Real-Time Polymerase Chain Reaction Detection of Angiostrongylus cantonensis DNA in Cerebrospinal Fluid from Patients with Eosinophilic Meningitis (original) (raw)
Abstract
Angiostrongylus cantonensis is the most common infectious cause of eosinophilic meningitis. Timely diagnosis of these infections is difficult, partly because reliable laboratory diagnostic methods are unavailable. The aim of this study was to evaluate the usefulness of a real-time polymerase chain reaction (PCR) assay for the detection of A. cantonensis DNA in human cerebrospinal fluid (CSF) specimens. A total of 49 CSF specimens from 33 patients with eosinophilic meningitis were included: A. cantonensis DNA was detected in 32 CSF specimens, from 22 patients. Four patients had intermittently positive and negative real-time PCR results on subsequent samples, indicating that the level of A. cantonensis DNA present in CSF may fluctuate during the course of the illness. Immunodiagnosis and/or supplemental PCR testing supported the real-time PCR findings for 30 patients. On the basis of these observations, this real-time PCR assay can be useful to detect A. cantonensis in the CSF from patients with eosinophilic meningitis.
Introduction
Infection by Angiostrongylus cantonensis (central nervous system [CNS] angiostrongyliasis) represents the most common cause of infectious eosinophilic meningitis in humans. This nematode, also known as rat lungworm, is now endemic in most tropical and subtropical regions of the world, including southeast Asia, the Pacific islands, South America, and the Caribbean islands.1–3 Humans become infected by ingesting the infectious third stage larvae from mollusks, by intentional or accidental ingestion of raw infected mollusks, via contaminated fresh vegetables, or possibly contaminated water.4–7 In addition, various species of fish, shrimp, amphibians, reptiles, and planarians serving as paratenic hosts have been implicated as the source of human infections.8–14 The majority of cases are self-limited with full recovery, but severe cases can be fatal or cause persistent neurological problems. Typical clinical manifestations include severe and persistent headache, neck stiffness, paresthesias, and cranial nerve palsy.15,16
Most cases of CNS angiostrongyliasis are diagnosed based on clinical symptoms, the presence of cerebrospinal fluid (CSF) eosinophilia, and exposure to a potential source of the infective larvae.7 Laboratory confirmation of infections is difficult since there are only few tests available. Finding the intact larvae during microscopic examination of the CSF is definitive, but this finding is rare, even in severe infections.17–19 Detection of antibodies produced in response to the infection (immunodiagnosis) can be performed on serum or CSF using enzyme-linked immunosorbent assay (ELISA) or western blot (WB) techniques. However, these methods are not standardized and their diagnostic performance may vary depending on the purity of the native antigenic preparation used. When crude antigens were used, the sensitivity was 100% for ELISA and 69% for WB, while specificity was only 67% for ELISA and 82% for WB.20 Purification of the 31-kDa antigens reportedly resulted in 100% specificity and sensitivity of both ELISA and WB,21 but the reliance of native antigens makes the assays difficult to reproduce in other laboratories. Thus, immunodiagnosis for angiostrongyliasis is only available in a few specialized research laboratories worldwide. Because of these diagnostic challenges, the disease can remain undiagnosed or be confused with infections that cause similar symptoms.16
Patients with syndromic diagnosis of meningitis routinely get lumbar puncture (LP) to collect CSF for diagnostic and therapeutic purposes. CSF is therefore a common type of sample available from patients with suspected CNS angiostrongyliasis. When laboratory rats were experimentally infected with A. cantonensis, 52% (11/21) had A. cantonensis DNA in their CSF as detected by real-time polymerase chain reaction (PCR) 60 days after infection, despite the absence of intact larvae in their CSF (unpublished data). These findings made it plausible that CSF from at least some infected humans may contain detectable levels of A. cantonensis DNA. A small study in Thailand indeed found Angiostrongylus DNA in CSF from four of 10 serologically confirmed angiostrongyliasis cases using a genus-level standard PCR.22 The aim of this work was to evaluate if a species-specific real-time PCR assay, originally developed to detect A. cantonensis in host animals,23 could be suitable for the detection of parasite DNA in CSF specimens from patients with CNS angiostrongyliasis.
Materials and Methods
Human samples.
From 33 patients with meningitis during 2000–2012, 49 CSF samples were included in this study. All patients except one met the following case definition for CNS angiostrongyliasis: patients who underwent LP, had evidence of pleocytosis with ≥ 6 leukocytes/mL and either eosinophil percentage ≥ 10% or absolute eosinophil count ≥ 10; had at least two of the following clinical manifestations: headache, neck stiffness or nuchal rigidity, visual disturbance, photophobia or hyperacusis, cranial nerve abnormality (e.g., palsy), abnormal skin sensation (e.g., paresthesia, hyperesthesia), sensory deficit, nausea or vomiting, documented fever, increased irritability (if age < 4 years), and bulging fontanelle (if age < 18 months); and had all other likely etiologies ruled out. One patient did not have CSF eosinophilia but did otherwise fit the case definition and was included because of known ingestion of raw snails. The patients were residents of Hawaii (N = 18), Jamaica (N = 2), Brazil (N = 3), or Cambodia (N = 3) or lived elsewhere in the United States but had recent travel history to Hawaii (N = 2), Jamaica (N = 3), Burma (N = 1), or Fiji (N = 1).
A clinical specificity panel was assembled from patients with non-angiostrongyliasis CNS conditions. The panel included 31 CSF samples from 31 continental U.S. patients and 36 CSF samples from 30 patients residing in Hawaii. Of patients, 59% (N = 36) presented with infection, including infections of unknown etiology (N = 25), cysticercosis (N = 5), toxoplasmosis (N = 2), enterovirus (N = 2), cytomegalovirus sepsis (N = 1), and chronic cryptococcal meningitis (N = 1). Fifteen percent (N = 9) presented with malignancy including leukemia (N = 5), brain cancer (N = 2), Burkitt's lymphoma (N = 1), and metastatic gastric cancer (N = 1). Ten percent (N = 6) presented with headache, 6% (N = 4) presented with altered mental status, and 10% (N = 6) presented with other conditions described as transverse myelitis, unknown hematological disorder, acute lower back pain, Guillain–Barre syndrome, multiorgan disease, or infant with low grade fever. The demographics of the continental U.S. patients were unknown. The Hawaii specificity panel consisted of 16 (53%) male and 14 (47%) female patients with ages ranging from 10 days to 74 years (mean = 33 years; median = 35 years). DNA from seven strains of Coccidioides immitis and Coccidioides posadasii isolated from human coccidioidomycosis patients were also available for specificity testing.
Serum for antibody testing was available from 13 of the 33 patients. Serology results from testing in other laboratories were available for four patients. All samples were collected and used in accordance with the Centers for Disease Control and Prevention Institutional Review Board (IRB) protocol entitled “Use of Human Specimens for Laboratory Methods Research,” Kaiser Permanente IRB approval, and/or Hawaii Department of Health IRB approval.
Immunodiagnosis.
Detection of anti-A. cantonensis antibodies was performed using ELISA and WB as described elsewhere.20 Samples were considered positive in WB when antibodies against the A. cantonensis 31-kDa antigen were detected. The availability of the two methods varied during the course of this study, so some samples were tested with both ELISA and WB, while other samples were tested with only one of the methods. For this reason, a sample was interpreted as being antibody positive if either tests was positive.
Molecular detection.
DNA was extracted from CSF using the DNeasy tissue and blood DNA extraction kit (QIAGEN, Valencia, CA), following the manufacturer's instructions for blood samples. Small volumes (i.e., < 0.2 mL) of CSF were adjusted with saline solution to a total volume of 0.2 mL. CSF samples larger than 0.2 mL were centrifuged at 2,500 g for 10 minutes and the excess supernatant removed so that 0.2 mL remained. Real-time PCR detection of the first internal transcribed spacer (ITS1) was performed using 5 μL of the DNA in 20 μL total volume as described elsewhere.23 CSF samples from patients without available immunodiagnostic results were tested by standard PCR to amplify and sequence 200 bp of the mitochondrial cytochrome oxidase subunit 1 gene (CO1). Primers used were CO1ACF7 (5′-TGC CTG CTT TTG GGA TTG TTA GAC-3′) and CO1ACR7 (5′-TCA CTC CCG TAG GAA CCG CA-3′), and the amplification mix consisted of 0.2 μM of each primer, the AmpliTaq Gold PCR Master Mix (Life Technologies, Grand Island, NY) and 5 μL of DNA in a total volume of 50 μL. After an initial incubation at 95°C for 15 minutes to activate the hot-start enzyme, the cycling structure was 94°C 30 seconds, 60°C 30 seconds, 72°C 1 minute 30 seconds for 45 cycles. The amplicons were sequenced using the amplification primers and the BigDye Terminator (Life Technologies) chemistry as previously described.24
Results
Patients and CSF samples.
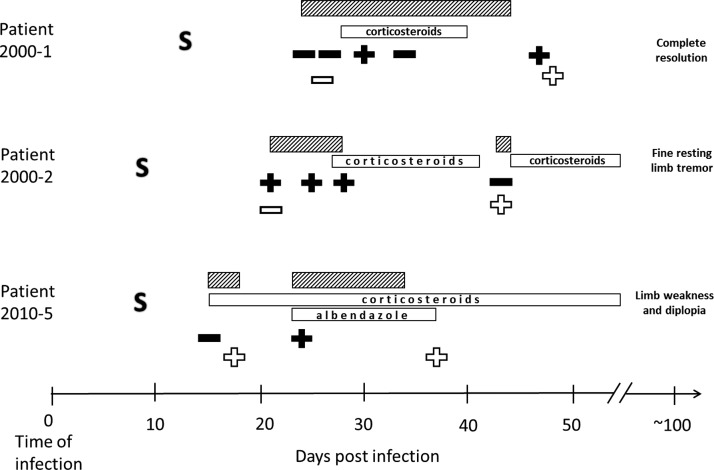
Table 1 summarizes selected demographic, clinical, and epidemiological findings related to 33 patients included in this study. The total number of CSF samples analyzed was 49; one single CSF sample from 23 patients (N = 23), two CSF samples collected at different days during their illness from seven patients (N = 14), and three or more samples collected at different days from three patients (N = 12). The date of infection was known for three patients; the clinical course, laboratory test results, and clinical outcome 3 months after infection for these three patients are illustrated in Figure 1.
Table 1.
Demographic, clinical, and epidemiologic findings of 33 patients with suspected CNS angiostrongyliasis
| Mean age (range) | 27 years (9 months to 69 years) |
|---|---|
| Male gender | 20 (61%) |
| Eosinophil percentage in CSF (range) | 26% (0–78) |
| Exposure location | |
| Hawaii | 19 (58%) |
| Jamaica | 5 (15%) |
| Brazil | 3 (9%) |
| Cambodia | 3 (9%) |
| Fiji | 1 (3%) |
| Burma | 1 (3%) |
| Primary clinical manifestation* | |
| Headache | 18 (54%) |
| Paresthesia | 11 (33%) |
| Lethargy | 6 (18%) |
| Myalgia/arthralgia/body pain | 5 (15%) |
| Fever† | 4 (12%) |
| Neck/back pain | 3 (9%) |
| Coma | 3 (9%) |
| Paralysis | 2 (6%) |
| Photophobia | 2 (6%) |
| Death | 1 (3%) |
| Exposure history | |
| Intentional ingestion of raw mollusks | 5 (15%) |
| Contaminated fresh produce‡ | 2 (6%) |
| Unknown | 26 (79%) |
Figure 1.
The clinical course and timing of laboratory tests of three patients whose date of infection was known. Serial lumbar punctures were performed at the discretion of the clinicians taking care of the patients to monitor the clinical course and relieve headaches. Number of days after time of infection is outlined on the x axis. S = first symptom onset. Filled symbols = collection of cerebrospinal fluid (CSF) that tested positive (plus sign) or negative (minus sign) in the real-time polymerase chain reaction (PCR) assay; open symbols = collection of serum that tested positive (plus sign) or negative (minus sign) in serology. Open boxes denote drug treatment (corticosteroids for all three plus albendazole for patient 2010-5). Striped boxes symbolize days of hospitalization; note that two of the patients were hospitalized twice, because of worsening symptoms due to too rapid corticosteroid dose reduction. The clinical outcome reported around 3 months after infection is outlined on the far right.
Real-time PCR results on CSF samples.
The real-time PCR results for all 33 patients are listed in Tables 2 and 3. Table 2 lists patients for whom immunodiagnosis was performed, whereas Table 3 lists the remaining seven patients. Overall, 32 of the 49 CSF samples tested positive for A. cantonensis DNA. Twenty-two patients had one or more CSF specimens that tested positive in real-time PCR. Of the 22 patients, 19 had positive real-time PCR result in the first CSF collected. The median time from symptom onset to collection of the first real-time-PCR-positive CSF was 21 days (range = 4–67 days). Six of the 10 patients who provided more than one CSF sample had positive real-time PCR results in all of their samples (persistently positive at all collection points). The remaining four patients had intermittently positive real-time PCR results, which means that some CSF samples tested positive while other CSF samples (from the same patient) tested negative.
Table 2.
Laboratory test results of 26 patients with available immunodiagnostic results
| Patient ID | Days post symptom onset | Real-time PCR (Ct*) | Immunodiagnosis results (method) | Reference |
|---|---|---|---|---|
| 2000-1 | 12 | Positive (38) | Acute serum negative (WB) | 25 |
| 15 | Positive (36) | Convalescent serum positive (WB) | ||
| 23 | Positive (38) | |||
| 34 | Negative | |||
| 2000-2 | 10 | Negative | Acute serum negative (WB) | 25 |
| 12 | Negative | Convalescent serum positive (WB) | ||
| 16 | Positive (38) | |||
| 20 | Negative | |||
| 33 | Positive (33) | |||
| 2006-1 | NA | Negative | Serum negative (WB) | |
| 2006-2 | NA | Negative | Serum negative (WB) | |
| 2008-1 | NA | Positive (31) | Serum positive (ELISA) | |
| 2008-2 | NA | Negative | Serum negative (ELISA) | |
| 2008-3 | 48 | Positive (32) | CSF positive (ELISA) | 26 |
| 2009-1 | 7 | Negative | CSF negative (ELISA) | |
| 2009-2 | 14 | Negative | CSF negative (ELISA) | |
| 2009-3 | 15 | Negative | CSF positive (ELISA)/negative (WB) | |
| 2009-4 | 67 | Positive (33) | Serum positive (ELISA, WB) | 27 |
| 2009-5 | 4 | Positive (30) | CSF positive (ELISA, WB) | |
| 2009-6 | 7 | Positive (35) | CSF negative (ELISA, WB) | |
| 2010-2 | NA | Positive (33) | Serum positive (ELISA) | |
| NA | Positive (34) | |||
| 2010-3 | NA | Negative | Serum negative (ELISA, WB) | |
| 2010-4 | 231 | Negative | CSF negative (ELISA, WB) | |
| 2010-5 | 6 | Negative | Acute serum negative (ELISA)/positive (WB) | 28 |
| 15 | Positive (29) | Convalescent serum indeterminate (ELISA)/positive (WB) | ||
| 2010-6 | 4 | Negative | CSF negative (ELISA, WB) | |
| 17 | Positive (27) | CSF positive (ELISA, WB) | ||
| 2010-7 | 10 | Positive (29) | CSF indeterminate (ELISA)/negative (WB) | |
| 15 | Positive (30) | CSF positive (ELISA)/negative (WB) | ||
| 2011-1 | 4 | Positive (30) | Serum positive (WB) | |
| 15 | Positive (28) | |||
| 2011-3 | 26 | Positive (35) | Serum positive (WB) | |
| 2011-4 | 16 | Negative | Serum negative (WB) | |
| 2011-5 | 48 | Positive (35) | Serum positive (WB) | 29 |
| 49 | Positive (35) | |||
| 2012-2 | 30 | Positive (33) | Serum positive (WB) | |
| 2012-3 | 27 | Positive (36) | Serum positive (WB) | |
| 2012-4 | 30 | Positive (35) | Serum positive (WB) |
Table 3.
PCR results of seven patients with no available immunodiagnostic results
| Patient ID | Days post symptom onset | Real-time PCR (Ct*) | CO1 sequencing |
|---|---|---|---|
| 2010-1 | 23 | Positive (35) | Angiostrongylus cantonensis |
| 2011-2 | 5 | Negative | Negative |
| 2011-6 | 10† | Positive (34) | A. cantonensis |
| 11 | Positive (35) | A. cantonensis | |
| 30 | Positive (36) | A. cantonensis | |
| 2011-7 | 21 | Positive (36) | Negative |
| 2012-1 | 21 | Positive (36) | Negative |
| 2012-5 | 23 | Positive (32) | A. cantonensis |
| 29 | Positive (32) | A. cantonensis | |
| 2012-6 | 33 | Negative | Negative |
Specificity controls included 67 CSF samples from 61 U.S. patients, representing non-Angiostrongylus CNS conditions of both infectious and noninfectious etiologies (see section Materials and Methods for details). All of these CSF samples were negative in the real-time PCR assay. The real-time PCR did not amplify DNA extracted from Coccidioides spp., which can also cause eosinophilic meningitis.
Comparison between real-time PCR and immunodiagnosis.
Anti-A. cantonensis antibody detection was performed for 26 patients (Table 2). Serology was performed on serum for 17 patients, either as part of this study (N = 13) or by another laboratory prior to this study (N = 4). Nine additional patients had antibody detection performed on CSF. The immunodiagnosis result was in agreement with the real-time PCR for 24 of the 26 patients; only two patients had discrepant test results and are discussed below (see section Discussion). The median time from symptom onset to collection of the first antibody-positive sample (either serum or CSF) was 27 days (range = 4–75 days).
Comparison between real-time PCR and other testing.
Immunodiagnosis could not be performed for seven patients because no serum was available, and the limited volume of CSF did not allow for additional testing after the PCR analysis. The 10 CSF samples from these seven patients were therefore further analyzed in a standard PCR followed by DNA sequencing of a part of the mitochondrial CO1 gene (CO1 assay). This approach confirmed the presence of A. cantonensis DNA in six of the eight real-time-PCR-positive CSF samples (Table 3).
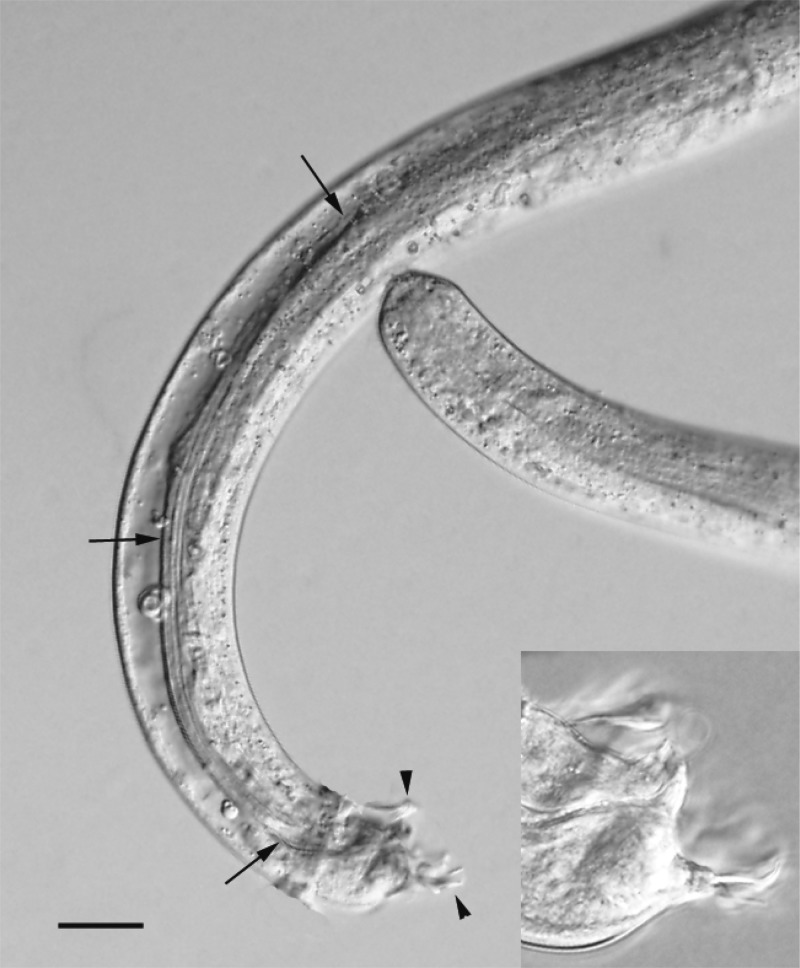
In one CSF sample, larvae were detected by microscopy (Figure 2). This CSF sample was also positive in real-time PCR. Two additional CSF samples from the same patient (2011-6; a 9-month-old boy) were also positive in real-time PCR and all three CSF samples from this patient were positive for A. cantonensis in the CO1 assay.
Figure 2.
Photomicrograph of a subadult male Angiostrongylus cantonensis worm isolated from the cerebrospinal fluid (CSF) of a 9-month-old boy (patient 2011-6). The anterior and posterior ends of the worm are present in the image, and the arrows point to the very long left spicule and the developing bursa (arrowheads) on the tail. Note that there is some host tissue encircling the worm to the right of the lower arrow. Scale bar = 50 μm. Inset is higher magnification of bursa showing greater level of detail. Scale bar = 15 μm.
Discussion
CNS angiostrongyliasis is usually difficult to diagnose. In most cases, symptoms are nonspecific, and the CSF reveals eosinophilia, but no larvae are detected. Only one patient in this study (a 9-month-old boy) had larvae detected in the CSF and was thus the only parasitologically confirmed case. In contrast, 22 of 33 patients (67%) were positive for A. cantonensis DNA in their CSF by real-time PCR. Immunodiagnosis or additional PCR-based testing corroborated the real-time PCR findings for all but three patients.
Quantitative assessment of A. cantonensis larvae in environmental samples using the same real-time PCR as applied to this study has shown that samples containing a single, stage-three larva are positive after an average of 23 PCR cycles (i.e., average Ct value of 23).30,31 Not unexpectedly, the PCR-positive CSF samples in this study had Ct values between 27 and 38, suggesting that these samples contained 1–4 log10 less DNA than that present in a specimen containing a single larva. This indicates that the real-time PCR can detect DNA from remnants of larvae, such as individual cells, nuclei, or chromosomal fragments, which may leak into the CSF from the brain. Presence of DNA-containing remnants is the likely reason why so many CSF samples that were devoid of intact parasites were positive in real-time PCR.
All 33 patients included in this study had clinical findings suggestive of CNS angiostrongyliasis. All had possible exposure to infective larvae, either as residents of or with recent travel history to endemic areas. Clinical and epidemiological details have been presented elsewhere for six of the patients.25–29 Five patients had knowingly ingested raw snails: four as part of exotic cuisine, one as a dare. Two patients were part of an outbreak in Jamaica where a Caesar salad was implicated as the source of infection.25 The source of infection for the other patients remains unknown. However, four patients were less than 2 years of age, and one patient was intellectually challenged, making it plausible that these became infected by putting infected mollusks in their mouth.
When available, serum samples were tested for the presence of antibodies against A. cantonensis using a crude antigen ELISA and/or WB technique that detects the 31-kDa antigen.20 These methods were also applied on some CSF samples with enough volume to accommodate both molecular and immunodiagnostic testing. The CSF from patient 2009-6 tested positive in real-time PCR but negative for antibodies and was initially considered a potential false-positive real-time PCR result. However, CO1 DNA sequencing verified that this CSF sample contained A. cantonensis DNA, so the explanation for the discordance was more likely failed antibody detection. PCR has the potential to become positive earlier than serology because A. cantonensis DNA has the potential to be present in the CSF in the acute phase, prior to the development of antibodies.
Patient 2009-3 had negative real-time PCR results but antibodies were detected in the CSF; this was likely a false-negative real-time PCR result. In this study, there was no clear correlation between date of collection of a real-time-PCR-positive CSF sample and other laboratory data (such as eosinophilia levels) or illness duration (time after symptom onset). However, CSF collections were performed at the discretion of the patients' clinicians, so the patients in this study all had different collection time points (based on their individual clinical course and medical management). More systematic studies, using standardized protocols, will be required to better understand the diagnostic value of this real-time PCR during the infection progress and to reveal possible correlations between real-time PCR positivity and other laboratory data or clinical signs.
Overall, seven CSF-samples from serologically confirmed patients tested negative in the real-time PCR assay, leading to a false-negative rate of 22%. At least some of those false-negative results may be due to fluctuating levels of parasite DNA in the CSF over time. It has therefore been suggested to test additional CSF samples, collected on other days, if the initial real-time PCR result is negative and the clinical suspicion remains high. Repeated CSF collections are sometimes performed on meningitis patients to monitor the course of infection or to measure the intracranial pressure. They may also have a therapeutic benefit because the pressure released during the LP tends to relieve symptoms. However, LP is an invasive procedure with potentially serious complications, such as CSF leak, worsening headache, and brain herniation. The risks versus benefits for each patient should be weighed when considering conducting repeated LPs. Another way to enhance the sensitivity of this real-time PCR assay could be to allow the patients to sit upright for some time before performing the LP, since this has been shown to increase the likelihood of finding larvae in the sample.32
Two patients (2011-7 and 2012-1) with positive real-time PCR results did not have specimens available for immunodiagnosis and the CO1 PCR testing was negative. This discrepancy between the two PCR-based tests is most likely because of a lower detection limit of the real-time PCR, thanks to a smaller amplicon size and more sensitive detection method (fluorescence).
All specificity controls tested with this real-time PCR assay were negative. Because no apparent false-positive real-time PCR results were observed, it thus far appears that other CNS conditions cause little or no interference with this assay. The original purpose of this particular real-time PCR assay was to specifically detect A. cantonensis in host animals. In silico investigations and analytic specificity testing during the initial test validation excluded cross-amplification of nematodes known to infect rodents, rats, and humans, including the closely related A. costaricensis.23 In this study, clinical isolates of Coccidioides spp., a group of fungi that can cause eosinophilic meningitis, were shown to be nonreactive with this assay. Specificity testing of this assay will continue whenever suitable samples become available.
Diagnosis of CNS angiostrongyliasis requires thorough patient history, good clinical examination, presence of some key laboratory values, and some indication that the parasite is/was present. This report describes the successful use of real-time PCR to detect A. cantonensis DNA in the CSF from 22 of 33 patients with clinical and epidemiological suspicion of CNS angiostrongyliasis.
ACKNOWLEDGMENTS
Specificity controls were generously provided by Cindy Press (Toxoplasma Serology Laboratory Research Institute, Palo Alto Medical Foundation), Xin Wang and Fang Hu (Meningitis Laboratory, CDC), Shawn Lockhart and Steve Hurst (Mycotic Diseases Branch, CDC), and Isabel McAuliffe and Leslie Henderson (Division of Parasitic Diseases and Malaria, CDC). Mark Eberhard and Henry Bishop (Division of Parasitic Diseases and Malaria, CDC) assisted in the identification of worm in CSF. Nichole Nirei assisted with verification testing in Hawaii. Koah Vierkoetter and Wichit Sae-Ow assisted in collecting clinical information at Kaiser Permanente, Honolulu, HI.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This study was funded by the U.S. National Food Safety Initiative and a CDC Epidemiology and Laboratory Capacity Cooperative Agreement.
Authors' addresses: Yvonne Qvarnstrom and LeAnne M. Fox, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: bvp2@cdc.gov and llf4@cdc.gov. Maniphet Xayavong, Life Source Biomedical LLC, Herndon, VA, E-mail: max1@cdc.gov. Ana Cristina Aramburu da Silva and Carlos Graeff-Teixeira, Laboratório de Parasitologia Molecular, Instituto de Pesquisas Biomédicas da PUCRS, Porto Alegre, Brazil, and Laboratório de Biologia Parasitária, Faculdade de Biociências da PUCRS, Porto Alegre, Brazil, E-mails: anacristina@pucrs.br and graeff.teixeira@gmail.com. Sarah Y. Park, Hawaii Department of Health, Honolulu, HI, E-mail: sarah.park@doh.hawaii.gov. A. Christian Whelen, Precilia S. Calimlim, and Rebecca H. Sciulli, Hawaii Department of Health, Pearl City, HI, E-mails: chris.whelen@doh.hawaii.gov, precilia.calimlim@doh.hawaii.gov, and rebecca.sciulli@doh.hawaii.gov. Stacey A. A. Honda, Hawaii Permanente Medical Group, Honolulu, HI, E-mail: stacey.honda@kp.org. Karen Higa, Kaiser Permanente Regional Laboratory, Honolulu, HI, E-mail: karen.higa@kp.org. Paul Kitsutani, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD, E-mail: paul.kitsutani@nih.gov. Nora Chea, Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: xdc7@cdc.gov. Seng Heng, Communicable Disease Department, Ministry of Health, Phnom Penh, Cambodia, E-mail: senghengmoh@gmail.com. Stuart Johnson, Loyola University Medical Center, Chicago, IL, and Edward Hines Jr. VA Hospital, Chicago, IL, E-mail: stuart.johnson2@va.gov. Alexandre J. da Silva, Center for Food Safety and Applied Nutrition, Food and Drug Administration, Laurel, MD, E-mail: alexandre.dasilva@fda.hhs.gov.
References
- 1.Evans-Gilbert T, Lindo JF, Henry S, Brown P, Christie CD. Severe eosinophilic meningitis owing to Angiostrongylus cantonensis in young Jamaican children: case report and literature review. Paediatr Int Child Health. 2013;34:148–152. doi: 10.1179/2046905513Y.0000000106. [DOI] [PubMed] [Google Scholar]
- 2.Kliks MM, Palumbo NE. Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med. 1992;34:199–212. doi: 10.1016/0277-9536(92)90097-a. [DOI] [PubMed] [Google Scholar]
- 3.Simoes RO, Monteiro FA, Sanchez E, Thiengo SC, Garcia JS, Costa-Neto SF, Luque JL, Maldonado A. Endemic angiostrongyliasis, Rio de Janeiro, Brazil. Emerg Infect Dis. 2011;17:1331–1333. doi: 10.3201/eid1707.101822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown FM, Mohareb EW, Yousif F, Sultan Y, Girgis NI. Angiostrongylus eosinophilic meningitis in Egypt. Lancet. 1996;348:964–965. doi: 10.1016/s0140-6736(05)65382-2. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg NS, Blackburn BG, Park SY, Sejvar JJ, Effler PV, Herwaldt BL. Eosinophilic meningitis attributable to Angiostrongylus cantonensis infection in Hawaii: clinical characteristics and potential exposures. Am J Trop Med Hyg. 2011;85:685–690. doi: 10.4269/ajtmh.2011.11-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai TH, Liu YC, Wann SR, Lin WR, Lee SJ, Lin HH, Chen YS, Yen MY, Yen CM. An outbreak of meningitis caused by Angiostrongylus cantonensis in Kaohsiung. J Microbiol Immunol Infect. 2001;34:50–56. [PubMed] [Google Scholar]
- 7.Wang Q-P, Wu Z-D, Wei J, Owen RL, Lun Z-R. Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis. 2012;31:389–395. doi: 10.1007/s10096-011-1328-5. [DOI] [PubMed] [Google Scholar]
- 8.Asato R, Taira K, Nakamura M, Kudaka J, Itokazu K, Kawanaka M. Changing epidemiology of angiostrongyliasis cantonensis in Okinawa prefecture, Japan. Jpn J Infect Dis. 2004;57:184–186. [PubMed] [Google Scholar]
- 9.Ash LR. The occurrence of Angiostrongylus cantonensis in frogs of New Caledonia with observations on paratenic hosts of metastrongyles. J Parasitol. 1968;54:432–436. [PubMed] [Google Scholar]
- 10.Ash LR. Observations on the role of mollusks and planarians in the transmission of Angiostrongylus cantonensis infection to man in New Caledonia. Rev Biol Trop. 1976;24:163–174. [Google Scholar]
- 11.Lai CH, Yen CM, Chin C, Chung HC, Kuo HC, Lin HH. Eosinophilic meningitis caused by Angiostrongylus cantonensis after ingestion of raw frogs. Am J Trop Med Hyg. 2007;76:399–402. [PubMed] [Google Scholar]
- 12.Panackel C, Vishad, Cherian G, Vijayakumar K, Sharma RN. Eosinophilic meningitis due to Angiostrongylus cantonensis. Indian J Med Microbiol. 2006;24:220–221. [PubMed] [Google Scholar]
- 13.Radomyos P, Tungtrongchitr A, Praewanich R, Khewwatchan P, Kantangkul T, Junlananto P, Ayudhya SIN. Occurrence of the infective stage of Angiostrongylus cantonensis in the yellow tree monitor (Varanus bengalensis) in five provinces of Thailand. Southeast Asian J Trop Med Public Health. 1994;25:498–500. [PubMed] [Google Scholar]
- 14.Wallace GD, Rosen L. Studies on eosinophilic meningitis. IV. Experimental infection of fresh-water and marine fish with Angiostrongylus cantonensis. Am J Epidemiol. 1967;85:395–402. doi: 10.1093/oxfordjournals.aje.a120701. [DOI] [PubMed] [Google Scholar]
- 15.Alto W. Human infections with Angiostrongylus cantonensis. Pac Health Dialog. 2001;8:176–182. [PubMed] [Google Scholar]
- 16.Wang Q-P, Lai D-H, Zhu X-Q, Chen X-G, Lun Z-R. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- 17.Kuberski T, Bart RD, Briley JM, Rosen L. Recovery of Angiostrongylus cantonensis from cerebrospinal fluid of a child with eosinophilic meningitis. J Clin Microbiol. 1979;9:629–631. doi: 10.1128/jcm.9.5.629-631.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen XG, Li H, Lun ZR. Angiostrongyliasis, Mainland China. Emerg Infect Dis. 2005;11:1645–1647. doi: 10.3201/eid1110.041338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochberg NS, Park SY, Blackburn BG, Sejvar JJ, Gaynor K, Chung H, Leniek K, Herwaldt BL, Effler PV. Distribution of eosinophilic meningitis cases attributable to Angiostrongylus cantonensis, Hawaii. Emerg Infect Dis. 2007;13:1675–1680. doi: 10.3201/eid1311.070367. [DOI] [PubMed] [Google Scholar]
- 20.Nuamtanong S. The evaluation of the 29 and 31 kDa antigens in female Angiostrongylus cantonensis for serodiagnosis of human angiostrongyliasis. Southeast Asian J Trop Med Public Health. 1996;27:291–296. [PubMed] [Google Scholar]
- 21.Eamsobhana P, Yoolek A, Suvouttho S, Suvouttho S. Purification of a specific immunodiagnostic Parastrongylus cantonensis antigen by electroelution from SDS-polyacrylamide gels. Southeast Asian J Trop Med Public Health. 2001;32:308–313. [PubMed] [Google Scholar]
- 22.Eamsobhana P, Wanachiwanawin D, Dechkum N, Parsartvit A, Yong HS. Molecular diagnosis of eosinophilic meningitis due to Angiostrongylus cantonensis (Nematoda: Metastrongyloidea) by polymerase chain reaction-DNA sequencing of cerebrospinal fluids of patients. Mem Inst Oswaldo Cruz. 2013;108:116–118. doi: 10.1590/S0074-02762013000100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qvarnstrom Y, da Silva ACA, Teem JL, Hollingsworth R, Bishop H, Graeff-Teixeira C, da Silva AJ. Improved molecular detection of Angiostrongylus cantonensis in mollusks and other environmental samples with a species-specific internal transcribed spacer 1-based TaqMan assay. Appl Environ Microbiol. 2010;76:5287–5289. doi: 10.1128/AEM.00546-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qvarnstrom Y, Sullivan JJ, Bishop HS, Hollingsworth R, da Silva AJ. PCR-based detection of Angiostrongylus cantonensis in tissue and mucus secretions from molluscan hosts. Appl Environ Microbiol. 2007;73:1415–1419. doi: 10.1128/AEM.01968-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, Zambrano CH, Sufit RL, Sakolvaree Y, Chaicumpa W, Herwaldt BL, Johnson S. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med. 2002;346:668–675. doi: 10.1056/NEJMoa012462. [DOI] [PubMed] [Google Scholar]
- 26.Lima AR, Mesquita SD, Santos SS, Aquino ER, Rosa Lda R, Duarte FS, Teixeira AO, Costa ZR, Ferreira ML. Alicata disease: neuroinfestation by Angiostrongylus cantonensis in Recife, Pernambuco, Brazil. Arq Neuropsiquiatr. 2009;67:1093–1096. doi: 10.1590/s0004-282x2009000600025. [DOI] [PubMed] [Google Scholar]
- 27.Howe K. A severe case of rat lungworm disease in Hawa'i. Hawaii J Med Public Health. 2013;72:46–48. [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon E, Ferguson TM, Park SY, Manuzak A, Qvarnstrom Y, Morgan S, Ciminera P, Murphy GS. A severe case of Angiostrongylus eosinophilic meningitis with encephalitis and neurologic sequelae in Hawa'i. Hawaii J Med Public Health. 2013;72:41–45. [PMC free article] [PubMed] [Google Scholar]
- 29.Thyssen A, Mitchell M, Qvarnstrom Y, Rao S, Benke TA, Glode MP. Eosinophilic meningitis in a previously healthy 13-year-old child. Pediatr Infect Dis J. 2013;32:194. doi: 10.1097/INF.0b013e31827c9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarvi SI, Farias ME, Howe K, Jacquier S, Hollingsworth R, Pitt W. Quantitative PCR estimates Angiostrongylus cantonensis (rat lungworm) infection levels in semi-slugs (Parmarion martensi) Mol Biochem Parasitol. 2012;185:174–176. doi: 10.1016/j.molbiopara.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qvarnstrom Y, Bishop HS, da Silva AJ. Detection of rat lungworm in intermediate, definitive, and paratenic hosts obtained from environmental sources. Hawaii J Med Public Health. 2013;72:63–69. [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai HC, Liu YC, Kunin CM, Lee SS, Chen YS, Lin HH, Tsai TH, Lin WR, Huang CK, Yen MY, Yen CM. Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am J Med. 2001;111:109–114. doi: 10.1016/s0002-9343(01)00766-5. [DOI] [PubMed] [Google Scholar]