Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk (original) (raw)
. Author manuscript; available in PMC: 2016 Jan 31.
Published in final edited form as: Mol Nutr Food Res. 2014 Dec 5;59(2):323–333. doi: 10.1002/mnfr.201400270
Abstract
Scope
Systemic inflammation, endothelial dysfunction, and oxidative stress are involved in the pathogenesis of the metabolic syndrome (MetS). Epidemiological evidence supports an association between whole soy food consumption and reduced risk of cardiovascular disease (CVD). The objective of this randomized, controlled, crossover study was to evaluate the effects of soy nut consumption on inflammatory biomarkers and endothelial function and to assess whether isoflavone metabolism to secondary products, equol and/or _O_-desmethylangolensin (ODMA), modifies these responses.
Methods and Results
n=17 adults at cardiometabolic risk were randomly assigned to the order of two snack interventions, soy nuts and macronutrient-matched control snack, for four weeks each, separated by a two week washout period. Outcome measures included biomarkers of inflammation, oxidative stress, and glycemic control (ELISA and clinical analyzers), endothelial function and arterial stiffness (peripheral arterial tonometry (PAT)), and isoflavone metabolites (LC-MS/MS). Results revealed that consuming soy nuts improved arterial stiffness as assessed by the augmentation index using PAT (_P_=0.03), despite lack of improvement in inflammatory biomarkers. Addition of equol and/ODMA production status as covariates did not significantly change these results.
Conclusions
Soy nuts when added to a usual diet for one month provide some benefit on arterial stiffness in adults at cardiometabolic risk.
Keywords: cardiometabolic risk, endothelial function, inflammation, isoflavone, soy
1) Introduction
Background
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the US [1]. Metabolic syndrome (MetS) is a clustering of risk factors for CVD and diabetes mellitus [2], affecting approximately 34% of adults in the US and its prevalence is increasing [1]. Because of the heterogeneity of MetS pathologies and more than one definition of MetS, a more broad term, cardiometabolic risk, has been coined that refers to CVD risk factors which contribute to MetS [3]. Traditional risk factors fail to predict up to 50% of the development of CVD, emphasizing the importance of other cardiometabolic risk factors [4]. Among these are multiple biological pathways that underlie the majority of vascular pathologies and involve inflammation, endothelial dysfunction [5], and oxidative stress [6].
Few investigations have studied the effects of whole foods on inflammatory biomarkers [7] and foods rich in polyphenols seem to provide significant benefit to endothelial function [8]. Epidemiological evidence demonstrates that whole soy food consumption is associated with reduced cardiometabolic risk in Asian populations [9, 10]. Further, there are limited data from randomized clinical trials on biological effects of whole soy foods consumed by Western populations. Soy nuts are soybeans that are minimally processed, non-fermented, and roasted. Soy nuts provide a concentrated source of the unique soy nutrient profile: high quality protein, PUFAs, minerals, dietary fiber, phytosterols, and isoflavones, among other bioactive compounds [11].
Of all whole soy foods, soy nuts contain the highest level of isoflavones [11–13]. In Asian countries, the mean isoflavone consumption is 25–50 mg/day [14, 15], whereas in Western countries 1–2 mg/day is typical [16, 17]. One of the primary isoflavones, daidzein, is metabolized by gut microflora and can undergo a ring cleavage to form _O_-desmethylangolensin (ODMA) and/or deoxygenation to convert to equol [18]. Not all individuals are equol producers [19] or ODMA producers [20]. Up to 60% of Asian populations and 35% Western populations are equol producers [21] and 80–95% of individuals are ODMA producers [20].
Further, the equol hypothesis has been proposed [19], which suggests that many of the biological effects of soy relate to metabolic actions due to equol, a metabolite produced by gut microflora in only some individuals [18, 19]. Equol is a biologically active isoflavone metabolite with estrogenic effects, affinity for estrogen receptors, and antioxidant activity [19]. The positive effects of soy on cardiometabolic risk factors may not be mediated primarily through estrogen receptors [22] and the food matrix may impact isoflavone metabolism [23] with additive or synergistic effects on health outcomes [11]. Few studies have examined effects of soy consumption on cardiometabolic risk factors by examining the relationship to equol and/or ODMA producers [24].
Objectives
Therefore, the purpose of this study was to determine whether soy consumption would influence cardiometabolic risk factors. The hypothesis was that the consumption of a whole soy food snack (soy nuts), compared to a macronutrient-matched control snack, would significantly attenuate inflammatory biomarkers and endothelial dysfunction in adults at cardiometabolic risk. A secondary hypothesis of this study was that participants who produce equol and/or ODMA would have significantly better inflammatory, vascular endothelial, and glycemic responses to soy consumption.
2) Materials and Methods
Study Participants
This study was approved by the University of California, Davis Institutional Review Board and informed consent of all participants was obtained. Participants were recruited from the surrounding county via flyers, newspapers, websites, and advertisements during community events. Interested participants were screened for inclusionary and exclusionary criteria. Participants who qualified after this initial screening were then invited to provide a fasting blood draw and anthropometric measurements to confirm cardiometabolic risk.
All participants were postmenopausal women and men over the age of 45 years, ensuring that participants had a low estrogen metabolic milieu. Participants were also required to meet the MetS blood pressure criteria of ≥ 130/85 mmHg and at least one of the remaining four criteria for MetS: elevated waist circumference by population definition, elevated triglycerides ≥ 150 mg/dL (1.7 mmol/L), reduced HDL-cholesterol < 50 mg/dL (1.3 mmol/L) for women and < 40 mg/dL (1.0 mmol/L) for men, or elevated fasting glucose ≥ 100 mg/dL (5.6 mmol/L). A risk factor was also met by use of medication for these parameters [2].
Exclusionary criteria included smoking, soy allergy, unwilling to avoid soy products during the entire study, other chronic diseases (diabetes, liver disease, etc.), > 2 medications per cardiometabolic risk factor, taking other medications or supplements that interfere with study outcomes (selective estrogen receptor modulators, hormone replacement therapy, etc.), reported difficulty with phlebotomy, regular blood donor, menses within the past year, acutely ill, elite athlete, vegetarian or vegan, and on a weight loss diet.
Study Design
The study was conducted at the University of California, Davis Ragle Human Nutrition Research Center. The study design was a randomized, controlled, crossover trial, so that each participant served as his or her own control and received both snacks in a random order. The snacks consisted of soy nuts or a macronutrient-matched control snack that were provided for four weeks, separated by a two week washout period to remove any potential carryover effect (Figure 1). There were four study days: before and after each snack intervention as baseline and follow-up visits, respectively. For each visit, participants arrived after an overnight fast to the research center. Reactive hyperemia peripheral arterial tonometry (PAT) measurements were obtained first, followed by anthropometrics and a blood draw. Participants returned unused snack containers and provided a spot urine sample at the two follow-up visits. Food records were obtained three days prior to each snack intervention and three days during each snack intervention for a total of 12 days of food records.
Figure 1.
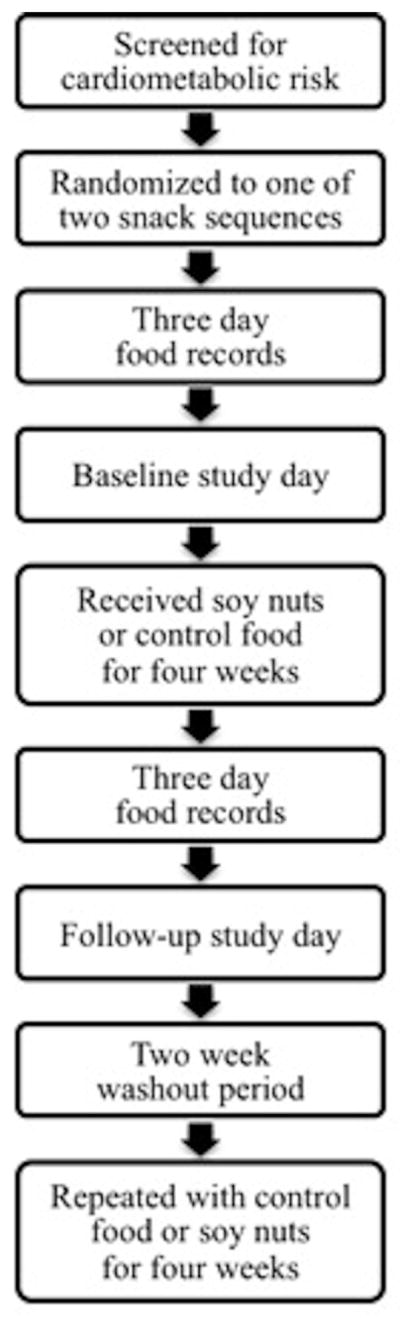
Study Flow
Snack Interventions
The soy nuts were donated in bulk (Genisoy® Food Company, Inc., Fairfield, CA) and were salted and oil roasted for palatability. The soy nuts were analyzed for isoflavone content using HPLC according to AOAC Method 2001.10. 101 mg was the target aglycone equivalent level due to its beneficial effects from other studies [25, 26]. Based on this analysis, 70 g of soy nuts provided 101 mg of aglycone equivalents that was distributed as 55 mg of genistein, 42 mg of daidzein, and 4 mg of glycitein. The soy nuts were weighed to the nearest gram and amounted to roughly 0.5 of a cup.
The control snack consisted of cookies developed and baked in the metabolic kitchen at our research center. The control snack was matched to soy nuts for kcals, macronutrients, fiber, and sodium and consisted of three small cookies comprised of vanilla whey protein powder (Body Fortress), sugar cookie mix (Betty Crocker), and generic unflavored fiber, prepackaged egg whites, and canola oil (Table 1).
Table 1.
Snack Composition
| Nutrient | Units | Soy Nutsa | Control Snackb |
|---|---|---|---|
| Calories | (kj) | 1,382 | 1,457 |
| (kcals) | 330 | 348 | |
| Fat | (g) | 18 | 17 |
| Sodium | (mg) | 114 | 113 |
| Potassium | (mg) | 1,030 | 170 |
| Carbohydrate | (g) | 23 | 27 |
| Sugars | (g) | 5 | 5 |
| Fiber | (g) | 12 | 12 |
| Protein | (g) | 25 | 25 |
| Isoflavonesc | (mg) | 101 | Non-detectable |
The snack interventions were portioned into 30 containers so that there were enough for four weeks plus extra to account for potential spillage. Participants were instructed not to consume other soy products (soy milk, tofu, etc.) during the study to avoid potential background diet confounding effects. Participants were also instructed to maintain their usual dietary patterns and physical activity levels throughout the study.
Biochemical Assays
Approximately 65 mL of blood were drawn into serum separator tubes, heparin plasma separator tubes, and EDTA tubes. Plasma blood samples were centrifuged at 1,800 g for 15 minutes at 4°C and serum at 1,000 g for 15 minutes at room temperature. Serum and plasma samples were then aliquoted and frozen at −80°C until lab analyses could be performed in batches. Biochemical assays were analyzed at the University of California, Davis, with select assays performed at the Food and Drug Administration laboratories at the Institute for Food Safety and Health (soy nut analysis) and University of Hawai’i, Mānoa (urinary isoflavone and creatinine analysis). All were performed according to manufacturers’ instructions and included appropriate quality controls.
Tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-10 were analyzed by ELISA (R&D Systems, Minneapolis, MN). IL-18 and oxidized LDL (oxLDL) were also analyzed by ELISAs (MBL International, Woburn, MA and Mercodia, Winston Salem, NC, respectively). High sensitivity CRP and insulin were analyzed by an Immulite 1000 Immunoassay Analyzer (Fisher Scientific, Pittsburgh, PA). Glucose, lipid panel, and clinical chemistries were analyzed by a Cobas c111 Analyzer (Fisher Scientific, Pittsburgh, PA). Baseline hemoglobin A1c was analyzed by a direct enzymatic assay (Diazyme, Poway, CA). Glucose and insulin levels were used to assess insulin resistance using the homeostasis model assessment (HOMA)-insulin resistance and HOMA-β cell function equations by Matthews et al. [27], quantitative insulin sensitivity check index (QUICKI) equation by Katz et al. [28], and the glucose: insulin ratio [29].
Urine samples were collected in individual urine collection containers, then aliquoted and frozen at −80°C until lab analyses could be performed in batches. Urine samples were analyzed for isoflavone metabolites using LC-MS/MS according to the protocol developed by Franke et al. [30, 31]; creatinine was determined with a test kit based on the Jaffé reaction (Randox Laboratories, Crumlink, UK) on a Cobas MiraPlus CC Clinical Analyzer (Roche Diagnostics, Indianapolis, IN). From the isoflavone metabolites, equol producers and ODMA producers were calculated using equol/daidzein ≥ 0.018 with a daidzein threshold of ≥ 2 nmol/mg creatinine for equol producers [21] and a detectable level of 339 nmol/L for ODMA producers [32].
Vascular Function
PAT measurements were performed using the EndoPAT 2000 system and software (Itamar Medical Inc., Caesarea, Israel). In brief, participants rested in a quiet, dimly lit, and temperature controlled room in a bed for at least 15 minutes. A PAT probe was placed on one finger on each hand to record pulse wave amplitude during baseline, occlusion, and reactive hyperemia. After a baseline period, the stimulus of occlusion was applied as a blood pressure cuff inflated to a suprasystolic blood pressure (50 mmHg above usual) for five minutes. Reactive hyperemia was induced with the blood pressure cuff deflation [33].
The PAT measurements included the reactive hyperemia index and augmentation index [34]: a higher reactive hyperemia index reflects flow mediated vasodilation [33] and a lower augmentation index reveals less arterial stiffness [35]. The reactive hyperemia index is defined as the ratio of the mean pulse wave amplitude during reactive hyperemia relative to the baseline period [36], normalized to the control arm [37]. A reactive hyperemia score of ≤ 1.67 represents endothelial dysfunction, 1.68–2.0 intermediate endothelial function, and > 2.1 protected endothelium (http://www.itamar-medical.com/). Augmentation index is a measure of arterial stiffness [35], which is defined as the percentage of the peak pressure attributed to the pressure of the inflection point corresponding to the reflected pulse wave [36]. A relatively lower augmentation index is considered beneficial, as a higher augmentation index has been significantly associated with more cardiometabolic risk factors [35].
Food Records
Food records were obtained to estimate current dietary intake and assess potential changes in dietary intake. The study dietitians instructed participants on how to fill out three day food records and the food records were analyzed using nutrition software (Food Processor SQL Version 10.2, ESHA Research, Salem, OR).
Statistical Analysis
A retrospective power analysis revealed adequate power for endothelial function using a sample size of n=18 per snack intervention, type I error rate of 0.05, observed differences, and observed variability. Primary outcome measures were endothelial function, inflammatory biomarkers, and glycemic control indices. Curve fitting and interpolation of ELISA values were obtained by Graphpad 4.03 (Graphpad Software, La Jolla, CA). Variables not meeting normality and/or homogeneity of variance assumptions were transformed, usually by log transformation. Data analysis was performed using a linear mixed model using Proc Mixed over the snack intervention relative differences, calculated as (follow-up visit – baseline visit) / baseline visit (SAS Version 9.3, Cary, NC). Main effects were snack, sequence, and snack*sequence interaction and random effects included participant. Covariates were tested using baseline outcome measurements as continuous variables and equol producers and ODMA producers as dichotomous variables. Statistical significance was considered with P<0.05 and data are presented as means ± SD or least squares means (LSM) ± SE.
3) Results
Participant Characteristics
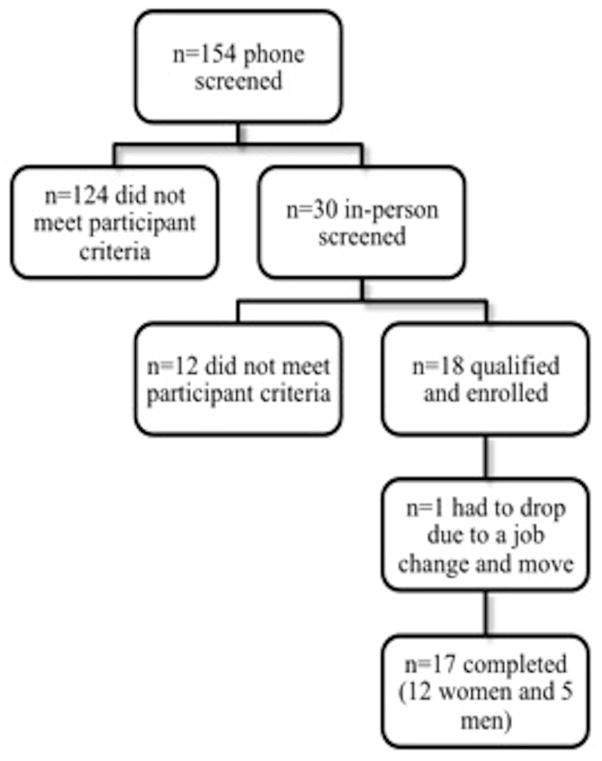
Out of the 18 participants who were enrolled, one participant withdrew from the study due to a job change that required a move out of the county (Figure 2). Of the 17 participants who completed the study, 15 met the criteria for MetS [2]. Despite the addition of ~340 calories a day for eight weeks, participants had no significant change in weight (Table 2).
Figure 2.
Participant Flow
Table 2.
Participant Characteristics (n=17)a
| Characteristic | Units | Mean ± SD |
|---|---|---|
| Age | (years) | 56 ± 5 |
| Gender | (% women) | 71 |
| Weight | (kg) | 86.7 ± 12.6 |
| BMI | (kg/m2) | 31.2 ± 4.0 |
| Ethnicity | (% Caucasian) | 59 |
| Metabolic syndromeb | (number of criteria out of 5) | 3.5 ± 1.1 |
| Waist circumference | (cm) | 106.5 ± 10.6 |
| Triglycerides | (mg/dL) | 168.4 ± 78.6 |
| HDL-cholesterol | (mg/dL) | 46.4 ± 12.7 |
| Systolic blood pressure | (mmHg) | 142 ± 12 |
| Diastolic blood pressure | (mmHg) | 87 ± 10 |
| Glucose | (mg/dL) | 96.4 ± 13.3 |
| Total cholesterol | (mg/dL) | 213.4 ± 30.7 |
| LDL-cholesterol | (mg/dL) | 129.0 ± 31.1 |
Biochemical Assays and Vascular Function
The inflammatory and oxidative stress biomarkers did not reveal any significant differences between soy nuts and control snack for CRP, TNF-α, IL-6, IL-18, IL-10, and oxLDL. Baseline levels of the inflammatory biomarkers, CRP and IL-6, suggest systemic inflammation [38, 39], yet the other inflammatory biomarkers fall within normal limits [39–42] (Table 4) except for IL-1β, which was non-detectable.
Table 4.
Inflammatory and Oxidative Stress Biomarkers (n=17)
| Biomarkers | Units | Baselinea or Changeb | Soy Nuts | Control Snack | P valuec |
|---|---|---|---|---|---|
| CRP | (mg/L) | Baseline | 3.4 ± 4.7 | 3.7 ± 5.5 | NS |
| Change | 1.0 ± 0.4 | 0.1 ± 0.4 | |||
| TNF-α | (pg/mL) | Baseline | 1.3 ± 0.8 | 1.3 ± 0.7 | NS |
| Change | 0.01 ± 0.05 | −0.03 ± 0.05 | |||
| IL-6 | (pg/mL) | Baseline | 2.0 ± 1.0 | 1.9 ± 1.1 | NS |
| Change | 0.08 ± 0.1 | 0.2 ± 0.1 | |||
| IL-18 | (pg/mL) | Baseline | 49.5 ± 15.2 | 55.5 ± 18.9 | NS |
| Change | 0.08 ± 0.04 | −0.01 ± 0.04 | |||
| IL-10 | (pg/mL) | Baseline | 0.2 ± 0.2 | 0.3 ± 0.1 | NS |
| Change | 1.5 ± 0.8 | 0.3 ± 0.8 | |||
| OxLDLd | (U/L) | Baseline | 53.0 ± 12.8 | 48.2 ± 8.4 | NS |
| Change | −0.1 ± 0.3 | 0.1 ± 0.3 |
Of the PAT measurements, baseline reactive hyperemia index demonstrated intermediate endothelial dysfunction and was not significantly different between soy nuts and control snack (Table 5). However, the change in augmentation index (%) was significantly different between snack intervention (_P_=0.03), with a lower LSM for soy nuts 19.7 ± 4.1, compared to the control snack 23.5 ± 4.1 (Figure 3).
Table 5.
Vascular and Glycemic Outcomes (n=17)
| Outcomes | Units | Baselinea or Changeb | Soy Nuts | Control Snack | P valuec |
|---|---|---|---|---|---|
| Reactive hyperemia index | (−) | Baseline | 2.0 ± 0.6 | 1.8 ± 0.6 | NS |
| Change | 0.06 ± 0.07 | 0.08 ± 0.07 | |||
| Augmentation index | (%) | Baseline | 25.2 ± 20.3 | 23.8 ± 16.5 | 0.03 |
| Change | −0.1 ± 0.2 | 0.08 ± 0.2 | |||
| Glucose | (mg/dL) | Baseline | 109.0 ± 8.0 | 107.8 ± 11.1 | 0.02 |
| Change | −0.03 ± 0.02 | −0.04 ± 0.02 | |||
| Insulin | (μIU/mL) | Baseline | 12.9 ± 6.5 | 12.7 ± 7.5 | NS |
| Change | 0.08 ± 0.08 | 0.1 ± 0.08 |
Figure 3.
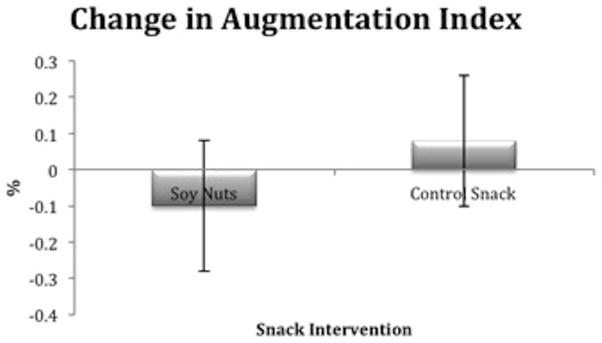
Relative change in augmentation index (%) was significantly different after soy nuts, compared to the control snack (_P_=0.03). The least squares means (LSM) ± SE after soy nuts was 19.7 ± 4.1 and after control snack, 23.5 ± 4.1.
There were no significant differences between the two snack interventions at baseline for fasting glucose. Fasting glucose (mg/dL) declined after both snack interventions, but the LSM remained significantly higher after soy nuts 107.7 ± 2.2, compared to the control snack 105.8 ± 2.2 (_P_=0.02) (Figure 4). Hemoglobin A1c revealed good long-term glycemic control and none of the participants reported a diagnosis of diabetes mellitus. Insulin was not significantly different between soy nuts and control snack, yet there was evidence of insulin resistance at baseline according to the HOMA equations [43], QUICKI equation [28], and glucose: insulin ratio [29], which did not change after the snack interventions (data not shown).
Figure 4.
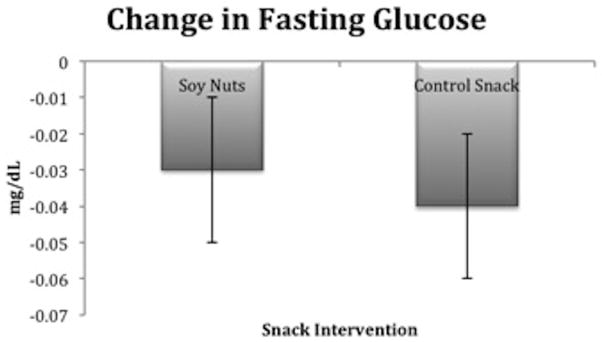
Relative change in glucose (mg/dL) was significantly different after soy nuts, compared to the control snack (_P_=0.02). The least squares means (LSM) ± SE after soy nuts was 107.7 ± 2.2 and after control snack, 105.8 ± 2.2.
Food Records
Averaging the baseline food records revealed that the participants consumed a diet consistent with the typical American diet: approximately 48% carbohydrate, 34% fat, and 17% protein; excessive intake of saturated fat, cholesterol, and sodium; and inadequate intake of dietary fiber, calcium, and potassium, as compared to the Dietary Reference Intakes. The food records during snack interventions revealed some significant differences in dietary intake between soy nuts and control snack, reflecting expected compositional differences between the two snack interventions (Table 6).
Table 6.
Food Records (n=17)
| Nutrient | Units | Baseline | Soy | Control | P valueb |
|---|---|---|---|---|---|
| Calories | (kj) | 8,884 ± 2,441 | 9,596 ± 3,848 | 8,667 ± 1,968 | NS |
| (kcals) | 2,122 ± 583 | 2,292 ± 919 | 2,070 ± 470 | NS | |
| Fat | (g) | 80.7 ± 31.4 | 87.4 ± 38.9 | 86.1 ± 28.5 | NS |
| Saturated fat | (g) | 24.6 ± 11.9 | 25.5 ± 13.8 | 20.6 ± 10.3 | NS |
| Trans fat | (g) | 0.9 ± 0.8 | 0.8 ± 1.1 | 0.7 ± 1.0 | NS |
| MUFA | (g) | 20.6 ± 9.4 | 20.7 ± 14.8 | 15.6 ± 6.5 | NS |
| PUFA | (g) | 11.4 ± 6.0 | 17.1 ± 8.0 | 8.5 ± 3.7 | 0.04 |
| Cholesterol | (mg) | 324 ± 162 | 286 ± 168 | 232 ± 151 | NS |
| Sugars | (g) | 86.4 ± 41.3 | 93.4 ± 53.5 | 83.1 ± 39.0 | NS |
| Sodium | (mg) | 3,008 ± 1,044 | 2,710 ± 1,057 | 2,646 ± 873 | NS |
| Carbohydrate | (g) | 254.5 ± 81.7 | 267.6 ± 129.0 | 232.2 ± 65.0 | NS |
| Fiber | (g) | 21.1 ± 7.7 | 34.4 ± 15.6 | 31.8 ± 10.5 | NS |
| Protein | (g) | 90.7 ± 26.3 | 108.7 ± 35.0 | 98.0 ± 20.2 | NS |
| Calcium | (mg) | 733 ± 264 | 849 ± 407 | 698 ± 333 | NS |
| Potassium | (mg) | 2,131 ± 782 | 3,220 ± 1,819 | 2,067 ± 935 | 0.04 |
Equol and/or ODMA Production
Six isoflavonoids were quantified: the native genistein and daidzein and their metabolites, dihydrogenistein, dihydrodaidzein, equol, and ODMA. As expected, levels of these isoflavonoids were significantly higher in response to soy nuts, compared to the control snack (P<0.05) (Table 3). 47% of participants (n=8) were equol producers and 71% (n=12), ODMA producers. Adding equol producers or ODMA producers as covariates in the linear mixed model did not result in any significant findings, suggesting that equol and/or ODMA production did not influence inflammatory or oxidative stress biomarkers, vascular function, or glycemic control in this population.
Table 3.
Isoflavone Metabolite Levels in Urine (n=17)
| Isoflavone | Units | Soy NutsMean ± SD | Control SnackMean ± SD |
|---|---|---|---|
| Genistein | (nmol/mg creatinine) | 24.4 ± 22.5 | 0.5 ± 0.9 |
| Dihydrogenistein | (nmol/mg creatinine) | 10.1 ± 18.1 | 0.8 ± 2.6 |
| Daidzein | (nmol/mg creatinine) | 54.9 ± 78.0 | 1.1 ± 2.0 |
| Dihydrodaidzein | (nmol/mg creatinine) | 32.3 ± 42.0 | 1.8 ± 3.9 |
| Equol | (nmol/mg creatinine) | 13.7 ± 19.9 | 1.1 ± 2.0 |
| ODMA | (nmol/mg creatinine) | 22.5 ± 36.1 | 0.2 ± 0.3 |
Compliance
Compliance to soy nuts and control snack was estimated at 91% based on counting returned snack containers. Compliance was also assessed by the food records and mean levels of isoflavonoids. As expected, a robust response in urinary isoflavones and metabolites occurred during the soy nut snack intervention. Additionally, small amounts of urinary isoflavones and metabolites were detected in samples during the control snack intervention, likely reflecting intakes of other legumes and dairy products [44]. Collectively, compliance to the snack interventions was good.
4) Discussion
Summary
This study demonstrated that soy nut consumption, compared to a control snack, significantly improved, i.e. reduced, arterial stiffness, independent of any significant effects on biomarkers of inflammation and oxidative stress. Two similarly designed studies have also investigated soy nuts and cardiometabolic risk factors [25, 26, 45–48]. Consistent with the current study, these studies found improvements in endothelial function, which were assessed by biochemical markers predictive of vascular reactivity, such as nitric oxide [45–47] and soluble vascular cell adhesion molecule-1 [25, 26]. These studies also found significant differences beyond endothelial markers, including reduced inflammatory biomarkers CRP [26, 45], TNF-α, and IL-18 [45]. Inflammation may have been modulated to a greater extent in that study because the participants were more at-risk for CVD by meeting all five of the MetS criteria and not receiving medications for cardiometabolic risk factors, so beneficial responses were more likely to occur [45–47].
Biochemical Assays and Vascular Function
The finding of improved augmentation index based on PAT measurement is consistent with the literature on soy and vascular function. A meta-analysis of 17 trials investigating isoflavone containing soy products revealed a modest improvement in endothelial dysfunction as assessed by flow mediated dilation [49], an older endothelial function measurement [8]. A separate meta-analysis examined endothelial function in postmenopausal women and found that positive responses to soy isoflavone supplementation were dependent on baseline vascular function levels [50]. Of particular relevance to the current study, a review on arterial stiffness found that four out of five studies investigating soy or isoflavones had beneficial effects using other validated measures of arterial stiffness [51]. The current study finding of improved arterial stiffness with consumption of soy nuts as measured by PAT adds to the growing literature on soy consumption and arterial compliance.
The dietary data supports the hypothesis that the soy was responsible for the beneficial changes in augmentation index in this study. Nutrient analysis of the food records confirmed that there were no significant changes in macronutrient intakes or total energy between the snack interventions that might influence vascular function. Although the PUFA content with the soy nuts was greater than the control snack, recent studies have not shown differential effects of chronic PUFA and MUFA intake on arterial stiffness [52–54]. A beneficial effect of a high potassium diet on endothelial function has been reported, but the relevance to the current snack interventions is unclear, as the levels of potassium were 1.5 fold higher than the dietary potassium during the soy nut intervention used in this study [55].
Recent studies analyzing biochemical inflammatory biomarkers and vascular adhesion molecules after soy consumption have shown mostly non-significant findings [56–61]. Two recent studies with modest favorable results in select biomarkers reflect interventions of 6–12 months and, in one, the response was apparent only in mildly hypertensive women [59, 61]. A meta-analysis of 14 trials studying postmenopausal women and soy foods containing isoflavones found that CRP was non-significantly different from controls, although a subgroup analysis revealed that soy isoflavones significantly reduced CRP in postmenopausal women with elevated CRP at baseline [56]. Another review found that only two of seven randomized clinical studies demonstrated that soy foods influenced pro-inflammatory cytokines [57] and one of these studies found that a high isoflavone soy diet actually increased IL-6 in women [62]. A daidzein rich supplement was reported to downregulate genes associated with inflammatory pathways, including Toll-like receptors, but were not mediated through the estrogen-responsive genes [22]. Despite this molecular finding, the preponderance of the evidence from multiple clinical trials and meta-analyses suggests that soy foods and components do not modulate inflammation as detected by these select circulating biochemical assays.
Some studies have found beneficial effects of soy on biomarkers of oxidative stress when investigating other soy products and different oxidative stress biomarkers: reduced plasma malondialdehyde levels [46, 60], increased total plasma antioxidant capacity [46, 63, 64], reduced LDL oxidative stress capacity lag time [23], and decreased advanced oxidation protein products [63]. Consistent with the current study, other studies have not demonstrated a protective effect against oxidative stress [63–65]. One of these studies found that neither a high or low soy diet revealed antioxidant effects, as assessed by urinary isoprostanes in women [65]. This finding is particularly important, as isoprostanes are stable metabolic products of in vivo lipid oxidation and are considered the gold standard as a marker for oxidative stress [66].
In the current study, blood glucose decreased after both snack interventions but to a greater extent following the control snack. Two recent studies on soy supplementation reported improved glycemic control [67, 68]: equol supplements significantly decreased hemoglobin A1c levels in adults with MetS [67] and genistein tablets decreased glucose, insulin, and HOMA-insulin resistance in postmenopausal women with MetS [68]. However, two recent meta-analyses on glycemic control reported no overall effect of soy intake on glycemic control [69, 70]. Notably, when only studies utilizing whole soy foods or a soy diet were analyzed for glycemic control outcomes, a significant decrease in glucose was found [69]. Although this subgroup finding contrasts with the current study, soy nuts did decrease glucose, but did not reach significance within four weeks. It is possible that with increased length of exposure, the results may have achieved statistical and clinical significance.
Limitations and Strengths
This study had several limitations. First, it is possible that the effects of soy on inflammation and endothelial dysfunction occur locally at the lesion site, rather than at the systemic level, and therefore are not detected by changes in circulating inflammatory biomarkers [71]. Furthermore, there is great variability in inflammatory biomarkers and the best one to use has not yet been identified. The current study had a small sample size and short duration as a pilot trial. A larger and longer-term study may detect true differences in outcomes.
The current study is now the third study to investigate soy nut consumption [25, 26, 45–48] and the first soy nut study to use a usual diet as the background diet. It has a strong study design as a randomized, controlled, crossover study. Additionally, it is the only soy nut study to assess vascular function utilizing PAT.
Conclusion
In conclusion, consumption of soy nuts attenuates arterial stiffness, as assessed by PAT. Soy nuts did not result in clinically significant effects on inflammatory biomarkers and glycemic control. Interestingly, the ability of the individual to synthesize the isoflavone metabolites, equol and/or ODMA, did not alter the study findings. Overall, this study suggests that soy nuts have modest effects on attenuating endothelial dysfunction over four weeks in adults with features of MetS, which may contribute to the beneficial cardiovascular effects of soy consumption.
Acknowledgments
We thank Neil Willits, PhD for his statistical consultation, Joseph Jablonski, PhD for the soy nut isoflavone analysis, Krystle Tagorda for the clinical analyzer runs, and James Graham for the hemoglobin A1c analysis. We also thank our undergraduate interns for analyzing the food records and managing study data and our participants for volunteering in the study. EJR, CDL, and FMS designed the study, EJR and CDL conducted the research, EJR, CDL, and AAF performed the lab work, EJR analyzed the data and wrote the manuscript, EJR and FMS edited the manuscript, and FMS had primary responsibility for the final manuscript. All authors read and approved the final manuscript.
Study funding was provided by the USDA Agricultural Research Service (CRIS # CA-D*NTR-6316-H), University of California, Davis Henry A. Jastro Research Awards (grant numbers GGNBEJ1 and GGNBEJ2), and University of California, Davis Humanities Research Award (grant number FMSEJGS). NIH grants P30 CA71789 and S10 RR020890 supported the LC-MS/MS analyses. Genisoy® Food Company, Inc. donated the soy nuts and had no role in the design, analysis, or writing of this study.
Abbreviations
CVD
Cardiovascular Disease
CRP
C-reactive protein
DASH
Dietary Approaches to Stop Hypertension
HOMA
homeostasis model assessment
LSM
least squares means
ODMA
_O_-desmethylangolensin
oxLDL
oxidized LDL
MetS
metabolic syndrome
PAT
peripheral arterial tonometry
QUICKI
quantitative insulin sensitivity check index
TNF-α
tumor necrosis factor-α
Footnotes
Conflict of Interest: The authors report no conflict of interest.
5) References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 3.Stern N, Izkhakov Y. The metabolic syndrome revisited: “cardiometabolic risk” emerges as common ground between differing views of the ADA and AHA. Journal of the cardiometabolic syndrome. 2006;1:362–363. doi: 10.1111/j.1524-6175.2006.05999.x. [DOI] [PubMed] [Google Scholar]
- 4.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 2010;4:351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Chen AF, Chen DD, Daiber A, Faraci FM, et al. Free radical biology of the cardiovascular system. Clin Sci (Lond) 2012;123:73–91. doi: 10.1042/CS20110562. [DOI] [PubMed] [Google Scholar]
- 7.Calder PC, Ahluwalia N, Brouns F, Buetler T, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(Suppl 3):S5–78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 8.Flammer AJ, Anderson T, Celermajer DS, Creager MA, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan A, Franco OH, Ye J, Demark-Wahnefried W, et al. Soy protein intake has sex-specific effects on the risk of metabolic syndrome in middle-aged and elderly Chinese. J Nutr. 2008;138:2413–2421. doi: 10.3945/jn.108.097519. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Shu XO, Gao YT, Yang G, et al. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133:2874–2878. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]
- 11.Reinwald S, Akabas SR, Weaver CM. Whole versus the piecemeal approach to evaluating soy. J Nutr. 2010;140:2335S–2343S. doi: 10.3945/jn.110.124925. [DOI] [PubMed] [Google Scholar]
- 12.Valentin-Blasini L, Sadowski MA, Walden D, Caltabiano L, et al. Urinary phytoestrogen concentrations in the U.S. population (1999–2000) Journal of exposure analysis and environmental epidemiology. 2005;15:509–523. doi: 10.1038/sj.jea.7500429. [DOI] [PubMed] [Google Scholar]
- 13.Selma MV, Espin JC, Tomas-Barberan FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 14.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutrition and cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 15.Kokubo Y, Iso H, Ishihara J, Okada K, et al. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007;116:2553–2562. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 16.van Erp-Baart MA, Brants HA, Kiely M, Mulligan A, et al. Isoflavone intake in four different European countries: the VENUS approach. Br J Nutr. 2003;89(Suppl 1):S25–30. doi: 10.1079/BJN2002793. [DOI] [PubMed] [Google Scholar]
- 17.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007;137:1244–1252. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 18.Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140:1355S–1362S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 20.Frankenfeld CL. O-desmethylangolensin: the importance of equol’s lesser known cousin to human health. Adv Nutr. 2011;2:317–324. doi: 10.3945/an.111.000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke AA, Lai JF, Halm BM, Pagano I, et al. Equol production changes over time in postmenopausal women. J Nutr Biochem. 2012;23:573–579. doi: 10.1016/j.jnutbio.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Velpen V, Geelen A, Schouten EG, Hollman PC, et al. Estrogen receptor-mediated effects of isoflavone supplementation were not observed in whole-genome gene expression profiles of peripheral blood mononuclear cells in postmenopausal, equol-producing women. J Nutr. 2013;143:774–780. doi: 10.3945/jn.113.174037. [DOI] [PubMed] [Google Scholar]
- 23.Ahn-Jarvis J, Clinton SK, Riedl KM, Vodovotz Y, Schwartz SJ. Impact of food matrix on isoflavone metabolism and cardiovascular biomarkers in adults with hypercholesterolemia. Food & function. 2012;3:1051–1058. doi: 10.1039/c2fo10284f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230:155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 25.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med. 2007;167:1060–1067. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 26.Nasca MM, Zhou JR, Welty FK. Effect of soy nuts on adhesion molecules and markers of inflammation in hypertensive and normotensive postmenopausal women. Am J Cardiol. 2008;102:84–86. doi: 10.1016/j.amjcard.2008.02.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Katz A, Nambi SS, Mather K, Baron AD, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 29.Monzillo LU, Hamdy O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr Rev. 2003;61:397–412. doi: 10.1301/nr.2003.dec.397-412. [DOI] [PubMed] [Google Scholar]
- 30.Franke AA, Halm BM, Kakazu K, Li X, Custer LJ. Phytoestrogenic isoflavonoids in epidemiologic and clinical research. Drug testing and analysis. 2009;1:14–21. doi: 10.1002/dta.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franke AA, Hebshi SM, Pagano I, Kono N, et al. Urine accurately reflects circulating isoflavonoids and ascertains compliance during soy intervention. Cancer Epidemiol Biomarkers Prev. 2010;19:1775–1783. doi: 10.1158/1055-9965.EPI-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkinson C, Newton KM, Bowles EJ, Yong M, Lampe JW. Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am J Clin Nutr. 2008;87:679–687. doi: 10.1093/ajcn/87.3.679. [DOI] [PubMed] [Google Scholar]
- 33.Axtell AL, Gomari FA, Cooke JP. Assessing endothelial vasodilator function with the Endo-PAT 2000. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moerland M, Kales AJ, Schrier L, van Dongen MG, et al. Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. International journal of vascular medicine. 2012;2012:904141. doi: 10.1155/2012/904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patvardhan E, Heffernan KS, Ruan J, Hession M, et al. Augmentation index derived from peripheral arterial tonometry correlates with cardiovascular risk factors. Cardiology research and practice. 2011;2011:253758. doi: 10.4061/2011/253758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lekakis J, Abraham P, Balbarini A, Blann A, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775–789. doi: 10.1177/1741826711398179. [DOI] [PubMed] [Google Scholar]
- 37.Kuvin J, Mammen A, Mooney P, Alsheikh-Ali A, Karas R. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 38.Myers GL, Christenson RH, Cushman M, Ballantyne CM, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice guidelines: emerging biomarkers for primary prevention of cardiovascular disease. Clin Chem. 2009;55:378–384. doi: 10.1373/clinchem.2008.115899. [DOI] [PubMed] [Google Scholar]
- 39.Calder PC, Ahluwalia N, Albers R, Bosco N, et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr. 2013;109(Suppl 1):S1–34. doi: 10.1017/S0007114512005119. [DOI] [PubMed] [Google Scholar]
- 40.Jefferis BJ, Papacosta O, Owen CG, Wannamethee SG, et al. Interleukin 18 and coronary heart disease: prospective study and systematic review. Atherosclerosis. 2011;217:227–233. doi: 10.1016/j.atherosclerosis.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jun L, Yanjun J, Xiaolin L, Ruixia X, et al. Serum interleukin-10 levels and adverse events in patients with acute coronary syndrome: a systematic review and meta-analysis. Chinese medical journal. 2014;127:150–156. [PubMed] [Google Scholar]
- 42.Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 44.Frankenfeld CL. Dairy consumption is a significant correlate of urinary equol concentration in a representative sample of US adults. Am J Clin Nutr. 2011;93:1109–1116. doi: 10.3945/ajcn.111.011825. [DOI] [PubMed] [Google Scholar]
- 45.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, et al. Soy consumption, markers of inflammation, and endothelial function: a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care. 2007;30:967–973. doi: 10.2337/dc06-2126. [DOI] [PubMed] [Google Scholar]
- 46.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, et al. Dietary soya intake alters plasma antioxidant status and lipid peroxidation in postmenopausal women with the metabolic syndrome. Br J Nutr. 2007;98:807–813. doi: 10.1017/S0007114507746871. [DOI] [PubMed] [Google Scholar]
- 47.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, et al. Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr. 2007;85:735–741. doi: 10.1093/ajcn/85.3.735. [DOI] [PubMed] [Google Scholar]
- 48.Bakhtiary A, Yassin Z, Hanachi P, Rahmat A, et al. Effects of soy on metabolic biomarkers of cardiovascular disease in elderly women with metabolic syndrome. Archives of Iranian medicine. 2012;15:462–468. [PubMed] [Google Scholar]
- 49.Beavers DP, Beavers KM, Miller M, Stamey J, Messina MJ. Exposure to isoflavone-containing soy products and endothelial function: a Bayesian meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2012;22:182–191. doi: 10.1016/j.numecd.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Li SH, Liu XX, Bai YY, Wang XJ, et al. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: a meta-analysis of randomized placebo-controlled trials. Am J Clin Nutr. 2010;91:480–486. doi: 10.3945/ajcn.2009.28203. [DOI] [PubMed] [Google Scholar]
- 51.Pase MP, Grima NA, Sarris J. The effects of dietary and nutrient interventions on arterial stiffness: a systematic review. Am J Clin Nutr. 2011;93:446–454. doi: 10.3945/ajcn.110.002725. [DOI] [PubMed] [Google Scholar]
- 52.Sanders TA, Lewis FJ, Goff LM, Chowienczyk PJ, Group RS. SFAs do not impair endothelial function and arterial stiffness. Am J Clin Nutr. 2013;98:677–683. doi: 10.3945/ajcn.113.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keogh JB, Grieger JA, Noakes M, Clifton PM. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2005;25:1274–1279. doi: 10.1161/01.ATV.0000163185.28245.a1. [DOI] [PubMed] [Google Scholar]
- 54.Hall WL. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutrition research reviews. 2009;22:18–38. doi: 10.1017/S095442240925846X. [DOI] [PubMed] [Google Scholar]
- 55.Blanch N, Clifton PM, Petersen KS, Willoughby SR, Keogh JB. Effect of high potassium diet on endothelial function. Nutr Metab Cardiovasc Dis. 2014;24:983–989. doi: 10.1016/j.numecd.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Dong JY, Wang P, He K, Qin LQ. Effect of soy isoflavones on circulating C-reactive protein in postmenopausal women: meta-analysis of randomized controlled trials. Menopause. 2011;18:1256–1262. doi: 10.1097/gme.0b013e31821bfa24. [DOI] [PubMed] [Google Scholar]
- 57.Beavers KM, Jonnalagadda SS, Messina MJ. Soy consumption, adhesion molecules, and pro-inflammatory cytokines: a brief review of the literature. Nutr Rev. 2009;67:213–221. doi: 10.1111/j.1753-4887.2009.00191.x. [DOI] [PubMed] [Google Scholar]
- 58.Rebholz CM, Reynolds K, Wofford MR, Chen J, et al. Effect of soybean protein on novel cardiovascular disease risk factors: a randomized controlled trial. Eur J Clin Nutr. 2013;67:58–63. doi: 10.1038/ejcn.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mangano KM, Hutchins-Wiese HL, Kenny AM, Walsh SJ, et al. Soy proteins and isoflavones reduce interleukin-6 but not serum lipids in older women: a randomized controlled trial. Nutr Res. 2013;33:1026–1033. doi: 10.1016/j.nutres.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pusparini, Dharma R, Suyatna FD, Mansyur M, Hidajat A. Effect of soy isoflavone supplementation on vascular endothelial function and oxidative stress in postmenopausal women: a community randomized controlled trial. Asia Pacific journal of clinical nutrition. 2013;22:357–364. doi: 10.6133/apjcn.2013.22.3.13. [DOI] [PubMed] [Google Scholar]
- 61.Liu ZM, Ho SC, Chen YM, Woo J. Effect of soy protein and isoflavones on blood pressure and endothelial cytokines: a 6-month randomized controlled trial among postmenopausal women. J Hypertens. 2013;31:384–392. doi: 10.1097/HJH.0b013e32835c0905. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins DJ, Kendall CW, Connelly PW, Jackson CJ, et al. Effects of high- and low-isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metabolism. 2002;51:919–924. doi: 10.1053/meta.2002.33352. [DOI] [PubMed] [Google Scholar]
- 63.Celec P, Hodosy J, Palffy R, Gardlik R, et al. The short-term effects of soybean intake on oxidative and carbonyl stress in men and women. Molecules. 2013;18:5190–5200. doi: 10.3390/molecules18055190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vega-Lopez S, Yeum KJ, Lecker JL, Ausman LM, et al. Plasma antioxidant capacity in response to diets high in soy or animal protein with or without isoflavones. Am J Clin Nutr. 2005;81:43–49. doi: 10.1093/ajcn/81.1.43. [DOI] [PubMed] [Google Scholar]
- 65.Sen C, Morimoto Y, Heak S, Cooney RV, et al. Soy foods and urinary isoprostanes: results from a randomized study in premenopausal women. Food & function. 2012;3:517–521. doi: 10.1039/c2fo10251j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang ZJ. Systematic review on the association between F2-isoprostanes and cardiovascular disease. Ann Clin Biochem. 2013;50:108–114. doi: 10.1258/acb.2012.011263. [DOI] [PubMed] [Google Scholar]
- 67.Usui T, Tochiya M, Sasaki Y, Muranaka K, et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clinical endocrinology. 2013;78:365–372. doi: 10.1111/j.1365-2265.2012.04400.x. [DOI] [PubMed] [Google Scholar]
- 68.Squadrito F, Marini H, Bitto A, Altavilla D, et al. Genistein in the Metabolic Syndrome: Results of a Randomized Clinical Trial. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-1180. [DOI] [PubMed] [Google Scholar]
- 69.Liu ZM, Chen YM, Ho SC. Effects of soy intake on glycemic control: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2011;93:1092–1101. doi: 10.3945/ajcn.110.007187. [DOI] [PubMed] [Google Scholar]
- 70.Ricci E, Cipriani S, Chiaffarino F, Malvezzi M, Parazzini F. Effects of soy isoflavones and genistein on glucose metabolism in perimenopausal and postmenopausal non-Asian women: a meta-analysis of randomized controlled trials. Menopause. 2010;17:1080–1086. doi: 10.1097/gme.0b013e3181dd05a9. [DOI] [PubMed] [Google Scholar]
- 71.Nagarajan S. Mechanisms of anti-atherosclerotic functions of soy-based diets. J Nutr Biochem. 2010;21:255–260. doi: 10.1016/j.jnutbio.2009.09.002. [DOI] [PubMed] [Google Scholar]