Curious kinetic behavior in silica polymorphs solves seifertite puzzle in shocked meteorite (original) (raw)
High-pressure experiments have revealed that seifertite metastably forms at much lower pressures than previously thought.
Keywords: shocked meteorite, silica polymorphs, seifertite, high pressure, kinetics, metastable phase
Abstract
The presence of seifertite, one of the high-pressure polymorphs of silica, in achondritic shocked meteorites has been problematic because this phase is thermodynamically stable at more than ~100 GPa, unrealistically high-pressure conditions for the shock events in the early solar system. We conducted in situ x-ray diffraction measurements at high pressure and temperatures, and found that it metastably appears down to ~11 GPa owing to the clear difference in kinetics between the metastable seifertite and stable stishovite formations. The temperature-insensitive but time-sensitive kinetics for the formation of seifertite uniquely constrains that the critical shock duration and size of the impactor on differentiated parental bodies are at least ~0.01 s and ~50 to 100 m, respectively, from the presence of seifertite.
INTRODUCTION
Silica, the most common silicate mineral on the Earth’s crust, has varieties of high-pressure polymorphs. Some of them have been found in achondritic shocked meteorites, which were formed by shock metamorphism on different parent bodies such as Mars, the Moon, and asteroids (1–4). Conditions envisaged by their presence could be an important clue to understand the collisional process in the early solar system. Seifertite is a high-pressure polymorph of silica with α-PbO2–type structure found in Martian and lunar shocked meteorites (1, 5, 6). However, it has been difficult to interpret the presence of seifertite straightforwardly because this phase is thermodynamically stable only at more than ~100 GPa (7–9), significantly higher pressures than those expected based on coexisting high-pressure assemblages. It is unlikely that such high pressures were generated during the impact on Mars and the Moon. Thus, its presence has been referred to as the seifertite puzzle.
The parent phase of seifertite is thought to be cristobalite or tridymite, two igneous-origin, high-temperature phases of silica, because these phases usually coexisted in shocked meteorites (1, 6). A previous study reported that seifertite metastably appears during compression of cristobalite to about 40 GPa at room temperature (10). On the basis of this result, the presence of seifertite in shocked meteorites is often used to infer a peak shock pressure in excess of ~40 GPa (5). Although the previous study provided one key to solving the seifertite puzzle, the criterion needs to be reconsidered because the appearance of a metastable phase should be temperature- and time-dependent. That is, if the quantitative kinetics are known, the pressure-temperature-time history of a shock event can be uniquely constrained from the presence of seifertite.
RESULTS AND DISCUSSION
To solve the seifertite puzzle and construct a new indicator for shock conditions based on the metastable transformation of silica, we carried out in situ x-ray diffraction (XRD) experiments using the Kawai-type high-pressure apparatus at the synchrotron radiation facilities of SPring-8 (BL04B1), Japan. The starting materials of synthetic SiO2 α-cristobalite and reagent-grade SiO2 quartz powders were first compressed up to ~30 GPa at room temperature, and then heated by 100 K step up to ~1450 K at a constant load. We kept temperature constant for ~10 to 50 min at each step while collecting diffraction patterns of the sample every 30 to 200 s.
Figure 1A shows changes of diffraction peaks in α-cristobalite during cold compression followed by heating. Compression of α-cristobalite to about 30 GPa at room temperature causes high-pressure transitions to cristobalite-II and X-I, which is consistent with previous studies (10–12). We did not observe seifertite during the cold compression; however, it metastably appeared when heating the sample at high pressures (Fig. 1B). Further heating leads to the transformation from seifertite to the stable phase of stishovite. We observed the formation of metastable seifertite down to ~11 GPa at around the coesite-stishovite transition boundary (fig. S1). The limit pressure is much lower than previously reported (~40 GPa).
Fig. 1. Changes of XRD patterns during cold compression followed by heating at high pressures in cristobalite.
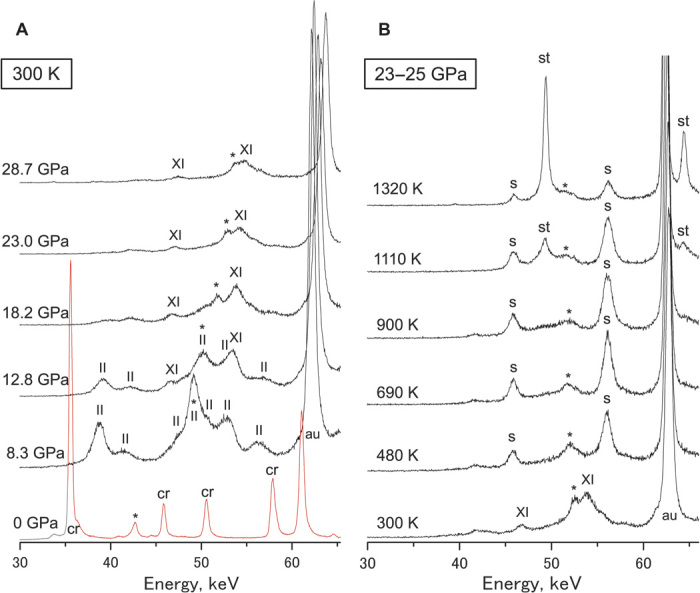
(A) Transformations of α-cristobalite (cr) to cristobalite-II (II) and X-I (XI) by compression at room temperature. (B) Formation of seifertite (s) and stishovite (st) by heating at 23 to 25 GPa. au, gold pressure marker; *, graphite sample capsule.
Pressure and temperature conditions for the formation of these silica polymorphs in experimental time scales (about tens of minutes) are summarized in Fig. 2. The kinetics of seifertite and stishovite formation depend on pressure and temperature differently, such that metastable seifertite was found over a wide range of temperatures at pressures greater than ~11 GPa (Fig. 2). It was an unexpected observation that seifertite, thermodynamically stable at more than ~100 GPa, metastably appears at pressures as low as ~11 GPa. We could recover the seifertite by quenching just before the transition to stishovite and decompressing the experimental charges over several hours. XRD pattern and lattice parameters are shown in fig. S2, and almost coincide with those obtained from shocked meteorites (6, 13).
Fig. 2. Pressure and temperature conditions for the seifertite and stishovite formations from cristobalite.
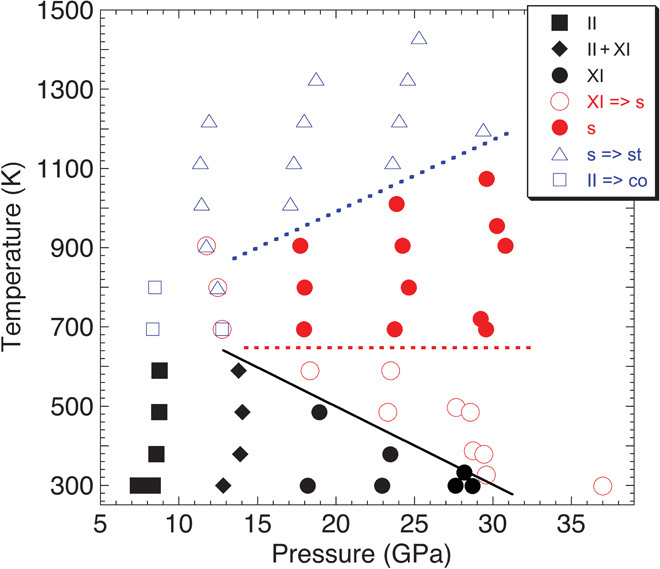
A datum point above 35 GPa was taken from the previous diamond-anvil cell experiment (10). II, cristobalite-II; XI, cristobalite X-I; s, seifertite; st, stishovite; co, coesite.
When using quartz powder as a starting material, we did not observe seifertite formation up to ~25 GPa and 900 K. Quartz directly transforms to stishovite at about 18 GPa and 800 K (fig. S3A). At pressures higher than ~23 GPa, the intensity of the diffraction peak in quartz decreases at 480 to 690 K before the appearance of stishovite at 800 to 900 K (fig. S3B), suggesting that quartz attains a partially amorphous state. The observed quartz amorphization condition is similar to that of the previous study (fig. S4), which may imply the metastable melting of quartz (14). Stishovite is formed from quartz at much lower temperatures compared to cristobalite (Fig. 2 and fig. S4).
We quantitatively observed the kinetics of the cristobalite X-I–to–seifertite and the seifertite-to-stishovite transformations in each temperature step by time-resolved XRD measurements (fig. S5). The reaction rates are summarized here in plots of volume fraction transformed as a function of time (fig. S6). We continuously collected kinetic data over several temperature steps. The kinetic data have been analyzed using the rate equation:
where V is the transformed volume fraction, k and n are constants, and t is time. Here, we have modified the Avrami rate equation (15) by adding a time-correction parameter _t_0 to analyze the kinetic data beginning in the middle of the transformation. Kinetic parameters in the rate equation (1) were estimated from nonlinear least-squares fitting as shown in fig. S6. The obtained k and n values are listed in table S1. The rate constant k can be described by k = k_0exp(−_H */RT), where _k_0 is constant, H * is activation enthalpy, R is gas constant, and T is absolute temperature. The activation enthalpy is described as H * = E* + PV*, where E* is activation energy, P is pressure, and V* is activation volume. Because the estimated n values for each reaction are largely unchanged by pressure and temperature conditions (table S1), we determined pressure and temperature dependencies of the rate constant k based on the averaged n values, as shown in Fig. 3A and table S2.
Fig. 3. Kinetics of the seifertite and stishovite formations.
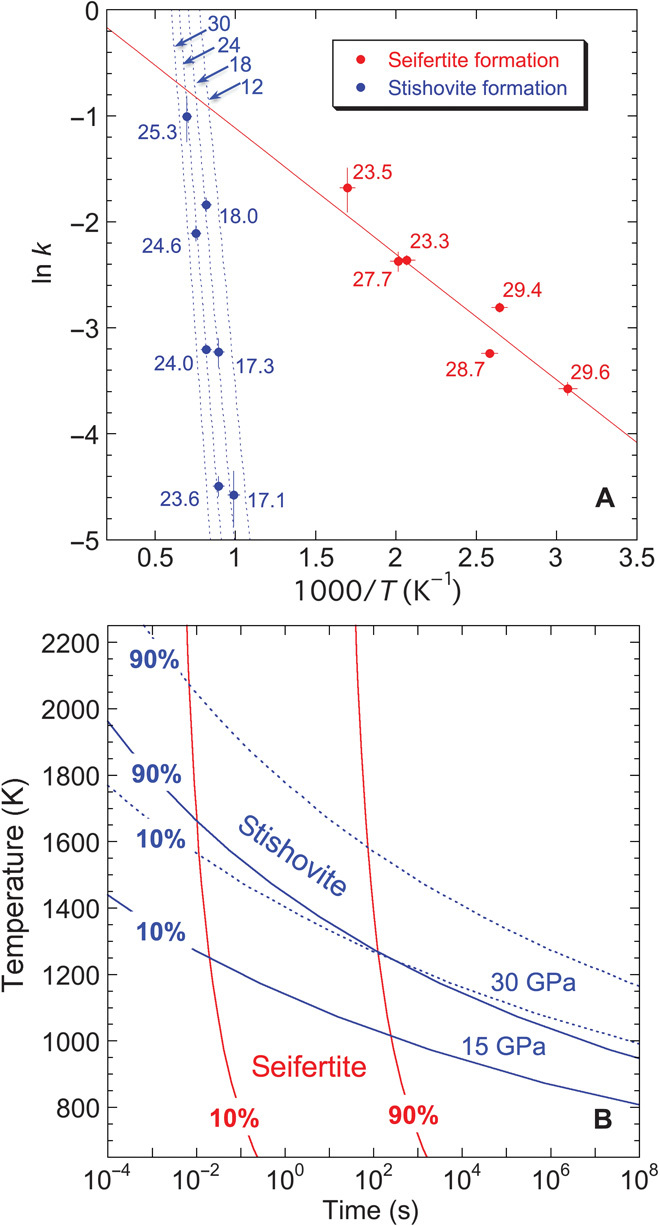
(A) Temperature dependence of the rate constant k. Numbers indicate the pressure condition for each datum point. In the case of the stishovite formation, the pressure dependence was also observed. Activation energies and volumes are summarized in table S2. Results of the fitting are also shown. (B) TTT curves constructed on the basis of kinetic parameters determined in (A).
Analysis of the kinetic data revealed clear differences in kinetics between seifertite and stishovite formation (Fig. 3A). In the case of the seifertite formation, the activation energy is very low (~10 kJ/mol) and there is no detectable pressure dependence. This indicates fast kinetics even at low temperatures, which is consistent with the diffusionless transformation mechanism from cristobalite to seifertite as proposed recently (16). On the contrary, the formation of stishovite from seifertite has relatively large activation energy (~110 kJ/mol) and activation volume, which is thought to have originated from a diffusion-controlled mechanism. These characteristics of reaction kinetics are represented in Fig. 2. Seifertite formation can start at rather low temperatures owing to its low activation energy. The apparent kinetic boundaries shown by dotted lines in Fig. 2 have zero and positive pressure dependencies for the formation of seifertite and stishovite, respectively. An exception is that the boundary for the development of seifertite from cristobalite X-I has a negative slope, although the kinetics have no detectable pressure dependence. We suggest that this boundary is thermodynamically, not kinetically, controlled; the Gibbs free energy relationships among stishovite (_G_st), seifertite (_G_sei), and cristobalite X-I (_G_cri) are _G_st < _G_cri < _G_sei and _G_st < _G_sei < _G_cri below and above the boundary, respectively. When crossing this boundary, seifertite becomes stable compared to cristobalite X-I, and can quickly form instead of the most stable phase of stishovite due to faster kinetics associated with the low activation energy.
Shock durations for Martian and lunar meteorites have so far been estimated as in the range ~0.01 to ~1 s (6, 17–19). To discuss reaction progress on such short time scales, we constructed the time-temperature-transformation (TTT) curves in Fig. 3B based on the kinetic parameters we obtained (table S2). The TTT curves for seifertite and stishovite formation are rather different. Seifertite formation is time-sensitive, requiring shock durations of at least ~0.01 s to start even at temperatures of ~2000 K and higher. Completion is difficult within the time scales of shock events. In contrast, stishovite formation is temperature-sensitive, requiring temperatures higher than ~1200 to 1500 K to start, and can complete at less than ~2000 K. Textural observations of shocked meteorites indicate that seifertite is not present as a single phase, but usually coexists with silica glass and/or stishovite (1, 5, 6). This may be due to a difference in the reaction kinetics, which can result in the coexistence of cristobalite X-I, seifertite, and stishovite under a range of _P_-_T_-t shock conditions. Amorphization of the cristobalite X-I phase may be the origin of the silica glass.
We previously proposed an indicator of shock conditions based on the plagioclase (albite and labradorite) breakdown (20). When used in combination with the present study, the shock metamorphism observed in achondritic meteorites can be consistently discussed. Figure 4 compares _P_-_T_-t kinetic boundaries of cristobalite and labradorite, both present in Martian meteorites of basaltic shergottites. Evidence for both labradorite amorphization and seifertite formation from cristobalite has been observed in basaltic shergottites (5, 21). Our experimental studies have demonstrated that both can occur at similar conditions of relatively low temperatures, although it has been suggested that amorphous plagioclase (maskelynite) forms by melting and quenching from plagioclase melt (22).
Fig. 4. Formation of high-pressure phases from cristobalite and plagioclase (20) on shock event time scales of 1 s (A) and 0.01 s (B).
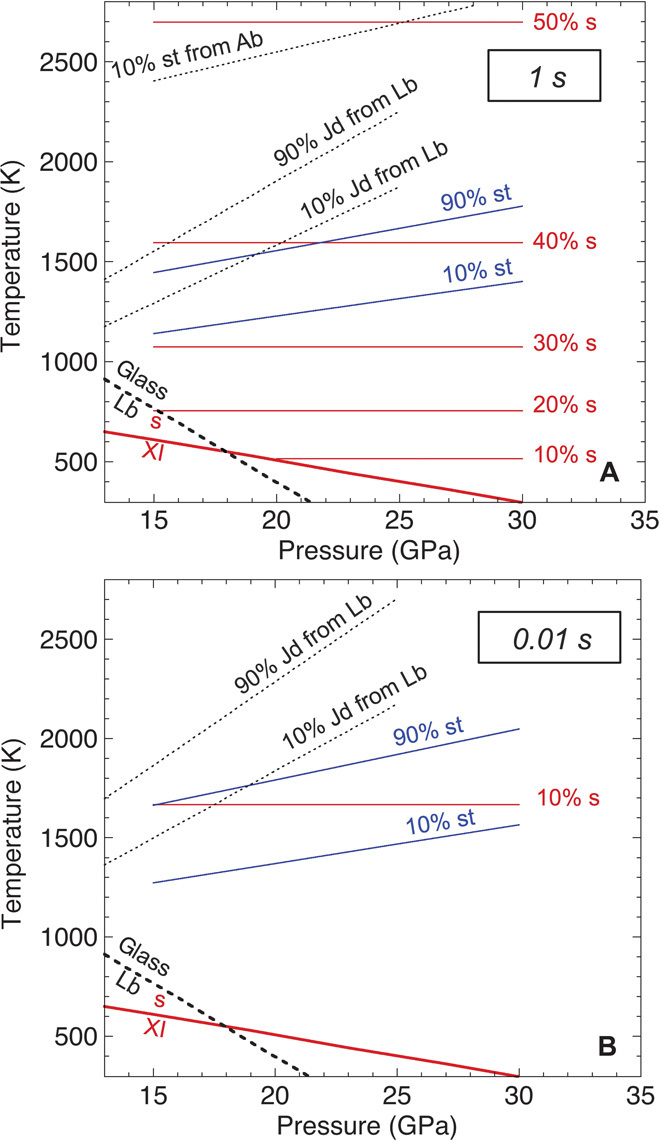
Metastable phase boundaries of the cristobalite X-I (XI)–to–seifertite (s) transformation and the labradorite (Lb) amorphization are shown in thick solid and dotted lines, respectively. _P_-_T_-t kinetic boundaries for the seifertite and stishovite (st) formations in cristobalite are shown in red and blue lines, respectively. Those for the jadeite (Jd) and stishovite formations in plagioclase (Ab: albite) are shown in dotted black lines.
Figure 4 also indicates that, with increasing temperature, seifertite and stishovite formations from cristobalite occur first, followed by jadeite formation from amorphous labradorite at higher temperatures. This explains another feature of shock metamorphism seen in shergottites: the coexistence of stishovite and seifertite without evidence of the formation of jadeite from plagioclase glass. Recently, a new mineral, tissintite, a vacancy-rich clinopyroxene with the composition of plagioclase, has been found in plagioclase glass in the Tissint Martian meteorite (23). This may correspond to the jadeite formation from labradorite glass in our previous experiment (20), implying that the Tissint meteorite had higher temperature history compared to the other shergottites (19, 23).
Additionally, stishovite should also be formed by plagioclase breakdown at more than ~10 GPa based on equilibrium phase relations. However, evidence for this has not been found (24, 25); existing research only confirms the development of stishovite from silica in shocked meteorites (1, 3, 4, 6). This can be reasonably explained by the kinetic differences between the two different reactions: Temperature changes required for 10% stishovite formation via the two mechanisms differ by more than 1000 K on the time scale of 1 s (Fig. 4A).
Note that, although we focused on the solid-state reaction in both present and previous (20) studies, various other processes involving melt phases have been recognized as important features of shock metamorphism in shergottites (22, 26). These include quenching to maskelynite and crystallization of stishovite + (Na, Ca)-hexaluminosilicate (Na-rich CAS phase) from plagioclase melt. It has also been suggested that tissintite is crystallized from plagioclase melt (19). To systematically explain these crystallization behaviors from melt over the short time scales of shock events, a consideration of kinetics is indispensable, as demonstrated for the solid-state reaction in our studies.
The study reported here revealed that a key factor in seifertite formation is shock duration, which depends on the impactor size. A recent study has reported that quartz and/or cristobalite in eucrite that probably originated from the differentiated asteroid Vesta partly transformed to coesite and stishovite, whereas seifertite has not been found (4). The authors suggested that both coesite and stishovite formed under similar pressure conditions of ~8 to 13 GPa, the former from the silica melt and the latter from solid-state reactions under variable temperature conditions. The missing seifertite in this eucrite, given that the estimated pressure and temperature conditions overlap those consistent with seifertite formation, may imply that the shock event recorded by this eucrite was different from those for Martian and lunar meteorites. For example, shock duration may have been shorter because the impactor size was smaller. However, detailed investigations of eucrites are insufficient to establish whether seifertite is missing in all of them.
When considering impact velocities of ~5 to 10 km/s (27) and shock durations of ~0.01 s in the context of our results (Fig. 3B), the critical size of an impactor that might produce seifertite is estimated to be ~50 to 100 m from the relationship of L = τ × v, where L is the impactor size, τ is the shock duration, and v is the impact velocity (28). Thus, the presence of metastable seifertite in shocked meteorites is potentially capable of being a simple index for the impactor size. The curious kinetic behaviors of silica and plagioclase observed in shocked meteorites can be used as a unique hybrid shock indicator recording the collisional history of differentiated parental bodies in the early solar system.
MATERIALS AND METHODS
In situ XRD experiments were conducted using a Kawai-type, high-pressure apparatus SPEED-1500 at SPring-8 (29). Tungsten carbide anvils of 26-mm edge lengths were used as the second-stage anvils. The truncated edge length of the second-stage anvil is 3.0 mm. Experimental details for the XRD system, the sample assembly, and pressure and temperature estimates were the same as those in the previous study (20). Powders of reagent-grade quartz and α-cristoballite, synthesized from the quartz at 1820 K for 7 hours, were used as starting material in the present study. Each starting material was mixed with gold (15:1 by weight) that was used as an internal pressure standard (30). The transformed fraction was estimated on the basis of the integrated intensities of diffraction lines 111, 202, 221 in seifertite, and 110 in stishovite, relative to the intensities after the complete reaction.
Because we continuously collected the kinetic data over several temperature steps, the transformed fraction was not zero at t = 0 in some steps as shown in fig. S6. To analyze the kinetic data starting in the middle of the transformation, we modified the Avrami rate equation (15) by adding a time-correction parameter _t_0 in Eq. 1. Because it was difficult to estimate all parameters from the kinetic data in the middle of the transformation, we mainly determined k and n values from the first step with _t_0 = 0. In some runs, it was possible to estimate all of the k, n, and _t_0 parameters from the kinetic data in the later steps. The estimated n values are largely unchanged with the temperature step in each run, and by pressure and temperature conditions for each reaction (table S1). The averaged n value for each reaction was then used to recalculate the rate constant k for each step to determine pressure and temperature dependencies.
Supplementary Material
http://advances.sciencemag.org/cgi/content/full/1/4/e1500075/DC1
Acknowledgments
We thank M. Miyahara and M. Kimura for valuable discussions, and N. Doi and Y. Shirose for their assistance in the experiments. We also thank A. El Goresy and an anonymous reviewer for Science Advances for valuable comments. In situ XRD experiments were carried out at BL04B1 of SPring-8 (proposal nos. 2011A1489, 2011B1421, and 2012A1470). Funding: This work was partially supported by a Grant-in-Aid for Scientific Research from the Japanese government to T. Kubo. Author contributions: T. Kubo organized the research project, analyzed the data, and completed the manuscript with the help of T. Kato. T. Kubo, Y.H., and K.F. carried out experimental work. All authors discussed the results and commented on the manuscript. Competing interests: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/4/e1500075/DC1
Fig. S1. Changes of XRD patterns in cristobalite during heating at around the coesite-stishovite transition boundary.
Fig. S2. XRD pattern of seifertite in the sample assembly recovered from 30.8 GPa and 900 K to ambient condition.
Fig. S3. Changes of XRD patterns in quartz during heating at high pressures.
Fig. S4. Pressure and temperature conditions for the partial amorphization and stishovite formation in quartz.
Fig. S5. Time-resolved XRD measurements for the appearance and disappearance of seifertite.
Fig. S6. Plots of transformed volume fraction as a function of time in the seifertite and stishovite formation.
Table S1. Estimated values of kinetic parameters for the seifertite and stishovite formations.
Table S2. Estimated values of kinetic parameters for pressure and temperature dependencies of the rate constant k.
REFERENCES AND NOTES
- 1.Sharp T. G., El Goresy A., Wopenka B., Chen M., A post-stishovite SiO2 polymorph in the meteorite Shergotty: Implications for impact events. Science 284, 1511–1513 (1999). [DOI] [PubMed] [Google Scholar]
- 2.El Goresy A., Dubrovinsky L., Sharp T. G., Saxena S. K., Chen M., A monoclinic post-stishovite polymorph of silica in the Shergotty meteorite. Science 288, 1632–1634 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Ohtani E., Ozawa S., Miyahara M., Ito Y., Mikouchi T., Kimura M., Arai T., Sato K., Hiraga K., Coesite and stishovite in a shocked lunar meteorite, Asuka-881757, and impact events in lunar surface. Proc. Natl. Acad. Sci. U.S.A. 108, 463–466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyahara M., Ohtani E., Yamaguchi A., Ozawa S., Sakai T., Hirao N., Discovery of coesite and stishovite in eucrite. Proc. Natl. Acad. Sci. U.S.A. 111, 10939–10942 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Goresy A., Dera P., Sharp T. G., Prewitt C. T., Chen M., Dubrovinsky L., Wopenka B., Boctor N. Z., Hemley R. J., Seifertite, a dense orthorhombic polymorph of silica from the Martian meteorites Shergotty and Zagami. Eur. J. Mineral. 20, 523–528 (2008). [Google Scholar]
- 6.Miyahara M., Kaneko S., Ohtani E., Sakai T., Nagase T., Kayama M., Nishido H., Hirao N., Discovery of seifertite in a shocked lunar meteorite. Nat. Commun. 4, 1737 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Murakami M., Hirose K., Ono S., Ohishi Y., Stability of CaCl2-type and -PbO2-type SiO2 at high pressure and temperature determined by in-situ x-ray measurements. Geophys. Res. Lett. 30, 1207 (2003). [Google Scholar]
- 8.Tsuchiya T., Caracas R., Tsuchiya J., First principles determination of the phase boundaries of high-pressure polymorphs of silica. Geophys. Res. Lett., 31, L11610 (2004). [Google Scholar]
- 9.Grocholski B., Shim S.-H., Prakapenka V. B., Stability, metastability, and elastic properties of a dense silica polymorph, seifertite. J. Geophys. Res. 118, 4745–4757 (2013). [Google Scholar]
- 10.Dubrovinsky L. S., Dubrovinskaia N. A., Saxena S. K., Tutti F., Rekhi S., Le Bihan T., Shen G., Hud J., Pressure-induced transformations of cristobalite, Chem. Phys. Lett. 333, 264–270 (2001). [Google Scholar]
- 11.Tsuchida Y., Yagi T., New pressure-induced transformations of silica at room temperature. Nature 347, 267–269 (1990). [Google Scholar]
- 12.Dera P., Lazarz J. D., Prakapenka V. B., Barkley M., Downs R. T., New insights into the high-pressure polymorphism of SiO2 cristobalite. Phys. Chem. Miner. 38, 517–529 (2011). [Google Scholar]
- 13.Dera P., Prewitt C. T., Boctor N. Z., Hemley R. J., Characterization of a high-pressure phase of silica from the Martian meteorite Shergotty. Am. Mineral. 87, 1018–1023 (2002). [Google Scholar]
- 14.Hemley R. J., Jephcoat A. P., Mao H. K., Ming L. C., Manghnani M. H., Pressure-induced amorphization of crystalline silica. Nature 334, 52–54 (1988). [Google Scholar]
- 15.J. W. Christian, Transformations in Metals and Alloys, Part I, Equilibrium and General Kinetic Theory (Pergamon, Oxford, 1975). [Google Scholar]
- 16.Blaß U. W., Shock-induced formation mechanism of seifertite in shergottites. Phys. Chem. Miner. 40, 425–437 (2013). [Google Scholar]
- 17.Beck P., Gillet P., El Goresy A., Mostefaoui S., Timescales of shock processes in chondritic and martian meteorites. Nature 435, 1071–1074 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Baziotis I. P., Liu Y., DeCarli P. S., Melosh H. J., McSween H. Y., Bodnar R. J., Taylor L. A., The Tissint Martian meteorite as evidence for the largest impact excavation. Nat. Commun. 4, 1404 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Walton E. L., Sharp T. G., Hu J., Filiberto J., Heterogeneous mineral assemblages in martian meteorite Tissint as a result of a recent small impact event on Mars. Geochim. Cosmochim. Acta 140, 334–348 (2014). [Google Scholar]
- 20.Kubo T., Kimura M., Kato T., Nishi M., Tominaga A., Kikegawa T., Funakoshi K., Plagioclase breakdown as an indicator for shock conditions of meteorites. Nat. Geosci. 3, 41–45 (2010). [Google Scholar]
- 21.H. Y. McSween Jr., A. H. Treiman, in Reviews in Mineralogy, J. J. Papike, Ed. (Mineralogical Society of America, Washington, DC, 1998) vol. 36, chap. 6. [Google Scholar]
- 22.El Goresy A., Gillet Ph., Miyahara M., Ohtani E., Ozawa S., Beck P., Montagna G., Shock-induced deformation of Shergottites: Shock-pressures and perturbations of magmatic ages on Mars. Geochim. Cosmochim. Acta 101, 233–262 (2013). [Google Scholar]
- 23.Ma C., Tschauner O., Liu Y., Beckett J. R., Rossman G. R., Zhuravlev K., Prakapenka V., Dera P., Sinogeikin S., Smith J., Taylor L. A., Discovery of ahrensite γ-Fe2SiO4 and tissintite (Ca,Na,□)AlSi2O6: Two new high pressure minerals from the Tissint Martian meteorite. Lunar Planet. Sci. Conf. 45, 1222 (2014). [Google Scholar]
- 24.Kimura M., Suzuki A., Kondo T., Ohtani E., El Goresy A., Natural occurrence of high-pressure phases jadeite, hollandite, wadsleyite, and majorite-pyrope garnet in an H chondrite. Meteorit. Planet. Sci. 35, A87–A88 (2000). [Google Scholar]
- 25.Miyahara M., Ozawa S., Ohtani E., Kimura M., Kubo T., Sakai T., Nagase T., Nishijima M., Hirao N., Jadeite formation in shocked ordinary chondrites. Earth Planet. Sci. Lett. 373, 102–108 (2013). [Google Scholar]
- 26.Beck P., Gillet P., Gautron L., Daniel I., El Goresy A., A new natural high-pressure (Na, Ca)-hexaluminosilicate [(CaxNa1-x)Al3+xSi3−xO11] in shocked Martian meteorites. Earth Planet. Sci. Lett. 219, 1–12 (2004). [Google Scholar]
- 27.Marchi S., Bottke W. F., Cohen B. A., Wuennemann K., Kring D. A., McSween H. Y., De Sanctis M. C., O’Brien D. P., Schenk P., Raymond C. A., Russell C. T., High-velocity collisions from the lunar cataclysm recorded in asteroidal meteorites. Nat. Geosci. 6, 303–307 (2013). [Google Scholar]
- 28.H. J. Melosh, in Impact Cratering: A Geologic Process (Oxford Univ. Press, New York, 1989). [Google Scholar]
- 29.Utsumi W., Funakoshi K., Urakawa S., Yamakata M., Tsuji K., Konishi H., Shimomura O., SPring-8 beamlines for high pressure science with multi-anvil apparatus. Rev. High Pressure Sci. Technol. 7, 1484–1486 (1998). [Google Scholar]
- 30.Tsuchiya T., First-principles prediction of the _P_-_V_-T equation of state of gold and the 660-km discontinuity in Earth’s mantle. J. Geophys. Res. 108, 2462. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
http://advances.sciencemag.org/cgi/content/full/1/4/e1500075/DC1
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/4/e1500075/DC1
Fig. S1. Changes of XRD patterns in cristobalite during heating at around the coesite-stishovite transition boundary.
Fig. S2. XRD pattern of seifertite in the sample assembly recovered from 30.8 GPa and 900 K to ambient condition.
Fig. S3. Changes of XRD patterns in quartz during heating at high pressures.
Fig. S4. Pressure and temperature conditions for the partial amorphization and stishovite formation in quartz.
Fig. S5. Time-resolved XRD measurements for the appearance and disappearance of seifertite.
Fig. S6. Plots of transformed volume fraction as a function of time in the seifertite and stishovite formation.
Table S1. Estimated values of kinetic parameters for the seifertite and stishovite formations.
Table S2. Estimated values of kinetic parameters for pressure and temperature dependencies of the rate constant k.