Effects of Low-Carbohydrate and Low-Fat Diets: A Randomized Trial (original) (raw)
. Author manuscript; available in PMC: 2015 Sep 2.
Published in final edited form as: Ann Intern Med. 2014 Sep 2;161(5):309–318. doi: 10.7326/M14-0180
Abstract
Background
Low-carbohydrate diets are popular for weight loss, but their cardiovascular effects have not been well-studied, particularly in diverse populations.
Objective
To examine the effects of a low-carbohydrate diet compared with a low-fat diet on body weight and cardiovascular risk factors.
Design
A randomized, parallel-group trial. (ClinicalTrials.gov: NCT00609271)
Setting
A large academic medical center.
Participants
148 men and women without clinical cardiovascular disease and diabetes.
Intervention
A low-carbohydrate (<40 g/d) or low-fat diet (<30% fat; <7% saturated fat). Both groups received dietary counseling at regular intervals throughout the trial.
Measurements
Data on weight, cardiovascular risk factors, and dietary composition were collected at 0, 3, 6, and 12 months.
Results
Sixty participants (82%) in the low-fat group and 59 (79%) in the low-carbohydrate group completed the intervention. At 12 months, participants on the low-carbohydrate diet had greater decreases in weight (mean difference in change, −3.5 kg [95% CI, −5.6 to −1.4 kg]; P < 0.001), fat mass (mean difference in change, −1.5% [CI, −2.6% to −0.4%]; P = 0.011), ratio of total to high-density lipoprotein (HDL) cholesterol (mean difference in change, −0.44 [CI, −0.71 to −0.16]; P = 0.002), and triglyceride level (mean difference in change, −0.16 mmol/L [−14.1 mg/dL] [CI, −0.31 to −0.01 mmol/L {−27.4 to −0.8 mg/dL}]; P = 0.038) and greater increases in HDL cholesterol level (mean difference in change, 0.18 mmol/L [7.0 mg/dL] [CI, 0.08 to 0.28 mmol/L {3.0 to 11.0 mg/dL}]; P< 0.001) than those on the low-fat diet.
Limitation
Lack of clinical cardiovascular disease end points.
Conclusion
The low-carbohydrate diet was more effective for weight loss and cardiovascular risk factor reduction than the low-fat diet. Restricting carbohydrate may be an option for persons seeking to lose weight and reduce cardiovascular risk factors.
Primary Funding Source
National Institutes of Health.
According to the latest estimates, more than one third of American adults have at least 1 form of cardiovascular disease (CVD) and one third of total deaths are due to CVD (1). The annual cost of caring for Americans with CVD was an estimated 312.9billionin2009andisprojectedtoincreasetoapproximately312.9 billion in 2009 and is projected to increase to approximately 312.9billionin2009andisprojectedtoincreasetoapproximately1.48 trillion by 2030 (1). Thus, CVD is one of the most important public health challenges in the United States.
Low-carbohydrate diets have become a popular strategy for weight loss and weight management in recent years; however, their cardiovascular effects are unknown. Prospective cohort studies have produced conflicting results regarding the association between low-carbohydrate dietary patterns and risk for CVD (2, 3). Few randomized, controlled trials thus far have examined the effects of carbohydrate restriction on CVD risk factors in a diverse population with a significant proportion of black persons. The few that have either did not assess a typical low-carbohydrate diet or included severely obese participants, most of whom had type 2 diabetes or the metabolic syndrome (4–6). Hence, we conducted a randomized, parallel-group trial to examine the effects of a 12-month low-carbohydrate diet compared with a low-fat diet (7–9) on body weight and CVD risk factors in a diverse population with a substantial proportion of black persons with no clinical comorbid conditions.
METHODS
Setting and Participants
Men and women aged 22 to 75 years with a body mass index of 30 to 45 kg/m2 were recruited from the general public by using mailing lists, fliers, work site and community screenings, and television advertisements. Major exclusion criteria were self-reported clinical CVD, type 2 diabetes, or kidney disease; use of prescription weight-loss medications; surgery; and weight loss greater than 6.8 kg within 6 months of study entry. A total of 148 participants (mean age, 46.8 years; 88% female; 51% black) were included (Table 1). We recruited, enrolled, and followed participants and collected data and specimens from 2008 through 2011 at the Tulane University Health Sciences Center in New Orleans, Louisiana. The study was approved by the institutional review board at Tulane University, and each participant signed an approved consent form.
Table 1.
Baseline Characteristics of Trial Participants
| Characteristic | Low-Fat Diet (n = 73) | Low-Carbohydrate Diet (n = 75) |
|---|---|---|
| Mean age (SD), y | 47.8 (10.4) | 45.8 (9.9) |
| Female, n (%) | 65 (89) | 66 (88) |
| Race/ethnicity, n (%) | ||
| White | 33 (45) | 34 (45) |
| Black | 36 (49) | 40 (53) |
| Asian | 0 (0) | 1 (1) |
| Hispanic | 3 (4) | 0 (0) |
| Other | 1 (1) | 0 (0) |
| Mean body weight (SD), kg* | 97.9 (13.5) | 96.3 (12.7) |
| Mean body composition (SD), % | ||
| Fat mass | 40 (10) | 40 (10) |
| Lean mass | 60 (10) | 60 (10) |
| Mean body mass index (SD), kg/m2 | 35.6 (4.5) | 35.2 (3.8) |
| Mean waist circumference (SD), cm | 111.0 (10.7) | 108.4 (9.3) |
| Mean systolic blood pressure (SD), mm Hg | 124.9 (13.8) | 120.3 (12.8) |
| Mean diastolic blood pressure (SD), mm Hg | 79.4 (8.3) | 77.5 (9.0) |
| Mean total cholesterol level (SD) | ||
| mmol/L | 5.3 (1.1) | 5.1 (1.1) |
| mg/dL | 204.3 (40.7) | 198.8 (42.2) |
| Mean LDL cholesterol level (SD) | ||
| mmol/L | 3.2 (1.0) | 3.2 (0.9) |
| mg/dL | 122.7 (38.6) | 122.5 (34.6) |
| Mean HDL cholesterol level (SD) | ||
| mmol/L | 1.5 (0.3) | 1.4 (0.3) |
| mg/dL | 56.5 (12.8) | 53.8 (13.3) |
| Mean total–HDL cholesterol ratio (SD) | 3.8 (1.0) | 3.8 (1.0) |
| Mean triglyceride level (SD) | ||
| mmol/L | 1.4 (0.9) | 1.3 (0.6) |
| mg/dL | 125.5 (81.3) | 112.6 (54.1) |
| Mean plasma glucose level (SD) | ||
| mmol/L | 5.2 (0.5) | 5.2 (0.6) |
| mg/dL | 93.4 (9.2) | 94.5 (10.9) |
| Mean serum insulin level (SD), pmol/L | 105.6 (54.9) | 102.8 (63.9) |
| Mean serum creatinine level (SD) | ||
| μmol/L | 97.2 (17.7) | 88.4 (17.7) |
| mg/dL | 1.1 (0.2) | 1.0 (0.2) |
| Mean C-reactive protein level (SD), nmol/L | 46.7 (48.6) | 46.7 (40.0) |
| Antihypertensive medication use, n (%) | 24 (32.9) | 21 (28.0) |
| Lipid-lowering medication use, n (%) | 9 (12.3) | 12 (16.0) |
| Mean physical activity level (SD), MET-h/wk† | 19.6 (35.5) | 16.3 (26.0) |
| Mean 10-y Framingham risk score (SD), % | 4.2 (3.3) | 3.9 (3.1) |
Study Design and Intervention
We used a computer-generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups. After randomization, 73 participants were assigned to the low-fat group and 75 were assigned to the low-carbohydrate group. Participants assigned to the low-carbohydrate diet were instructed to maintain an intake of digestible carbohydrate (total carbohydrate minus total fiber) of less than 40 g/d. Those assigned to the low-fat diet were instructed to maintain less than 30% of their daily energy intake from total fat (with <7% from saturated fat) and 55% from carbohydrate, based on National Cholesterol Education Program guidelines (7–9). Neither diet included a specific calorie or energy goal. Participants in each group were asked to refrain from changing their physical activity levels during the intervention. A handbook was given to participants that contained recipes, sample menus for 1 week of food intake at various energy levels, food lists, shopping lists, meal planners, and guides on counting macronutrients and reading nutrition labels. We also provided 1 low-carbohydrate or low-fat meal replacement (bar or shake) per day to participants in each group for the duration of the study.
Participants met with a dietitian in weekly individual counseling sessions for the first 4 weeks, followed by small group counseling sessions every other week for the next 5 months (a total of 10 sessions) and monthly for the last 6 months of the intervention period. Individual sessions generally lasted about 1 hour and included dietary instruction and supportive counseling. Group counseling sessions were held separately for participants in the low-fat and low-carbohydrate groups but followed a common behavioral curriculum.
Staff provided a single set of instructions that were not altered over the course of the study. Participants in each diet group received the same information on dietary fiber (recommended intake of 25 g/d) and types of dietary fats. These common instructions included education on saturated, monounsaturated, and trans fats, with emphasis on the benefits of monounsaturated fats and recommendations to limit or eliminate trans fats.
Data Collection
Two 24-hour dietary recalls were obtained from participants at baseline and 3, 6, and 12 months to characterize and monitor individual dietary intake of macronutrients. One recall reflected consumption on a weekday, and the other reflected consumption on a weekend day. All dietary recalls were obtained by a trained and certified staff member. We calculated dietary nutrient intakes using the food composition tables of the Nutrition Data System for Research (10). Five percent of the dietary recalls were recorded and reviewed for quality control purposes.
A detailed medical history that included assessment of hypertension, diabetes, CVD, medication use, and health behaviors (smoking habits, alcohol use, and physical activity) was obtained at the screening visit. We collected anthropometric measures, blood pressure, and blood and urine samples at the screening visit, randomization, and each follow-up visit. Body weight and height (without shoes) were measured to the nearest 0.1 kg and 0.1 cm, respectively, using a single calibrated scale (Detecto, model 6855) and a wall-mounted stadiometer. We measured body composition using whole-body bioelectrical impedance analysis (RJL Systems) while the participant was in a supine position. We measured blood pressure 3 times with a mercury sphygmomanometer using procedures recommended by the American Heart Association (11). The systolic and diastolic blood pressures were recorded as the first and fifth Korotkoff sounds, respectively. Blood samples were collected after the participant had fasted for 12 hours. We assayed serum total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels according to procedures recommended by the National Heart, Lung, and Blood Institute and the Centers for Disease Control and Prevention (12). Low-density lipoprotein (LDL) cholesterol level was calculated using the Friedewald formula (13). We measured plasma glucose, serum creatinine, and high-sensitivity C-reactive protein (CRP) levels using standard methods. We calculated physical activity as the sum of hours of moderate to vigorous activities per week (walking, sports, dance, and conditioning) multiplied by each activity’s individual metabolic equivalent value. Urinary ketone levels were measured by dipstick at each behavioral session attended and each clinic visit for data collection. A range of adverse effects was assessed using closed-ended questions at each counseling session.
Statistical Analysis
The power assessment for the primary end point (body weight) was based on data abstracted from trials similar to this one (4, 14–16). Assuming a 2-sided significance level of 0.05, we needed 55 participants per group to provide 80% power to detect differences in weight change of at least 3% (SD, 5%) between the groups. The sample size of 148 participants allowed for a 25% dropout rate after randomization.
Data on baseline characteristics of study participants were expressed as means (SDs) or numbers (percentages). Eleven participants (5 in the low-fat group and 6 in the low-carbohydrate group) declined to have their body weight measured at randomization and were not included in the analysis of our primary outcome. We used t tests or chi-square tests to compare baseline characteristics between the groups. Dietary composition data were expressed as means (SDs) and compared using t tests. We used a random-effects linear model that was fitted to continuous outcomes (primary and secondary). Each random-effects model consisted of a random intercept and a random slope to adjust for the within-participant correlation among the observed longitudinal data. To examine the change in each study end point, the model included an indicator variable for time (3, 6, and 12 months), diet group, an interaction term for diet group by time, and baseline level of the corresponding end point. In a post hoc analysis, we also examined the estimated 10-year risk for coronary heart disease (CHD) by Framingham risk score between groups (17). To examine adverse effects (binary outcomes) over time while accounting for the repeated measurements within individuals, we used generalized estimating equations under the logistic regression model.
The random-effects model allows the assumption of data missing at random (MAR). We performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption. We used Markovchain Monte Carlo techniques to impute missing values, including additional covariates (age, sex, race, marital status, education, and employment status), in the model to make the MAR assumption more plausible (18). All P values were 2-sided, and no adjustment was made for multiple comparisons. We used SAS, version 9.2 (SAS Institute), for all analyses.
Role of the Funding Source
The study was funded by the National Center for Research Resources of the National Institutes of Health. The funding source had no role in the design, conduct, analysis, or reporting of the study.
RESULTS
Baseline Characteristics
Baseline characteristics of the trial participants are shown in Table 1. Demographic characteristics and cardiovascular risk factors were similar between groups. The proportions of participants completing assessments at months 3, 6, and 12 were 93.2%, 87.7%, and 82.2%, respectively, in the low-fat group and 92.0%, 82.7%, and 78.7%, respectively, in the low-carbohydrate group (Figure 1).
Figure 1. Study flow diagram.
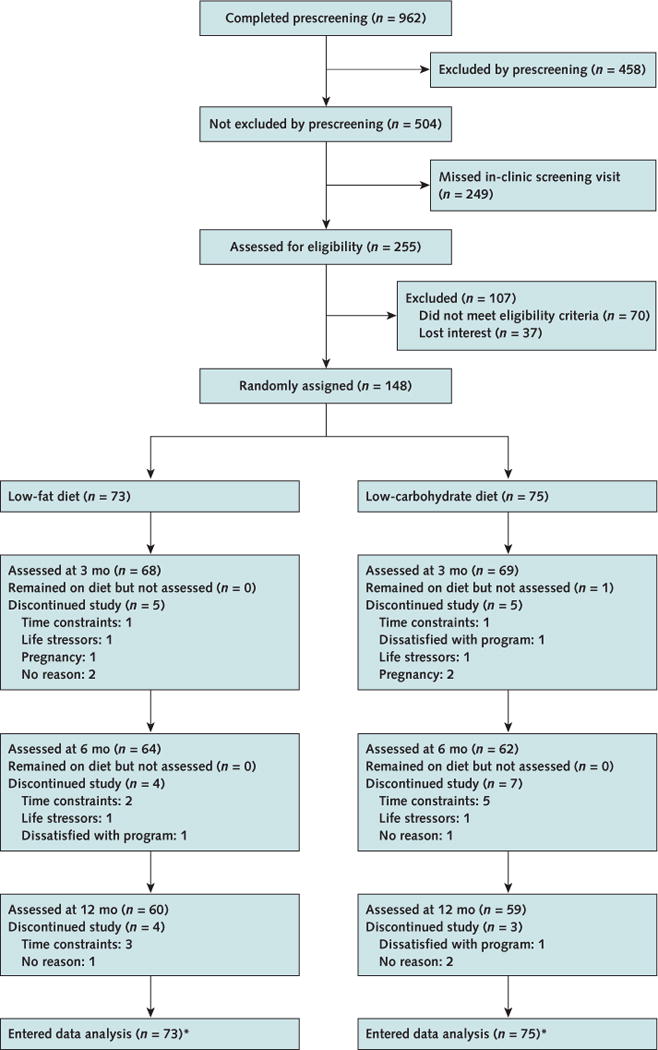
* Five participants in the low-fat group and 6 in the low-carbohydrate group had no data on body weight at randomization.
Dietary Intake and Physical Activity
Dietary composition data for participants who remained on each diet and also provided 24-hour recalls are summarized in Table 2. At baseline, reported dietary composition in the low-fat group was similar to that in the low-carbohydrate group. During the follow-up period, total energy intake was similar between groups. The intake of total carbohydrate was significantly higher and intakes of protein and total, saturated, and monounsaturated fat (as percentages of kilocalories) were significantly lower in the low-fat group at 12 months (P < 0.001 for these comparisons). Physical activity levels were similar throughout the study.
Table 2.
Daily Dietary Composition in the Low-Fat and Low-Carbohydrate Diet Groups Over the Course of the Study*
| Variable | Baseline | 3 mo | 6 mo | 12 mo | ||||
|---|---|---|---|---|---|---|---|---|
| Low-Fat Diet (n = 69) | Low-Carbohydrate Diet (n = 70) | Low-Fat Diet (n = 61) | Low-Carbohydrate Diet (n = 62) | Low-Fat Diet (n = 50) | Low-Carbohydrate Diet (n = 54) | Low-Fat Diet (n = 49) | Low-Carbohydrate Diet (n = 54) | |
| Energy, kcal | 2034 (702) | 1998 (740) | 1418 (468) | 1258 (409) | 1481 (483) | 1324 (537) | 1527 (522) | 1448 (610) |
| Carbohydrate, g | 242 (100) | 242 (92) | 193 (75) | 97 (45) | 202 (79) | 93 (46) | 198 (78) | 127 (69) |
| Total fiber, g | 16.7 (6.6) | 18.5 (8.7) | 16.9 (8.9) | 16.2 (8.9) | 16.4 (8.1) | 15.1 (7.5) | 15.6 (7.7) | 15.1 (8.7) |
| Soluble fiber, g | 5.1 (2.0) | 5.8 (2.9) | 5.1 (2.5) | 6.0 (4.4) | 5.1 (2.9) | 5.2 (3.4) | 5.1 (2.8) | 5.2 (3.9) |
| Insoluble fiber, g | 11.5 (5.1) | 12.5 (6.5) | 11.8 (6.9) | 10.0 (5.5) | 11.3 (6.0) | 9.9 (5.0) | 10.4 (5.5) | 9.9 (5.6) |
| Fat, g | 80.7 (32.4) | 75.6 (36.4) | 45.3 (21.7) | 62.6 (28.6) | 46.4 (18.9) | 67.2 (38.2) | 52.4 (24.3) | 69.0 (36.8) |
| SFA, g | 27.6 (13.6) | 24.7 (14.4) | 13.5 (6.8) | 19.9 (9.8) | 14.0 (6.7) | 21.2 (15.6) | 15.8 (8.4) | 23.3 (15.1) |
| MUFA, g | 29.3 (12.5) | 28.1 (13.7) | 17.0 (9.5) | 24.0 (12.0) | 17.1 (7.3) | 25.1 (13.6) | 20.0 (9.9) | 25.8 (14.3) |
| PUFA, g | 17.1 (8.1) | 16.7 (9.2) | 10.9 (6.2) | 13.3 (6.9) | 11.3 (5.5) | 15.0 (8.8) | 12.2 (7.7) | 14.2 (7.4) |
| ω-3 Fatty acid, g | 1.97 (1.13) | 1.88 (1.31) | 1.22 (0.69) | 1.63 (1.63) | 1.33 (0.68) | 1.88 (1.45) | 1.27 (0.76) | 1.63 (0.99) |
| Carbohydrate, % kcal | 46.0 (7.8) | 48.1 (8.8) | 52.9 (10.7) | 28.9 (12.6) | 52.4 (8.9) | 27.5 (12.1) | 54.0 (9.6) | 34.0 (13.9) |
| Protein, % kcal | 17.6 (5.2) | 17.3 (5.0) | 19.0 (5.7) | 25.6 (7.7) | 18.3 (5.0) | 26.3 (5.6) | 18.6 (5.8) | 23.6 (7.4) |
| Fat, % kcal | 34.7 (6.6) | 32.5 (7.2) | 27.5 (8.8) | 42.7 (10.0) | 27.9 (7.3) | 43.4 (11.8) | 29.8 (8.8) | 40.7 (10.6) |
| SFA, % kcal | 11.6 (2.9) | 10.5 (3.4) | 8.1 (3.0) | 13.6 (4.2) | 8.4 (2.9) | 13.4 (4.5) | 9.0 (3.2) | 13.4 (4.8) |
| MUFA, % kcal | 12.7 (3.0) | 12.0 (3.1) | 10.3 (4.2) | 16.3 (4.4) | 10.3 (3.1) | 16.3 (4.9) | 11.3 (3.7) | 15.3 (4.7) |
| PUFA, % kcal | 7.5 (2.7) | 7.3 (2.7) | 6.7 (3.1) | 9.1 (3.1) | 6.7 (2.2) | 9.8 (4.3) | 6.9 (3.5) | 8.6 (3.3) |
| Folate, mg | 0.40 (0.17) | 0.41 (0.19) | 0.36 (0.23) | 0.29 (0.13) | 0.38 (0.22) | 0.32 (0.17) | 0.35 (0.20) | 0.31 (0.15) |
| Median _β_-carotene (IQR), mg | 0.75 (2.04) | 0.49 (1.31) | 0.99 (2.03) | 0.89 (1.99) | 0.74 (2.44) | 0.60 (1.50) | 1.00 (2.35) | 0.42 (1.42) |
| Vitamin C, mg | 78.4 (47.0) | 88.6 (60.0) | 82.7 (68.1) | 67.8 (45.2) | 85.4 (66.0) | 81.1 (54.8) | 82.5 (61.1) | 72.5 (67.9) |
Body Weight and Composition and Waist Circumference
Weight loss from baseline values was greater in the low-carbohydrate group than in the low-fat group at 3, 6, and 12 months (Table 3). The reduction in body weight was significantly greater in the low-carbohydrate group (mean difference in change at 12 months, −3.5 kg [95% CI, −5.6 to −1.4 kg]; P = 0.002). Compared with participants on the low-fat diet, those on the low-carbohydrate diet had significantly greater proportional reductions in fat mass (mean difference in change at 12 months, −1.5% [CI, −2.6% to −0.4%]; P = 0.011) and significantly greater proportional increases in lean mass (mean difference in change at 12 months, 1.7% [CI, 0.6% to 2.8%]; P = 0.003). Participants in both groups significantly reduced their waist circumference. Changes in waist circumference were more favorable in the low-carbohydrate group at 3 and 6 months but did not differ significantly from those in the low-fat group at 12 months (Table 3; Figure 2; and Appendix Figure, available at www.annals.org).
Table 3.
Predicted Mean Differences in Changes in Cardiovascular Risk Factors From Baseline, by Assigned Dietary Group
| Variable | Predicted Mean Difference (95% CI)* | P Value† | ||
|---|---|---|---|---|
| Low-Fat Diet (n = 73) | Low-Carbohydrate Diet (n = 75) | Mean Difference in Change | ||
| Body weight, kg | ||||
| 3 mo | −2.6 (−3.4 to −1.7) | −5.7 (−6.5 to −4.9) | −3.1 (−4.3 to −1.9) | <0.001 |
| 6 mo | −2.3 (−3.3 to −1.3) | −5.6 (−6.5 to −4.6) | −3.2 (−4.6 to −1.9) | <0.001 |
| 12 mo | −1.8 (−3.3 to −0.3) | −5.3 (−6.8 to −3.8) | −3.5 (−5.6 to −1.4) | 0.002 |
| Waist circumference, cm | ||||
| 3 mo | −3.5 (−4.6 to −2.4) | −5.5 (−6.6 to −4.4) | −2.0 (−3.6 to −0.5) | 0.012 |
| 6 mo | −4.0 (−5.2 to −2.8) | −5.9 (−7.1 to −4.7) | −1.9 (−3.6 to −0.3) | 0.024 |
| 12 mo | −5.0 (−6.8 to −3.2) | −6.7 (−8.5 to −4.9) | −1.7 (−4.2 to 0.9) | 0.197 |
| Lean mass, % | ||||
| 3 mo | 0.4 (−0.2 to 1.1) | 1.6 (1.0 to 2.2) | 1.2 (0.3 to 2.0) | 0.010 |
| 6 mo | 0.2 (−0.4 to 0.7) | 1.5 (0.9 to 2.1) | 1.3 (0.5 to 2.2) | 0.002 |
| 12 mo | −0.4 (−1.2 to 0.4) | 1.3 (0.5 to 2.0) | 1.7 (0.6 to 2.8) | 0.003 |
| Fat mass, % | ||||
| 3 mo | −0.3 (−0.9 to 0.3) | −1.1 (−1.7 to −0.5) | −0.8 (−1.6 to 0.1) | 0.066 |
| 6 mo | −0.1 (−0.6 to 0.5) | −1.1 (−1.7 to −0.6) | −1.0 (−1.8 to −0.3) | 0.011 |
| 12 mo | 0.3 (−0.5 to 1.1) | −1.2 (−2.0 to −0.4) | −1.5 (−2.6 to −0.4) | 0.011 |
| Total cholesterol level, mmol/L‡ | ||||
| 3 mo | 0.03 (−0.10 to 0.16) | −0.09 (−0.21 to 0.04) | −0.12 (−0.30 to 0.06) | 0.20 |
| 6 mo | 0.03 (−0.09 to 0.15) | −0.04 (−0.16 to 0.07) | −0.07 (−0.23 to 0.09) | 0.38 |
| 12 mo | 0.03 (−0.13 to 0.18) | 0.05 (−0.11 to 0.20) | 0.02 (−0.20 to 0.24) | 0.86 |
| LDL cholesterol level, mmol/L‡ | ||||
| 3 mo | 0.05 (−0.06 to 0.18) | −0.02 (−0.14 to 0.10) | −0.07 (−0.24 to 0.10) | 0.40 |
| 6 mo | 0.02 (−0.08 to 0.13) | −0.04 (−0.15 to 0.06) | −0.06 (−0.21 to 0.09) | 0.42 |
| 12 mo | −0.05 (−0.20 to 0.11) | −0.08 (−0.24 to 0.08) | −0.04 (−0.26 to 0.19) | 0.74 |
| HDL cholesterol level, mmol/L‡ | ||||
| 3 mo | −0.03 (−0.09 to 0.02) | 0.03 (−0.02 to 0.09) | 0.06 (−0.01 to 0.14) | 0.106 |
| 6 mo | −0.00 (−0.05 to 0.05) | 0.10 (0.05 to 0.15) | 0.10 (0.03 to 0.17) | 0.004 |
| 12 mo | 0.06 (−0.01 to 0.13) | 0.24 (0.17 to 0.31) | 0.18 (0.08 to 0.28) | <0.001 |
| Total–HDL cholesterol ratio | ||||
| 3 mo | 0.13 (−0.02 to 0.29) | −0.13 (−0.28 to 0.03) | −0.26 (−0.48 to −0.04) | 0.020 |
| 6 mo | 0.07 (−0.06 to 0.21) | −0.25 (−0.38 to −0.11) | −0.32 (−0.51 to −0.13) | 0.001 |
| 12 mo | −0.05 (−0.24 to 0.14) | −0.49 (−0.68 to −0.29) | −0.44 (−0.71 to −0.16) | 0.002 |
| Triglyceride level, mmol/L§ | ||||
| 3 mo | 0.03 (−0.08 to 0.14) | −0.21 (−0.32 to −0.11) | −0.25 (−0.40 to −0.09) | 0.002 |
| 6 mo | −0.01 (−0.10 to 0.09) | −0.22 (−0.31 to −0.13) | −0.22 (−0.35 to −0.08) | 0.002 |
| 12 mo | −0.07 (−0.18 to 0.04) | −0.23 (−0.34 to −0.12) | −0.16 (−0.31 to −0.01) | 0.038 |
| Systolic blood pressure, mm Hg | ||||
| 3 mo | −2.6 (−4.3 to −0.9) | −4.2 (−5.9 to −2.5) | −1.6 (−4.0 to 0.9) | 0.20 |
| 6 mo | −2.2 (−3.8 to −0.6) | −2.9 (−4.5 to −1.3) | −0.7 (−3.0 to 1.7) | 0.54 |
| 12 mo | −1.3 (−3.6 to 1.0) | −0.2 (−2.6 to 2.1) | 1.2 (−2.2 to 4.6) | 0.52 |
| Diastolic blood pressure, mm Hg | ||||
| 3 mo | −0.9 (−2.1 to 0.4) | −2.3 (−3.5 to −1.1) | −1.4 (−3.2 to 0.4) | 0.112 |
| 6 mo | −0.5 (−1.7 to 0.6) | −1.7 (−2.8 to −0.5) | −1.1 (−2.8 to 0.6) | 0.177 |
| 12 mo | 0.2 (−1.5 to 1.9) | −0.5 (−2.2 to 1.3) | −0.6 (−3.1 to 1.9) | 0.61 |
| Plasma glucose level, mmol/L‖ | ||||
| 3 mo | −0.10 (−0.21 to 0.01) | −0.05 (−0.16 to 0.05) | 0.04 (−0.11 to 0.19) | 0.52 |
| 6 mo | −0.10 (−0.20 to 0.01) | 0.03 (−0.13 to 0.07) | 0.07 (−0.08 to 0.21) | 0.32 |
| 12 mo | −0.10 (−0.22 to 0.03) | 0.02 (−0.11 to 0.14) | 0.12 (−0.09 to 0.32) | 0.21 |
| Serum insulin level, pmol/L | ||||
| 3 mo | −18.8 (−29.9 to −7.0) | −25.0 (−36.1 to −13.9) | −7.0 (−22.2 to 8.3) | 0.42 |
| 6 mo | −20.8 (−30.6 to −11.1) | −21.5 (−31.3 to −11.8) | −1.4 (−13.9 to 11.8) | 0.90 |
| 12 mo | −24.3 (−36.1 to −13.2) | −13.9 (−25.7 to −2.8) | 10.4 (−10.4 to 31.3) | 0.20 |
| C-reactive protein level, nmol/L | ||||
| 3 mo | 5.7 (−2.9 to 13.3) | −4.8 (−13.3 to 3.8) | −10.5 (−21.9 to 1.9) | 0.099 |
| 6 mo | 6.7 (−1.0 to 13.3) | −4.8 (−12.4 to 1.9) | −11.4 (−21.9 to −1.9) | 0.019 |
| 12 mo | 8.6 (−1.0 to 18.1) | −6.7 (−16.2 to 2.9) | −15.2 (−27.6 to −1.9) | 0.024 |
| Serum creatinine level, μmol/L¶ | ||||
| 3 mo | 1.8 (−1.7 to 5.2) | −0.1 (−3.4 to 3.3) | −1.8 (−6.6 to 2.9) | 0.45 |
| 6 mo | −1.7 (−4.7 to 1.3) | −3.1 (−6.1 to −0.2) | −1.5 (−5.7 to 2.5) | 0.49 |
| 12 mo | −8.5 (−12.3 to −4.6) | −9.2 (−13.1 to −5.4) | −0.7 (−6.2 to 4.7) | 0.79 |
| 10-y Framingham risk score, % | ||||
| 3 mo | 0.4 (−0.1 to 0.9) | −0.5 (−1.0 to 0.0) | −0.9 (−1.6 to −0.2) | 0.019 |
| 6 mo | 0.4 (0.0 to 0.8) | −0.7 (−1.0 to −0.3) | −1.0 (−1.6 to −0.5) | <0.001 |
| 12 mo | 0.4 (−0.2 to 0.9) | −1.0 (−1.6 to −0.5) | −1.4 (−2.1 to −0.6) | <0.001 |
Figure 2. Predicted mean changes in body weight, fat mass, total–HDL cholesterol ratio, and triglyceride level in the low-fat and low-carbohydrate diet groups.
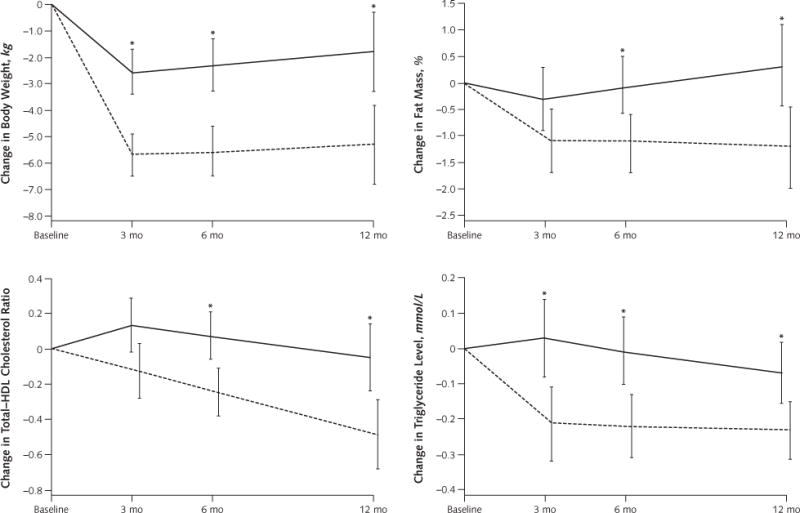
Results are from random-effects models and are expressed as means, with error bars representing 95% CIs. To convert triglyceride values to mg/dL, divide by 0.0113. HDL = high-density lipoprotein.
* P < 0.05 for between-group difference.
Serum Lipid Levels
At 12 months, serum levels of total and LDL cholesterol had not significantly changed among participants in either group. High-density lipoprotein cholesterol level increased significantly more in the low-carbohydrate group than in the low-fat group (mean difference in change at 12 months, 0.18 mmol/L [7.0 mg/dL] [CI, 0.08 to 0.28 mmol/L {3.0 to 11.0 mg/dL}]; P < 0.001). Ratios of total to HDL cholesterol decreased significantly only among participants in the low-carbohydrate group, and the decreases were significantly greater than those in the low-fat group (mean difference in change at 12 months, −0.44 [CI, −0.71 to −0.16]; P = 0.002). Serum levels of triglycerides also decreased significantly in both groups, with greater decreases among participants in the low-carbohydrate group (mean difference in change at 12 months, −0.16 mmol/L [−14.1 mg/dL] [CI, −0.31 to −0.01 mmol/L {−27.4 to −0.8 mg/dL}]; P = 0.038) (Table 3, Figure 2, and Appendix Figure).
Blood Pressure and CRP, Plasma Glucose, Insulin, and Serum Creatinine Levels
At 12 months, participants in the low-carbohydrate group had significantly greater decreases in CRP level than those in the low-fat group (mean difference in change at 12 months, −15.2 nmol/L [CI, −27.6 to −1.9 nmol/L]; P = 0.024). Systolic and diastolic blood pressures did not significantly decrease among participants in either group, and mean differences in change between the groups were also not significant at 12 months. Plasma glucose levels also did not significantly change in either group. Although serum levels of insulin and creatinine decreased significantly in each group, the decreases did not differ significantly between groups (Table 3).
10-Year Framingham CHD Risk Score
Participants in the low-carbohydrate group had significant decreases in estimated 10-year risk for CHD at 6 and 12 months, whereas those in the low-fat group did not (Table 3 and Appendix Figure). The reductions in estimated 10-year risk for CHD were significantly greater in the low-carbohydrate group at 12 months (mean difference in change, −1.4% [CI, −2.1% to −0.6%]; P < 0.001).
We examined differences among white and black participants and found that the results were consistent with those of the overall population (Appendix Tables 1 and 2, available at www.annals.org), except HDL cholesterol levels increased slightly with the low-fat diet among black participants at 12 months. Small sample sizes precluded meaningful assessments of other racial and ethnic groups individually.
Sensitivity Analyses
Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses. Specifically, participants in the low-carbohydrate group lost significantly more weight than those in the low-fat group (mean difference in change at 12 months, −3.6 kg [CI, −5.7 to −1.4 kg]; P = 0.001).
Adverse Events
No serious adverse events were reported during the course of the study. The number of participants who had symptoms, including constipation, fatigue, thirst, polyuria, diarrhea, heartburn, gas, nausea, vomiting, appetite changes, or headache, did not differ significantly between the low-carbohydrate and low-fat groups, except significantly more participants on the low-fat diet reported headaches at 3 months (18 [25%] vs. 6 [8%] participants; P = 0.030 for between-group difference) (Appendix Table 3, available at www.annals.org).
DISCUSSION
Our study found that a low-carbohydrate diet induced greater weight loss and reductions in cardiovascular risk factors at 12 months than a low-fat diet among black and white obese adults who did not have diabetes, CVD, or kidney disease at baseline. Compared with a low-fat diet, a low-carbohydrate diet resulted in greater improvements in body composition, HDL cholesterol level, ratio of total to HDL cholesterol, triglyceride level, CRP level, and estimated 10-year CHD risk. Because CVD is the most common cause of death in the United States and obesity is a particularly prevalent risk factor, our study has important clinical and public health implications. Findings from this trial may offer new evidence for the recommendation of a low-carbohydrate diet to obese persons as an additional nonpharmacologic approach for weight loss and reduction of CVD risk factors.
Previous studies have examined the effects of low-carbohydrate diets on CVD risk factors, but most of these trials had small sample sizes or low completion rates, did not assess a typical low-carbohydrate diet for weight loss, or did not include diverse populations (19–24). In contrast, our study tested the effects of a typical low-carbohydrate dietary intervention, with a high completion rate (approximately 80%) over 12 months of follow-up and a substantial sample of black persons (a group under-represented in previous trials). Although 2 trials have examined cardiovascular effects in samples with a majority of black persons, they included only diabetic patients or those with severe obesity, most of whom (83%) also had type 2 diabetes or the metabolic syndrome (4, 5). The POUNDS LOST (Preventing Overweight Using Novel Dietary Strategies) study, which examined the effects of 4 diets with different macronutrient compositions, included a substantial number of black persons but did not test a typical low-carbohydrate diet. In that study, participants on the low-carbohydrate diet (which was high in protein and fat) aimed for 35% of daily energy intake from carbohydrate and achieved approximately 43%. Typical low-carbohydrate diets for weight loss restrict carbohydrate to less than 20% of daily energy intake (6). Over the course of 12 months, participants in the low-carbohydrate group in our study achieved an average of 30% of daily energy from carbohydrate. Unlike some previous studies, our trial included men and women who did not have diabetes and CVD at baseline and comprehensively measured cardiovascular risk profiles.
Our results with regard to body weight are consistent with those of other trials (23, 24) and a recent meta-analysis (25). The underlying mechanisms that may account for differences in weight loss by diet are still not fully identified, but a recent study indicated that low-carbohydrate diets may have a more favorable effect on resting energy expenditure and total energy expenditure than low-fat diets (26). In addition, our findings suggest that the loss of fat mass accounts for most of the reduction in body weight on a low-carbohydrate diet, which is consistent with other study findings (19, 21).
We found that a low-carbohydrate diet resulted in a significantly greater reduction in the ratio of total to HDL cholesterol, which has been identified as a strong and independent predictor of CHD (27). This finding is consistent with at least 1 previous study (23) but not others that had small sample sizes or high rates of loss to follow-up (20, 21). The decreases in HDL cholesterol and triglyceride levels that we observed were within the range reported in previous weight-loss studies (25).
A major concern that has been frequently raised about low-carbohydrate diets is their potential to elevate LDL cholesterol levels, an established risk factor for CVD (8, 28). In contrast, a recent meta-analysis showed that both low-fat and low-carbohydrate diets reduced LDL cholesterol levels, although the reduction was less for persons assigned to low-carbohydrate diets (25). Our study also found reductions in LDL cholesterol level among participants in both groups, with no significant difference between the groups.
We also observed moderate reductions in blood pressure and plasma glucose, serum insulin, and serum creatinine levels that did not differ significantly between groups. In our study, participants on the low-carbohydrate diet had greater decreases in CRP levels than those on the low-fat diet. Two previous studies that examined CRP levels found no difference between the diets (19, 29); however, both had relatively small sample sizes and may have been underpowered.
The Framingham risk score is a global index of CHD risk used in clinical settings (8, 17, 30). Although it was not a prespecified outcome in our study, we prospectively collected data needed to calculate it. Brinkworth and colleagues (19) reported a nonsignificant difference in Framingham risk score between a modified Atkins-style low-carbohydrate diet and a low-fat diet among 118 participants with abdominal obesity and other metabolic syndrome components. In contrast, in our study, participants randomly assigned to the low-carbohydrate diet had greater decreases in 10-year CHD risk score than those assigned to the low-fat diet; however, the overall level of risk was low in our sample (about 4% over 10 years at baseline). Thus, the clinical significance of this difference is not clear. These different findings may be due to different population characteristics or completion rates (roughly 80% in our study vs. 58% in Brinkworth and colleagues’ study) (19). Moreover, these results should be interpreted with caution because of difficulty quantifying the exact amount of uncertainty around an individual’s risk score.
Our conclusions are subject to limitations. First, self-reported dietary information may be subject to memory and recall issues, and participants who complete the dietary recall may be more likely to report adhering to the interventions. However, we collected these within 24 hours of consumption and used multiple 24-hour diet recalls to reflect weekday and weekend eating patterns. Second, dietitians were not blinded to the study hypothesis. In order to avoid potential differences in dietary counseling due to this, we used specific and detailed scripts for all counseling sessions and trained staff to deliver the scripts without deviation. Dietary sessions for both groups were intermittently observed for consistency by an independent registered dietitian consultant who was not a regular part of the study staff, and all outcome assessors were blinded to the diet group assignment. Third, conclusions from our study are limited by the lack of CVD clinical end points; however, we assessed CVD risk factors extensively. Because of the number of tests performed in the primary analyses, statistically significant results should be interpreted with caution, particularly P values denoting significance levels between 0.01 and 0.05. Finally, although our findings show what can be achieved, they may not be generalizable to more common situations where intensive and repeated dietary counseling is not available.
Our study has several strengths. All data were collected by trained and certified staff using rigorous quality control protocols. Also, the completion rate was approximately 80% in both diet groups. In addition, this study had high rates of dietary adherence, as shown by 24-hour recall and urinary ketone levels (31). The proportion of participants with detectable urinary ketone levels was significantly higher in the low-carbohydrate group than in the low-fat group at 3, 6, and 12 months (data not shown). Finally, our study included a substantial proportion of black participants, a group underrepresented in previous trials.
In summary, this 12-month randomized, parallel-group trial showed that a low-carbohydrate diet resulted in greater weight loss and reduction in cardiovascular risk factors than a low-fat diet among obese black and white adults. Restricting carbohydrate may be an option for persons who are seeking to lose weight and reduce cardiovascular risk factors and should be studied further.
See also:
Summary for Patients.........................1
Context
The relative benefits of low-carbohydrate and low-fat diets have not been well-studied in populations that included a substantial proportion of black persons.
Contribution
Participants in this 12-month study who were randomly assigned to a low-carbohydrate diet lost more weight and had greater reductions in certain markers of cardiovascular disease than those assigned to a low-fat diet. About half of the study’s participants were black.
Implication
A low-carbohydrate diet may be beneficial for weight loss and reduction of cardiovascular risk factors.
—The Editors
Acknowledgments
The authors thank the study participants for their cooperation.
Grant Support: By NIH/NCRR P20-RR017659 to the Tulane University Hypertension and Renal Center of Excellence.
Appendix Figure.
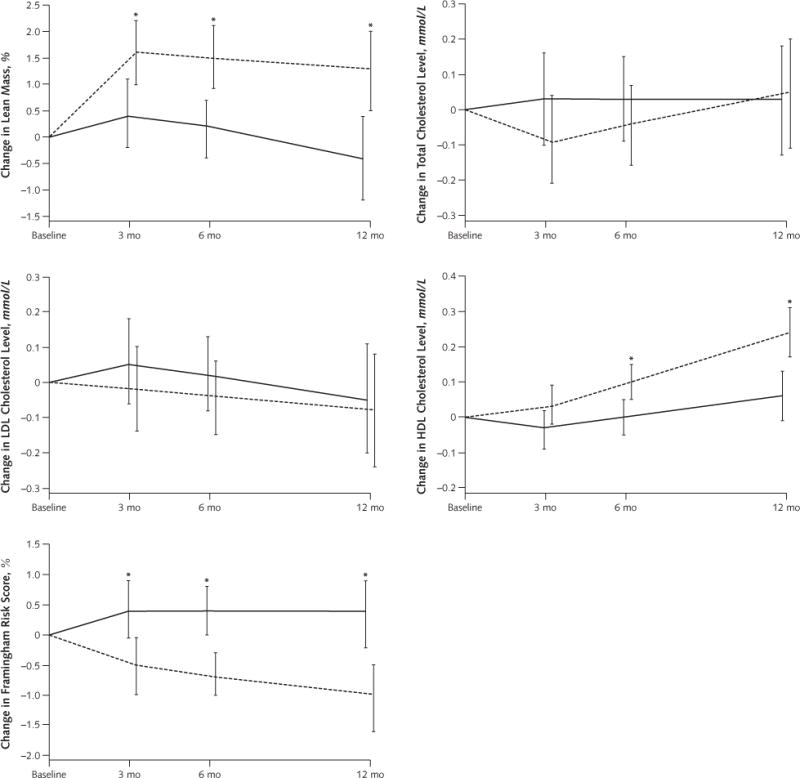
Predicted mean changes in lean mass, total cholesterol level, LDL cholesterol level, HDL cholesterol level, and 10-y Framingham risk score in the low-fat and low-carbohydrate diet groups
Results are from random-effects models and are expressed as means, with error bars representing 95% CIs. To convert cholesterol values to mg/dL, divide by 0.0259. HDL = high-density lipoprotein; LDL = low-density lipoprotein.
* P < 0.05 for between-group difference.
Appendix Table 1.
Predicted Mean Differences in Changes in Cardiovascular Risk Factors From Baseline, by Assigned Dietary Group: White Persons
| Variable | Predicted Mean Difference (95% CI)* | P Value† | ||
|---|---|---|---|---|
| Low-Fat Diet (n = 73) | Low-Carbohydrate Diet (n = 75) | Mean Difference in Change | ||
| Body weight, kg | ||||
| 3 mo | −3.5 (−4.8 to −2.3) | −6.4 (−7.6 to −5.1) | −2.8 (−4.6 to −1.1) | 0.002 |
| 6 mo | −3.2 (−4.7 to −1.7) | −6.4 (−7.9 to −4.9) | −3.2 (−5.3 to −1.0) | 0.004 |
| 12 mo | −2.6 (−5.1 to −0.1) | −6.5 (−9.0 to −4.0) | −3.9 (−7.4 to −0.4) | 0.032 |
| Waist circumference, cm | ||||
| 3 mo | −2.7 (−4.3 to −1.0) | −6.1 (−7.7 to −4.6) | −3.5 (−5.7 to −1.2) | 0.004 |
| 6 mo | −4.1 (−5.8 to −2.3) | −6.4 (−8.1 to −4.7) | −2.4 (−4.8 to 0.1) | 0.053 |
| 12 mo | −6.8 (−9.6 to −4.1) | −7.0 (−9.8 to −4.3) | −0.2 (−4.1 to 3.7) | 0.91 |
| Fat mass, % | ||||
| 3 mo | −0.3 (−1.2 to 0.7) | −1.4 (−2.4 to −0.4) | −1.1 (−2.5 to 0.3) | 0.119 |
| 6 mo | −0.2 (−1.1 to 0.7) | −1.5 (−2.5 to −0.6) | −1.3 (−2.6 to 0.0) | 0.050 |
| 12 mo | −0.10 (−1.4 to 1.4) | −1.8 (−3.2 to −0.4) | −1.8 (−3.8 to 0.2) | 0.083 |
| Lean mass, % | ||||
| 3 mo | 0.7 (−0.4 to 1.7) | 1.7 (0.7 to 2.8) | 1.1 (−0.4 to 2.6) | 0.156 |
| 6 mo | 0.2 (−0.7 to 1.2) | 1.6 (0.6 to 2.7) | 1.4 (−0.0 to 2.8) | 0.052 |
| 12 mo | −0.6 (−1.9 to 0.8) | 1.5 (0.1 to 2.8) | 2.1 (0.1 to 4.0) | 0.037 |
| Systolic blood pressure, mm Hg | ||||
| 3 mo | −2.0 (−4.7 to 0.6) | −6.2 (−8.9 to −3.6) | −4.2 (−8.1 to −0.3) | 0.034 |
| 6 mo | −2.8 (−5.4 to −0.3) | −4.4 (−7.0 to −1.9) | −1.6 (−5.3 to 2.1) | 0.39 |
| 12 mo | −4.4 (−7.9 to −1.0) | −0.8 (−4.3 to 2.7) | 3.7 (−1.3 to 8.6) | 0.146 |
| LDL cholesterol level, mmol/L‡ | ||||
| 3 mo | 0.09 (−0.10 to 0.28) | −0.04 (−0.23 to 0.16) | −0.13 (−0.40 to 0.14) | 0.34 |
| 6 mo | 0.08 (−0.09 to 0.24) | −0.02 (−0.18 to 0.15) | −0.09 (−0.32 to 0.14) | 0.42 |
| 12 mo | 0.04 (−0.20 to 0.29) | 0.03 (−0.22 to 0.27) | −0.02 (−0.37 to 0.33) | 0.91 |
| HDL cholesterol level, mmol/L‡ | ||||
| 3 mo | −0.08 (−0.15 to −0.01) | 0.03 (−0.04 to 0.10) | 0.11 (0.01 to 0.21) | 0.031 |
| 6 mo | −0.05 (−0.12 to 0.01) | 0.11 (0.05 to 0.18) | 0.17 (0.08 to 0.26) | <0.001 |
| 12 mo | −0.01 (−0.11 to 0.09) | 0.27 (0.17 to 0.38) | 0.28 (0.14 to 0.42) | <0.001 |
| Total–HDL cholesterol ratio | ||||
| 3 mo | 0.3 (0.1 to 0.5) | −0.2 (−0.4 to 0.1) | −0.4 (−0.7 to −0.1) | 0.007 |
| 6 mo | 0.2 (0.1 to 0.4) | −0.3 (−0.5 to −0.1) | −0.5 (−0.8 to −0.2) | <0.001 |
| 12 mo | 0.1 (−0.2 to 0.4) | −0.5 (−0.8 to −0.2) | −0.6 (−1.1 to −0.1) | 0.017 |
| Triglyceride level, mmol/L§ | ||||
| 3 mo | −0.02 (−0.23 to 0.19) | −0.29 (−0.50 to −0.08) | −0.28 (−0.57 to 0.02) | 0.069 |
| 6 mo | −0.06 (−0.24 to 0.13) | −0.31 (−0.49 to −0.13) | −0.26 (−0.51 to 0.00) | 0.051 |
| 12 mo | −0.13 (−0.33 to 0.07) | −0.34 (−0.55 to −0.14) | −0.21 (−0.50 to 0.07) | 0.140 |
| C-reactive protein level, nmol/L | ||||
| 3 mo | 5.7 (−5.7 to 18.1) | −10.5 (−21.9 to 1.9) | −16.2 (−33.3 to 1.0) | 0.062 |
| 6 mo | 3.8 (−6.7 to 14.3) | −8.6 (−19.0 to 1.0) | −13.3 (−27.6 to 1.9) | 0.078 |
| 12 mo | −0.0 (−17.1 to 16.2) | −6.7 (−23.8 to 9.5) | −6.7 (−29.5 to 17.1) | 0.57 |
| 10-y Framingham risk score, % | ||||
| 3 mo | 0.9 (−0.1 to 1.9) | −0.6 (−1.5 to 0.4) | −1.5 (−2.9 to −0.1) | 0.037 |
| 6 mo | 0.7 (−0.1 to 1.4) | −0.8 (−1.5 to −0.1) | −1.5 (−2.5 to −0.4) | 0.006 |
| 12 mo | 0.4 (−0.6 to 1.4) | −1.0 (−2.0 to −0.0) | −1.4 (−2.8 to 0.0) | 0.053 |
Appendix Table 2.
Predicted Mean Differences in Changes in Cardiovascular Risk Factors From Baseline, by Assigned Dietary Group: Black Persons
| Variable | Predicted Mean Difference (95% CI)* | P Value† | ||
|---|---|---|---|---|
| Low−Fat Diet (n = 73) | Low−Carbohydrate Diet (n = 75) | Mean Difference in Change | ||
| Body weight, kg | ||||
| 3 mo | −1.8 (−3.1 to −0.6) | −5.2 (−6.4 to −4.0) | −3.3 (−5.1 to −1.6) | <0.001 |
| 6 mo | −1.6 (−2.9 to −0.2) | −4.9 (−6.2 to −3.6) | −3.3 (−5.2 to −1.5) | <0.001 |
| 12 mo | −1.1 (−3.1 to 0.9) | −4.4 (−6.3 to −2.5) | −3.3 (−6.1 to −0.5) | 0.019 |
| Waist circumference, cm | ||||
| 3 mo | −4.3 (−6.0 to −2.7) | −5.0 (−6.6 to −3.4) | −0.6 (−3.0 to 1.7) | 0.59 |
| 6 mo | −4.1 (−5.9 to −2.4) | −5.5 (−7.2 to −3.8) | −1.4 (−3.9 to 1.1) | 0.27 |
| 12 mo | −3.7 (−6.3 to −1.1) | −6.6 (−9.1 to −4.1) | −2.9 (−6.5 to 0.8) | 0.119 |
| Fat mass, % | ||||
| 3 mo | −0.2 (−1.0 to 0.6) | −0.9 (−1.6 to −0.1) | −0.7 (−1.8 to 0.4) | 0.193 |
| 6 mo | 0.1 (−0.6 to 0.9) | −0.8 (−1.5 to −0.1) | −1.0 (−2.0 to 0.0) | 0.060 |
| 12 mo | 0.7 (−0.3 to 1.8) | −0.8 (−1.7 to 0.2) | −1.5 (−2.9 to −0.1) | 0.037 |
| Lean mass, % | ||||
| 3 mo | 0.2 (−0.6 to 1.0) | 1.5 (0.7 to 2.3) | 1.3 (0.2 to 2.4) | 0.026 |
| 6 mo | 0.0 (−0.7 to 0.8) | 1.4 (0.7 to 2.1) | 1.4 (0.3 to 2.4) | 0.013 |
| 12 mo | −0.3 (−1.3 to 0.7) | 1.2 (0.2 to 2.1) | 1.5 (0.1 to 2.9) | 0.039 |
| Systolic blood pressure, mm Hg | ||||
| 3 mo | −2.8 (−5.2 to −0.3) | −2.6 (−4.9 to −0.3) | 0.1 (−3.3 to 3.6) | 0.93 |
| 6 mo | −1.5 (−3.7 to 0.8) | −1.7 (−3.8 to 0.5) | −0.2 (−3.3 to 3.0) | 0.91 |
| 12 mo | 1.1 (−2.2 to 4.4) | 0.3 (−3.0 to 3.5) | −0.9 (−5.5 to 3.8) | 0.71 |
| LDL cholesterol level, mmol/L‡ | ||||
| 3 mo | −0.01 (−0.16 to 0.16) | 0.04 (−0.11 to 0.19) | 0.04 (−0.18 to 0.26) | 0.72 |
| 6 mo | −0.05 (−0.19 to 0.09) | −0.03 (−0.16 to 0.11) | 0.02 (−0.17 to 0.21) | 0.83 |
| 12 mo | −0.13 (−0.35 to 0.08) | −0.15 (−0.36 to 0.06) | −0.02 (−0.32 to 0.28) | 0.91 |
| HDL cholesterol level, mmol/L‡ | ||||
| 3 mo | 0.01 (−0.08 to 0.10) | 0.04 (−0.05 to 0.12) | 0.02 (−0.10 to 0.15) | 0.69 |
| 6 mo | 0.02 (−0.03 to 0.12) | 0.10 (0.02 to 0.17) | 0.05 (−0.06 to 0.16) | 0.37 |
| 12 mo | 0.11 (0.01 to 0.22) | 0.21 (0.11 to 0.32) | 0.10 (−0.05 to 0.26) | 0.190 |
| Overall | – | – | – | 0.98 |
| Total–HDL cholesterol ratio | ||||
| 3 mo | −0.0 (−0.2 to 0.2) | −0.1 (−0.3 to 0.1) | −0.1 (−0.4 to 0.3) | 0.70 |
| 6 mo | −0.1 (−0.3 to 0.1) | −0.2 (−0.4 to −0.0) | −0.1 (−0.4 to 0.1) | 0.34 |
| 12 mo | −0.2 (−0.4 to 0.1) | −0.5 (−0.7 to −0.2) | −0.3 (−0.6 to 0.1) | 0.112 |
| Triglyceride level, mmol/L§ | ||||
| 3 mo | 0.10 (−0.01 to 0.22) | −0.14 (−0.25 to −0.03) | −0.24 (−0.40 to −0.08) | 0.003 |
| 6 mo | 0.07 (−0.03 to 0.17) | −0.13 (−0.23 to −0.04) | −0.20 (−0.34 to −0.07) | 0.004 |
| 12 mo | −0.00 (−0.12 to 0.12) | −0.13 (−0.25 to −0.01) | −0.13 (−0.30 to 0.04) | 0.137 |
| C−reactive protein level, nmol/L | ||||
| 3 mo | 1.9 (−12.4 to 16.2) | 1.9 (−11.4 to 15.2) | −0.0 (−19.0 to 19.0) | 0.99 |
| 6 mo | 6.7 (−4.8 to 17.1) | −1.0 (−10.5 to 9.5) | −6.7 (−21.9 to 8.6) | 0.36 |
| 12 mo | 16.2 (4.8 to 26.7) | −4.8 (−15.2 to 5.7) | −21.0 (−36.2 to −4.8) | 0.010 |
| 10−y Framingham risk score, % | ||||
| 3 mo | 0.0 (−0.6 to 0.6) | −0.3 (−0.8 to 0.2) | −0.3 (−1.1 to 0.5) | 0.44 |
| 6 mo | 0.1 (−0.3 to 0.6) | −0.5 (−0.9 to −0.1) | 0.6 (−1.2 to 0.0) | 0.050 |
| 12 mo | 0.3 (−0.3 to 1.0) | −0.8 (−1.4 to −0.2) | 1.2 (−2.0 to −0.3) | 0.009 |
Appendix Table 3.
Symptoms Reported by Participants
| Symptom | Participants (95% CI), n | P Value* | |
|---|---|---|---|
| Low-Fat Diet (n = 73) | Low-Carbohydrate Diet (n = 75) | ||
| Constipation | |||
| 3 mo | 13 (7–24) | 19 (11–30) | 0.38 |
| 6 mo | 19 (11–31) | 18 (10–29) | 0.84 |
| 12 mo | 17 (9–28) | 11 (5–22) | 0.40 |
| Fatigue | |||
| 3 mo | 9 (4–19) | 17 (10–28) | 0.156 |
| 6 mo | 22 (13–33) | 18 (10–29) | 0.57 |
| 12 mo | 16 (8–27) | 15 (8–26) | 0.91 |
| Headache | |||
| 3 mo | 18 (11–30) | 6 (2–15) | 0.030 |
| 6 mo | 16 (8–27) | 12 (6–23) | 0.57 |
| 12 mo | 22 (13–34) | 11 (5–21) | 0.110 |
| Thirst | |||
| 3 mo | 6 (2–15) | 14 (8–25) | 0.125 |
| 6 mo | 9 (4–20) | 12 (6–23) | 0.65 |
| 12 mo | 10 (5–20) | 12 (6–23) | 0.81 |
| Polyuria | |||
| 3 mo | 8 (3–17) | 3 (1–10) | 0.23 |
| 6 mo | 5 (1–15) | 4 (1–12) | 0.76 |
| 12 mo | 4 (1–14) | 2 (0–10) | 0.39 |
| Diarrhea | |||
| 3 mo | 5 (2–14) | 4 (1–13) | 0.94 |
| 6 mo | 5 (2–15) | 5 (2–15) | 0.99 |
| 12 mo | 3 (1–13) | 1 (0–13) | 0.60 |
| Heartburn | |||
| 3 mo | 12 (6–23) | 7 (3–16) | 0.40 |
| 6 mo | 11 (5–22) | 6 (2–17) | 0.38 |
| 12 mo | 18 (10–31) | 9 (4–19) | 0.141 |
| Gas | |||
| 3 mo | 15 (9–26) | 19 (11–30) | 0.59 |
| 6 mo | 22 (13–35) | 17 (10–29) | 0.51 |
| 12 mo | 23 (14–35) | 16 (9–27) | 0.32 |
| Nausea† | |||
| 3 mo | 2 | 4 | – |
| 6 mo | 2 | 0 | – |
| 12 mo | 3 | 0 | – |
| Vomiting† | |||
| 3 mo | 0 | 1 | – |
| 6 mo | 0 | 0 | – |
| 12 mo | 0 | 0 | – |
| Decreased appetite† | |||
| 3 mo | 1 | 3 | – |
| 6 mo | 2 | 0 | – |
| 12 mo | 2 | 1 | – |
Footnotes
Reproducible Research Statement: Study protocol and data set: Not available. Statistical code: Available from Dr. Bazzano (lbazzano@tulane.edu).
Author Contributions: Conception and design: L.A. Bazzano, T. Hu, K. Reynolds, L. Yao, M.J. Klag, J. He.
Analysis and interpretation of the data: L.A. Bazzano, T. Hu, Y. Liu, C.S. Chen, M.J. Klag, P.K. Whelton, J. He.
Drafting of the article: L.A. Bazzano, T. Hu.
Critical revision of the article for important intellectual content: L.A. Bazzano, T. Hu, K. Reynolds, L. Yao, P.K. Whelton, J. He.
Final approval of the article: L.A. Bazzano, K. Reynolds, M.J. Klag, P.K. Whelton, J. He.
Provision of study materials or patients: L.A. Bazzano, C. Bunol.
Statistical expertise: T. Hu, C.S. Chen.
Obtaining of funding: L.A. Bazzano.
Administrative, technical, or logistic support: L.A. Bazzano, L. Yao, C. Bunol.
Collection and assembly of data: L.A. Bazzano, L. Yao, C.S. Chen, J. He. Fa, Ta1-a3
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 3.Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 5.Davis NJ, Tomuta N, Schechter C, Isasi CR, Segal-Isaacson CJ, Stein D, et al. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care. 2009;32:1147–52. doi: 10.2337/dc08-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–99. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 8.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 9.Stone NJ, Van Horn L. Therapeutic lifestyle change and Adult Treatment Panel III: evidence then and now. Curr Atheroscler Rep. 2002;4:433–43. doi: 10.1007/s11883-002-0047-x. [DOI] [PubMed] [Google Scholar]
- 10.Nutrition Data System for Research. The Minnesota Nutrition Data System. Minneapolis, MN: University of Minnesota; 2005. [Google Scholar]
- 11.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 12.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–81. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 15.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–23. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 16.Seshadri P, Iqbal N, Stern L, Williams M, Chicano KL, Daily DA, et al. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. Am J Med. 2004;117:398–405. doi: 10.1016/j.amjmed.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 18.Molenberghs G, Kenward M. Missing Data in Clinical Studies. Hoboken, NJ: J Wiley; 2007. [Google Scholar]
- 19.Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90:23–32. doi: 10.3945/ajcn.2008.27326. [DOI] [PubMed] [Google Scholar]
- 20.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–57. doi: 10.7326/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 23.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Dietary Intervention Randomized Controlled Trial (DIRECT) Group Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 24.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 25.Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS, Jr, et al. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2012;176(Suppl 7):S44–54. doi: 10.1093/aje/kws264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–34. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–81. [PubMed] [Google Scholar]
- 28.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 29.Cardillo S, Seshadri P, Iqbal N. The effects of a low-carbohydrate versus low-fat diet on adipocytokines in severely obese adults: three-year follow-up of a randomized trial. Eur Rev Med Pharmacol Sci. 2006;10:99–106. [PubMed] [Google Scholar]
- 30.US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 31.Larosa JC, Fry AG, Muesing R, Rosing DR. Effects of high-protein, low-carbohydrate dieting on plasma lipoproteins and body weight. J Am Diet Assoc. 1980;77:264–70. [PubMed] [Google Scholar]