Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects (original) (raw)
Abstract
Sodium-glucose cotransporter 2 inhibition with canagliflozin decreases HbA1c, body weight, BP, and albuminuria, implying that canagliflozin confers renoprotection. We determined whether canagliflozin decreases albuminuria and reduces renal function decline independently of its glycemic effects in a secondary analysis of a clinical trial in 1450 patients with type 2 diabetes receiving metformin and randomly assigned to either once-daily canagliflozin 100 mg, canagliflozin 300 mg, or glimepiride uptitrated to 6–8 mg. End points were annual change in eGFR and albuminuria over 2 years of follow-up. Glimepiride, canagliflozin 100 mg, and canagliflozin 300 mg groups had eGFR declines of 3.3 ml/min per 1.73 m2 per year (95% confidence interval [95% CI], 2.8 to 3.8), 0.5 ml/min per 1.73 m2 per year (95% CI, 0.0 to 1.0), and 0.9 ml/min per 1.73 m2 per year (95% CI, 0.4 to 1.4), respectively (P<0.01 for each canagliflozin group versus glimepiride). In the subgroup of patients with baseline urinary albumin-to-creatinine ratio ≥30 mg/g, urinary albumin-to-creatinine ratio decreased more with canagliflozin 100 mg (31.7%; 95% CI, 8.6% to 48.9%; _P_=0.01) or canagliflozin 300 mg (49.3%; 95% CI, 31.9% to 62.2%; P<0.001) than with glimepiride. Patients receiving glimepiride, canagliflozin 100 mg, or canagliflozin 300 mg had reductions in HbA1c of 0.81%, 0.82%, and 0.93%, respectively, at 1 year and 0.55%, 0.65%, and 0.74%, respectively, at 2 years. In conclusion, canagliflozin 100 or 300 mg/d, compared with glimepiride, slowed the progression of renal disease over 2 years in patients with type 2 diabetes, and canagliflozin may confer renoprotective effects independently of its glycemic effects.
Keywords: SGLT2, canagliflozin, renal function, diabetic nephropathy
Many patients with type 2 diabetes present with hyperglycemia, hypertension, and excess weight, and develop increased albuminuria, all of which increase the risk of micro- and macrovascular complications. Current practice guidelines recommend targeting these risk factors with a range of different drugs.1 However, despite the many drugs used, many patients do not reach treatment targets and develop potentially preventable micro- and macrovascular complications.
Emerging interest in the role of the kidney in glucose homeostasis has led to the development of sodium-glucose cotransporter inhibitors.2 These drugs are designed to inhibit sodium-glucose cotransporter 2 (SGLT2), which is located in the S1 segment of the proximal tubule. Previous phase 2 and phase 3 studies have shown that administration of SGLT2 inhibitors augments urinary glucose and sodium excretion and decreases glycated hemoglobin A1c (HbA1c), body weight, and BP in patients with type 2 diabetes.3,4 Moreover, reductions in albuminuria have been observed after administration of SGLT2 inhibitors.5,6
The beneficial effects of SGLT2 inhibitors on multiple cardiovascular and renal risk parameters suggest that SGLT2 inhibitors may confer cardiovascular and renal protection. A recent large clinical trial of the SGLT2 inhibitor empagliflozin demonstrated marked reductions in cardiovascular morbidity and mortality and suggested possible renoprotective effects.7,8 Whether SGLT2 inhibition slows the progression of kidney function decline independent of its glucose-lowering effect, however, is unknown.
Therefore, the aim of this study was to determine whether SGLT2 inhibition decreases albuminuria and slows the progression of kidney function decline independent of glycemic control by analyzing 2 years of data from a randomized controlled trial comparing canagliflozin and glimepiride.3
Results
The study ran from August 28, 2009 until January 30, 2013. As previously reported, 482 patients were assigned to glimepiride, 483 to canagliflozin 100 mg, and 485 to canagliflozin 300 mg. A total of 1161 patients (80.1%) completed the 104-week, double-blind treatment period. At baseline, 230 (15.9%) patients had a urinary albumin-to-creatinine ratio (UACR) ≥30 mg/g. The baseline demographics, clinical and biochemical characteristics, and concomitant medications were similar between the three treatment groups, both in the overall population as well as in the subgroup of patients with UACR≥30 mg/g (Table 1).
Table 1.
Baseline characteristics of the overall population and a subgroup of patients with UACR≥30 mg/g
| Characteristic | Overall Population | UACR≥30 mg/g Subgroup | ||||
|---|---|---|---|---|---|---|
| Glimepiride (_n_=482) | Canagliflozin | Glimepiride (_n_=76) | Canagliflozin | |||
| 100 mg (_n_=483) | 300 mg (_n_=485) | 100 mg (_n_=76) | 300 mg (_n_=78) | |||
| Age, yr | 56.3 (9.0) | 56.4 (9.5) | 55.8 (9.2) | 58.5 (8.0) | 55.6 (10.2) | 55.8 (9.6) |
| Female, n (%) | 219 (45.4) | 231 (47.8) | 244 (50.3) | 28 (36.8) | 30 (39.5) | 36 (46.2) |
| HbA1c, % | 7.8 (0.8) | 7.8 (0.8) | 7.8 (0.8) | 7.9 (0.8) | 7.9 (0.9) | 8.0 (0.8) |
| Diabetes duration, yr | 6.6 (5.0) | 6.5 (5.5) | 6.7 (5.5) | 6.9 (4.9) | 7.7 (6.6) | 8.2 (6.4) |
| SBP, mmHg | 129.5 (13.52) | 130.0 (12.40) | 129.9 (13.72) | 136.7 (12.52) | 133.7 (11.79) | 133.0 (15.32) |
| DBP, mmHg | 78.9 (8.40) | 78.8 (7.98) | 79.1 (8.34) | 81.7 (9.05) | 80.6 (8.98) | 80.9 (8.42) |
| eGFR, ml/min per 1.73 m2 | 89.5 (17.5) | 89.7 (19.3) | 91.4 (19.4) | 86.5 (18.5) | 91.0 (23.9) | 91.1 (23.9) |
| UACR, mg/ga | 8.2 (5.75; 17.98) | 8.7 (5.74; 17.52) | 8.6 (5.28; 20.64) | 60.1 (41.29; 124.91) | 56.5 (40.17; 115.49) | 75.2 (43.35; 173.11) |
| ACEI/ARB use, n (%) | 303 (62.9) | 287 (59.4) | 291 (60.0) | 49 (64.5) | 44 (57.9) | 45 (57.7) |
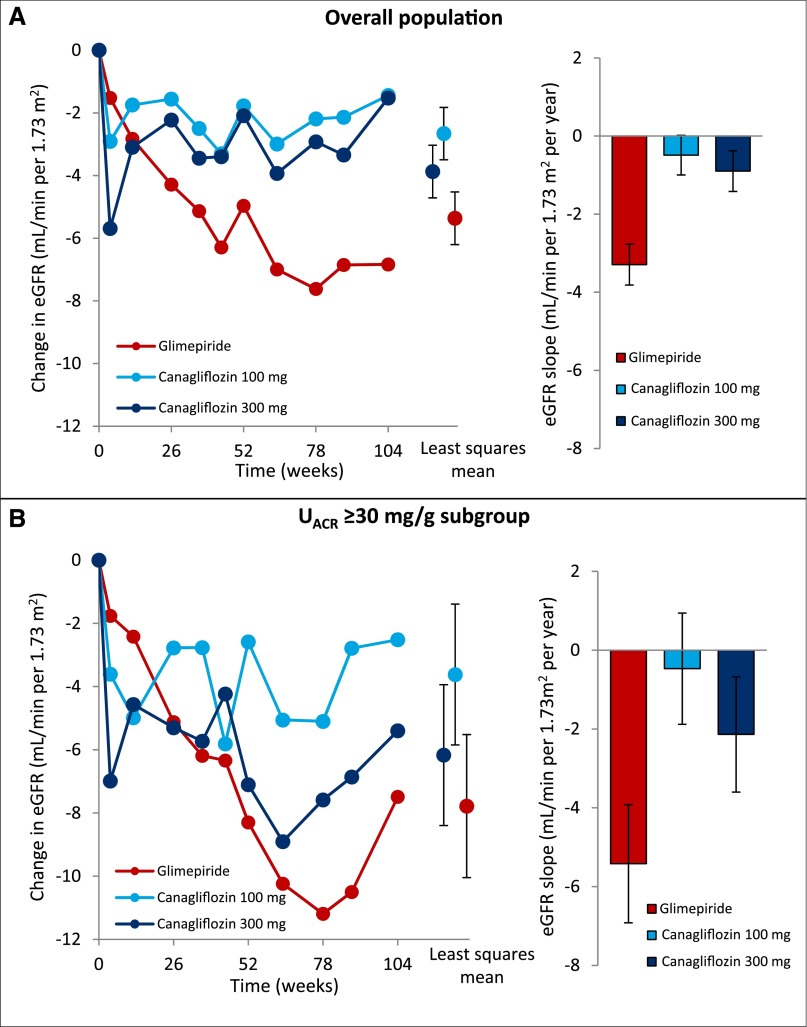
The annual slope of eGFR decline was 3.3 ml/min per 1.73 m2 per year (95% confidence interval [95% CI], 2.8 to 3.8) in the glimepiride group, 0.5 ml/min per 1.73 m2 per year (95% CI, 0.0 to 1.0) in the canagliflozin 100 mg group (P<0.001 versus glimepiride; Figure 1A), and 0.9 ml/min per 1.73 m2 per year (95% CI, 0.4 to 1.4) in the canagliflozin 300 mg group (_P_=0.002 versus glimepiride; Figure 1A). Repeated measures analysis showed a least squares mean change in eGFR from baseline of −5.4 ml/min per 1.73 m2 (95% CI, −6.2 to −4.5) in the glimepiride group versus −2.7 ml/min per 1.73 m2 (95% CI, −3.5 to −1.9) and −3.9 ml/min per 1.73 m2 (95% CI, −4.7 to −3.0) in the canagliflozin 100 and 300 mg groups, respectively (Figure 1A). The eGFR decline was also significantly lower with canagliflozin 100 and 300 mg compared with glimepiride in the subgroup of patients with UACR≥30 mg/g at baseline (Figure 1B). A sensitivity analysis was conducted using the 4-week follow-up visit (i.e., the first postrandomization visit where eGFR was measured) as a baseline to assess the effect of the acute eGFR outcome of canagliflozin on the subsequent eGFR slope. In the glimepiride group eGFR decline was −2.7 ml/min per 1.73 m2 per year (95% CI, −3.3 to −2.1), 0.1 ml/min per 1.73 m2 per year (95% CI, −0.5 to 0.6) in the canagliflozin 100 mg group (P<0.001 versus glimepiride), and 0.1 ml/min per 1.73 m2 per year (95% CI, −0.5 to 0.6) in the canagliflozin 300 mg group (P<0.001 versus glimepiride).
Figure 1.
Canagliflozin slows the progression of eGFR decline in patients with type 2 diabetes compared with glimepiride. (A) Changes in eGFR in the canagliflozin and glimepiride treatment arms in the overall population, and the rate of eGFR decline per year. (B) Changes in eGFR in the canagliflozin and glimepiride treatment arms in patients with UACR≥30 mg/g, and the rate of eGFR decline per year in patients with UACR≥30 mg/g.
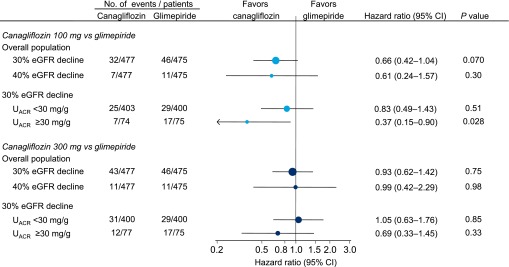
In the overall population, the 30% eGFR decline end point was observed in 46 (9.7%) patients in the glimepiride group, 32 (6.7%) in the canagliflozin 100 mg group (hazard ratio, 0.66; 95% CI, 0.42 to 1.04; _P_=0.07), and 43 (9.0%) patients in the canagliflozin 300 mg group (hazard ratio, 0.93; 95% CI, 0.62 to 1.42; _P_=0.75; Figure 2). In the subgroup of patients with UACR≥30 mg/g, the hazard ratios for the 30% eGFR decline end point were 0.37 (95% CI, 0.15 to 0.90; _P_=0.03) and 0.69 (95% CI, 0.33 to 1.45; _P_=0.33) for the canagliflozin 100 and 300 mg groups, respectively, compared with glimepiride.
Figure 2.
eGFR decline >30% or >40% was generally less frequent with canagliflozin compared with glimepiride.
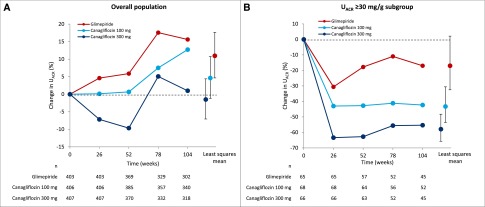
UACR progressively increased over time in the glimepiride group, remained stable in the canagliflozin 100 mg group, and decreased in the canagliflozin 300 mg group in the first year, and then returned to baseline at 2 years (Figure 3A). Relative to glimepiride, canagliflozin 100 mg decreased UACR by 5.7% (95% CI, −2.3 to 13.1; _P_=0.16) and canagliflozin 300 mg decreased UACR by 11.2% (95% CI, 3.6 to 18.3; P<0.01). In the subgroup of patients with UACR≥30 mg/g, canagliflozin 100 and 300 mg significantly decreased UACR over time, by 31.7% (95% CI, 8.6 to 48.9; _P_=0.01) and 49.3% (95% CI, 31.9 to 62.2; P<0.001), respectively, relative to glimepiride (Figure 3B). Effects of canagliflozin on UACR were consistent regardless of baseline angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use (Supplemental Table 1).
Figure 3.
Canagliflozin decreases albuminuria compared with glimepiride. (A) Changes in UACR in the canagliflozin and glimepiride treatment arms in the overall population. (B) Changes in UACR in the canagliflozin and glimepiride treatment arms in a subgroup of patients with UACR≥30 mg/g at baseline.
Reductions in HbA1c with glimepiride, canagliflozin 100 mg, and canagliflozin 300 mg were 0.81%, 0.82%, and 0.93%, respectively, at 1 year, and 0.55%, 0.65%, and 0.74%, respectively, at 2 years. Changes in systolic BP with glimepiride, canagliflozin 100 mg, and canagliflozin 300 mg were 0.2 mmHg, −3.3 mmHg, and −4.6 mmHg, respectively, at 1 year, and 1.7 mmHg, −2.0 mmHg, and −3.1 mmHg, respectively, at 2 years. Adjusting the treatment effects of canagliflozin on albuminuria for the differences in HbA1c reductions at 1 and 2 years did not alter the results, nor did adjustment for changes in systolic BP or body weight, or the combined changes in HbA1c, systolic BP, and body weight (Table 2).
Table 2.
Effect of canagliflozin versus glimepiride on albuminuria and eGFR with and without adjustments for covariates
| Covariates | Canagliflozin 100 mg Versus Glimepiride | Canagliflozin 300 mg Versus Glimepiride | ||
|---|---|---|---|---|
| Overall Population | UACR≥30 mg/g Subgroup | Overall Population | UACR≥30 mg/g Subgroup | |
| eGFR, ml/min per 1.73 m2 per yeara | ||||
| None | 2.8 (2.1 to 3.5) | 5.0 (2.9 to 7.0) | 2.4 (1.7 to 3.1) | 3.3 (1.2 to 5.4) |
| _Δ_HbA1c | 2.8 (2.1 to 3.5) | 5.0 (2.9 to 7.1) | 2.4 (1.7 to 3.1) | 3.3 (1.3 to 5.4) |
| _Δ_SBP | 2.8 (2.1 to 3.5) | 5.0 (2.9 to 7.0) | 2.4 (1.7 to 3.1) | 3.3 (1.2 to 5.4) |
| _Δ_BW | 2.8 (2.1 to 3.5) | 5.0 (2.9 to 7.0) | 2.4 (1.7 to 3.1) | 3.3 (1.2 to 5.4) |
| _Δ_HbA1c; _Δ_SBP; _Δ_BW | 2.8 (2.1 to 3.5) | 5.0 (2.9 to 7.1) | 2.4 (1.6 to 3.1) | 3.4 (1.3 to 5.5) |
| eGFR, ml/min per 1.73 m2b | ||||
| None | 2.7 (1.5 to 3.9) | 4.2 (1.6 to 7.4) | 1.5 (0.3 to 2.7) | 1.6 (–1.6 to 4.8) |
| _Δ_HbA1c | 3.3 (2.1 to 4.5) | 4.8 (1.5 to 8.1) | 2.1 (0.9 to 3.4) | 2.2 (–1.1 to 5.5) |
| _Δ_SBP | 3.0 (1.9 to 4.2) | 4.5 (1.3 to 7.7) | 1.9 (0.7 to 3.1) | 2.0 (–1.2 to 5.2) |
| _Δ_BW | 2.8 (1.6 to 4.0) | 3.9 (0.7 to 7.2) | 1.6 (0.3 to 2.8) | 1.4 (–1.9 to 4.6) |
| _Δ_HbA1c; _Δ_SBP; _Δ_BW | 3.7 (2.4 to 5.0) | 4.8 (1.5 to 8.2) | 2.6 (1.3 to 3.9) | 2.2 (–1.2 to 5.6) |
| Albuminuria, mg/g | ||||
| None | −5.7 (−13.1 to 2.3) | −31.7 (−48.9 to −8.6) | −11.2 (−18.3 to −3.6) | −49.3 (−62.2 to −31.9) |
| _Δ_HbA1c | −6.2 (−13.5 to 1.7) | −32.3 (−49.2 to −9.7) | −10.9 (−17.8 to −3.3) | −48.6 (−61.6 to −31.2) |
| _Δ_SBP | −3.5 (−11.1 to 4.8) | −28.9 (−46.7 to −5.3) | −8.3 (−15.6 to −0.4) | −47.1 (−60.4 to −29.4) |
| _Δ_BW | −5.3 (−13.3 to 3.3) | −30.3 (−48.6 to −5.5) | −10.9 (−18.6 to −2.6) | −48.8 (−62.5 to −30.0) |
| _Δ_HbA1c; _Δ_SBP; _Δ_BW | −5.7 (−13.6 to 2.8) | −28.7 (−47.0 to −4.1) | −9.9 (−17.6 to −1.5) | −46.3 (−60.4 to −27.2) |
With respect to safety, similar proportions of patients across treatment groups experienced adverse events potentially related to kidney function (Table 3). Five patients, all in the canagliflozin groups, experienced an acute renal failure (ARF) or renal failure event. Four of these events were asymptomatic decreases in eGFR that recovered or resolved. One patient, in whom a serious adverse ARF event was reported, died and autopsy revealed a massive pulmonary embolism as a cause of death.
Table 3.
Treatment-emergent kidney-related adverse events during the entire double-blind treatment period
| Adverse Event | Glimepiride (_n_=482), n (%) | Canagliflozin 100 mg (_n_=483), n (%) | Canagliflozin 300 mg (_n_=485), n (%) |
|---|---|---|---|
| Total number of subjects with kidney-related adverse events | 16 (3.3) | 15 (3.1) | 16 (3.3) |
| Blood creatinine increased | 5 (1.0) | 2 (0.4) | 1 (0.2) |
| GFR decreased | 10 (2.1) | 11 (2.3) | 10 (2.1) |
| Renal failure | 0 | 1 (0.2) | 3 (0.6) |
| Renal failure acute | 0 | 1 (0.2) | 0 |
| Renal impairment | 3 (0.6) | 0 | 3 (0.6) |
Discussion
This analysis of 2 years of follow-up data from a randomized controlled trial comparing treatment with the SGLT2 inhibitor canagliflozin to glimepiride showed that canagliflozin slows the progression of kidney function decline in patients with type 2 diabetes who are already receiving metformin. In addition, canagliflozin 300 mg significantly decreases albuminuria, particularly in people with increased urinary albumin excretion. The beneficial effects of canagliflozin on kidney function and albuminuria are unlikely to be explained by the modest differential effect on glucose levels, so these findings suggest that SGLT2 inhibitors may be renoprotective, independent of their effects on glycemic control.
As a result of the use of an active comparator arm (glimepiride) in this study, the differences in HbA1c during the trial were modest, and adjustment for changes in HbA1c, BP, and body weight did not alter the results. Although improved glycemic control has been proven to be an important method for reducing the risk of microvascular complications in patients with type 1 and type 2 diabetes on the basis of data from a number of trials,9–11 these and other trials were not able to show any differences in average kidney function between the treatment arms, despite substantial differences in glycemic control throughout the trials.12,13 In this study, canagliflozin is superior to glimepiride in preserving kidney function, as defined by eGFR levels, despite smaller between-group differences in glycemic control compared with previous placebo-controlled studies.
The magnitude of the reduction in albuminuria with canagliflozin relative to glimepiride was also similar to that previously described with other SGLT2 inhibitors, as compared with placebo. In patients with type 2 diabetes and CKD, empagliflozin decreased albuminuria by approximately 30%, whereas dapagliflozin decreased albuminuria by 33% compared with placebo in patients with type 2 diabetes and micro- or macroalbuminuria treated with an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.6,14 The albuminuria reduction achieved by empagliflozin was completely reversible 4 weeks after treatment discontinuation, which suggests that albuminuria reduction is mediated through changes in renal hemodynamics.6 The consistency in results between these studies and the present analysis strongly supports the concept that the reduction in albuminuria has a predominantly glucose-independent mechanism.
What could be the underlying mechanism of any renoprotective effects? Blockade of the SGLT2 transporter inhibits sodium and glucose reabsorption. As a result, the delivery of glucose and sodium is increased in the distal tubule and the juxtaglomerular apparatus, which is sensed as an increase in glomerular perfusion. This leads to a feedback signal that causes afferent arteriolar vasoconstriction, an acute fall in glomerular perfusion and pressure, as well as a diminished extracellular plasma volume and BP.15 Additionally, these effects reduce atrial natriuretic peptide secretion that may also be important in reducing intraglomerular pressure.16,17 These effects are clinically manifested as acute reductions in albuminuria and eGFR, followed by stabilization in eGFR in the longer term, and these effects are not observed with other classes of glucose-lowering agents. Thus, SGLT2 inhibitors specifically alter renal hemodynamics and reduce intraglomerular pressure, which could be expected to translate into improved long-term kidney outcomes.
Meta-analyses of large clinical trials have suggested that a 30% reduction in albuminuria might translate into a 30% reduction in the risk of ESRD, assuming no changes in other markers of kidney disease.18 The effects on eGFR in this study were also similar to those in the placebo-controlled EMPA-REG outcomes trial, where a 55% relative reduction in the risk of ESRD has been reported in a post hoc analysis, albeit with a limited number of ESRD cases.8 These supportive findings will be definitively tested in the ongoing Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (ClinicalTrials.gov identifier: NCT02065791) trial, which is designed to assess whether canagliflozin 100 mg delays or prevents kidney failure or cardiovascular death in patients with type 2 diabetes and established nephropathy.
This study has several limitations. It was not designed nor powered to compare the renoprotective effects of canagliflozin versus glimepiride, so the results should be interpreted as hypothesis-generating. Unfortunately, eGFR was not measured after drug discontinuation in order to verify whether the initial fall in eGFR in the canagliflozin groups was reversible. However, other studies with SGLT2 inhibitors have shown that eGFR returns to baseline values after drug discontinuation.6,19 In addition, the lack of a placebo arm in this study means that no definitive conclusions can be drawn regarding whether canagliflozin is renoprotective or whether glimepiride worsens the progression of kidney disease. The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation trial showed that lowering HbA1c with a sulfonylurea derivative–based regimen confers renoprotection, suggesting that sulfonylurea derivatives do not accelerate kidney function decline.20 We note, however, that the rate of eGFR decline in the glimepiride group is higher than what could be expected in a population with preserved kidney function and normoalbuminuria, which may offer an alternative explanation for the beneficial effects of canagliflozin. Also, the effects on albuminuria were particularly marked in people with higher UACR levels at baseline, and were smaller in those with normal UACR levels. This is perhaps not surprising, as individuals with high UACR are more likely to show a reduction in albuminuria compared with people in whom albuminuria levels are already low. Moreover, 61% of all patients received background renin-angiotensin system (RAS) inhibition. Whether the beneficial effects of canagliflozin remain present if all patients receive optimal doses of RAS inhibition remains to be studied, but is likely, since our data showed that the effects of canagliflozin were present regardless of the use of background RAS inhibition. The eGFR course indicates a two-slope model of an acute fall in eGFR, observed after 4 weeks, followed by an attenuation of long-term eGFR decline. eGFR slopes were calculated from baseline in the main analyses instead of using the week-4 eGFR data as baseline to avoid violation of the randomization principle. Finally, eGFR decline was slightly higher with canagliflozin 300 mg versus 100 mg. This may be explained by the larger acute eGFR effects of the 300 mg dose and may also explain why the larger albuminuria reduction in the canagliflozin 300 mg group did not correspond with less kidney function decline.21,22 A longer follow-up period would be required to completely characterize the effects of canagliflozin on long-term kidney function decline.
In conclusion, in patients with type 2 diabetes, canagliflozin 100 and 300 mg as add-on to metformin slows the progression of disease compared with glimepiride during 2 years of follow-up. Therefore, canagliflozin may offer a novel therapeutic option for patients with type 2 diabetes who are at a high risk of kidney failure.
Concise Methods
Study Design and Participants
The study design has been published previously.3 In short, this randomized, double-blind, active-controlled trial was conducted in 157 research centers in 19 countries. The study consisted of a 2-week, single-blind, placebo run-in period; a 52-week, double-blind, core treatment period; and a 52-week, prespecified, double-blind, extension treatment period during which patients continued their originally assigned treatment. The 52-week, double-blind, extension period was prespecified to provide a more complete characterization of the efficacy and safety of canagliflozin over 104 weeks of treatment. The prespecified primary efficacy end point was the change in HbA1c from baseline to week 52. The sample size calculation assumed that 277 patients per group would be needed to provide approximately 90% power to show noninferiority of canagliflozin compared with glimepiride for the lowering of HbA1c, with an assumed between-group difference of 0.0% and an SD of 1.0. The primary efficacy results showed that canagliflozin 100 mg was noninferior to glimepiride in reducing HbA1c whereas canagliflozin 300 mg was superior to glimepiride.3 Eligible patients were between 18 and 80 years of age and had type 2 diabetes with an HbA1c between 7.0% and 9.5% while receiving metformin ≥2000 mg/d (or ≥1500 mg/d if higher doses were not tolerated). Patients with an eGFR of <55 ml/min per 1.73 m2 (or <60 ml/min per 1.73 m2 if based on restriction of metformin use in the local label) or serum creatinine concentrations ≥124 _μ_mol/L for men and ≥115 _μ_mol/L for women were excluded. No inclusion or exclusion criteria were specified for albuminuria. Glimepiride was uptitrated if patients met protocol-specified glycemic criteria after ≥2 weeks at the current dose, and uptitration could occur at any time during the study; patients receiving canagliflozin were mock uptitrated. Glycemic rescue therapy was initiated with pioglitazone (where approved) for patients who were at the maximum level of study drug titration and met prespecified glycemic criteria.
The trial was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. Ethics committees and institutional review boards approved the research protocol. All patients provided written informed consent before entering the trial. The study was registered with ClinicalTrials.gov (identifier: NCT00968812).
Randomization and Masking
During the run-in period, all patients received single-blind placebo capsules matching the study drug once daily. After the run-in period, patients were stratified according to whether they were taking a stable, protocol-defined dose of metformin before screening versus whether they had undergone dose adjustments in their metformin dose during the run-in period or had discontinued use of a second glucose-lowering drug. Patients were randomly assigned to receive oral, once-daily glimepiride (uptitrated to 6 or 8 mg/d based on the maximum approved dose in the country of the investigational site), canagliflozin 100 mg/d, or canagliflozin 300 mg/d at a 1:1:1 ratio by a computer-generated schedule prepared by the sponsor. Patients and all study personnel (except the safety monitoring committee) were masked to treatment allocation. To allow for masked increases or decreases in the glimepiride dose throughout the double-blind treatment, study drugs were supplied in five levels (levels 1–5).
Procedures
After randomization, patients collected first morning void urine at baseline and weeks 12, 26, 52, 78, and 104 for assessment of UACR. Urine albumin was measured by a nephelometric assay and creatinine was measured by a rate-blanked colorimetric assay in a central laboratory (Covance [Indianapolis, IN; Meyrin-Geneva, Switzerland; Singapore]). Serum creatinine and HbA1c were measured at baseline and weeks 4, 12, 18 (HbA1c only), 26, 36, 44, 52, 64, 78, 88, and 104. Serum creatinine was used to calculate eGFR with the Modification of Diet in Renal Disease equation: eGFR = 175×(serum creatinine [mg/dl])−1.154×(age [years])−0.203×(0.742 if female)×(1.212 if black).21
Safety was monitored throughout the study by assessing adverse events, laboratory data, vital sign measurements, physical examinations, self-monitoring of blood glucose, and 12-lead electrocardiograms. Reported adverse events were recorded during the trial and analyzed with a standard coding dictionary (Medical Dictionary for Regulatory Activities) to classify adverse event terms. Serious adverse events were defined as any adverse event that resulted in death, was immediately life-threatening, required hospital admission or prolonged existing hospitalization, resulted in persistent or significant disability or incapacity, was a birth defect, or was an event that jeopardized the patient’s health or required intervention.
Outcomes
The main efficacy end point for this post hoc analysis was the yearly rate of eGFR decline over the 104 weeks of follow-up. Exploratory efficacy end points were the least squares mean change from baseline in eGFR and UACR to week 104. The effects on eGFR and UACR were assessed by comparing mean levels across the treatment period and by assessing the number of individuals who developed a 30% or 40% reduction in eGFR during the study period, as recommended in a recent National Kidney Foundation–sponsored workshop.22 The effect of the interventions on these end points was assessed in the overall population and in a subgroup that was defined by baseline UACR≥30 mg/g (_n_=230). Since all patients had a baseline eGFR >55 ml/min per 1.73 m2, eGFR subgroups were not examined.
Statistical Analyses
Differences in eGFR and UACR between randomized groups during follow-up were estimated from repeated measures analysis. Differences between randomized groups in eGFR decline during follow-up were estimated from a mixed-effects model for repeated measures (MMRM). The model included treatment, visit (expressed in time in years since randomization), and treatment-by-visit interaction as factors. The latter was used to estimate the overall between-group difference in eGFR over the course of the study. The MMRM model was also used to calculate the least squares mean change from baseline in eGFR and UACR over time. For these analyses, baseline eGFR and UACR were entered as covariates in the model. UACR was log-transformed before entering in the MMRM analysis to alleviate the skewness of the data. To allow generality for the covariance structure for repeated measures, the variance-covariance matrix was assumed to be unstructured (i.e., purely data-dependent). In the MMRM model, all patients and all data points were included. No patients were excluded due to missing data and no imputation was carried out for missing data.
The effects of randomized treatment on the 30% or 40% eGFR decline end points were estimated from Cox proportional hazard models. Cox proportional hazard models were conducted on the basis of the intention to treat principle. For patients who experienced more than one event during follow-up, survival time to the first 30% or 40% eGFR decline end point was used in each analysis. Patients were censored at their date of death or, for those still alive at the end of follow-up, the date of their last clinic visit before the termination of this study arm. Patients with unknown vital status were censored when they were last known to be alive. A P value <0.05 (two-sided) was considered to indicate a statistically significant difference. Analyses were performed using SAS 9.2 for Windows (SAS Institute, Cary, NC).
Disclosures
H.J.L.H. has consultancy agreements with the following companies: AbbVie (North Chicago, IL), Astellas (Tokyo, Japan), AstraZeneca (London, United Kingdom), Boehringer Ingelheim (Ingelheim am Main, Germany), Janssen (Raritan, NJ), and ZS Pharma (San Mateo, CA), and has a policy of honoraria going to his employer. M.D., D.B., and G.M. are full-time employees of Janssen Research & Development, LLC (Raritan, NJ). M.J. is supported by a Career Development Fellowship from the National Health and Medical Research Council of Australia (Canberra, Australia) and the National Heart Foundation (Canberra, Australia); has received speaker’s fees from Amgen (Thousand Oaks, CA) and Roche (Basel, Switzerland); advisory board funding from Boehringer Ingelheim (Ingelheim am Main, Germany), unrestricted research funding from Gambro (Lund, Sweden), Baxter (Deerfield, IL), CSL (King of Prussia, PA), Amgen (Thousand Oaks, CA), Eli Lilly (Indianapolis, IN), and Merck (Kenilworth, NJ); and serves on a steering committee for a trial funded by Janssen; all honoraria are contributed directly to clinical research programs. V.P. is supported by a Senior Research Fellowship from the National Health and Medical Research Council of Australia (Canberra, Australia); has served on advisory boards and/or spoken at scientific meetings sponsored by Janssen (Raritan, NJ), Baxter (Deerfield, IL), AbbVie (North Chicago, IL), Astellas (Tokyo, Japan), Bayer (Leverkusen, Germany), Bristol-Myers Squibb (New York City, NY), Boehringer Ingelheim (Ingelheim am Main, Germany), AstraZeneca (London, United Kingdom), Eli Lilly (Indianapolis, IN), Merck (Kenilworth, NJ), and GlaxoSmithKline (Brentford, United Kingdom); and has a policy of honoraria going to his employer.
Supplementary Material
Supplemental Data
Acknowledgments
We thank the supportive role of all site investigators, support staff, and participating patients.
Study funding was provided by Janssen Research & Development, LLC.
The sponsor was involved in the design of the study, in the collection and analysis of the data, and in the writing of the manuscript. All authors had access to study results, and reviewed, edited, and approved the manuscript for submission. The lead author vouches for the accuracy and completeness of the data reported. The lead author had the final decision to submit to the publication. The authors prepared the manuscript with editorial assistance from Shannon O’Sullivan, ELS, of MedErgy, which was funded by Janssen Global Services, LLC.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “SGLT2 Inhibitors—Sweet Success for Diabetic Kidney Disease?,” on pages 7–10.
References
- 1.American Diabetes Association : Standards of medical care in diabetes–2015. Diabetes Care 38[Suppl]: S1–S93, 2015 [Google Scholar]
- 2.Mudaliar S, Polidori D, Zambrowicz B, Henry RR: Sodium-glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care 38: 2344–2353, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G: Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382: 941–950, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G: Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 15: 463–473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewster UC, Setaro JF, Perazella MA: The renin-angiotensin-aldosterone system: cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci 326: 15–24, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC; EMPA-REG RENAL trial investigators : Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2: 369–384, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators : Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Wanner C, Inzucchi SE, Lachin JM, Fitchett DH, von Eynatten M, Mattheus M, Johansen O-E, Woerle H-J, Broedl UC, Zinman B: Empagliflozin and progression of kidney disease in type 2 diabetes [published online ahead of print June 14, 2016]. N Engl J Med doi:10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT; Action to Control Cardiovascular Risk in Diabetes Study Group : Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators : Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129–139, 2009 [DOI] [PubMed] [Google Scholar]
- 11.de Bour IH, Sun W, Clearly PA, Lachin JM, Molitch ME, Steffes MW, Zinman B; DCCT/EDIC research group : Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol 2: 793–800, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M: Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Lambers Heerspink HJ, Johnsson E, Gause-Nilsson I, Sjöström C: Erratum to: Abstracts of the 51st Annual Meeting of the EASD, Stockholm 2015. Dapagliflozin reduces albuminuria on top of renin-angiotensin system blockade in hypertensive patients with diabetes. Diabetologia 58: 2901, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Ballermann BJ, Brenner BM: Atrial natriuretic peptide and the kidney. Am J Kidney Dis 10[Suppl 1]: 7–12, 1987 [PubMed] [Google Scholar]
- 15.Ortola FV, Ballermann BJ, Anderson S, Mendez RE, Brenner BM: Elevated plasma atrial natriuretic peptide levels in diabetic rats. Potential mediator of hyperfiltration. J Clin Invest 80: 670–674, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heerspink HJ, Kröpelin TF, Hoekman J, de Zeeuw D; Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium : Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. J Am Soc Nephrol 26: 2055–2064, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, MacMahon S, Cooper ME, Hamet P, Marre M, Mogensen CE, Poulter N, Mancia G, Cass A, Patel A, Zoungas S; ADVANCE Collaborative Group : Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 83: 517–523, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Lambers Heerspink HJ, Weldegiorgis M, Inker LA, Gansevoort R, Parving HH, Dwyer JP, Mondal H, Coresh J, Greene T, Levey AS, de Zeeuw D: Estimated GFR decline as a surrogate end point for kidney failure: a post hoc analysis from the Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) study and Irbesartan Diabetic Nephropathy Trial (IDNT). Am J Kidney Dis 63: 244–250, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD: Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab 18: 590–597, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene T, Teng CC, Inker LA, Redd A, Ying J, Woodward M, Coresh J, Levey AS: Utility and validity of estimated GFR-based surrogate time-to-event end points in CKD: a simulation study. Am J Kidney Dis 64: 867–879, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J: GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data