Role of Host Cell-Derived Amino Acids in Nutrition of Intracellular Salmonella enterica (original) (raw)
Abstract
The facultative intracellular pathogen Salmonella enterica resides in a specific membrane-bound compartment termed the _Salmonella_-containing vacuole (SCV). Despite being segregated from access to metabolites in the host cell cytosol, Salmonella is able to efficiently proliferate within the SCV. We set out to unravel the nutritional supply of Salmonella in the SCV with focus on amino acids. We studied the availability of amino acids by the generation of auxotrophic strains for alanine, asparagine, aspartate, glutamine, and proline in a macrophage cell line (RAW264.7) and an epithelial cell line (HeLa) and examined access to extracellular nutrients for nutrition. Auxotrophies for alanine, asparagine, or proline attenuated intracellular replication in HeLa cells, while aspartate, asparagine, or proline auxotrophies attenuated intracellular replication in RAW264.7 macrophages. The different patterns of intracellular attenuation of alanine- or aspartate-auxotrophic strains support distinct nutritional conditions in HeLa cells and RAW264.7 macrophages. Supplementation of medium with individual amino acids restored the intracellular replication of mutant strains auxotrophic for asparagine, proline, or glutamine. Similarly, a mutant strain deficient in succinate dehydrogenase was complemented by the extracellular addition of succinate. Complementation of the intracellular replication of auxotrophic Salmonella by external amino acids was possible if bacteria were proficient in the induction of _Salmonella_-induced filaments (SIFs) but failed in a SIF-deficient background. We propose that the ability of intracellular Salmonella to redirect host cell vesicular transport provides access of amino acids to auxotrophic strains and, more generally, is essential to continuously supply bacteria within the SCV with nutrients.
INTRODUCTION
The facultative intracellular life-style is a common virulence strategy among bacterial pathogens, and intracellular life-styles are as diverse as the diseases caused by these pathogens. While some bacteria lyse the host cell membrane compartment and initiate replication in the cytosol, others remain in a host cell-derived membrane compartment that is modified to allow intravacuolar survival and replication. To understand the life-style of bacterial pathogens, it is of central importance to analyze which nutritional limitations are experienced by the pathogen in its intracellular habitat and how the pathogen adapts its metabolism in order to survive and proliferate despite these limitations.
Salmonella enterica is an invasive, facultative, intracellular pathogen responsible for foodborne diseases ranging from localized gastroenteritis to systemic typhoid fever. Inside mammalian host cells, S. enterica resides in a specialized membrane-bound compartment, the _Salmonella_-containing vacuole (SCV). The SCV is modified by the action of virulence factors of S. enterica, and the function of the type III secretion system (T3SS) encoded by Salmonella pathogenicity island 2 (SPI2) and its effector proteins is of central importance (reviewed in reference 1). Despite being secluded from direct access to host cell metabolites, Salmonella is able to obtain all nutrients required for rapid proliferation within the SCV. The mechanisms of how Salmonella cells within the SCV are able to obtain these nutrients are largely unknown.
Salmonella enterica serovar Typhimurium is commonly used as a model organism for systemic Salmonella infections, and various cell culture models and murine infection models can be employed. S. Typhimurium is a prototrophic species able to synthesize all cellular macromolecules from simple C sources. S. Typhimurium can also synthesize all proteinogenic amino acids from metabolites of the central metabolism. Mutant strains deficient in amino acid biosynthesis are attenuated in intracellular proliferation, such as the aroA strain that is deficient in the synthesis of aromatic amino acids (2). This indicates that access to host-derived or extracellular metabolites is limited for these amino acids.
In addition to being building blocks for protein biosynthesis, amino acids have been shown to be important C sources for various intracellular pathogens, such as Legionella, Francisella, or Chlamydia (3–5). Legionella pneumophila, for example, exploits the host cell degradation machinery by injecting effector proteins into its host cells, mimicking typical eukaryotic domains to increase the cytosolic level of free amino acids. The presence of intracellular pathogens such as Shigella spp. in the cytosol can lead to amino acid starvation of the host cell and was shown to stimulate host cell responses leading to autophagy (6).
Intracellular S. Typhimurium cells efficiently manipulate their host cells by a complex set of effector proteins injected via the SPI2-encoded T3SS (SPI2-T3SS) (reviewed in reference 7). Coinciding with the onset of intracellular replication is the formation of tubular membrane compartments that are derived from host cell endosomes, and the most prominent type of _Salmonella_-induced tubules is termed _Salmonella_-induced filaments (SIFs) (reviewed in reference 8). SIFs and the SCV membranes contain characteristic organelle markers that indicate the origin of vesicles mainly from the endolysosomal pathway. Furthermore, interference with the secretory pathway and the Golgi apparatus was observed (7). The various interactions of intracellular pathogens with host cell vesicular transport and their role in the efficient intracellular replication of S. Typhimurium raise the question of a role for intracellular Salmonella in nutrition. These interactions might play an important role in intracellular nutrition by delivering extracellular or host cell-derived material, and by this strategy, Salmonella might also acquire host cell transporters for transportation of nutrients such as amino acids from the host cell cytosol to the SCV lumen.
We wanted to shed light on the relevance of amino acids as a nutritional source for intracellular Salmonella and addressed the following three questions. (i) Can auxotrophy for nonessential amino acids be compensated for by the uptake of amino acids from host cells? (ii) Is SIF formation required to access amino acids for growth in the SCV? (iii) Are requirements for amino acids distinct for Salmonella strains in various mammalian cell line infection models? We investigated the availability of amino acids for the intracellular growth of S. Typhimurium within the SCV. For this purpose, a set of defined mutant strains defective in the biosynthesis of amino acids not essential for the mammalian host cell was generated and analyzed for intracellular replication in an epithelial cell line (HeLa) and a macrophage cell line (RAW264.7). A subset of these auxotrophic strains was attenuated in intracellular replication, and we investigated whether this defect would be restored by the addition of individual amino acids to the culture medium of host cells. Our data demonstrate that intracellular Salmonella cells utilize medium-derived amino acids rather than host cell-derived amino acids. The dependency of auxotrophic Salmonella on amino acid supplementation was distinct in different host cells. The access to such amino acids is dependent on the function of the SPI2-T3SS, indicating a role in manipulation of host cell transport for intracellular nutrition.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Salmonella enterica serovar Typhimurium NCTC 12023 was used as the wild-type (WT) strain, and isogenic mutant strains were constructed by λ Red-mediated mutagenesis (9) (Table 1). Primers and plasmids required to introduce the FLP recombination target (FRT)-flanked resistance cassette and to check for the correct insertion and removal of the cassette by FLP recombinase afterwards are listed in Table S1 in the supplemental material. Mutant alleles were transferred into the fresh strain background or for combination with other mutations by P22 transduction according to standard procedures (10). Transductants were streak purified twice on Luria broth (LB) agar containing selective antibiotics and 10 mM EGTA to prevent the formation of lysogenic strains. Control for lysogeny in transduced strains was performed by streaking onto green plates and by streaking over a P22 lysate to prove that strains can still be infected by phage and therefore are nonlysogenic. Positive clones were verified by PCR. Multiple gene deletions were introduced by repeated rounds of λ Red mutagenesis.
TABLE 1.
Salmonella enterica serovar Typhimurium strains used in this study
| Strain | Genotype | Relevant defect(s) and/or phenotypea | Source or reference |
|---|---|---|---|
| NCTC 12023 | Wild type | NCTC, Colindale, UK | |
| MvP377 | Δ_sseJ_::aph | Effector SseJ; Kmr | 50 |
| MvP505 | Δ_sifA_::FRT | aph deleted from MvP509 | This study |
| MvP509 | Δ_sifA_::aph | Effector SifA; Kmr | 51 |
| MvP1165 | Δ_sucAB_::FRT | α-Ketoglutarate dehydrogenase | Popp and Hensel, unpublished |
| MvP1573 | Δ_ssaV_::FRT | SPI2-T3SS | This study |
| MvP2096 | Δ_avtA_::FRT Δ_yfbQ_::FRT Δ_yfdZ_::aph | Valine-pyruvate aminotransferase, putative aminotransferase, putative PLP-dependent aminotransferase; Kmr | This study |
| MvP1540 | Δ_aspC_::aph | PLP-dependent aspartate aminotransferase, Kmr | This study |
| MvP1670 | Δ_aspC_::FRT | PLP-dependent aspartate aminotransferase | This study |
| MvP1541 | Δ_glnA_::aph | Glutamine synthetase; Kmr | This study |
| MvP1671 | Δ_asnA_::aph | Asparagine synthetase A; Kmr | This study |
| MvP1672 | Δ_asnA_::FRT | Asparagine synthetase A | This study |
| MvP1673 | Δ_asnB_::aph | Asparagine synthetase B; Kmr | This study |
| MvP1674 | Δ_asnB_::FRT | Asparagine synthetase B | This study |
| MvP1675 | Δ_proB_::aph | Glutamate 5-kinase; Kmr | This study |
| MvP1676 | Δ_proB_::FRT | Glutamate 5-kinase | This study |
| MvP1677 | Δ_orf32_::aph | Proline iminopeptidase; Kmr | This study |
| MvP1678 | Δ_orf32_::FRT | Proline iminopeptidase | This study |
| MvP1679 | Δ_proC_::aph | Pyrroline-5-carboxylate reductase; Kmr | This study |
| MvP1680 | Δ_proC_::FRT | Pyrroline-5-carboxylate reductase | This study |
| MvP1683 | Δ_asnA_::FRT Δ_asnB_::aph | Asparagine synthetases A and B; Kmr | This study |
| MvP1684 | Δ_asnA_::FRT Δ_asnB_::FRT | Asparagine synthetases A and B | This study |
| MvP1685 | Δ_proB_::FRT Δ_proC_::aph | Glutamate 5-kinase; Kmr; pyrroline-5-carboxylate reductase | This study |
| MvP1900 | Δ_sifA_::FRT Δ_sseJ_::aph | P22 transduction of MvP377 to MvP503, effector SifA, SseJ; Kmr | This study |
| MvP2164 | Δ_sifA_::FRT Δ_sseJ_::FRT | Deletion of aph cassette from MvP1900 | This study |
| MvP2192 | Δ_sifA_::FRT Δ_sseJ_::FRT Δ_proC_::aph | P22 transduction of MvP1679 in MvP2164 | This study |
| MvP2203 | Δ_sifA_::FRT Δ_sseJ_::FRT Δ_proC_::FRT | Deletion of aph cassette from MvP2192, effectors SifA, SseJ, pyrroline-5-carboxylate reductase | This study |
Bacteria were grown at 37°C in LB (10 g tryptone, 5 g yeast extract, and 10 g NaCl per liter) or on LB agar plates (additionally 15 g agar) containing carbenicillin and/or kanamycin at 50 μg/ml if required for selection of antibiotic resistance or maintenance of plasmids. For growth measurements, we used LB and minimal medium, referred to as PCN medium (11), containing 10 mM glucose, 4 mM tricine, 100 μM FeCl3, 376 μM K2SO4, 50 mM NaCl, 10 nM Na2MoO4, 10 nM NaSeO3, 4 nM H3BO3, 0.3 mM CoCl2, 0.1 mM CuSO4, 0.8 mM MnCl2, 0.1 mM ZnSO4, 15 mM NH4Cl, 1 mM MgSO4, and 10 μM CaCl2. Here, neutral PCN medium (pH 7.4) containing 80 mM morpholinepropanesulfonic acid (MOPS) and 1 mM phosphate was used.
Growth kinetics.
Cultures of bacteria were grown overnight in neutral PCN medium with 1 mM phosphate. Minimal medium for the Δ_asnAB_ strain was supplemented with 0.8 mM asparagine (Sigma), and medium for the Δ_proB_ and Δ_proC_ strains contained 0.8 mM proline (Sigma). Cultures grown overnight were inoculated into neutral PCN medium with 1 mM phosphate to start at an optical density at 600 nm (OD600) of 0.01. The cultures were shaken aerobically in a water bath at 37°C. For growth tests at increased osmolarity, LB was adjusted with 0.4 or 0.65 M NaCl. At time intervals of 1 h, the optical density at 600 nm was measured by using a Shimadzu UV-1202 photometer.
Gentamicin protection assay.
RAW264.7 cells were routinely cultured in high-glucose (4.5 g/liter) Dulbecco's modified Eagle's medium (DMEM) containing 4 mM glutamine (Gln) (PAA, Cölbe, Germany) and 6% fetal calf serum (FCS) (Sigma) in a cell culture incubator (37°C in 5% CO2). HeLa cells were cultured in DMEM containing 4.5 g/liter glucose, 4 mM glutamine (PAA, Cölbe, Germany), and 10% FCS (Sigma) in a cell culture incubator (37°C in 5% CO2). For infection experiments, cells were seeded 24 or 48 h in advance in surface-treated 24-well dishes (Nunc, Thermo Fisher Scientific, Waltham, MA, USA, or TPP, Trasadingen, Switzerland) to confluence (∼4 × 105 and 2 × 105 cells for RAW264.7 and HeLa cells, respectively) on the day of infection. With the onset of infection, FCS-free medium was used for the remainder of the experiment. Cells were infected at a multiplicity of infection (MOI) of 1, and infection was performed with cultures of Salmonella strains grown aerobically in LB overnight (for RAW264.7 cells) or for 3.5 h to late log phase (for HeLa cells). Bacteria were centrifuged onto the cells for 5 min at 370 × g, and infection was allowed to proceed for a further 25 min. Afterwards, cells were washed twice with phosphate-buffered saline (PBS), medium containing 100 μg/ml gentamicin (Genta 100) was added, and cells were incubated for 1 h to kill extracellular bacteria. The concentration of gentamicin was reduced to 10 μg/ml for the rest of the experiment (Genta 10). To determine replication, cells were washed twice with PBS and lysed at 2 or 16 h postinfection (p.i.) by the addition of PBS with 0.1% Triton X-100. Serial dilutions of lysates were plated onto Mueller-Hinton II agar plates and incubated overnight at 37°C. CFU at various time points p.i. were determined.
Extracellular supplementation experiments. (i) Glutamine.
For glutamine, gentamicin protection assays were performed, with the exception that glutamine was left out during infection and incubation with Genta 100 medium. A total of 4 mM glutamine (PAA, Cölbe, Germany) or alanyl-glutamine (Glx) (PAA, Cölbe, Germany) was added to Genta 10 medium and maintained for the remaining period of culturing.
(ii) Asparagine and proline.
For asparagine and proline, experiments were performed as described above for the gentamicin protection assays except that 0.8 or 2.0 mM asparagine or 0.8 mM proline (Sigma) was added to Genta 10 medium where indicated.
(iii) Succinate.
For succinate, experiments were performed as described above for the gentamicin protection assays except that hybridoma express medium (HEM) (PAA, Cölbe, Germany) was used and 24.6 mM succinate was added to the cells with Genta 10 medium where indicated. For one experiment, RAW264.7 cells were precultured in DMEM with additional sodium pyruvate (PAA, Cölbe, Germany) instead of medium without sodium pyruvate.
RESULTS
Generation of auxotrophic S. Typhimurium mutant strains.
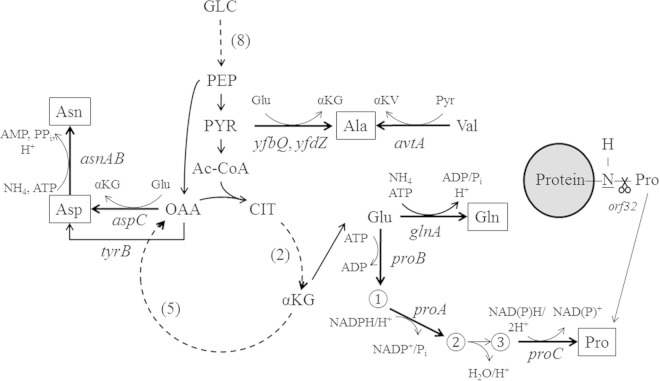
Based on the knowledge of amino acid biosynthesis pathways in S. enterica and related bacteria (Fig. 1), S. Typhimurium strains auxotrophic for the nonessential amino acids alanine, asparagine, aspartate, glutamine, and proline were constructed. For Escherichia coli, it has been shown that the major enzymes contributing to alanine biosynthesis are encoded by avtA, alaA (yfbQ), and alaC (yfdZ). Deletion of these genes led to severe growth defects in minimal medium containing the C source glycerol, glucose, acetate, or succinate without the addition of alanine (12). An avtA yfbQ yfdZ triple-deletion strain of S. Typhimurium was generated. Asparagine biosynthesis starts from aspartate and is performed by the gene products of asnA and asnB. In E. coli, the loss of either enzyme is compensated for by the other, as long as ammonia is not limiting (13). Thus, we constructed an asnA asnB double mutant strain. Glutamine derives from glutamate in a reaction catalyzed by glutamine synthetase (GlnA). For the synthesis of glutamine, neither an alternative pathway nor an alternative enzyme with glutamine synthase activity is known. Glutamine is a proteinogenic amino acid but serves as a donor for amino groups in biosynthetic processes and plays an important role in nitrogen metabolism. It has been shown that nitrogen-limiting conditions are sensed by changes in the glutamine pool (14). The loss of GlnA therefore resulted in glutamine auxotrophy. Like glutamine, proline derives from glutamate in a three-step reaction process catalyzed by ProB, ProA, and ProC. As these three enzymes work sequentially in one pathway and do not represent alternative pathways, knockout of any of these enzymes should result in proline auxotrophy. We generated Δ_proB_ and Δ_proC_ strains to block the first and last steps of proline biosynthesis. We also tested a strain deficient in the gene for a putative proline iminopeptidase (orf32), an enzyme that removes the N-terminal proline from a peptide (15). Proline iminopeptidase contributes to the virulence of the plant pathogen Xanthomonas campestris and is highly active in Treponema denticola, a pathogen causing human periodontal disease (16, 17). For the generation of an aspartate-auxotrophic strain, aspC was deleted. The aspC gene product acts as an aminotransferase, preferentially as an aspartate aminotransferase producing aspartate from oxaloacetate, but also takes part in the biosynthesis of the aromatic amino acids Phe and Tyr by performing transamination reactions. Furthermore, aspartate is a precursor for Thr, Ile, Leu, Met, and Asn. The aromatic amino acid transferase TyrB is mainly involved in the synthesis of aromatic amino acids but was also reported to act as an aspartate aminotransferase (18) (Fig. 1). However, only low levels of aspartate aminotransferase activity were observed in cell extracts from Δ_aspC_ strains of E. coli and Salmonella, indicating a key role of aspC in aspartate biosynthesis (18, 19).
FIG 1.
Schematic overview of selected amino acid biosynthesis pathways in S. Typhimurium and their origin from central metabolic pathways. Genes that encode the relevant enzymes are indicated and are further referred to in the text. Intermediates in the proline biosynthesis pathway are indicated by numbers, as follows: ①, γ-glutamyl phosphate; ②, glutamate γ-semialdehyde; ③, Δ1-pyrroline-5-carboxylate. Solid arrows indicate single reaction steps, while dotted arrows represent several reactions, with the number of steps given in parentheses. The reaction from ② to ③ occurs spontaneously. αKG, α-ketoglutarate; αKV, α-keto-isovalerate; CIT, citrate; GLC, glucose; OAA, oxaloacetate; PEP, phosphoenolpyruvate; PYR, pyruvate.
All mutant strains generated grew well in LB medium. With the exception of strains with a single deletion of asnA, asnB, or orf32, no growth overnight was detected in cultures in minimal medium with glucose (data not shown). Growth of the mutant strains in minimal medium was restored by the addition of Casamino Acids or specific single amino acids (data not shown).
Intracellular replication of auxotrophic S. Typhimurium strains in RAW264.7 and HeLa cells.
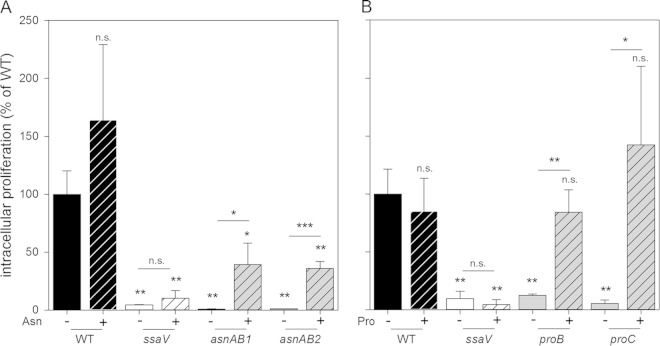
The intracellular proliferation of mutant strains with defects in amino acid biosynthesis pathways in comparison to the S. Typhimurium WT was analyzed by using the macrophage-like cell line RAW264.7 as the host cell line. The ssaV mutant strain deficient in the SPI2-encoded T3SS served as a negative control, and this strain showed highly attenuated intracellular proliferation (Fig. 2). The avtA yfbQ yfdZ, asnA, asnB, and orf32 mutant strains showed intracellular proliferation comparable to that of the WT, whereas the glnA, aspC, proB, and proC mutant strains showed reduced intracellular proliferation. The Δ_proC_ strain was more severely attenuated than the Δ_proB_ strain blocked in the introducing step of proline synthesis (Fig. 2A). Proline uptake is one mechanism of osmoprotection in bacteria (reviewed in reference 20). The Δ_proB_ and Δ_proC_ strains grew similarly to or even better than WT Salmonella in medium with increased osmolarity (see Fig. S1 in the supplemental material). This indicates that increased sensitivity to high osmolarity is unlikely to cause attenuated intracellular proliferation of the Δ_proB_ or Δ_proC_ strain. The most severe attenuation of intracellular proliferation was observed for the asnAB mutant strain, with an intracellular replication rate 4-fold lower than that of the ssaV strain (Fig. 2A).
FIG 2.
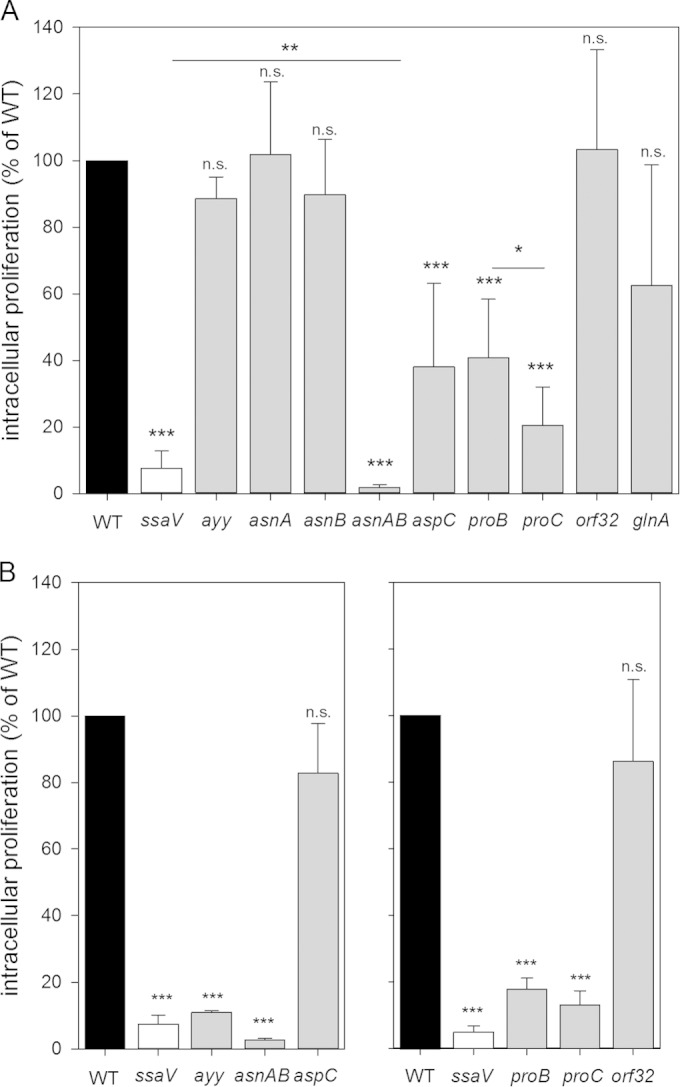
Role of amino acid biosynthesis pathways in intracellular proliferation of S. Typhimurium. Intracellular replication of S. Typhimurium WT, Δ_ssaV_, and various mutant strains impaired in amino acid biosynthesis in RAW264.7 (A) and HeLa (B) cells was determined by gentamicin protection assays. The Δ_ssaV_ strain defective in the SPI2-encoded T3SS served as a negative control. The Δ_avtA_ Δ_yfbQ_ Δ_yfdZ_ strain is abbreviated ayy. Infected cells were lysed at 2 h and 16 h p.i., and serial dilutions were plated onto agar plates for determination of CFU. _x_-fold intracellular proliferation is calculated as the ratio of CFU at 16 h p.i./CFU at 2 h p.i. The x_-fold proliferation of the WT strain was set as 100%, and intracellular proliferation of mutant strains is expressed as a percentage of the value for the WT. Experiments were performed in triplicates, and mean values and standard deviations are given for at least three biological replicates (two biological replicates for the Δ_asnAB strain in HeLa cells). Statistical significances were calculated by Student's t test and are indicated as follows: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
It was reported previously that strains impaired in the biosynthesis of aromatic amino acids and purine biosynthesis showed distinct intracellular replication defects, dependent on the cell line used for infection experiments (21). We also tested the replication of the avtA yfbQ yfdZ, asnAB, aspC, and proC mutant strains in the human epithelial cell line HeLa. The asnAB and proC mutant strains showed similarly reduced intracellular proliferation in HeLa cells as well as in RAW264.7. In contrast, the avtA yfbQ yfdZ mutant strain was highly attenuated in replication in HeLa cells, while the aspC mutant strain showed replication similar to that of the WT strain, with both phenotypes being reverted in RAW264.7 cells (Fig. 2B). Since identical formulations of DMEM were used for RAW264.7 and HeLa cell cultures, the differences observed are features of the host cells.
Medium amino acids can supplement intracellular proliferation of auxotrophic mutant strains.
We next investigated the effect of medium supplementation with specific amino acids on the intracellular proliferation of auxotrophic strains in RAW264.7 cells. Tests for culture growth indicated that the asnAB, proB, and proC mutant strains showed growth rates comparable to that of the WT strain in minimal medium supplemented with asparagine or proline (Table 2).
TABLE 2.
Generation times for S. Typhimurium WT and mutant strains auxotrophic for asparagine or proline
| Strain | Generation time (min)a | |
|---|---|---|
| Expt 1 | Expt 2 | |
| WT | 41.9 | 45.9 |
| Δ_asnAB_b | 42.6 | 45.0 |
| Δ_proB_c | 45.0 | 44.9 |
| Δ_proC_c | 45.7 | 46.9 |
The cell culture medium DMEM contains all amino acids apart from alanine, asparagine, aspartate, glutamate, and proline. To investigate supplementation in infection experiments, asparagine or proline was added to DMEM 1 h after infection. For the asnAB mutant strain, the addition of 0.8 mM asparagine to DMEM cell culture medium resulted in a 35- to 40-fold increase in intracellular proliferation (Fig. 3A). Asparagine supplementation also led to increased intracellular proliferation of the WT and the ssaV mutant strain, but these effects were not statistically significant. The addition of larger amounts of asparagine (2 mM) further increased intracellular replication of the asnAB mutant strain without significant effects on WT and ssaV strains (see Fig. S2 in the supplemental material). For proB and proC mutant strains, supplementation of medium with 0.8 mM proline restored intracellular proliferation to levels of the WT strain (Fig. 3B). Proline supplementation did not affect the intracellular proliferation of WT and ssaV deletion strains, while proliferation of proB and proC mutant strains was increased 9-fold and 26-fold, respectively.
FIG 3.
Complementation of asparagine- and proline-auxotrophic strains by extracellular amino acids. Intracellular replication of the S. Typhimurium WT strain, the Δ_ssaV_ strain, and mutant strains auxotrophic for asparagine (two independently generated strains) (A) or proline (B) in RAW264.7 cells was determined as described in the legend of Fig. 2. Cell culture medium was not supplemented (−) or was supplemented (+) with 0.8 mM asparagine (A) or 0.8 mM proline (B). Amino acids were added at 1 h p.i., with a change to medium containing 10 μg/ml gentamicin. Experiments were performed in triplicates, and mean values and standard deviations are given for one biological experiment. Statistical significances were calculated by Student's t test and are indicated as follows: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Glutamine but not alanyl-glutamine restores intracellular replication of glutamine-auxotrophic Salmonella.
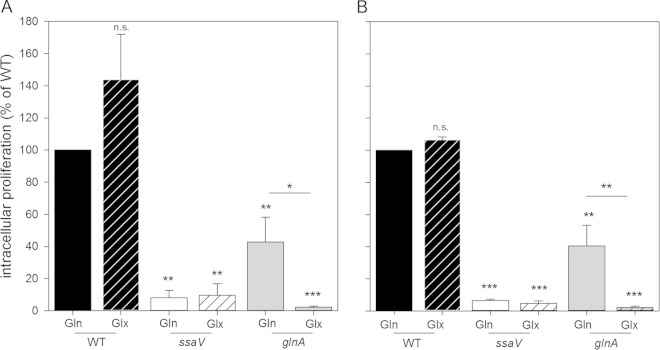
While Gln is a nonessential amino acid for mammalian cells, tumor cell lines are unable to meet the demand of their own biosynthesis and depend on large amounts of extracellular glutamine in the medium (22–24). Besides being a nitrogen and carbon source, the most recently reported results postulate an important role for glutamine in counteracting acid stress due to high glycolysis rates and the level of lactate production of tumor cell lines (25). For tumor cell lines like RAW264.7 and HeLa cells, glutamine is an essential amino acid, and therefore, cell culture media usually contain 4 mM Gln. Glx is often preferred over Gln in media due to its greater stability, avoiding the formation of harmful ammonia (26). Using cell culture medium containing 4 mM Gln, we observed replication of the Δ_glnA_ strain varying from 30% to 100% of WT replication (Fig. 2A). Interestingly, we found that virtually no intracellular replication was observed for the Δ_glnA_ strain when cell culture medium was supplemented with 4 mM Glx instead of Gln (Fig. 4). Intracellular proliferation in RAW264.7 (Fig. 4A) and HeLa (Fig. 4B) cells was 18.6-fold and 18.4-fold higher, respectively, with Gln than with Glx. During extracellular growth, Salmonella used both Glx and Gln as amino acid sources, since the glnA mutant strain grew with similar rates in vitro in DMEM supplemented with 4 mM Gln and in DMEM supplemented with Glx (data not shown). The presence of the additional alanine therefore has no detrimental effect on growth in vitro. Replication of the WT strain in host cells was not negatively affected by the use of Glx instead of Gln (Fig. 4).
FIG 4.
Complementation of the glutamine-auxotrophic strain by extracellular amino acids. The effect of the addition of glutamine (Gln) or alanyl-glutamine (Glx) on the intracellular replication of S. Typhimurium in RAW264.7 cells (A) or HeLa cells (B) was determined as described in the legend of Fig. 2. S. Typhimurium WT, Δ_ssaV_, and glutamine-auxotrophic Δ_glnA_ strains were used for infection. Cell culture medium was supplemented with 4 mM glutamine (Gln) or alanyl-glutamine (Glx). Amino acids were added at 1 h p.i., i.e., with a change to medium containing 10 μg/ml gentamicin. Experiments were performed in triplicates, and mean values and standard deviations are given for two (WT strain with Glx, ssaV mutant with Gln, and ssaV mutant with Glx) or three (Δ_glnA_ strain with Gln and Δ_glnA_ strain with Glx) independent biological experiments. Statistical significances were calculated by Student's t test and are indicated as follows: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Medium succinate supplements intracellular proliferation of a tricarboxylic acid (TCA) cycle-deficient strain.
To further address the direct access of intracellular Salmonella bacteria to nutrients in medium, we considered central metabolites as possible reporters. Succinate was a candidate since a S. Typhimurium Δ_sucAB_ strain is dependent on supplementation with succinate for growth in minimal medium with glucose (J. Popp and M. Hensel, unpublished observations). A sucAB mutant strain showed WT levels of intracellular replication in RAW264.7 cells when cultured in DMEM but highly reduced intracellular replication when host cells were cultured in fully synthetic hybridoma express medium (HEM) (Popp and Hensel, unpublished). Analysis of HEM showed that histidine, cysteine, and lysine are absent (data not shown), but they are present in the DMEM formulation used here. At least lysine biosynthesis in E. coli depends on succinyl coenzyme A (succinyl-CoA) (27). We determined the intracellular replication of Salmonella WT, Δ_ssaV_, and Δ_sucAB_ strains in RAW264.7 cells cultured in HEM with and without the addition of succinate (Fig. 5). The addition of succinate to the extracellular medium partially restored the intracellular replication of the Δ_sucAB_ strain but had no or only a small effect on the intracellular proliferation of the ssaV or WT strain, respectively. This observation indicates that access of intracellular Salmonella to molecules present in the external medium is a more general phenomenon.
FIG 5.
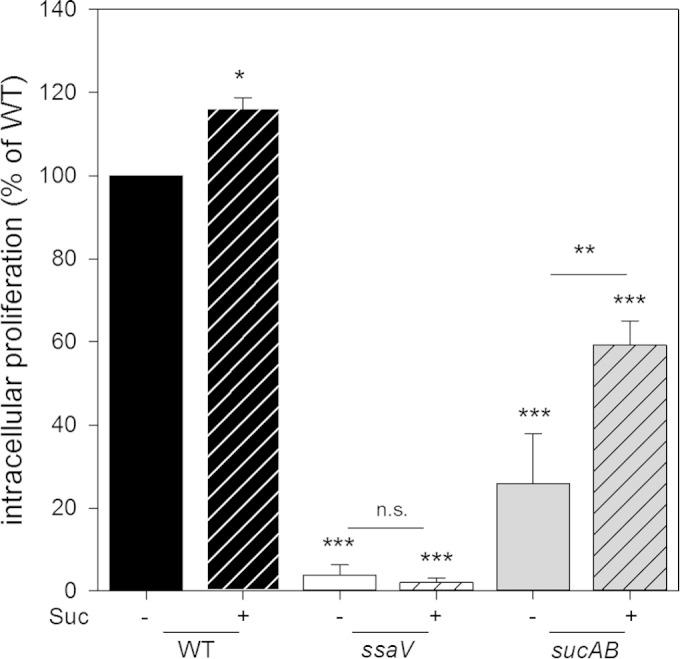
Effect of availability of extracellular succinate on intracellular replication of S. Typhimurium. RAW264.7 cells were infected with WT, Δ_ssaV_, and Δ_sucAB_ strains, and intracellular proliferation was determined as described in the legend of Fig. 2. Hybridoma express medium was used for the period of the infection experiment without (−) or with (+) the addition of 24.6 mM succinate (Suc), added at 1 h p.i. WT replication was set as 100%, and replication of the mutant strains is expressed as a percentage of WT replication. Experiments were performed in triplicates, and mean values and standard deviations are given for at least three biological experiments, with the exception of experiments with the WT strain with succinate and the Δ_ssaV_ strain with succinate (two replicates). Statistical significances were calculated by Student's t test and are indicated as follows: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Role of SIF induction in access to extracellular amino acids.
We demonstrated that extracellular amino acid supplementation can complement the intracellular proliferation of auxotrophic Salmonella strains. A possible mechanism is the remodeling of the endosomal system in host cells as a function of the SPI2-encoded T3SS. To test this hypothesis, we investigated the effects of ssaV or sifA defects on the extracellular supplementation of the proC strain. SsaV is a subunit essential for the function of the SPI2-T3SS, while the effector protein SifA is required for the maintenance of the SCV and the formation of a network of so-called Salmonella_-induced filaments (SIFs). We investigated whether the ability to induce SIF formation affects the intracellular complementation of proline-auxotrophic strains by supplementation with extracellular proline. A sifA_-deficient strain is unable to induce SIF formation and to maintain the integrity of the SCV (28). As a consequence, release of Salmonella bacteria into the host cell cytosol and cytosolic replication was observed. A double mutant strain defective in the SPI2-T3SS effectors SifA and SseJ also fails to induce SIFs but is not released into the cytosol (29). Cytosolic replication of the sifA mutant strain may obscure potential nutritional limitation of the bacterial population in the SCV. Thus, we used the Δ_sifA Δ_sseJ strain background to investigate the fate of Salmonella in the SCV dependent on SIF formation. Intracellular proliferation rates of the WT, Δ_ssaV_, Δ_sifA_ Δ_sseJ_, Δ_proC_, and Δ_sifA_ Δ_sseJ_ Δ_proC_ strains with and without the addition of 0.8 mM proline to the medium were determined (Fig. 6). Proline supplementation slightly increased the intracellular proliferation of WT Salmonella but had no effect on the ssaV or sifA sseJ mutant strain. Intracellular replication of the proC mutant strain was augmented 7.8-fold by proline supplementation, while no significant effect on the sifA sseJ proC strain was observed (1.08-fold increased replication). These data suggest that the SPI2-T3SS-mediated redirection of endosomal transport in host cells contributes to the access of intracellular Salmonella to external amino acids.
FIG 6.
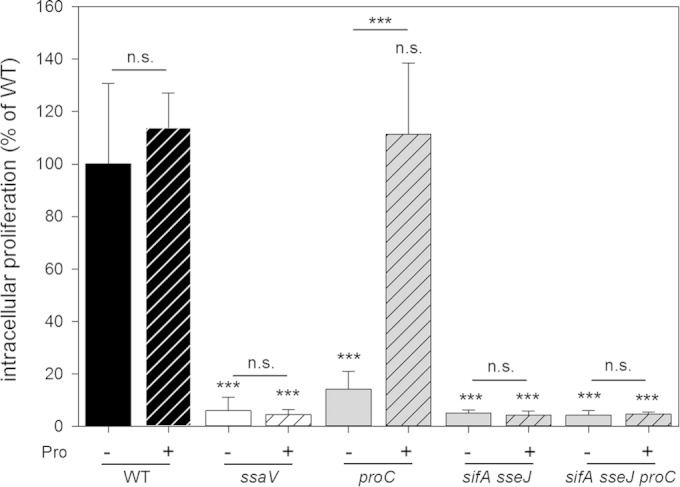
Effect of Salmonella_-induced filament formation on complementation by extracellular proline. The intracellular replication of S. Typhimurium WT, Δ_ssaV, Δ_sifA_ Δ_sseJ_, and proline-auxotrophic Δ_proC_ or Δ_proC_ Δ_sifA_ Δ_sseJ_ strains in RAW264.7 cells was determined as described in the legend of Fig. 2. WT replication was set as 100%, and replication of the mutant strains was calculated as a percentage of WT replication. Cell culture media were used without (−) or with (+) 0.8 mM proline added to the medium at 1 h p.i. Experiments were performed in triplicates, and mean values and standard deviations are given for three biological replicates. Statistical significances were calculated by Student's t test and are indicated as follows: n.s., not significant; ***, P < 0.001.
DISCUSSION
Various forms of dependency of intracellular pathogens on their host cells are known. Obligate intracellular bacteria such as Rickettsia spp. and Coxiella spp. (30, 31) have reduced genome sizes and also restricted metabolic capacities, leaving the bacteria to acquire relevant building blocks like amino acids from the host (32). Some facultative intracellular pathogens such as Legionella pneumophila and Francisella tularensis are auxotrophic for several amino acids and have evolved strategies to increase the intracellular abundance of free amino acids (4, 33). In contrast, S. Typhimurium appears to be independent from its host cell, since this pathogen possesses all relevant central metabolic pathways (glycolysis, pentose-phosphate pathway, and the TCA cycle) and biosynthetic pathways for all amino acids. However, host defense mechanisms like nitric oxide production might provoke Lys and Met auxotrophies, and this would cause the need for uptake from the host cell (34). Accordingly, a defect of the methionine transporter MetD attenuated virulence in a mouse model (34). Furthermore, attenuated virulence was also shown for a S. Typhimurium strain deficient in the arginine transporter argT (35). 13C isotopologue profiling studies further supported the access of S. Typhimurium to host cell-derived amino acids in epithelial and macrophage cell lines (37; Popp and Hensel, unpublished). Therefore, the uptake of amino acids may play an important role in the intracellular life of Salmonella, and we analyzed the intracellular phenotypes of various Salmonella mutant strains with auxotrophies for nonessential amino acids. Of these, auxotrophies for alanine, asparagine, or proline attenuated intracellular replication in HeLa cells, while aspartate, asparagine, or proline auxotrophies attenuated intracellular replication in RAW264.7 macrophages. Our work demonstrated that a subset of amino-acid-auxotrophic mutant strains of S. Typhimurium can be complemented in intracellular proliferation in mammalian host cells by adding amino acids externally to the medium. These amino acids are not present in most cell culture media such as DMEM, used here.
Our data show a strong dependency of Salmonella on de novo amino acid biosynthesis of asparagine and proline if the respective amino acid is not available in the extracellular medium. In turn, this dependency indicates that intracellular Salmonella bacteria are not able to obtain sufficient amounts of the amino acids from the host cell. This finding is in accordance with previously reported observations by the Groisman group, who suggest that proline-poor conditions in the SCV to which Salmonella adapt, mediated by the small amount of Pro-charged tRNAs, are an inductor of expression of the operon for the virulence genes mgtCBR (38). Despite the ability to utilize a multiplicity of nutrients for intracellular proliferation (39), the ability to sense the precise composition of the intracellular environment allows rapid and efficient adaptation. Our results do not exclude host cell-derived amino acids as possible nutrients, yet the amounts are likely insufficient to fulfill the requirements for proliferation of Salmonella.
The different degrees of attenuation for the auxotrophic strains suggest differences in the intracellular availabilities of amino acids. Interestingly, we found that the alanine-auxotrophic Δ_avtA_ Δ_yfbQ_ Δ_yfdZ_ strain was not affected in replication in RAW264.7 cells but exhibited highly reduced replication in HeLa cells. Therefore, Salmonella might have access to alanine in RAW264.7 cells but not in HeLa cells, or the host cell alanine pool is larger in RAW264.7 than in HeLa cells. Alternatively, access to pyruvate could explain the observed difference, since previous work showed that the Δ_avtA_ Δ_yfbQ_ Δ_yfdZ_ mutant had no growth defect if pyruvate is available as a C source (12). There are alternative pathways and enzymes that can compensate for the loss of the major transaminases in this case. For E. coli, it was shown that several minor aminotransferases could contribute to alanine biosynthesis that can in the best case compensate for the loss of the major enzymes if pyruvate is available as a carbon source (12). Therefore, this difference might not be due to different abundances of alanine in the two cell lines but rather could be caused by different access to the pyruvate present in the host cell cytosol or internalized from the culture medium.
Mammalian host cells possess transporters for monocarboxylates, like pyruvate and lactate, in the plasma membrane (monocarboxylate transporter [MCT]) and a pyruvate transporter in the mitochondrial membrane (mitochondrial pyruvate carrier [MPC]) (40). By manipulation of host cell trafficking, Salmonella might recruit these transporters and integrate them into the SCV to access cytosolic pyruvate. Tumor cell lines have highly upregulated glycolysis, known as the Warburg effect (41), thus producing pyruvate that is subsequently converted to lactate and excreted. Furthermore, we have shown that infection with Salmonella triggers increased glycolysis in RAW264.7 cells (Popp and Hensel, unpublished), so pyruvate might be available as a carbon source. It is also possible that Salmonella has access to extracellular excreted lactate that is converted into pyruvate by lactate dehydrogenase. Higher glycolytic fluxes and/or lactate excretion in macrophages stimulated by Salmonella infection than in HeLa cells may explain the differences. Further work should address this point by analyzing metabolite profiles of uninfected and infected host cells. Furthermore, it would be of interest to determine the growth of the Δ_avtA_ Δ_yfbQ_ Δ_yfdZ_ strain in cell culture medium and cell culture supernatants removed from infected and uninfected RAW264.7 and HeLa cells. Also, the Δ_avtA_ Δ_yfbQ_ Δ_yfdZ_ strain that is additionally defective in lactate dehydrogenase could be tested for further attenuation in RAW264.7 cells.
The opposite situation was observed for the aspC mutant strains, showing WT levels of replication in HeLa cells and reduced proliferation in RAW264.7 cells. Salmonella might gain efficiently the required amounts of aspartate and amino acids deriving from aspartate from the host cell in HeLa cells yet not in RAW264.7 cells. Also, a higher level of activity of TyrB could explain this phenotype.
The greater intracellular attenuation of the Δ_asnAB_ strain than of proline-auxotrophic Salmonella strains indicates more limited access to host-derived asparagine than to proline or a greater requirement for asparagine than for proline. Interestingly, reduced intracellular proliferation of an Δ_asnAB_ strain was predicted by a systems biology approach, and a murine systemic infection model confirmed the severe attenuation of the Δ_asnAB_ strain (43). The more severe attenuation of the Δ_proC_ strain could be caused by the harmful accumulation of Δ1-pyrroline-5-carboxylate (P5C) or other side effects, whereas a Δ_proB_ strain may partially complete proline biosynthesis from intermediates of the biosynthesis pathway. The addition of larger amounts of extracellular asparagine resulted in a gradual increase in the intracellular proliferation of the asnAB mutant strain, indicating a limiting availability of asparagine. The knockout of asnA and asnB might also have further side effects leading to attenuated replication. However, since asparagine is formed from aspartate in one enzymatic step, it is unlikely that intermediates required for other pathways are missing or that a harmful intermediate(s) accumulates.
Intracellular S. Typhimurium bacteria induce the massive remodeling of the host cell endosomal system in a SPI2-T3SS-dependent manner. One remarkable consequence is the formation of extensive tubular membrane compartments, termed SIFs. SIF formation coincides with intracellular replication (44), and mutant strains deficient in SIF induction are also highly attenuated in systemic virulence in a murine model and in intracellular proliferation. However, the role of SIF induction in the physiology of intracellular S. Typhimurium is not clear. Continuous interactions of the SCV with the endolysosomal network have been observed (45), and live-cell imaging revealed that the SCV is connected with an extensive network of SIFs (46). These activities may indicate that continuous exchange of the SCV with host cell endosomes is the strategy for the acquisition of nutrients that are limiting intracellular replication. Complementation of amino acid auxotrophies by extracellular amino acids was observed for a SPI2-T3SS-proficient S. Typhimurium strain but not for an sifA sseJ mutant strain that is unable to induce tubular endosomal aggregates. From these data, we conclude that access to portions of media endocytosed by the host cell is an important part of the nutrition of intracellular S. Typhimurium.
The glutamine-auxotrophic strain could be complemented by glutamine or alanyl-glutamine during extracellular growth; however, only glutamine restored intracellular proliferation of the Δ_glnA_ strain. At present, we cannot explain the molecular basis of this remarkable difference. For E. coli and Salmonella, the presence of various peptide permeases for the uptake of di-, tri-, and oligopeptides was reported previously (47, 48). Furthermore, the dipeptide permease Dpp seemed to be functional at a broad range of pHs since the protein binding part of DppA showed similar substrate binding between pH 3.0 and pH 9.5 (48). Therefore, even under the acidic conditions within the SCV, the uptake of alanyl-glutamine by Salmonella should be possible, provided that this dipeptide reaches the lumen of the SCV. If manipulation of endolysosomal trafficking is a major pathway to gain nutrients, the impact of the access to glutamine or alanyl-glutamine in medium should be similar for the intracellular growth of the S. Typhimurium Δ_glnA_ strain. An alternative explanation might be the putative recruiting of host cell transporters for glutamine to the SCV, similar to the intracellular strategy of S. Typhimurium for the acquisition of arginine described previously (35). Such a recruitment mechanism would call for host cell transporters for the uptake of glutamine from the medium. In contrast to glutamine, alanyl-glutamine is hydrolyzed to alanine and glutamine by extracellular aminopeptidases that are released into the medium by the host cell and taken up after cleavage (GlutaMAX; Life Technologies) (49). This results in a constantly lower concentration of available glutamine in the extracellular medium (GlutaMAX; Life Technologies) (49), possibly provoking less uptake of glutamine and lower cytosolic levels in the host cell. Further work has to reveal how S. Typhimurium can utilize glutamine and alanyl-glutamine under intracellular conditions.
To conclude, our work demonstrates the requirement of prototrophic metabolism and the full biosynthetic repertoire for the intracellular life-style of S. Typhimurium in mammalian host cells. The availabilities of amino acids are distinct in HeLa cells and murine macrophages, and our data support that the SPI2-T3SS-dependent manipulation of host cell endosomal transport is a means of continuous supply of the SCV with medium compounds internalized by the host cell. As a metabolic all-rounder, intracellular S. Typhimurium can efficiently utilize internalized amino acids and various C sources for efficient intracellular proliferation.
Supplementary Material
Supplemental material
ACKNOWLEDGMENT
This work was supported by grant HE1964/14-2 as part of the DFG priority program 1316, Host-Adapted Metabolism of Bacteria Pathogens.
Footnotes
REFERENCES
- 1.Figueira R, Holden DW. 2012. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 158:1147–1161. doi: 10.1099/mic.0.058115-0. [DOI] [PubMed] [Google Scholar]
- 2.Hoiseth SK, Stocker BA. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 3.Al-Quadan T, Price CT, Abu Kwaik Y. 2012. Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella. Trends Microbiol 20:299–306. doi: 10.1016/j.tim.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steele S, Brunton J, Ziehr B, Taft-Benz S, Moorman N, Kawula T. 2013. Francisella tularensis harvests nutrients derived via ATG5-independent autophagy to support intracellular growth. PLoS Pathog 9:e1003562. doi: 10.1371/journal.ppat.1003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun PR, Al-Younes H, Gussmann J, Klein J, Schneider E, Meyer TF. 2008. Competitive inhibition of amino acid uptake suppresses chlamydial growth: involvement of the chlamydial amino acid transporter BrnQ. J Bacteriol 190:1822–1830. doi: 10.1128/JB.01240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tattoli I, Sorbara MT, Vuckovic D, Ling A, Soares F, Carneiro LA, Yang C, Emili A, Philpott DJ, Girardin SE. 2012. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 11:563–575. doi: 10.1016/j.chom.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 7.LaRock DL, Chaudhary A, Miller SI. 2015. Salmonellae interactions with host processes. Nat Rev Microbiol 13:191–205. doi: 10.1038/nrmicro3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liss V, Hensel M. 2015. Take the tube: remodelling of the endosomal system by intracellular Salmonella enterica. Cell Microbiol 17:639–647. doi: 10.1111/cmi.12441. [DOI] [PubMed] [Google Scholar]
- 9.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maloy SR, Stewart VL, Taylor RK. 1996. Genetic analysis of pathogenic bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 11.Neidhardt FC, Bloch PL, Smith DF. 1974. Culture medium for enterobacteria. J Bacteriol 119:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Schneider BL, Reitzer L. 2010. Genetics and regulation of the major enzymes of alanine synthesis in Escherichia coli. J Bacteriol 192:5304–5311. doi: 10.1128/JB.00738-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felton J, Michaelis S, Wright A. 1980. Mutations in two unlinked genes are required to produce asparagine auxotrophy in Escherichia coli. J Bacteriol 142:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda TP, Shauger AE, Kustu S. 1996. Salmonella typhimurium apparently perceives external nitrogen limitation as internal glutamine limitation. J Mol Biol 259:589–607. doi: 10.1006/jmbi.1996.0342. [DOI] [PubMed] [Google Scholar]
- 15.Sarid S, Berger A, Katchalski E. 1959. Proline iminopeptidase. J Biol Chem 234:1740–1746. [PubMed] [Google Scholar]
- 16.Makinen KK, Chen CY, Makinen PL. 1996. Proline iminopeptidase from the outer cell envelope of the human oral spirochete Treponema denticola ATCC 35405. Infect Immun 64:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Jia Y, Wang L, Fang R. 2007. A proline iminopeptidase gene upregulated in planta by a LuxR homologue is essential for pathogenicity of Xanthomonas campestris pv. campestris. Mol Microbiol 65:121–136. doi: 10.1111/j.1365-2958.2007.05775.x. [DOI] [PubMed] [Google Scholar]
- 18.Gelfand DH, Rudo N. 1977. Mapping of the aspartate and aromatic amino acid aminotransferase genes tyrB and aspC. J Bacteriol 130:441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Dyk TK, LaRossa RA. 1986. Sensitivity of a Salmonella typhimurium aspC mutant to sulfometuron methyl, a potent inhibitor of acetolactate synthase II. J Bacteriol 165:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood JM. 1988. Proline porters effect the utilization of proline as nutrient or osmoprotectant for bacteria. J Membr Biol 106:183–202. doi: 10.1007/BF01872157. [DOI] [PubMed] [Google Scholar]
- 21.Hölzer SU, Hensel M. 2012. Divergent roles of Salmonella pathogenicity island 2 and metabolic traits during interaction of S. enterica serovar Typhimurium with host cells. PLoS One 7:e33220. doi: 10.1371/journal.pone.0033220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs BC, Bode BP. 2006. Stressing out over survival: glutamine as an apoptotic modulator. J Surg Res 131:26–40. doi: 10.1016/j.jss.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Eagle H. 1955. Nutrition needs of mammalian cells in tissue culture. Science 122:501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 24.Wise DR, Thompson CB. 2010. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang W, Choi W, Chen Y, Zhang Q, Deng H, He W, Shi Y. 2013. A proposed role for glutamine in cancer cell growth through acid resistance. Cell Res 23:724–727. doi: 10.1038/cr.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tritsch GL, Moore GE. 1962. Spontaneous decomposition of glutamine in cell culture media. Exp Cell Res 28:360–364. doi: 10.1016/0014-4827(62)90290-2. [DOI] [PubMed] [Google Scholar]
- 27.Patte J-C. 1996. Biosynthesis of threonine and lysine, p 528–541. _In_Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 28.Beuzon CR, Meresse S, Unsworth KE, Ruiz-Albert J, Garvis S, Waterman SR, Ryder TA, Boucrot E, Holden DW. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J 19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Albert J, Yu XJ, Beuzon CR, Blakey AN, Galyov EE, Holden DW. 2002. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol Microbiol 44:645–661. doi: 10.1046/j.1365-2958.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 30.Omsland A, Hackstadt T, Heinzen RA. 2013. Bringing culture to the uncultured: Coxiella burnetii and lessons for obligate intracellular bacterial pathogens. PLoS Pathog 9:e1003540. doi: 10.1371/journal.ppat.1003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hackstadt T. 1996. The biology of rickettsiae. Infect Agents Dis 5:127–143. [PubMed] [Google Scholar]
- 32.Casadevall A. 2008. Evolution of intracellular pathogens. Annu Rev Microbiol 62:19–33. doi: 10.1146/annurev.micro.61.080706.093305. [DOI] [PubMed] [Google Scholar]
- 33.Isberg RR, O'Connor TJ, Heidtman M. 2009. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol 7:13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson AR, Payne EC, Younger N, Karlinsey JE, Thomas VC, Becker LA, Navarre WW, Castor ME, Libby SJ, Fang FC. 2011. Multiple targets of nitric oxide in the tricarboxylic acid cycle of Salmonella enterica serovar Typhimurium. Cell Host Microbe 10:33–43. doi: 10.1016/j.chom.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das P, Lahiri A, Lahiri A, Sen M, Iyer N, Kapoor N, Balaji KN, Chakravortty D. 2010. Cationic amino acid transporters and Salmonella Typhimurium ArgT collectively regulate arginine availability towards intracellular Salmonella growth. PLoS One 5:e15466. doi: 10.1371/journal.pone.0015466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reference deleted.
- 37.Gotz A, Eylert E, Eisenreich W, Goebel W. 2010. Carbon metabolism of enterobacterial human pathogens growing in epithelial colorectal adenocarcinoma (Caco-2) cells. PLoS One 5:e10586. doi: 10.1371/journal.pone.0010586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee EJ, Choi J, Groisman EA. 2014. Control of a Salmonella virulence operon by proline-charged tRNAPro. Proc Natl Acad Sci U S A 111:3140–3145. doi: 10.1073/pnas.1316209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steeb B, Claudi B, Burton NA, Tienz P, Schmidt A, Farhan H, Maze A, Bumann D. 2013. Parallel exploitation of diverse host nutrients enhances Salmonella virulence. PLoS Pathog 9:e1003301. doi: 10.1371/journal.ppat.1003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schell JC, Rutter J. 2013. The long and winding road to the mitochondrial pyruvate carrier. Cancer Metab 1:6. doi: 10.1186/2049-3002-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Lazaro M. 2008. The Warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents Med Chem 8:305–312. doi: 10.2174/187152008783961932. [DOI] [PubMed] [Google Scholar]
- 42.Reference deleted.
- 43.Jelsbak L, Hartman H, Schroll C, Rosenkrantz JT, Lemire S, Wallrodt I, Thomsen LE, Poolman M, Kilstrup M, Jensen PR, Olsen JE. 2014. Identification of metabolic pathways essential for fitness of Salmonella Typhimurium in vivo. PLoS One 9:e101869. doi: 10.1371/journal.pone.0101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-del Portillo F, Zwick MB, Leung KY, Finlay BB. 1993. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc Natl Acad Sci U S A 90:10544–10548. doi: 10.1073/pnas.90.22.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drecktrah D, Knodler LA, Howe D, Steele-Mortimer O. 2007. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic 8:212–225. doi: 10.1111/j.1600-0854.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Hensel M. 2013. Evaluation of nanoparticles as endocytic tracers in cellular microbiology. Nanoscale 5:9296–9309. doi: 10.1039/c3nr01550e. [DOI] [PubMed] [Google Scholar]
- 47.Payne JW, Smith MW. 1994. Peptide transport by microorganisms. Adv Microb Physiol 36:1–80. doi: 10.1016/S0065-2911(08)60176-9. [DOI] [PubMed] [Google Scholar]
- 48.Smith MW, Tyreman DR, Payne GM, Marshall NJ, Payne JW. 1999. Substrate specificity of the periplasmic dipeptide-binding protein from Escherichia coli: experimental basis for the design of peptide prodrugs. Microbiology 145:2891–2901. doi: 10.1099/00221287-145-10-2891. [DOI] [PubMed] [Google Scholar]
- 49.Christie A, Butler M. 1994. Glutamine-based dipeptides are utilized in mammalian cell culture by extracellular hydrolysis catalyzed by a specific peptidase. J Biotechnol 37:277–290. doi: 10.1016/0168-1656(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 50.Chakravortty D, Hansen-Wester I, Hensel M. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J Exp Med 195:1155–1166. doi: 10.1084/jem.20011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halici S, Zenk SF, Jantsch J, Hensel M. 2008. Functional analysis of the Salmonella pathogenicity island 2-mediated inhibition of antigen presentation in dendritic cells. Infect Immun 76:4924–4933. doi: 10.1128/IAI.00531-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material