The Association between the Use of Proton Pump Inhibitors and the Risk of Hypomagnesemia: A Systematic Review and Meta-Analysis (original) (raw)
Abstract
Background
Although many case reports have described patients with proton pump inhibitor (PPI)-induced hypomagnesemia, the impact of PPI use on hypomagnesemia has not been fully clarified through comparative studies. We aimed to evaluate the association between the use of PPI and the risk of developing hypomagnesemia by conducting a systematic review with meta-analysis.
Methods
We conducted a systematic search of MEDLINE, EMBASE, and the Cochrane Library using the primary keywords “proton pump,” “dexlansoprazole,” “esomeprazole,” “ilaprazole,” “lansoprazole,” “omeprazole,” “pantoprazole,” “rabeprazole,” “hypomagnesemia,” “hypomagnesaemia,” and “magnesium.” Studies were included if they evaluated the association between PPI use and hypomagnesemia and reported relative risks or odds ratios or provided data for their estimation. Pooled odds ratios with 95% confidence intervals were calculated using the random effects model. Statistical heterogeneity was assessed with Cochran’s Q test and I 2 statistics.
Results
Nine studies including 115,455 patients were analyzed. The median Newcastle-Ottawa quality score for the included studies was seven (range, 6–9). Among patients taking PPIs, the median proportion of patients with hypomagnesemia was 27.1% (range, 11.3–55.2%) across all included studies. Among patients not taking PPIs, the median proportion of patients with hypomagnesemia was 18.4% (range, 4.3–52.7%). On meta-analysis, pooled odds ratio for PPI use was found to be 1.775 (95% confidence interval 1.077–2.924). Significant heterogeneity was identified using Cochran’s Q test (df = 7, P<0.001, I 2 = 98.0%).
Conclusions
PPI use may increase the risk of hypomagnesemia. However, significant heterogeneity among the included studies prevented us from reaching a definitive conclusion.
Introduction
Proton pump inhibitors (PPIs) are a mainstay of treatment for acid-related diseases, including gastroesophageal reflux disease, functional dyspepsia, and peptic ulcer disease [1]–[5]. PPIs have an excellent safety profile, and have become one of the most commonly prescribed class of drugs in both primary and specialty care [6]. However, PPI use may induce some adverse events, including interstitial nephritis [7], respiratory infections [8], Clostridium difficile colitis [9], and hip fractures [10]. More recently, it has been reported that hypomagnesemia may also be induced by PPIs. The association between symptomatic hypomagnesemia and PPI use was first described in two patients in 2006 [11]. Since this report, many case reports have accumulated, supporting the association between PPI use and induced hypomagnesemia [12]–[16]. The Food and Drug Administration issued a Drug Safety Communication in 2011 [17], emphasizing the importance of long term use of prescription PPIs in this association. Moreover, a systematic review of 18 case reports of PPI-induced hypomagnesemia found that discontinuation of PPI use resulted in recovery from PPI-induced hypomagnesemia [18]. Although the pathophysiology of PPI-induced hypomagnesemia has not been definitively determined, impaired absorption of intestinal magnesium, due to administration of PPIs, may be one possible mechanism [19]. Decreased luminal pH in the intestine, caused by PPI use, may alter the affinity of the transient receptor potential melastatin-6 and transient receptor potential melastatin-7 (TRPM6/7) channel for Mg2+, reducing active transport of Mg2+ [20], [21].
Although many case reports have described patients with PPI-induced hypomagnesemia, the impact of PPI use on hypomagnesemia has not been fully clarified in comparative studies [22]–[30]. In some studies, the association between PPI use and hypomagnesemia was not identified [23]–[25], [29], while in others, PPI use was found to increase the risk of hypomagnesemia [22], [26]–[28], [30]. The discrepancy between these studies may be attributed to various patient characteristics, and/or underlying conditions.
To examine this topic, we performed a systematic review with meta-analysis, of existing comparative studies that investigated the association between PPI use and the risk of developing hypomagnesemia.
Materials and Methods
Search strategy
We searched for all relevant studies published between January 1990 and May 2014 that examined the effect of PPI use on the risk of hypomagnesemia, using MEDLINE, EMBASE, and the Cochrane Library. The terms “proton pump,” “dexlansoprazole,” “esomeprazole,” “ilaprazole,” “lansoprazole,” “omeprazole,” “pantoprazole,” “rabeprazole,” “hypomagnesemia,” “hypomagnesaemia,” and “magnesium” were used in our search. We also examined the references of screened articles, in order to identify additional studies. All human studies published in English were considered, and the latest date for updating our search was August 19, 2014.
Study selection
In the first stage of the study selection, the titles and abstracts of papers returned by our keyword search were examined to exclude irrelevant articles. Next, the full-text of all selected studies was screened, according to our inclusion and exclusion criteria. The inclusion criteria specified (1) studies regarding PPIs and hypomagnesemia, and (2) studies reporting relative risks or odds ratios (ORs) of PPI use, or provided data for their calculation. The exclusion criteria ruled out publications in a language other than English. Two investigators (C.H.P. and E.H.K.) independently evaluated studies for eligibility, resolved any disagreements through discussion and consensus. If no agreement could be reached, a third investigator (S.K.L.) decided eligibility.
To understand the risk of bias in individual studies, a formal quality assessment of studies was performed, along with subgroup analysis. The methodological quality of observational studies was independently assessed by two investigators (C.H.P. and E.H.K.), using the Newcastle-Ottawa scale [31], [32]. Using this scale, observational studies were scored across three categories: selection (4 questions), comparability of study groups (2 questions), and ascertainment of the exposure or outcome (3 questions). All questions were assigned a score of one point, with the exception of comparability of study groups, in which a maximum of two points were awarded. Studies with a cumulative score ≥7 were considered high quality [33], [34].
Data extraction
Using a data extraction form developed in advance, two reviewers (C.H.P. and E.H.K.) independently extracted the following information: first author, year of publication, study design, country, enrollment period, study population, age and sex of patients, definition of hypomagnesemia, total number of patients in each group (exposed vs. not exposed), ORs, and 95% confidence intervals (CIs). When incomplete information was available, attempts were made to contact the corresponding authors of the studies for additional information.
Outcomes assessed
Our primary analysis focused on assessing the risk of hypomagnesemia among users of PPIs. Our a priori hypothesis included study population (inpatients vs. others) as a potential explanation for heterogeneity in the direction and magnitude of effect. For exploratory analysis we conducted subgroup analysis according to the cut-off value of serum magnesium level (1.7 mg/dL vs. 1.8 mg/dL).
Statistical analysis
Meta-analyses were performed to calculate pooled ORs with 95% CIs [35]. Taking a conservative approach, we used a random effects model, which produces wider CIs than a fixed effect model. We assessed heterogeneity using two methods: Cochran’s Q test, which was considered statistically significant for heterogeneity if P<0.1, and _I_ 2 statistics, with values >50% suggestive of significant heterogeneity [36]. The tests for funnel plot asymmetry was not conducted when the included studies were less than 10 [37]. All _P_-values were 2-tailed, and a value of P<0.05 was considered statistically significant for all tests (except heterogeneity). Analysis and reporting were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [38]. Statistical analyses were conducted using the statistical software Comprehensive Meta Analysis (version 2.2.064; Biostat Inc., Englewood, NJ, USA).
Results
Study selection
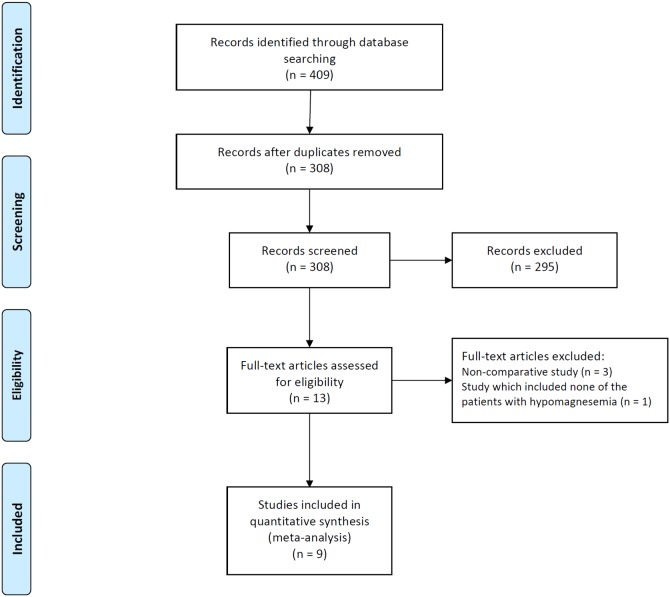
A flow diagram for our systematic review is shown in Fig. 1. In summary, 409 studies were identified by our literature search. After scanning titles and abstracts, we discarded 101 duplicate articles retrieved through multiple search engines. Another 295 irrelevant articles were excluded based on the titles and abstracts. The full texts of the 13 remaining articles were reviewed, and 4 non-pertinent articles were excluded. Of 4 excluded studies, three were non-comparative studies without control group and remaining one included none of the patients with hypomagnesemia in both treatment and control group.
Figure 1. Flow diagram of studies included in the meta-analysis.
PPI, proton pump inhibitor.
Of the original 409 studies, nine studies were deemed appropriate for our meta-analysis [22]–[25], [27], [28]. These studies included a total of 115,455 patients, and all had been published in the past 2 years, between 2012 and 2014. The enrollment period for these studies ranged from 2000 to 2013, and all were retrospective in nature. Of the included studies, five included only hospitalized patients [22]–[25], [30], two included outpatients only [28], [29], one included both inpatients and outpatients [26], and one included patients with end-stage renal disease on hemodialysis, but did not provide data regarding the hospitalization of patients [27]. The median Newcastle-Ottawa quality score for these studies was seven (range, 6–9), and all studies except one were considered high quality. The characteristics of the studies included in the meta-analysis are summarized in Table 1.
Table 1. Characteristics of studies included in the meta-analysis.
| Author | Year of | Study design | Country | Enrollment | Study population | Number of | Study quality | ||
|---|---|---|---|---|---|---|---|---|---|
| publication | period | (Inpatients vs. Out patients) | patients | Selection | Comparability | Exposure/Outcome | |||
| Gau et al. [22] | 2012 | Cross-sectional | USA | 2004–2008 | Inpatients | 487 | *** | ** | ** |
| El-Charabaty et al. [23] | 2013 | Cross-sectional | USA | 2007–2012 | Inpatientsa | 421 | *** | * | ** |
| Koulouridis et al. [24] | 2013 | Case-control | USA | 2000–2007 | Inpatients | 804 | *** | ** | *** |
| Danziger et al. [25] | 2013 | Cross-sectional | USA | 2001–2008 | Inpatientsb | 11,490 | *** | ** | ** |
| Kim et al. [26] | 2013 (in press) | Retrospective cohort | Korea | 2007–2012 | Inpatients & Outpatients | 1,356 | *** | ** | *** |
| Alhosaini et al. [27] | 2014 | Retrospective cohort | USA | 2012–2013 | NAc | 62 | *** | ** | *** |
| Markovitis et al. [28] | 2014 (in press) | Cross-sectional | Israel | 2008–2011 | Outpatients | 95,205 | **** | ** | *** |
| Van Ende et al. [29] | 2014 (in press) | Cross-sectional | Belgium | 2004–2006 | Outpatientsd | 512 | *** | ** | ** |
| Lindner et al. [30] | 2014 (in press) | Cross-sectional | Switzerland | 2009–2010 | Inpatients | 5,118 | *** | ** | ** |
Risk of hypomagnesemia
The cut-off value of serum magnesium level for defining hypomagnesemia was 1.6, 1.7, and 1.8 mg/dL in one [25], five [22], [24], [26], [28], [29], and three studies [23], [27], [30], respectively (Table 2). Among patients taking PPIs, the median proportion of patients with hypomagnesemia in all included studies was 27.1% (range, 11.3–55.2%). In addition, the median of the proportion of patients with hypomagnesemia in those not taking PPIs was 18.4% (range, 4.3–52.7%) across the studies. Of 9 included studies, six reported both unadjusted OR and adjusted OR. Other two studies showed unadjusted OR only. Remaining one study showed adjusted OR only. Most studies adjusted for the following confounders: use of diuretics (6/7), renal function (5/7), age (4/7), diabetes mellitus (4/7), and comorbidities (4/7). On meta-analysis, pooled unadjusted OR for PPI use was found to be 1.775 (95% CI = 1.077–2.924). Significant heterogeneity was identified (Cochran’s Q test, df = 7, P<0.001, I 2 = 98.0%). This risk increase with PPI use persisted even after adjusting for potential confounders where reported in studies (pooled adjusted OR [95% CI] = 1.484 [1.103–1.997], Fig. 2), although the heterogeneity persisted (Cochran’s Q test, df = 6, P<0.001, I 2 = 89.1%).
Table 2. Characteristics of individuals in included studies.
| Author | Age, mean ± SD | Male, n (%) | Definitionof | HypoMg inPPI users | HypoMg innon-PPI users | UnadjustedOR | AdjustedOR | Adjusted variables | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HypoMg | Non-HypoMg | HypoMg | Non-HypoMg | hypoMg | HypoMg | PPI user | HypoMg | PPI user | (95% CI) | (95% CI) | ||
| mg/dL | n (%) | N | n (%) | n | ||||||||
| Gau et al. [22] | 73.3±11.5 | 76.3±12.1 | 23(29.5) | 150 (36.7) | <1.7 | 48 (23.2) | 207 | 30(10.7) | 280 | 2.52(1.53–4.14) | 2.50(1.43–4.36) | Age, sex, DM, past medication history of congestive heart failure, diuretics, supplementation of potassium and magnesium, discharge diagnosis of any acute gastrointestinal illness, serum albumin, serum potassium, and serum creatinine. |
| El-Charabaty_et al._ [23] | NA | NA | NA | NA | <1.8 | 47 (25.5) | 184 | 48(20.3) | 237 | 1.35(0.85–2.14) | NA | |
| Koulouridis_et al._ [24] | 70.0±14.4 | 70.0±14.4 | 161(40.0) | 161 (40.0) | <1.7 | 219 (47.9) | 457 | 183(52.7) | 347 | 0.82(0.62–1.09) | 0.82(0.61–1.11) | Charlson-Deyo comorbidity index, DM, GERD, diuretics, and eGFR. |
| Danziger_et al._ [25] | PPI group: 67.8±15.4 H2 blocker group: 66.9±15.9 Control group: 61.1±19.2 | PPI group:1,403 (53.3) H2 blocker group: 368 (56.3) Control group: 4,796 (58.5) | <1.6 | 405 (15.4) | 2,632 | 1,456(16.4) | 8,858 | 0.92(0.82–1.04) | 1.10(0.96–1.25) | Age, sex, ethnicity, comorbidities, diuretics, renal function, systolic blood pressure, heart rate, temperature, serum calcium, serum phosphate, serum glucose, and serum hematocrit. | ||
| Kim et al. [26] | PPI group: 56.2±14.3 Control group: 55.2±14.1 | PPI group: 65(58.0) Control group:462 (37.1) | ≤1.7 | 32 (28.6) | 112 | 177(14.2)a | 1,244 | 2.41(1.55–3.74) | NA | |||
| Alhosaini_et al._ [27] | 64.8±7.2 | 64.0±9.6 | 24(100.0) | 38(100.0) | <1.8 | 16 (55.2) | 29 | 8(24.2) | 33 | 3.85(1.30–11.34) | 4.20(1.16–15.2) | Age, DM, diuretics, plasma albumin, protein intake, duration of dialysis, and dialytic urea clearance. |
| Markovitis_et al._ [28] | 58.1±18.5 | 47.4±20.3 | 2,031(35.7) | 33,003(36.9) | ≤1.7 | 2,532 (11.3) | 22,458 | 2,890(4.1) | 69,714 | 2.94(2.78–3.11) | 1.66(1.55–1.78) | Age, sex, comorbidities(hypertension, DM, heart failure, and malignancy), eGFR, drugs (diuretics, imunosuppressants, lithium, and digoxin), and recent hospitalization. |
| Van Ende et al. [29] | NA | NA | NA | NA | <1.7 | NA | 101 | NA | 411 | NA | 0.84(0.26–2.71) | Hemoglobin, tacrolimus, vitamin D substitution, and eGFR. |
| Lindner et al. [30] | 56±20 | 54±20 | 798 (66) | 2,565 (64) | <1.8 | 155 (36.6) | 423 | 1,091 (23.2) | 4,695 | 1.91(1.55–2.35) | 2.19(1.54–2.86) | Charlson comorbidity index score, diuretics, and eGFR. |
Figure 2. Forest plots for risk of hypomagnesemia.
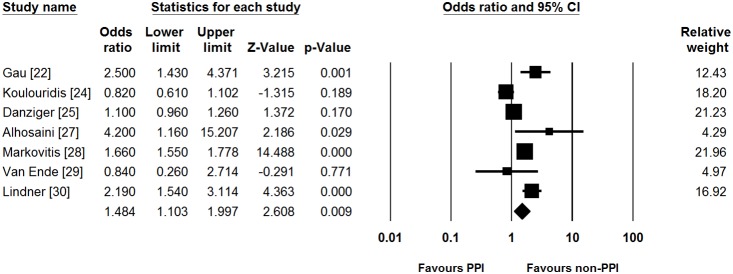
PPI, proton pump inhibitor; CI, confidence interval.
Subgroup analysis
We performed pre-planned subgroup analysis of studies, based on the hospitalization of patients. In five studies which included only hospitalized patients [22]–[25], [30], PPI use was not associated with hypomagnesemia (pooled unadjusted OR [95% CI] = 1.342 [0.895–2.011]). Significant heterogeneity did exist in the inpatient subgroup (Cochran’s Q test, df = 4, P<0.001, I 2 = 92.0%). Pooled adjusted OR showed similar results between PPI use and incidence of hypomagnesemia compared to pooled unadjusted OR (pooled adjusted OR [95% CI] = 1.424 [0.924–2.196], Fig. 3). Significant heterogeneity persisted in this analysis (Cochran’s Q test, df = 3, P<0.001, I 2 = 88.3%).
Figure 3. Subgroup analysis for studies which included only hospitalized patients.
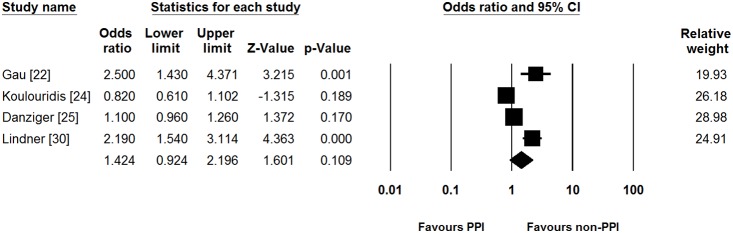
PPI, proton pump inhibitor; CI, confidence interval.
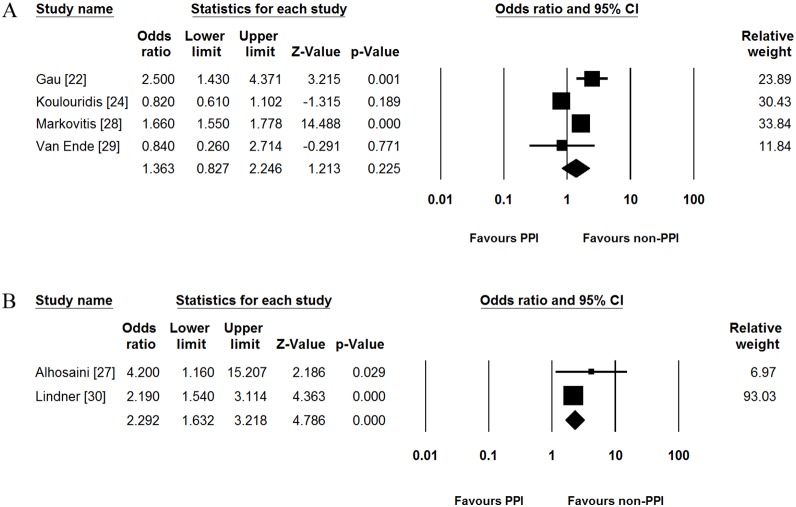
For exploratory analysis, we performed additional subgroup analysis according to the cut-off value of serum magnesium level. In 4 studies which reported adjusted OR based on the 1.7 mg/dL of cut-off value [22], [24], [28], [29], PPI use was not associated with incidence of hypomagnesemia (pooled adjusted OR [95% CI] = 1.363 [0.827–2.246], Fig. 4A). Significant heterogeneity was identified in this subgroup (Cochran’s Q test, df = 3, P<0.001, I 2 = 87.7%). In 2 studies which showed adjusted ORs based on the 1.8 mg/dL of cut-off value [27], [30], on the contrary, PPI use increased the risk of hypomagnesemia (pooled adjusted OR [95% CI] = 2.292 [1.632–3.218], Fig. 4B). There was no heterogeneity in this subgroup (Cochran’s Q test, df = 1, P = 0.339, I 2 = 0.0%).
Figure 4. Subgroup analysis according to the cut-off value of serum magnesium levels.
(A) Subgroup analysis for studies based on the 1.7 mg/dL of cut-off value. (B) Subgroup analysis for studies based on the 1.8 mg/dL of cut-off value. PPI, proton pump inhibitor; CI, confidence interval.
Discussion
Intracellular magnesium is an important cofactor for enzymatic reactions, and is critical for energy metabolism involving adenosine triphosphate [19]. Magnesium homeostasis is determined primarily by two processes, gastrointestinal absorption and renal excretion [39]. Gastrointestinal magnesium absorption occurs through both passive paracellular movement, and active transport into the portal venous system [19]. Active magnesium transport in the gut occurs through the combined action of TRPM6/7 channels, which are present in the apical membrane of enterocytes [39]. Previous studies have found that renal excretion of magnesium is reduced appropriately in patients with PPI-induced hypomagnesemia [40], [41], and that a PPI-induced decrease in the luminal pH of the intestine might alter the TRPM6/7 channel’s affinity for magnesium [21]. Therefore, impaired intestinal absorption rather than renal excretion is considered the primary cause of PPI-induced hypomagnesemia.
Many previous case reports [12]–[16], and proposed mechanisms of PPI-induced hypomagnesemia, have promoted awareness of the risk of hypomagnesemia in patients taking PPIs. However, the risk for PPI-induced hypomagnesemia should be evaluated by comparative studies, since the prevalence of hypomagnesemia is not rare. One previous population-based study found that 2% of subjects (104 of 5,179) have hypomagnesemia [42]. Other population-based studies also have suggested that hypomagnesemia is not rare in the general population [43], [44]. Moreover, in hospitalized patients, electrolyte disorders (including hypomagnesemia) are often acute and severe. Therefore, case reports alone cannot analyze the effect of PPI use on hypomagnesemia, and well-designed, comparative studies are needed to clarify the risk of PPI-induced hypomagnesemia.
Our meta-analysis showed statistical significance between PPI use and the risk of hypomagnesemia. The risk of hypomagnesemia in PPI users persisted even after adjusting for confounding variables. However, the significant heterogeneity among the included studies may be a concern. The significance of heterogeneity implied that the effect of PPI use on hypomagnesemia was varied. The observed heterogeneity may be due to the various types of study design and population among our included studies. For example, Gau _et al._’s study [22], Koulouridis _et al._’s study [24], and Lindner _et al._’s study [30] included hospitalized patients, regardless of disease type. However, Koulouridis _et al._’s study was designed as case-control study, while other two studies were designed as cross-sectional study. In contrast, Danziger _et al._’s study [25] included only patients admitted to intensive care units. El-Charabaty _et al._’s study [23] included only hospitalized patients with unstable angina, non-ST elevation myocardial infarction, and ST elevation myocardial infarction. Kim _et al._’s study [26] included both inpatients and outpatients. Alhosaini _et al._’s study [27] included only patients with end-stage renal disease on hemodialysis. Van Ende _et al._’s study [29] included only renal transplant recipients. Finally, Markovitis _et al._’s study [28] included only outpatients in the community setting. In addition, the definition of hypomagnesemia was varied among the included studies. These variations in study design may be reflected in the proportion of patients with hypomagnesemia for each group (patients taking PPIs vs. those not taking PPIs). The proportion of patients with hypomagnesemia ranged from 11.3% to 55.2% (PPI user group), and from 4.1% to 52.7% (non-PPI user group), and therefore, we believe that the incidence or prevalence of hypomagnesemia may depend on the study design.
One of the clinical concerns on PPI-induced hypomagnesemia is whether the test for serum magnesium level should be performed before initiating PPIs or not. Although it has not been fully evaluated, experts usually recommend the test for serum magnesium level prior to initiation of PPIs when patients are expected to be on treatment for long period of time [45], [46]. While short-term standard dose PPI treatment has low risk, long-term PPI use may complicate health conditions in high risk patients for hypomagnesemia including elderly patients [46]. The inter-study differences in the proportion of patients with hypomagnesemia in our meta-analysis may suggest that there are patients with increased susceptibility to PPI-induced hypomagnesemia. Considering the excellent safety profile of PPIs, risk identification and stratification for PPI-induced hypomagnesemia may be more helpful for clinical practice, rather than investigation of PPI-induced hypomagnesemia in the general population. Kim _et al._’s study, which evaluated associated factors for hypomagnesemia, found that long-term PPI use (>1 year), young age (<45 years), and concurrent cisplatin or carboplatin use were associated with hypomagnesemia in PPI users [26]. However, the study only included PPI users whose serum magnesium levels were available, and therefore, selection bias may be a concern. A population based study with regular checkup for serum magnesium level may be needed for clarifying the high risk patients for PPI-induced hypomagnesemia.
In our study, we conducted the two types of subgroup analysis. The former was subgroup analysis for studies which included hospitalized patients only, while the latter was subgroup analysis according to the cut-off value of serum magnesium level. In the former subgroup analysis, statistical significance between PPI use and the risk of hypomagnesemia was not shown. Although the subgroup included only hospitalized patients, the variation among the studies was still existed. In the latter subgroup analysis, PPI use increased the risk of hypomagnesemia in the studies whose cut-off value was 1.8 mg/dL rather than 1.7 mg/dL. These results implied that PPI-induced hypomagnesemia might not be as severe as we were concerned; however, further studies would be needed for assessing the severity of PPI-induced hypomagnesemia.
Although this is the first meta-analysis which demonstrated that PPI use could increase the risk of hypomagnesemia, there are several limitations. First, this is the meta-analysis for observational studies rather than randomized controlled trials. Magnesium assessment in a large database is usually healthcare-driven and potentially biased. In addition, dyspepsia may lead to prescribing PPIs as well as deficient food intake with low magnesium ingestion. Furthermore, serum magnesium was not usually evaluated in clinical practice. Although we conducted a meta-analysis using the adjusted ORs in the included studies, potential issues of confounding variables may be exist. The conclusion from the meta-analysis for observational studies should be interpreted carefully. Second, the significant heterogeneity among the included studies was additional obvious limitation. Through our systematic review and meta-analysis, we found that the proportion of patients with hypomagnesemia depended on the study settings including patient population and characteristics. Prospective cohort studies will be needed to evaluate severity of PPI-induced hypomagnesemia and to identify high risk group for PPI-induced hypomagnesemia. Third, we could not assess the risk of PPI-induced hypomagnesemia according to the amount or duration of usage of PPIs because available data were limited in the included studies.
Despite of these limitations, our meta-analysis showed that PPI use may increase the risk of hypomagnesemia. However, significant heterogeneity among the included studies prevented us from reaching a definitive conclusion. Well-designed, prospective cohort studies, which include regular serum magnesium monitoring, would provide more conclusive results.
Supporting Information
Checklist S1
PRISMA checklist of the study.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no funding or support to report.
References
- 1.Lacy BE, Talley NJ, Locke GR, Bouras EP, DiBaise JK, et al. (2012) Review article: current treatment options and management of functional dyspepsia. Alimentary pharmacology & therapeutics 36: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeomans ND, Tulassay Z, Juhasz L, RÃcz I, Howard JM, et al. (1998) A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid Suppression Trial: Ranitidine versus Omeprazole for NSAID-associated Ulcer Treatment (ASTRONAUT) Study Group. The New England journal of medicine 338: 719–726. [DOI] [PubMed] [Google Scholar]
- 3.Hawkey CJ, Karrasch JA, Szczepanski L, Walker DG, Barkun A, et al. (1998) Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. The New England journal of medicine 338: 727–734. [DOI] [PubMed] [Google Scholar]
- 4.Kahrilas PJ, Shaheen NJ, Vaezi MF, Hiltz SW, Black E, et al. (2008) American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 135: 1383–1391 1391: e1381. [DOI] [PubMed] [Google Scholar]
- 5.Xiao Y, Peng S, Tao J, Wang A, Lin J, et al. (2010) Prevalence and symptom pattern of pathologic esophageal acid reflux in patients with functional dyspepsia based on the Rome III criteria. The American journal of gastroenterology 105: 2626–2631. [DOI] [PubMed] [Google Scholar]
- 6.Sheen E, Triadafilopoulos G (2011) Adverse effects of long-term proton pump inhibitor therapy. Digestive diseases and sciences 56: 931–950. [DOI] [PubMed] [Google Scholar]
- 7.Sierra F, Suarez M, Rey M, Vela MF (2007) Systematic review: Proton pump inhibitor-associated acute interstitial nephritis. Alimentary pharmacology & therapeutics 26: 545–553. [DOI] [PubMed] [Google Scholar]
- 8.Herzig SJ, Howell MD, Ngo LH, Marcantonio ER (2009) Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA: the Journal of the American Medical Association 301: 2120–2128. [DOI] [PubMed] [Google Scholar]
- 9.Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, et al. (2010) Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Archives of internal medicine 170: 784–790. [DOI] [PubMed] [Google Scholar]
- 10.Gray SL, LaCroix AZ, Larson J, Robbins J, Cauley JA, et al. (2010) Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Archives of internal medicine 170: 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein M, McGrath S, Law F (2006) Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med 355: 1834–1836. [DOI] [PubMed] [Google Scholar]
- 12.Cundy T, Dissanayake A (2008) Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clinical endocrinology 69: 338–341. [DOI] [PubMed] [Google Scholar]
- 13.Broeren MA, Geerdink EA, Vader HL, van den Wall Bake AW (2009) Hypomagnesemia induced by several proton-pump inhibitors. Annals of Internal Medicine 151: 755–756. [DOI] [PubMed] [Google Scholar]
- 14.Kuipers MT, Thang HD, Arntzenius AB (2009) Hypomagnesaemia due to use of proton pump inhibitors–a review. Netherlands journal of medicine 67: 169–172. [PubMed] [Google Scholar]
- 15.Hoorn EJ, van der Hoek J, de Man RA, Kuipers EJ, Bolwerk C, et al. (2010) A case series of proton pump inhibitor-induced hypomagnesemia. American journal of kidney diseases 56: 112–116. [DOI] [PubMed] [Google Scholar]
- 16.Regolisti G, Cabassi A, Parenti E, Maggiore U, Fiaccadori E (2010) Severe hypomagnesemia during long-term treatment with a proton pump inhibitor. American journal of kidney diseases 56: 168–174. [DOI] [PubMed] [Google Scholar]
- 17.FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm245011. Accessed May 25, 2014.
- 18.Hess MW, Hoenderop JG, Bindels RJ, Drenth JP (2012) Systematic review: hypomagnesaemia induced by proton pump inhibition. Alimentary pharmacology & therapeutics 36: 405–413. [DOI] [PubMed] [Google Scholar]
- 19.Perazella MA (2013) Proton pump inhibitors and hypomagnesemia: a rare but serious complication. Kidney international 83: 553–556. [DOI] [PubMed] [Google Scholar]
- 20.Michalek W, Semler JR, Kuo B (2011) Impact of acid suppression on upper gastrointestinal pH and motility. Digestive diseases and sciences 56: 1735–1742. [DOI] [PubMed] [Google Scholar]
- 21.Bai JP, Hausman E, Lionberger R, Zhang X (2012) Modeling and simulation of the effect of proton pump inhibitors on magnesium homeostasis. 1. Oral absorption of magnesium. Mol Pharm 9: 3495–3505. [DOI] [PubMed] [Google Scholar]
- 22.Gau JT, Yang YX, Chen R, Kao TC (2012) Uses of proton pump inhibitors and hypomagnesemia. Pharmacoepidemiol Drug Saf 21: 553–559. [DOI] [PubMed] [Google Scholar]
- 23.El-Charabaty E, Saifan C, Abdallah M, Naboush A, Glass D, et al. (2013) Effects of proton pump inhibitors and electrolyte disturbances on arrhythmias. Int J Gen Med 6: 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koulouridis I, Alfayez M, Tighiouart H, Madias NE, Kent DM, et al. (2013) Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. American journal of kidney diseases 62: 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danziger J, William JH, Scott DJ, Lee J, Lehman L, et al. (2013) Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney international 83: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, Lee H, Park CH, Shim CN, Lee HJ, et al. (2013) Clinical Predictors Associated With Proton Pump Inhibitor-Induced Hypomagnesemia. Am J Ther. [DOI] [PubMed]
- 27.Alhosaini M, Walter JS, Singh S, Dieter RS, Hsieh A, et al. (2014) Hypomagnesemia in hemodialysis patients: role of proton pump inhibitors. American journal of nephrology 39: 204–209. [DOI] [PubMed] [Google Scholar]
- 28.Markovits N, Loebstein R, Halkin H, Bialik M, Landes-Westerman J, et al. (2014) The association of proton pump inhibitors and hypomagnesemia in the community setting. J Clin Pharmacol. [DOI] [PubMed]
- 29.Van Ende C, Van Laecke S, Marechal C, Verbeke F, Kanaan N, et al. (2014) Proton-pump inhibitors do not influence serum magnesium levels in renal transplant recipients. J Nephrol. [DOI] [PubMed]
- 30.Lindner G, Funk GC, Leichtle AB, Fiedler GM, Schwarz C, et al. (2014) Impact of proton pump inhibitor use on magnesium homoeostasis: a cross-sectional study in a tertiary emergency department. Int J Clin Pract. [DOI] [PubMed]
- 31.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 25, 2014.
- 32.Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 33.Castillo J, Mull N, Reagan JL, Nemr S, Mitri J (2012) Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood 119: 4845–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W (2013) Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 144: 323–332. [DOI] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled clinical trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks J, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ. British medical journal 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins J, Green S (2011) Cochrane handbook for systematic reviews of interventions, Version 5.1.0, Available at: http://handbook.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm. Accessed August 29, 2014.
- 38.Liberati A, Altman DG, Tetzlaff J, Mulrow C, GÃtzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology 62: e1–34. [DOI] [PubMed] [Google Scholar]
- 39.Rondón LJ, Groenestege WM, Rayssiguier Y, Mazur A (2008) Relationship between low magnesium status and TRPM6 expression in the kidney and large intestine. American journal of physiology. Regulatory, integrative and comparative physiology 294: R2001–R2007. [DOI] [PubMed] [Google Scholar]
- 40.Mackay JD, Bladon PT (2010) Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM 103: 387–395. [DOI] [PubMed] [Google Scholar]
- 41.Furlanetto TW, Faulhaber GA (2011) Hypomagnesemia and proton pump inhibitors: below the tip of the iceberg. Archives of internal medicine 171: 1391–1392. [DOI] [PubMed] [Google Scholar]
- 42.Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, et al. (2013) Electrolyte disorders in community subjects: prevalence and risk factors. The American journal of medicine 126: 256–263. [DOI] [PubMed] [Google Scholar]
- 43.Khan AM, Lubitz SA, Sullivan LM, Sun JX, Levy D, et al. (2013) Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation 127: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reffelmann T, DÃrr M, Ittermann T, Schwahn C, VÃlzke H, et al. (2010) Low serum magnesium concentrations predict increase in left ventricular mass over 5 years independently of common cardiovascular risk factors. Atherosclerosis 213: 563–569. [DOI] [PubMed] [Google Scholar]
- 45.Famularo G, Gasbarrone L, Minisola G (2013) Hypomagnesemia and proton-pump inhibitors. Expert Opinion on Drug Safety 12: 709–716. [DOI] [PubMed] [Google Scholar]
- 46.Corleto VD, Festa S, Di Giulio E, Annibale B (2014) Proton pump inhibitor therapy and potential long-term harm. Current opinion in endocrinology, diabetes and obesity 21: 3–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Checklist S1
PRISMA checklist of the study.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.