55–1.23 |
| Glucose half-saturation constant (mg/L) | 0.0053–99 | |||
| Varma and Palsson (1994) | E. coli K-12 W3110 | Aerobic batch growth, 38 °C, M9 minimal media, 2 g/L glucose | Maximum growth rate on glucose (h−1) | 0.68 |
| Biomass yield (CDW[g]/g of glucose) | 0.36 | |||
| Varma and Palsson (1994) | E. coli K-12 W3110 | Anaerobic batch growth, 38 °C, M9 minimal media, 2 g/L glucose | Maximum growth rate on glucose (h−1) | 0.43 |
| Biomass yield (CDW[g]/g of glucose) | 0.13 | |||
| Xu et al. (1999) | E. coli K-12 W3110 | Aerobic batch growth, 35 °C, mineral medium, 4–14 g/L glucose | Maximum growth rate on glucose (h−1) | 0.55 |
| Biomass yield (CDW[g]/g of glucose) | 0.52 | |||
| Maximum growth rate on acetate (h−1) | 0.05 | |||
| Xu et al. (1999) | E. coli K-12 W3110 | Aerobic batch growth, 35 °C, mineral medium, 5 g/L glucose, 0–8 g/L acetate | Acetate product inhibition constant (g/L) | 9 |
| Biomass yield (CDW[g]/g of acetate) | 0.4 | |||
| Xu et al. (1999) | E. coli K-12 W3110 | Aerobic fed-batch (exponential feeding rate) with target μ = 0:3 h−1, mineral medium, 35 °C | Maximum growth rate on glucose (h−1) | 0.54 |
| Glucose half-saturation constant (mg/L) | 50 | |||
| Acetate product inhibition constant (g/L) | 4, 5 | |||
| Aerobic biomass yield (CDW[g]/g of glucose) | 0.49, 0.51 | |||
| Anaerobic biomass yield (CDW[g]/g of glucose) | 0.15 | |||
| Maximum growth rate on acetate (h−1) | 0.06, 0.08 | |||
| Acetate half-saturation constant (g/L) | 0.05 | |||
| Biomass yield (CDW[g]/g of acetate) | 0.4 |
Footnotes
Uncited reference
Contributor Information
Emily Harvey, Email: e.p.harvey@massey.ac.nz.
Jeffrey Heys, Email: jeffrey.heys@coe.montana.edu.
TomáS̆ Gedeon, Email: gedeon@math.montana.edu.
References
- Abrams P. The functional responses of adaptive consumers of two resources. Theor Popul Biol. 1987;32:262–288. [Google Scholar]
- Aota Y, Nakajima H. Mutualistic relationships between phytoplankton and bacteria caused by carbon excretion from phytoplankton. Ecol Res. 2001;16:289–299. [Google Scholar]
- Bernstein H, Paulson S, Carlson R. Synthetic Escherichia coli consortia engineered for syntrophy demonstrate enhanced biomass productivity. J Biotechnol. 2012;157:159–166. doi: 10.1016/j.jbiotec.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TDK, Jones-Mortimer MC, Kornberg HL. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977;102:327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- Burchard A. Substrate degradation by a mutualistic association of two species in the chemostat. J Math Biol. 1994;32:465–489. [Google Scholar]
- Carlson R, Srienc F. Fundamental Escherichia coli biochemical pathways for biomass and energy production: creation of overall flux states. Biotechnol Bioeng. 2004;85:149–162. doi: 10.1002/bit.20044. [DOI] [PubMed] [Google Scholar]
- Chen R, Yap W, Postma P, Bailey J. Comparative studies of Escherichia coli strains using different glucose uptake systems: metabolism and energetics. Biotechnol Bioeng. 1997;56:583–590. doi: 10.1002/(SICI)1097-0290(19971205)56:5<583::AID-BIT12>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Costa E, Perez J, Kreft J. Why is metabolic labour divided in nitrification? TRENDS Microbiol. 2006;14:213–219. doi: 10.1016/j.tim.2006.03.006. [DOI] [PubMed] [Google Scholar]
- DeLong J. The maximum power principle predicts the outcomes of two-species competition experiments. Oikos. 2008;117:1329–1336. [Google Scholar]
- Doebeli M. A model for the evolutionary dynamics of cross-feeding polymorphisms in microorganisms. Popul Ecol. 2002;44:59–70. [Google Scholar]
- Dykhuizen D. Selection for tryptophan auxotrophs of Escherichia coli in glucose-limited chemostats as a test of the energy conservation hypothesis of evolution. Evolution. 1978;32:125–150. doi: 10.1111/j.1558-5646.1978.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Edwards JS, Ibarra RU, Palsson BO. In silico predictions of Escherichia coli metabolic capabilities are consistent with experimental data. Nat Biotechnol. 2001;19:125–130. doi: 10.1038/84379. [DOI] [PubMed] [Google Scholar]
- Egli T, Lendenmann U, Snozzi M. Kinetics of microbial growth with mixtures of carbon sources. Antonie van Leeuwenhoek. 1993;63:289–298. doi: 10.1007/BF00871224. [DOI] [PubMed] [Google Scholar]
- Eiteman M, Lee S, Altman E. A co-fermentation strategy to consume sugar mixtures effectively. J Biol Eng. 2008;2:3. doi: 10.1186/1754-1611-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhader A. Global stability in a synthrophic chain model. Math Biosci. 1991;104:203–245. doi: 10.1016/0025-5564(91)90062-n. [DOI] [PubMed] [Google Scholar]
- Estrela S, Gudejl I. Evolution of cooperative cross-feeding could be less challenging than originally thought. PLoS One. 2010;5:e14121. doi: 10.1371/journal.pone.0014121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer WR, Liao JC. Reduction of aerobic acetate production by Escherichia coli. Appl Environ Microbiol. 1997;63:3205–3210. doi: 10.1128/aem.63.8.3205-3210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T. Adaptation to life at micromolar nutrient levels: the regulation of Escherichia coli glucose transport by endoinduction and cAMP. FEMS Microbiol Rev. 1996;18:301–317. doi: 10.1111/j.1574-6976.1996.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Fierer N, Jackson R. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer T, Fischer E, Sauer U. Experimental identification and quantification of glucose metabolism in seven bacterial species. J Bacteriol. 2005;187:1581–1590. doi: 10.1128/JB.187.5.1581-1590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S, Hillis S, Heilmann K, Segre J, Grice E. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62:923–930. doi: 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Pop M, DeBoy R, Eckburg P, Turnbaugh P, Samuel B, Gorden J, Relman D, Fraser-Liggett C, Nelson K. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia MJ, Calvo EG. Modeling of Escherichia coli growth and acetate formation under different operational conditions. Enzyme Microbial Technol. 2001;29:449–455. [Google Scholar]
- Han K, Levenspiel O. Extended Monod kinetics for substrate, product, and cell inhibition. Biotechnol Bioeng. 1988;32:430–447. doi: 10.1002/bit.260320404. [DOI] [PubMed] [Google Scholar]
- Han K, Lim H, Hong J. Acetic acid formation in Escherichia coli fermentation. Biotechnol Bioeng. 1992;39:663–671. doi: 10.1002/bit.260390611. [DOI] [PubMed] [Google Scholar]
- Hardin G. The competitive exclusion principle. Science. 1960;131:1292–1297. doi: 10.1126/science.131.3409.1292. [DOI] [PubMed] [Google Scholar]
- Helling RB, Vargas CN, Adams J. Evolution of Escherichia coli during growth in a constant environment. Genetics. 1987;116:349–358. doi: 10.1093/genetics/116.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra RU, Edwards J, Palsson B. Escherichia coli K-12 undergoes adaptive evolution to achieve in silico predicted optimal growth. Nature. 2002;420:186–189. doi: 10.1038/nature01149. [DOI] [PubMed] [Google Scholar]
- James G, Swogger E, Wolcott R, deLancey Pulcini E, Secor P, Sestrich J, Costerton J, Stewart P. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Johnson D, Goldschmidt F, Lilja E, Ackermann M. Metabolic specialization and the assembly of microbial communities. ISME J. 2012:1–7. doi: 10.1038/ismej.2012.46. http://dx.doi.org/10.1038/ismej.2012.46. [DOI] [PMC free article] [PubMed]
- Katsuyama C, Nakaoka S, Takeuchi Y, Tago K, Hayatsu M, Kato K. Complementary cooperation between two syntrophic bacteria in pesticide degradation. J Theor Biol. 2009;256:644–654. doi: 10.1016/j.jtbi.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Kleman GL, Strohl WR. Acetate metabolism by Escherichia coli in high-cell-density fermentation. Appl Environ Microbiol. 1994;60:3952–3958. doi: 10.1128/aem.60.11.3952-3958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreikenbohm R, Bohl E. A mathematical model of syntrophic cocultures in the chemostat. FEMS Microbiol Ecol. 1986;38:131–140. [Google Scholar]
- Kumari S, Tichel R, Eisenbach M, Wolfe AJ. Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol. 1995;177:2878–2886. doi: 10.1128/jb.177.10.2878-2886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendenmann U, Egli T. Kinetic models for the growth of Escherichia coli with mixtures of sugars under carbon-limited conditions. Biotechnol Bioeng. 1922;59:99–107. doi: 10.1002/(sici)1097-0290(19980705)59:1<99::aid-bit13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lotka A. Contribution to the energetics of evolution. Proc Natl Acad Sci. 1922;8:147–151. doi: 10.1073/pnas.8.6.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur R, Levins R. Competition, habitat selection, and character displacement in a patchy environment. Proc Natl Acad Sci. 1964;51:1207–1210. doi: 10.1073/pnas.51.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mazancourt C, Schwartz MW. A resource ratio theory of cooperation. Ecol Lett. 2010;13:349–359. doi: 10.1111/j.1461-0248.2009.01431.x. [DOI] [PubMed] [Google Scholar]
- Miller T, Burns J, Munguia P, Walters E, Kneitel J, Richards P, Mouquet N, Buckley H. A critical review of twenty years’ use of the resource-ratio theory. Am Nat. 2005;165:439–448. doi: 10.1086/428681. [DOI] [PubMed] [Google Scholar]
- Natarajan A, Srienc F. Glucose uptake rates of single Escherichia coli cells grown in glucose-limited chemostat cultures. J Microbiol Methods. 2000;42:87–96. doi: 10.1016/s0167-7012(00)00180-9. [DOI] [PubMed] [Google Scholar]
- Nicolaou SA, Gaida SM, Papoutsakis ET. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: from biofuels and chemicals, to biocatalysis and bioremediation. Metab Eng. 2010;12:307–331. doi: 10.1016/j.ymben.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Oh M, Rohlin L, Kao K, Liao J. Global expression profiling of acetate-grown Escherichia coli. J Biol Chem. 2002;277:13175–13183. doi: 10.1074/jbc.M110809200. [DOI] [PubMed] [Google Scholar]
- Paalme T, Elken R, Kahru A, Vanatalu K, Vilu R. The growth rate control in Escherichia coli at near to maximum growth rates: the A-stat approach. Antonie van Leeuwenhoek. 1997;71:217–230. doi: 10.1023/a:1000198404007. [DOI] [PubMed] [Google Scholar]
- Peralta-Yahya P, Zhang F, del Cardayre S, Keasling J. Microbial engineering for the production of advanced biofuels. Nature. 2012;488:320–328. doi: 10.1038/nature11478. [DOI] [PubMed] [Google Scholar]
- Pfeiffer T, Bonhoeffer S. Evolution of cross-feeding in microbial populations. Am Nat. 2004;163:E126–E135. doi: 10.1086/383593. [DOI] [PubMed] [Google Scholar]
- Powell G. Stable coexistence of syntrophic associations in continuous culture. J Chem Technol Biotechnol. 1985;35B:46–50. [Google Scholar]
- Powell G. Stable coexistence of synthrophic chains in continuous culture. Theor Popul Biol. 1986;30:17–25. [Google Scholar]
- Reilly P. Stability of commensalistic systems. Biotechnol Bioeng. 1974;16:1373–1392. doi: 10.1002/bit.260161006. [DOI] [PubMed] [Google Scholar]
- Rescigno A, Richardson I. On the competitive exclusion principle. Bull Math Biol. 1965;27:85–89. doi: 10.1007/BF02477264. [DOI] [PubMed] [Google Scholar]
- Rosenzweig F, Sharp R, Treves D, Adams J. Microbial evolution in a simple unstructured environment: genetic differentiation in Escherichia coli. Genetics. 1994;137:903–917. doi: 10.1093/genetics/137.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen D, Lenski R. Long-term experimental evolution in Escherichia coli. VIII: dynamics of a balanced polymorphism. Am Nat. 2000;155:24–35. doi: 10.1086/303299. [DOI] [PubMed] [Google Scholar]
- Sari T, El Hajji M, Harmand J. The mathematical analysis of a syntrophic relationship between two microbial species in a chemostat. Math Biosci Eng. 2012;9:627–645. doi: 10.3934/mbe.2012.9.627. [DOI] [PubMed] [Google Scholar]
- Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B. Synergistic interactions in the microbial world. Antonie van Leeuwenhoek. 2002;81:257–261. doi: 10.1023/a:1020579004534. [DOI] [PubMed] [Google Scholar]
- Schuster S, Pfeiffer T, Fell D. Is maximization of molar yield in metabolic networks favoured by evolution? J Theor Biol. 2008;252:497–504. doi: 10.1016/j.jtbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Sciubba E. What did Lotka really say? A critical reassessment of the “maximum power principle”. Ecol Model. 2011;222:1347–1353. [Google Scholar]
- Seitz H, Schink B, Pfennig N, Conrad R. Energetics of syntrophic ethanol oxidation in defined chemostat cocultures: 1. Energy requirement for H2 production and H2 oxidation. Arch Microbiol. 1990a;155:82–88. [Google Scholar]
- Seitz H, Schink B, Pfennig N, Conrad R. Energetics of syntrophic ethanol oxidation in defined chemostat cocultures: 2. Energy sharing in biomass production. Arch Microbiol. 1990;155:89–93. [Google Scholar]
- Senn H, Lendenmann U, Snozzi M, Hamer G, Egli T. The growth of Escherichia coli in glucose-limited chemostat cultures: a re-examination of the kinetics. Biochim Biophys Acta. 1994;1201:424–436. doi: 10.1016/0304-4165(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Shong J, Diaz M, Collins C. Towards synthetic microbial consortia for bioprocessing. Curr Opin Biotechnol. 2012;23:1–5. doi: 10.1016/j.copbio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Smith H, Waltman P. The Theory of the Chemostat. Cambridge University Press; 1995. [Google Scholar]
- Tilman D. Resource Competition and Community Structure. Princeton University Press; Princeton, NJ, USA: 1982. [PubMed] [Google Scholar]
- Tilman D. Dynamics and Structure of Plant Communities. Princeton University Press; Princeton, NJ, USA: 1988. [Google Scholar]
- Treves D, Manning S, Adams J. Repeated evolution of an acetate-crossfeeding polymorphism in long-term populations of Escherichia coli. Mol Biol Evol. 1998;15:789–797. doi: 10.1093/oxfordjournals.molbev.a025984. [DOI] [PubMed] [Google Scholar]
- Varma A, Palsson BO. Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110. Appl Environ Microbiol. 1994;60:3724–3731. doi: 10.1128/aem.60.10.3724-3731.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter J, Remingtion K, Heidelberg J, Halpern A, Rusch D, Eisen J, Wu D, Paulsen I, Nelson K, Nelson W, Fouts D, Levy S, Knap A, Lomas M, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkock C, Rogers Y, Smith HO. Environmental genome shotgun sequencing of the sargasso sea. Science. 2004;304:66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- Wintermute E, Silver P. Emergent cooperation in microbial metabolism. Mol Syst Biol. 2010;6:407. doi: 10.1038/msb.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. The acetate switch. Microbiol Mol Biol Rev. 2005;69:12. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Jahic M, Enfors S. Modeling of overflow metabolism in batch and fed-batch cultures of Escherichia coli. Biotechnol Prog. 1999;15:81–90. doi: 10.1021/bp9801087. [DOI] [PubMed] [Google Scholar]
- Zuroff T, Curtis W. Developing symbiotic consortia for lignocellulosic biofuel production. Appl Microbiol Biotechnol. 2012;93:1423–1435. doi: 10.1007/s00253-011-3762-9. [DOI] [PubMed] [Google Scholar]