Patient-initiated appointments compared with standard outpatient care for rheumatoid arthritis: a randomised controlled trial (original) (raw)
Abstract
Objectives
To test the hypothesis that implementing a patient-initiated system of care could improve clinical outcome in rheumatoid arthritis (RA) using disease activity guided management.
Methods
An 18-month controlled blinded end point two-centre study with 131 patients with RA randomised to intervention (n=64) or control (n=67). The intervention group participants were guaranteed appointments to a rheumatologist within 10 working days if they subjectively experienced a flare in disease activity. The control group participants were booked in advance according to guidelines. Independent assessments were performed in the two groups at 0, 3, 6, 12 and 18 months. Outcome measures included: Disease Activity Score 28 (DAS28), a Visual Analogue Scale (satisfaction with care, confidence in care), number of appointments with a rheumatologist.
Results
DAS28 decreased. Median satisfaction and confidence in care were >90 mm on Visual Analog Scale. Median number of appointments was 3. There were no significant differences between the groups among these outcomes. Visits in the intervention group more often resulted in change of treatment than in the control group (p<0.001).
Conclusions
Patient-initiated care was neither better nor inferior to traditional care in terms of outcomes analysed. Patient-initiated appointments can safely be used in everyday outpatient care of RA to empower the patient, if disease activity guided management is applied. Further research should investigate if this intervention can target a subgroup of patients and hence also result in released resources.
Keywords: Rheumatoid Arthritis, Early Rheumatoid Arthritis, Health services research, Outcomes research
Key messages.
What is already known about this subject?
- Patient-initiated tight control is known to improve outcomes in established rheumatoid arthritis (RA) and physician-initiated tight control is known to improve outcomes in early and established RA.
What does this study add?
- This study shows that patient-initiated tight control results in the same level of decreased disease activity compared to traditional appointments in early and established RA.
- Patient-initiated tight control can be used without affecting the patient experience of care.
How might this impact on clinical practice?
- Implementation of Open-Tight clinics is a safe way to empower the patients in daily clinical practice.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that causes chronic synovitis and progressive joint damage.1 Current treatment strategies aim to induce and maintain remission2 or low disease activity in the individual patient.3 Tight control to reach low disease activity or remission within a reasonable period of time4 has been demonstrated using Disease Activity Score 28 (DAS28).3 This enables a disease activity guided management with tight outpatient appointments.
RA is a disease with variation in disease activity but patients are traditionally seen at preplanned appointments by a specialist in rheumatology. Preplanned appointments may take place when the patient is well, and flares in disease activity may occur when there are no appointments available. Thus, there is incongruence between the natural variation of the disease activity and the preplanned appointments at the clinic. Thirty per cent of routine physician appointments of patients with RA result in no changed strategy of treatment.5 With many unnecessary appointments, prioritising patients based on disease activity guided management constitutes a challenge. Rapid specialist access when needed has been highlighted by general practitioners as more important than routine appointments.6 Studies introducing nurses for routine appointments showed an increased satisfaction with care and empowerment of the patient.7 8
The patient may initiate appointments with the rheumatologist in between prebooked appointments, if necessary based on disease activity. Therefore, patient-initiated appointments are of interest for the management of patients with RA. Patients who believe in their own capacity and ability to participate in their healthcare report less physical problems and enhanced well-being.9 Thereby, healthcare can be delivered more effectively and efficiently.10 Since patients are the ultimate beneficiaries of care, patient involvement has a value of its own. In addition, patient involvement in chronic diseases has positive effects on health status, self-esteem and satisfaction with care.10 Further, chronically ill patients who experience that they have an active role in their treatment are more likely to adhere to treatment.11
Earlier studies12 are not directly generalisable to daily clinical practice. The challenge is to still ascertain whether disease activity guided management of the inflammatory response can be achieved and sustained in patients with RA in daily clinical practice. We hypothesised that patient-initiated appointments would reduce unnecessary appointments, be more responsive and empower the patient to improve their own health. The aim of this study was to test the hypothesis that implementing a patient-initiated system of care could improve clinical outcome in RA using disease activity guided management.
Methods
Study design
An 18-month controlled randomised blinded end point parallel-group trial with patients with RA recruited from the outpatient clinics at Karolinska University Hospital in Solna and Huddinge, Sweden. All patients were scheduled to monitor appointments with the study nurse and the independent joint count assessor at the department of rheumatology at 0, 3, 6, 12 and 18 months. The primary end point was DAS28. Secondary end points are listed under a separate headline below. The full trial protocol is available on request.
Study population
From 2008 to 2010, patients aged between 18 and 80 years, who fulfilled the American College of Rheumatology criteria for RA from 1987,13 were recruited for inclusion in the study when changing an attending rheumatologist. The study ended in 2012 as decided on before the study start. In total, 39 persons declined participation, and of the 139 assessed for eligibility, 131 were randomised. Patients unable to make contact with healthcare in case of increased disease activity, who participated in another research project or who had other serious diseases that were hypothesised to influence the outcome of this study, were excluded. Previous or concomitant antirheumatic therapies did not affect participation.
After obtaining written informed consent, patients were randomly assigned to the intervention or control group by using a computer-generated random-number sequence prepared by an independent party (ie, simple randomisation). Allocation was concealed in envelopes held in a locked location during the study. Participants received no financial compensation or gifts. The study has been approved by the local ethics committee in Stockholm, registration number 2008/1226–32/3.
Power calculation
Minimum clinically important difference was chosen as 0.6 in the DAS28 score, in accordance with the European League Against Rheumatism (EULAR) response criteria.14 A sample of 54 patients in each randomisation group was needed to obtain 80% power and 5% significance level.
Study interventions
The study involved patients, rheumatologists, clinic nurses, study nurses and independent joint count assessors. At baseline, all patients were screened with the American College of Rheumatology classification criteria, disease onset, previously tried disease modifying antirheumatic drugs (DMARDs), comorbidity and extra-articular manifestations. Data were collected on: sex, age, disease duration, rheumatoid factor and anticyclic citrullinic peptide. The independent joint count assessor performed blinded tender and swollen joint counts based on the 28-joint count according to the EULAR handbook.15 The patients were not allowed to discuss their disease with the independent joint count assessor.
Disease activity evaluation by the study nurse
The study nurse collected all measurements including disease activity and discussed adverse drug effects, general health as well as educated the patients about injections. This structured monitoring of disease activity in all patients was conducted as a safety net since this study even included patients with early RA. The data were entered in The Swedish Rheumatology Quality register that offered a possibility for rheumatologists to evaluate disease activity in a web-based decision support system. Study nurses and patients were aware of the group assignment.
The intervention group
If the patients experienced a flare in disease activity in between the monitor appointments as defined by the individual patient themselves, they were told to contact the study nurse for an appointment with a rheumatologist within 10 working days, which worked as Open access in the study. Also, if DAS28 was >3.2 at the monitor appointment, the study nurse determined if there was active disease and arranged an appointment with a rheumatologist within 10 working days. At the rheumatologist's visit, the treatment was changed if it was believed to decrease disease activity, and the patient was given a follow-up appointment to evaluate the treatment as soon as it was considered to have reached effect—that is, tight control. The treatment goal was DAS28<3.2. When the treatment goal was achieved or the physician considered the disease activity as optimal, the patient was not booked for any further appointments with their rheumatologist. Instead, they relied on their own detection of signs of a disease flare to ask for an immediate return visit within 10 days—returning to be an Open access patient.
The control group
Appointments were regulated and booked in advance by the physician in the standard way at the two rheumatology study centres. If DAS28>3.2 at the monitor appointment and the patient experienced a flare, the study nurse told the patient to contact the clinic nurse. An appointment with a rheumatologist was arranged. This was done as quickly as possible but was subject to the standard waiting time at the clinic, typically at least 6 weeks.
Treatment
Swedish national guidelines16 comprised the drug treatment strategy. A single therapy of methotrexate, sulfasalazine or chloroquine phosphate was used for patients with DAS28<3.2. Higher DAS28 mandated combinations with methotrexate, biological DMARDs and steroids. Intra-articular corticosteroid injections were allowed, but not later than 4 weeks prior to a monitor appointment. There was no difference in drug treatment strategy between the groups.
Secondary outcome measures
Secondary outcome measures for exploratory analysis were: number of appointments with a rheumatologist, Visual Analogue Scale (VAS; satisfaction with care, confidence in care, general health, pain), erythrocyte sedimentation rate (ESR), C reactive protein (CRP), number of tender joints, number of swollen joints, the proportion of patients with DAS28<3.2 at 0, 3, 6, 12 and 18 months, and Health Assessment Questionnaire (HAQ) at 18 months versus baseline, changes in DMARD or prednisone treatment.
Statistical analysis
The DAS28 mean was analysed using an intention to treat approach with the last available observation carried forward in a longitudinal linear regression model. An unstructured mixed model was selected on the basis of an information-theoretic approach. Assumptions of linear regression were met. Group assignment and time point were treated as fixed effects, and participants as random effects. The model was further adjusted for baseline differences in covariates that demonstrated a difference between the groups when analysed categorically (age, gender and disease duration). Owing to non-normal distributions in most outcomes, non-parametric tests were used for secondary outcomes. Data are reported as medians, IQRs and for categorical variables as percentages. The Mann-Whitney U test was used for comparisons of ordinal and interval scales, and the χ2 test for nominal scales. The level of significance was set at α=0.05 and tested using SPSS V.20.0. Data handling and statistical analysis were performed unblinded.
Results
Characteristics of patients
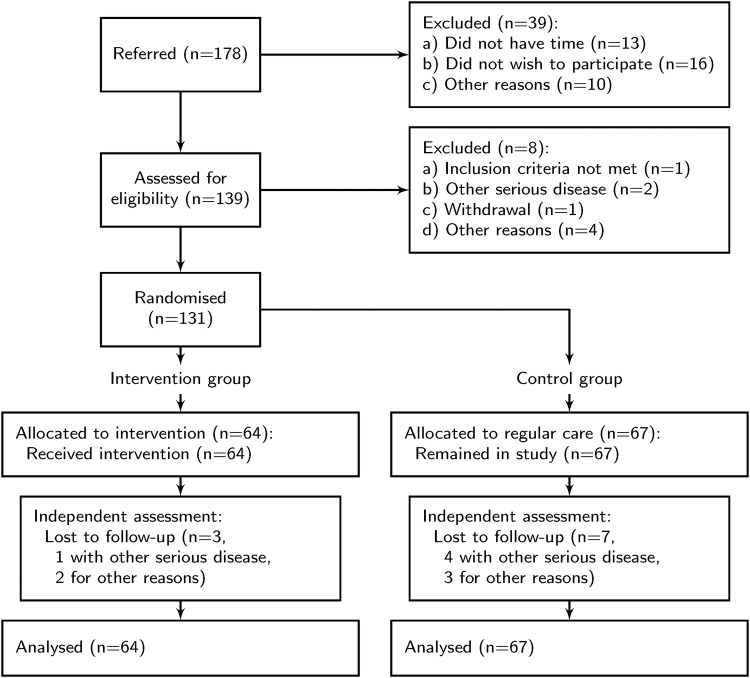
Enrolment and allocation of patients can be seen in figure 1. In total, three patients in the intervention group and seven in the control group did not complete the study. They were similar in baseline features compared to those who completed the study. Using the last available observation, no significant difference for clinical outcomes between the groups of non-completers was found.
Figure 1.
Flowchart of participants’ progress through the trial.
Baseline characteristics in the two groups were similar, although patients in the intervention group notably had a narrower IQR in disease duration and slightly more women, while patients in the control group were more often treated with leflunomide (table 1).
Table 1.
Baseline demographic and disease characteristics
| Total (n=131) | Intervention (n=64) | Control (n=67) | |
|---|---|---|---|
| Age (years), median (IQR) | 65 (55 to 71) | 65 (55 to 71) | 64 (55 to 71) |
| Female, n (%) | 90 (69) | 49 (77) | 41 (61) |
| Disease duration (years), median (IQR) | 3 (1 to 22) | 3 (1 to 9) | 5 (2 to 12) |
| RF positive, n (%) | 67 (51) | 34 (51) | 33 (49) |
| Positive anti-CCP, n (%) | 95 (73) | 46 (72) | 49 (73) |
| DAS28, median (IQR) | 3.5 (2.6 to 5.0) | 3.5 (2.6 to 5.0) | 3.4 (2.7 to 4.7) |
| HAQ, median (IQR) | 0.4 (0.1 to 0.8) | 0.4 (0.1 to 0.8) | 0.5 (0.1 to 1.0) |
| CRP (mg/L), median (IQR) | 3 (2 to 5) | 3 (2 to 5) | 3 (1 to 7) |
| ESR (mm/h), median (IQR) | 12 (8 to 18) | 12 (8 to 18) | 14 (8 to 24) |
| VAS general health, median (IQR) | 22 (11 to 43) | 22 (10 to 42) | 26 (11 to 42) |
| VAS pain, median (IQR) | 23 (11 to 43) | 23 (11 to 43) | 24 (10 to 42) |
| Methotrexate treatment, n (%) | 105 (80) | 51 (80) | 54 (81) |
| Hydroxychloroquine treatment, n (%) | 6 (5) | 2 (3) | 4 (6) |
| Sulfasalazine treatment, n (%) | 13 (10) | 8 (13) | 5 (7) |
| Leflunomide treatment, n (%) | 3 (2) | 0 (0) | 3 (4) |
| Biologics treatment, n (%) | 31 (23.7) | 11 (18) | 20 (30) |
| Prednisone treatment, n (%) | 51 (39) | 28 (44) | 23 (34) |
| Prednisone dose*, mg, median (IQR) | 5 (2.5 to 7.5) | 5 (2.5 to 7.5) | 5 (5 to 6.25) |
| Combination treatment†, n (%) | 7 (5.3) | 3 (4.7) | 4 (6.0) |
Clinical outcome
There were no significant differences between the group mean differences in DAS28 and the disease activity of both groups decreased during the study. The mean DAS28 decreased with 0.24 in the intervention group and with 0.59 in the control group between the first and the last months of the study (significance of difference p=0.055; 95% CI −0.01 to 0.91; table 2). In the exploratory analysis, there was no significant difference in the proportion of patients with low disease activity between the groups (figure 2) and no difference in analysis of DAS28 stratified by disease duration (data not shown). The median decrease of HAQ scores and difference between the groups at the end of the study were not significant. Patients in the intervention and control groups differed significantly in view of the change in ESR (U=1725, p=0.03), with patients in the intervention group showing higher ESR changes (table 3). Medication at the end of the trial was not significantly different between the groups (data not shown).
Table 2.
Comparison of mean disease activity score over 18 months
| Time point (month) | Difference in DAS28 (95% CI) | Effect size (Cohen's d) | p Value |
|---|---|---|---|
| 0 | 0.10 (−0.36 to 0.57) | 0.079 | 0.66 |
| 3 | 0.24 (−0.20 to 0.68) | 0.19 | 0.29 |
| 6 | −0.11 (−0.57 to 0.35) | −0.087 | 0.63 |
| 12 | 0.26 (−0.14 to 0.66) | 0.20 | 0.19 |
| 18 | 0.45 (−0.01 to 0.91) | 0.35 | 0.055 |
Figure 2.
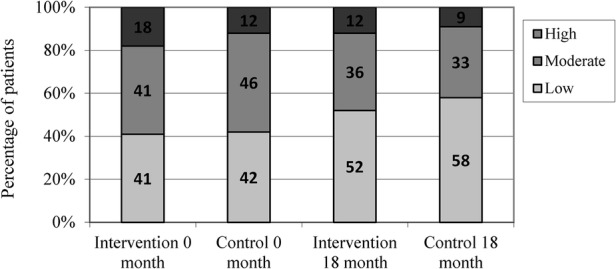
Percentage of patients in the disease activity index categories at 0 and 18 months.
Table 3.
Average change from baseline to 18 months in exploratory analysed variables
| Intervention groupN=64 | Control groupN=67 | p Value | |
|---|---|---|---|
| CRP (mg/L) | 0 (−2 to 1) | 0 (−3 to 1) | 0.70 |
| ESR (mm/h) | 4 (−2 to 10) | 0 (−4 to 5) | 0.03* |
| VAS—general health | −1 (−12 to 8) | −4 (−17 to 9) | 0.57 |
| VAS—pain | −3 (−13 to 5) | −4 (−18 to 8) | 0.75 |
| Number of tender joints | −1 (−4 to 0) | −1 (−3 to 0) | 0.81 |
| Number of swollen joints | −3 (−5 to 0) | −1 (−3 to 1) | 0.40 |
| HAQ | −0.12 (−0.25 to 0.13) | −0.11 (−0.25 to 0.12) | 0.91 |
| VAS—Satisfaction with care | 1 (−4 to 5) | 1 (−2 to 4) | 0.47 |
| VAS—Confidence in care | 1 (−2 to 7) | 0 (−4 to 7) | 0.97 |
Appointments with the rheumatologist
The number of appointments with the rheumatologist for completers was not significantly different between the groups during the study (U=1579; p=0.2; table 4). The intervention group (n=61) requested 165 appointments, median 3 (IQR 1 to 4); the control group (n=60) requested 185 appointments, median 3 (IQR 2 to 4) per patient. The 10 patients who did not complete the study requested 0–2 appointments each. The nine patients who did not request any appointments during the study had a median age of 65 years (IQR 55 to 71), median disease duration of 3 years (IQR 1 to 9) and median DAS28 of 2.44 (IQR 1.77 to 3.22). After the trial, their median DAS28 was 2.29 (IQR 1.65 to 3.18). In the intervention group, 33% had 0 to 1 appointment compared to 5% in the control group. In the intervention group, individual treatment was changed 112 times (68% of visits), and in the control group 89 times (48% of visits). This difference was significant (χ2=13.9439; p=0.0002).
Table 4.
Number of appointments with the rheumatologist
| Number of visits | Intervention group | Control group |
|---|---|---|
| 0 | 9 (15) | 0 (0) |
| 1 | 11 (18) | 3 (5) |
| 2 | 7 (11) | 18 (30) |
| 3 | 4 (23) | 18 (30) |
| 4 | 9 (15) | 16 (26) |
| 5 | 7 (11) | 4 (7) |
| 6 | 2 (3) | 0 (0) |
| 7 | 1 (2) | 0 (0) |
| 8 | 1 (2) | 1 (2) |
At the start and end of the study, median satisfaction and confidence in care were >90 mm with no difference between the groups.
Discussion
This study was designed to align with everyday frontline care and tested the outcome of care guided by patient subjective experience as well as by disease activity. At the conclusion of the study, disease activity in patients who themselves initiated appointments with the rheumatologist did not differ from that patients receiving appointments initiated by the rheumatologist. The study thus demonstrated that a patient-initiated system of care can be safely introduced in everyday practice. However, it is important to note that patients not willing to participate in patient-initiated care were excluded from this study.
This study explores a combination of two mechanisms: patient-initiated appointments and disease activity guided management. The intervention group had a higher ESR at 18 months and ESR is one component of DAS28. Elevated ESR might have led to an intervention more often in the control group since appointments frequency was decided by the rheumatologist who had access to the ESR, whereas the patients in the intervention group did not decide to seek care based on ESR, but only if they subjectively perceived a flare. This demonstrates that patient outcomes can be improved equally well through two different mechanisms, patient subjective experience as well as clinical monitoring of disease activity. The intervention group had a lower percentage of patients in low disease activity after 18 months. This result may be due to the presence of more women in the intervention group and a poorer prognosis in women,17 as well as more patients with early RA in the intervention group requiring a longer time to reach optimal treatment outcome. Visits in the intervention group seemed to more often result in change of treatment. This could either be due to visits being timed at moments of disease activity, or the patient being more likely to expect and ask for a change of treatment when they initiated the appointment. However, there was no difference in treatment regime at the end of the trial, excluding the possibility of patient-initiated appointments becoming more costly due to more expensive treatment.
Several clinical trials of disease activity guided treatment have shown that applying a tight control strategy is effective in reducing the disease activity and progression of joint damage.12 18–20 Studies have shown that tight monitoring of disease activity followed by treatment adjustment helps reach low disease activity in clinical trials.5 18–20 In the present study, patient-initiated appointments and disease activity guided management were combined.
One trial by Hewlett et al12 on patient-initiated appointments reported that patients who initiated their appointments through direct access were clinically and psychologically healthy as well as patients with traditional appointments initiated by a rheumatologist. Patients with direct access had 38% fewer hospital appointments over the 6 years, thus making more efficient use of finite resources. The data also showed that patients had more confidence and satisfaction in such a system compared to traditional care.12 Comparing the study designs, the study by Hewlett had a longer follow-up, did not collect data on DAS28, and monitored the patients once a year using postal questionnaires and monitor visits every other year. The longer follow-up can explain the difference in number of appointments with the rheumatologist compared to that in our study. The two groups in this study had a satisfaction level similar to the intervention group in the study by Hewlett, which is higher than that in their control group.
The disease duration of this study ranged from 1 to 59 years compared to the study by Hewlett et al,12 which only included patients with established RA, and earlier studies of tight control that only concern early RA.5 18–20 This study therefore better represents the everyday situation at the rheumatology clinics in Sweden. It would be reasonable to believe that persons with established RA are better at judging whether they have a disease flare, however the results of this trial could not support that. In our study, the two groups were treated according to the guidelines of the clinic using no protocol beyond current national guidelines, that is, a pragmatic trial. This resembles the clinical setting in an outpatient clinic in rheumatology. Thus, the results of this study have an improved external validity compared to previous studies.
The study design has several limitations. Patients were recruited when changing a rheumatologist. This was done for feasibility, and constitutes a selection bias. In addition, the new rheumatologist presumably did a thorough evaluation of the patient at the first appointment, which may have influenced satisfaction positively. The rheumatologists in the study were not blinded to group assignment of the patients, and this may have influenced the care since no protocol was used. The randomisation of the groups resulted in a shorter disease duration in the intervention group. This could have increased the risk of high DAS28 in the control, but in the baseline measurements DAS28 was equal. The frequent nurse monitoring in this study was necessary to receive ethical approval. It may, however, have introduced such a strong bias on all outcome measures that it rendered actual differences between the groups unnoticeable. This study thus unintentionally introduced an additional intervention in the two groups. The importance of this additional intervention is further supported by an earlier study that demonstrated a positive effect on outcomes when pharmacological treatment is combined with coordination and close monitoring by a rheumatology nurse specialist.21 The frequent nurse visits may also explain the high satisfaction. This has implications for selection of monitor frequency in studies of care delivery in rheumatology. Other scales suitable for home assessment with similar capacity to distinguish disease activity in clinical trials and clinical care could be an alternative solution (eg, RAPID322). The used outcome DAS28 has further limitations. It has not been validated for use in individual patients, but in clinical studies in combination with EULAR response criteria.23 Further, DAS28 does not include joints of the feet, which often are involved in RA,4 and tender joints have a higher relative contribution than swollen joints, despite swelling being more specific to RA.4 However, since DAS28 is frequently used in clinical settings, this outcome measurement was deemed the most appropriate.
In conclusion, this study shows that consenting patients can safely be given open access to clinics if care is guided by disease activity monitoring. This means Tight control of disease flares and return to Open access when the disease activity is low. Participating in this type of Open-Tight clinic, nearly half of the intervention group requested two appointments or less over 18 months. A challenge for further research is to identify this subgroup to enable the release of resources that can be used to improve care and reduce waiting time for other patients. Further, future research should verify the findings in lager cohorts, and could investigate whether this intervention is of greater value from a satisfaction perspective for certain subgroups, such as young patients. Since this management strategy can be used without reducing medical outcomes, patient's trust or satisfaction Open-Tight clinics may be a safe way to empower the patients to have a greater influence in their care.
Acknowledgments
The authors thank Eva Hagel for invaluable help with statistical calculations.
Footnotes
Twitter: Follow David Ebbevi at @DEbbevi
Contributors: CF and DE contributed equally to the study. All authors of this study meet the criteria for authorship as stated in your policy.
Funding: This work was supported by Forte (The Swedish Research Council for Health, Work Life and Welfare, grant number 2014–4238) and through the regional agreement on medical training and clinical research (ALF) between the Stockholm County Council and the Karolinska Institute.
Competing interests: None declared.
Ethics approval: Local ethics committee in Stockholm, registration number 2008/1226-32/3.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Pollard L, Choy EH, Scott DL. The consequences of rheumatoid arthritis: quality of life measures in the individual patient. Clin Exp Rheumatol 2005;23(Suppl 39):S43–52. [PubMed] [Google Scholar]
- 2.Emery P, Breedveld FC, Hall S et al. . Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82. 10.1016/S0140-6736(08)61000-4 [DOI] [PubMed] [Google Scholar]
- 3.Fransen J, Moens HB, Speyer I et al. . Effectiveness of systematic monitoring of rheumatoid arthritis disease activity in daily practice: a multicentre, cluster randomised controlled trial. Ann Rheum Dis 2005;64:1294–8. 10.1136/ard.2004.030924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker MF, Jacobs JW, Verstappen SM et al. . Tight control in the treatment of rheumatoid arthritis: efficacy and feasibility. Ann Rheum Dis 2007;66(Suppl 3):iii56–60. 10.1136/ard.2007.078360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verstappen SM, Jacobs JW, van der Veen MJ et al. . Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis 2007;66:1443–9. 10.1136/ard.2007.071092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hehir M, Hewlett S, Mitchell K et al. . What happens in rheumatoid arthritis (RA) out-patient clinics? Rheumatology 2001. Oxford Univ Press Great clarendon ST, Oxford OX2 6DP, England. [Google Scholar]
- 7.Mowat AG, Nichols PJ, Hollings EM et al. . A comparison of follow-up regimes in rheumatoid arthritis. Ann Rheum Dis 1980;39:12–17. 10.1136/ard.39.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvidsson SB, Petersson A, Nilsson I et al. . A nurse-led rheumatology clinic's impact on empowering patients with rheumatoid arthritis: A qualitative study. Nurs Health Sci 2006;8:133–9. 10.1111/j.1442-2018.2006.00269.x [DOI] [PubMed] [Google Scholar]
- 9.Newman S. Coping with rheumatoid arthritis. Ann Rheum Dis 1993;52:553–4. 10.1136/ard.52.8.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McWilliam CL. Patients, persons or partners? Involving those with chronic disease in their care. Chronic Illn 2009;5: 277–92. 10.1177/1742395309349315 [DOI] [PubMed] [Google Scholar]
- 11.Mosen DM, Schmittdiel J, Hibbard J et al. . Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage 2007;30:21–9. 10.1097/00004479-200701000-00005 [DOI] [PubMed] [Google Scholar]
- 12.Hewlett S, Kirwan J, Pollock J et al. . Patient initiated outpatient follow up in rheumatoid arthritis: six year randomised controlled trial. BMJ 2005;330:171 10.1136/bmj.38265.493773.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA et al. . The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 14.van Gestel AM, Prevoo ML, van ‘t Hof MA et al. . Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996;39:34–40. 10.1002/art.1780390105 [DOI] [PubMed] [Google Scholar]
- 15.van Riel PLCM, van Gestel AM, Scott DL et al. . EULAR Handbook of Clinical Assessments in Rheumatoid Arthritis: On Behalf of the EULAR Standing Committee for International Clinical Studies Including Therapeutic Trials -ESCISIT (chairman, Piet L.C.M Van Riel): Van Zuiden Communications B.V., 2001.
- 16.Baecklund E, d'Elia HF, Turesson C.2011. Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Secondary Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis. http://www.svenskreumatologi.se/sites/default/files/49/Guidelines_for_the_Pharmaceutical_Management_of_Rheumatoid_Arthritis.pdf.
- 17.Forslind K, Hafström I, Ahlmén M et al. . Sex: a major predictor of remission in early rheumatoid arthritis? Ann Rheum Dis 2007;66:46–52. 10.1136/ard.2006.056937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Möttönen T, Hannonen P, Leirisalo-Repo M et al. . Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet 1999;353:1568–73. 10.1016/S0140-6736(98)08513-4 [DOI] [PubMed] [Google Scholar]
- 19.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF et al. . Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2008;58(2 Suppl):S126–35. 10.1002/art.23364 [DOI] [PubMed] [Google Scholar]
- 20.Grigor C, Capell H, Stirling A et al. . Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9. 10.1016/S0140-6736(04)16676-2 [DOI] [PubMed] [Google Scholar]
- 21.Esselens G, Westhovens R, Verschueren P. Effectiveness of an integrated outpatient care programme compared with present-day standard care in early rheumatoid arthritis. Musculoskeletal Care 2009;7:1–16. 10.1002/msc.136 [DOI] [PubMed] [Google Scholar]
- 22.Pincus T, Yazici Y, Bergman MJ. RAPID3, an index to assess and monitor patients with rheumatoid arthritis, without formal joint counts: similar results to DAS28 and CDAI in clinical trials and clinical care. Rheum Dis Clin North Am 2009;35:773–8, viii 10.1016/j.rdc.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 23.Wells G, Becker JC, Teng J et al. . Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. 10.1136/ard.2007.084459 [DOI] [PMC free article] [PubMed] [Google Scholar]