Atezolizumab as first-line therapy in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial (original) (raw)
. Author manuscript; available in PMC: 2017 Aug 23.
Summary
Background
First-line chemotherapy for patients with cisplatin-ineligible locally-advanced or metastatic urothelial carcinoma (mUC) is associated with short response duration, poor survival, and high toxicity. This multicenter, 2-cohort phase 2 study evaluated atezolizumab (anti–programmed death-ligand 1 [PD-L1]) as treatment for mUC in this setting, as well as in later lines.
Methods
In a cohort of previously untreated patients who were cisplatin ineligible, atezolizumab was given 1200 mg every 3 weeks until progression. The primary endpoint was independently confirmed objective response rate per Response Evaluation Criteria In Solid Tumors v1.1 (central review), evaluated in pre-specified subgroups based on PD-L1 expression and in all patients. Secondary endpoints included response duration, progression-free survival, overall survival, and safety. Exploratory analyses included biomarker correlates of response and survival. This study is registered with ClinicalTrials.gov, number NCT02108652.
Findings
Of 119 patients who received atezolizumab in the first-line setting, 83 (70%) had baseline renal impairment, and 24 (20%) had Eastern Cooperative Oncology Group performance status 2. At 17·2 months’ median follow-up, the objective response rate was 23% (95% CI 16–31), the complete response rate was 9%, and 19 of 27 responses were ongoing. Median response duration was not reached. Responses occurred across all PD-L1 and poor prognostic factor subgroups. Median progression-free survival was 2·7 months. Median overall survival was 15·9 months. Tumour mutation load was associated with response. Treatment-related adverse events ≥10% were fatigue, diarrhoea, and pruritus. One treatment-related death (sepsis) occurred. Nine patients (8%) had an adverse event leading to treatment discontinuation. Immune-mediated events occurred in 14 (12%) patients.
Interpretation
Atezolizumab demonstrated encouraging durable response rates, survival, and tolerability, supporting its therapeutic use in untreated mUC.
Funding
F. Hoffmann-La Roche Ltd./Genentech, Inc., a member of the Roche Group.
Introduction
Urothelial cancer (UC) is an aggressive malignancy with ≈165,084 global deaths annually and a 5-year survival of ≈5% in the metastatic setting.1,2 Cisplatin-based chemotherapy, a first-line treatment standard, provides overall survival benefit;3 however, up to two-thirds of patients are ineligible4 due to impaired performance status or comorbidities (e.g., renal dysfunction). Treatment alternatives include carboplatin-based combinations and single-agent chemotherapy5–8 but are associated with shorter overall survival.9 In clinical practice, many patients do not receive systemic chemotherapy and are offered supportive care,5,6,10 further underscoring the need for more efficacious and tolerable therapies in cisplatin-ineligible patients.10,11
Atezolizumab is a humanised engineered immunoglobulin G1 monoclonal antibody that inhibits binding of programmed death-ligand 1 (PD-L1) to receptors programmed death-1 (PD-1) and B7.1, thereby restoring anti-cancer T-cell activity and reinvigorating suppressed immune cells.12,13 Atezolizumab has demonstrated efficacy and a tolerable safety profile in a range of cancers, including locally advanced or metastatic UC (mUC).12–16 In the IMvigor210 cohort of patients who progressed during or following platinum-based therapy, atezolizumab conferred significant clinical benefit,16 leading to accelerated regulatory approval, and several biomarkers associated with response were identified.16 Here we present clinical data from the first-line cisplatin-ineligible IMvigor210 cohort—the first report of an anti–PD-L1/PD-1 checkpoint inhibitor in this setting—along with exploratory analyses to validate biomarker correlates of clinical outcomes.
Methods
Study design
IMvigor210 was a multicentre, single-arm, 2-cohort phase 2 trial that investigated efficacy and safety of atezolizumab in mUC. This trial was conducted in 47 academic medical centres and community oncology practices across 7 countries, in North America and Europe. Cohort 1 enrolled patients without prior treatment for mUC. Eligible patients had inoperable, locally advanced or metastatic UC (renal pelvis, ureters, bladder, or urethra), measurable disease per Response Evaluation Criteria In Solid Tumors (RECIST) v1.1, Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2, and tumour sample for PD-L1 testing. (Neo)adjuvant chemotherapy or radiation was permitted if >12 months had elapsed between treatment and recurrence. Patients were required to be cisplatin ineligible per ≥1 of the following: glomerular filtration rate >30 and <60 mL/min (Cockcroft-Gault formula), grade ≥2 hearing loss or peripheral neuropathy, or ECOG PS 2.17 Complete inclusion and exclusion criteria are listed in the protocol (with statistical analysis plan) at thelancet.com. Patients received 1200 mg intravenous atezolizumab every 21 days until unacceptable toxicity or investigator-assessed radiographic progression. Dose interruptions, but not reductions, were permitted. Cohort 2 (described previously)16 enrolled patients previously treated with platinum-based chemotherapy. This study is registered with ClinicalTrials.gov, number NCT02108652.
Study assessments
Patients underwent response assessments at baseline, every 9 weeks for 12 months, and then every 12 weeks until disease progression, withdrawal of consent, or death; assessments were performed by local investigators and reviewed by a central independent facility (BioClinica; Princeton, NJ, USA). These assessments included measurement of tumor burden, including change over time in sum of longest diameters. Additionally, the ORR estimates in key subgroups defined by demographic and baseline characteristics were assessed. Safety was assessed per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Archival tumour tissue was collected for biomarker assessments. The VENTANA SP142 immunohistochemistry assay (Ventana Medical Systems, Inc.; Tucson, AZ, USA) was used to prospectively evaluate PD-L1 expression on tumour-infiltrating immune cells (IC) via a central laboratory (HistoGeneX; Brussels, Belgium). Scoring criteria designated tumours as IC0, IC1, or IC2/3 (PD-L1 expression on <1%; ≥1% and <5%; or ≥5% of IC, respectively).16 Patients, investigators, and sponsor were blinded to PD-L1 status. Somatic mutation and tumour mutation load assessments were made using a FoundationOne DNA-based panel (Foundation Medicine, Cambridge, MA, USA). Microsatellite status was centrally confirmed by next-generation sequencing-based scoring (Foundation Medicine, Cambridge, MA, USA). Gene expression was quantified for a T-effector gene signature (consisting of CD8A, GZMA, GZMB, PRF1, INFG, and TBX21) and for subtyping using The Cancer Genome Atlas18 (TCGA) categories.16
Statistical analysis
The cisplatin-ineligible patient cohort of IMvigor210 was initially planned as an exploratory subgroup of 30 patients. Subsequently, a protocol amendment increased sample size to ≈100 patients to provide a better estimate of the response rate (RECIST v1.1) in UC patients who were cisplatin ineligible, assessed by independent central review. Determination of sample size was based on the assumption of 30% IC2/3 prevalence. The 95% CI (calculated using the Clopper-Pearson method) for an overall response rate of 40% would be 22·7% to 59·4%, resulting in 98% power to detect a 30% increase in the overall response rate from 10% to 40%.
The primary efficacy analysis of the cisplatin-ineligible IMvigor210 cohort (data cutoff: September 14, 2015) was performed when the last patient enrolled had a minimum of 6 months of follow-up. An interim efficacy analysis was also performed when patients had ≥24 weeks of follow-up (see appendix for details). A hierarchical fixed-sequence testing procedure (previously described and details in appendix)16 to compare the observed primary endpoint for three pre-specified subgroups (PD-L1 IC2/3, followed by IC1/2/3, and followed by all patients) versus a control response rate of 10%. The hypothesis tests were conducted sequentially using IRF-assessed RECIST v1.1 at a specific two-sided α level of 0·05 for each test. If no statistical significance was detected at a specific level of the hierarchy, then no further testing was done. The study was expected to attract patients who would not be candidates for combination chemotherapy, including those not eligible for any cytotoxic chemotherapy, reflective of the heterogeneous cisplatin-ineligible population.10 Therefore, the 10% rate was approximated by a composite average of 75% of patients enrolled who would otherwise not be candidates for any cytotoxic chemotherapy (expected ORR 0%) and 25% who would be candidates for carboplatin-based combination chemotherapy (expected ORR 36%).9 The exact binomial test evaluated whether atezolizumab treatment results in a statistically significant difference between the observed and control response rates in the pre-specified subgroups. The tests were performed in a sequential order such that the subsequent hypothesis would not be performed if the preceding test was rejected (further described and presented in supplementary Methods S1). Clinical significance was assessed in an ongoing manner, and subsequent analyses did not use hypothesis testing as described for the primary analysis. This report uses a later cutoff (July 4, 2016) to provide updated efficacy and safety data.
Secondary endpoints included investigator-assessed objective response rate; duration of response and progression-free survival, both assessed by independent review and investigator (RECIST v1.1); and overall survival. Unless otherwise specified, RECIST results reported herein are per independent review.
Role of the funding source
The protocol, developed by the sponsor (F. Hoffmann-La Roche Ltd.) and advisors, was approved by the institutional review boards or independent ethics committees at each participating centre. All patients provided written informed consent before study entry. The study was conducted in accordance with the Declaration of Helsinki and International Conference of Harmonization Good Clinical Practice guidelines. Data were collected and analysed in collaboration between sponsor and clinical investigators. An independent data monitoring committee evaluated safety approximately every 6 months, in addition to a pre-specified futility analysis of efficacy data. All authors had access to the data, contributed to development and review of the manuscript (with editorial assistance from a sponsor-funded professional medical writer), approved submission, verified the study conduct in accordance with the protocol, and attested for data accuracy and completeness.
Results
Patients and treatment
Between June 9, 2014, and March 30, 2015, 167 patients were screened, and 123 patients were enrolled (supplementary figure S1), 4 of whom subsequently did not meet eligibility criteria and did not receive study drug. One-hundred nineteen patients received ≥1 dose of atezolizumab. One-hundred two patients (86%) discontinued treatment, either due to disease progression (n=77), patient withdrawal (n=12), an adverse event (n=11), or other reasons (n=2). The median treatment duration was 15 weeks (range 0–102), and 25 patients (21%) had been treated for >52 weeks at time of data cutoff (median follow-up 17·2 months [range 0·2–23·5]). Table 1 summarises baseline characteristics of the safety and efficacy population. Seventy percent were cisplatin ineligible due to renal impairment. Poor performance status and visceral metastatic disease are independent prognostic factors (Bajorin risk factors) that predict survival in metastatic UC.19 Fifty-six percent of patients in this study had 1 Bajorin risk factor and 15% had both Bajorin risk factors. Most patients (87%) had comorbidities. The distribution of PD-L1 subgroups matched prior study populations.16
Table 1.
Baseline characteristics and prior therapy
| Characteristic | All patients*(N=119) |
|---|---|
| Age, years | |
| Median | 73 |
| Range | 51–92 |
| Age ≥80 years | 25 (21%) |
| Male sex | 96 (81%) |
| PD-L1 status on immune cells | |
| IC2/3 | 32 (27%) |
| IC1 | 48 (40%) |
| IC0 | 39 (33%) |
| Primary tumour site† | |
| Bladder/urethra | 85 (71%) |
| Renal pelvis/ureter | 33 (28%) |
| Metastatic disease | 110 (92%) |
| Lymph node only | 31 (26%) |
| Visceral sites‡ | 78 (66%) |
| Liver sites | 25 (21%) |
| Prior tobacco use | |
| Current | 7 (6%) |
| Former | 77 (65%) |
| Never | 35 (29%) |
| Prior therapy | |
| Radiotherapy | 12 (10%) |
| Perioperative chemotherapy§ | 22 (19%) |
| Cisplatin ineligibility criteria | |
| Renal impairment‖ | 83 (70%) |
| Hearing loss, 25 dB¶ | 17 (14%) |
| Peripheral neuropathy, grade ≥2 | 7 (6%) |
| ECOG PS 2 | 24 (20%) |
| Renal impairment and ECOG PS 2 | 8 (7%) |
Efficacy
The primary efficacy analysis (Methods S1) was designed to be performed when patients had a minimum of 6 months’ follow-up. In that analysis (with a median follow-up duration of 8·5 months [range 0·2–14·3]), hierarchal testing did not reach significance in the IC2/3 patient subgroup (objective response rate 22% 95% CI (9–40%), compared with the pre-specified 10% response rate, precluding further statistical tests (Methods S1). However, after a 17·2-month median follow-up duration, the objective response rate was 23% (95% confidence interval [CI] 16–31; table 2) in all patients, with the lower bound of the 95% CI exceeding 10%. Furthermore, the updated objective response rate by PD-L1 subgroup rose to 28% (95% CI 14–47) in IC2/3, 24% (95% CI 15–35) in IC1/2/3, 21% (95% CI 10–35) in IC1, and 21% (95% CI 9–36) in IC0 patients. Complete responses were seen in 11 patients (9%). Concordance between responses assessed by investigators vs independent review was >90% (supplementary table S2).
Table 2.
Objective response rate by PD-L1 status on tumour-infiltrating immune cells
| Patients | Completeresponse | Partialresponse | Objective responserate, n (% [95% CI])* | Median durationof response (95% CI), | |
|---|---|---|---|---|---|
| All patients | 119 | 11 | 16 | 27 (23% (16–31) | NE (14·1–NE) |
| IC2/3 | 32 | 4 | 5 | 9 (28% (14–47]) | NE (11·1–NE) |
| IC1/2/3 | 80 | 8 | 11 | 19 (24% [15–35]) | NE (NE) |
| IC1 | 48 | 4 | 6 | 10 (21% ([11–35]) | NE (NE) |
| IC0 | 39 | 3 | 5 | 8 (21% [9–37])) | NE (12·8–NE) |
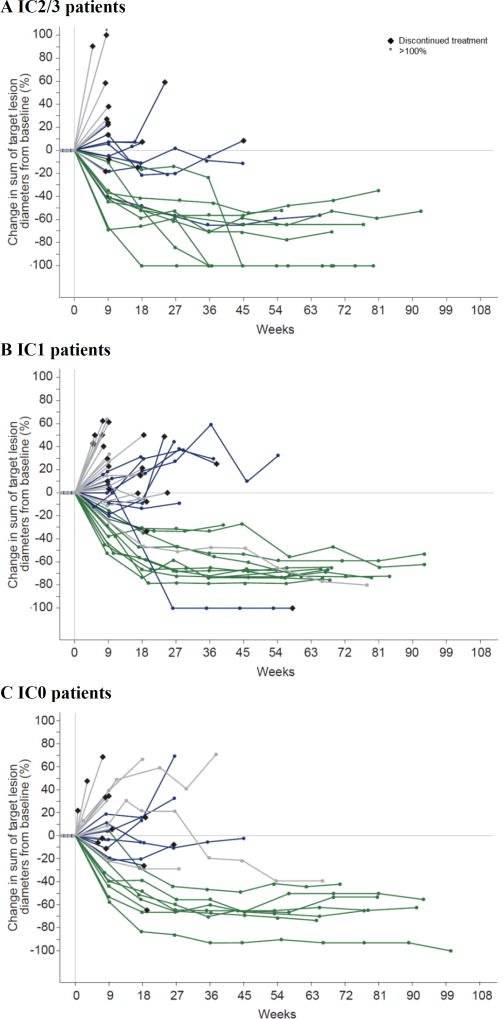
Median time to onset of first response was 2·1 months (range 1·8–10·5), but late responses were also observed (after 6 months in 2 patients; figure 1 and supplementary figure S2). Median response duration had not been reached in all patients or in pre-defined PD-L1 subgroups (range 3·7 to 21·0+), and 19 of 27 responses (70%) were ongoing. Median progression-free survival was 2·7 months (95% CI 2·1–4·2) in all patients, 4·1 months (95% CI 2·3–11·8) in IC2/3 patients, 2·1 months (95% CI 2·1–5·4) in IC1 patients, and 2·6 months (95% CI 2·1–5·7) in IC0 patients. The clinical benefit rate in all patients was 30% (95% CI 22–39; supplementary table S3).
Figure 1. Change from baseline tumour burden by PD-L1 status.
Spider plots depict changes from baseline tumour burden, defined as the sum of target lesion diameters, in patients who received atezolizumab, based on baseline PD-L1 status on immune cells of (A) IC2/3, (B) IC1, and (C) IC0. Grey colour denotes progressive disease; blue denotes stable disease; green denotes complete and partial responses (per RECIST v1.1). Data cutoff: July 4, 2016. PD-L1=programmed death-ligand 1. IC=tumour-infiltrating immune cell.
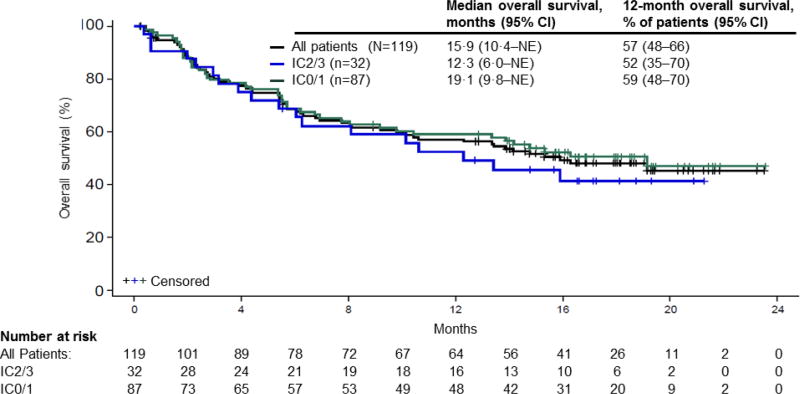
The median overall survival was 15·9 months (95% CI 10·4 to not estimable; figure 2) in all patients, 12·3 months (95% CI 6·0 to not estimable) in IC2/3 patients, and 19·1 months (95% CI 9·8 to not estimable) in IC0/1 patients. The 12-month landmark survival rate was 57% (95% CI 48–66) in all patients.
Figure 2. Overall survival in patients treated with atezolizumab.
Kaplan-Meier estimates of overall survival according to PD-L1 status on immune cells. A total of 59 events occurred in all patients by the data cutoff date (18 in IC2/3 patients; 41 in IC0/1 patients). Data cutoff: July 4, 2016. PD-L1=programmed death-ligand 1. NE=not estimable. IC=tumour-infiltrating immune cell.
Subgroup analyses
Responses to atezolizumab occurred in all clinical subgroups evaluated (table 3). Notably, 39% of patients with upper-tract primary tumours (renal pelvis/ureter; n=33) had an objective response. Patient subgroups with lower response rates (e.g., those with liver metastases [n=25]; 8%) still had durable responses, with median response duration also not reached in any of these subgroups. Bajorin risk factors also appeared to maintain prognostic utility.19 Median survival was not reached in patients with no risk factors (supplementary table S4), was 13·4 months in those with 1 risk factor (either visceral metastases or ECOG PS 2), and was 6·2 months in those with 2 risk factors. Patients with liver metastases had a 5·5-month median survival. Furthermore, patients aged ≥80 years (n=25) and those with renal dysfunction (n=83) had median survival durations of 14·8 and 14·1 months, respectively. Patients who achieved stable disease (n=29) had a median survival of 19·1 months (supplementary table S5).
Table 3.
Objective response rates by baseline subgroups
| Subgroup | Patients | Objective responserate, n (% [95% CI])* |
|---|---|---|
| All patients | 119 | 27 (23% [16–31]) |
| Demographics and prior treatment | ||
| Age ≥80 years | 25 | 7 (28% [12–49]) |
| Perioperative chemotherapy† | 22 | 8 (36% [17–59]) |
| Primary tumour sites‡ | ||
| Bladder/urethra | 85 | 14 (17% [9–26]) |
| Upper tract | 33 | 13 (39% [23–58]) |
| Metastatic sites at baseline | ||
| Lymph node only | 31 | 10 (32% [17–51]) |
| Visceral§ | 78 | 11 (14% [7–24]) |
| Liver | 25 | 2 (8% [1–26]) |
| Cisplatin ineligibility criteria | ||
| Impaired renal function | 83 | 21 (25% [16–36]) |
| ECOG PS 2 | 24 | 6 (25% [10–47]) |
| Hearing loss, 25 dB | 17 | 2 (12% [2–36]) |
| Peripheral neuropathy, grade ≥2 | 7 | 1 (14% [0–58]) |
| Renal impairment and ECOG PS 2 | 8 | 2 (25% [3–65]) |
| Bajorin risk factors‖ | ||
| 0 | 35 | 12 (34% [19–52]) |
| 1 | 66 | 13 (20% [11–31]) |
| 2 | 18 | 2 (11% [1–35]) |
Median survival in patients with upper-tract primary tumours had not been reached. To investigate a possible basis for improved outcomes in these patients, we assessed baseline covariates, including anatomic sites of metastases, tumour mutation load, T-effector gene expression, TCGA subtype, and baseline tumour burden; however, we found no significant differences in these factors between patients with upper- and lower-tract disease (supplementary figure S3). Microsatellite instability was observed only in two patients with upper-tract primary tumours (and two with lower-tract primary tumours), suggesting that this factor was not a primary determinant.
Exploratory analyses of biomarkers of efficacy
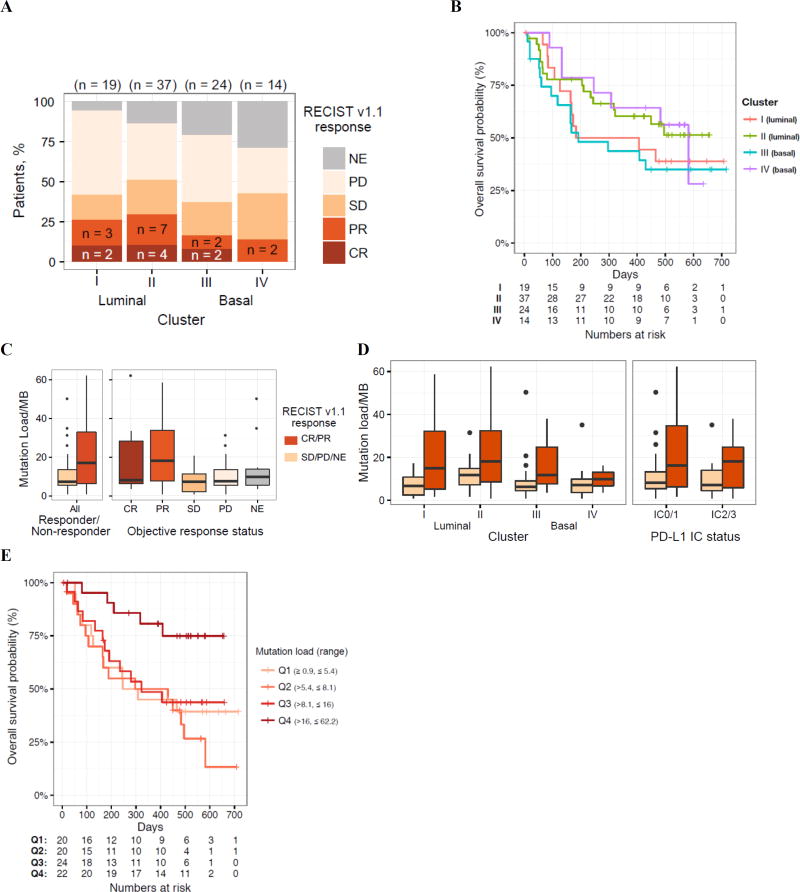
Exploratory biomarker assessments that were not pre-specified included expression of individual genes and gene sets, subtyping according to TCGA (supplementary figure S4), and quantification of mutation load.16 Overall, 72 (61%) of samples obtained for these analyses were from primary tumor samples and 47 (39%) were from metastatic tumors. Responses were seen across all subtypes and were more frequent with the luminal II subtype (figure 3A). Survival by TCGA subtype is presented in figure 3B. Tumour mutation load was significantly higher in responding patients than in non-responders, and this relationship was consistent across TCGA subtypes and PD-L1 subgroups (figures 3C and 3D). Mutation load was also associated with overall survival (figure 3E); patients with the highest mutation load (quartile 4) had significantly longer survival compared with those in quartiles 1 to 3.
Figure 3. Associations among TCGA subtype, mutation load, and clinical activity.
(A) Response as a function of The Cancer Genome Atlas subtype. (B) Kaplan-Meier plot of overall survival by subtype (luminal I, papillary-like; luminal II; basal III, squamous-like; and basal IV). (C) Mutation load as a function of response (Wilcoxon rank sum p=0·0180 for responding vs non-responding patients). (D) Mutation load versus response disaggregated by subtype or PD-L1 IC score. (E) Kaplan-Meier estimate of overall survival according to estimated mutation load (per megabase), binned into quartiles (log-rank p=0·0041 for a difference in overall survival between quartiles 1 to 3 and quartile 4). P values are for descriptive purposes only. TCGA=The Cancer Genome Atlas. PD-L1=programmed death-ligand 1. Data cutoff: July 4, 2016. IC=tumour-infiltrating immune cell. Lum=luminal. Bas=basal. RECIST=Response Evaluation Criteria In Solid Tumors. NE=not estimable. PD=progressive disease. SD=stable disease. PR=partial response. CR=complete response. MB=megabase.
Safety
One hundred fourteen patients (96%) experienced an adverse event (see supplementary table S6 for list of adverse events regardless of attribution), and 79 patients (66%) experienced a treatment-related event (table 4). No major safety differences were observed across PD-L1 subgroups. Treatment-related adverse events observed in ≥10% of patients (any grade) were fatigue, diarrhoea, and pruritus. Grade 3 or 4 treatment-related events occurred in 19 patients (16%), most frequently fatigue (3·4%, n=4), increased alanine aminotransferase (3·4%, n=4), and increased aspartate aminotransferase (2·5%, n=3). Only 1 of 4 reported grade 5 adverse events (supplementary table S6) was considered treatment related by the investigator (sepsis, in a patient with an unidentified source of infection).
Table 4.
Treatment-related adverse events
| Adverse event | Any grade | Grade 3–4 |
|---|---|---|
| Overall | 79 (66%) | 19 (16%) |
| Fatigue | 36 (30%) | 4 (3%) |
| Diarrhoea | 14 (12%) | 2 (2%) |
| Pruritus | 13 (11%) | 1 (1%) |
| Decreased appetite | 11 (9%) | 1 (1%) |
| Hypothyroidism | 8 (7%) | 0 |
| Anaemia | 6 (5%) | 1 (1%) |
| Chills | 6 (5%) | 0 |
| Nausea | 6 (5%) | 0 |
| Pyrexia | 6 (5%) | 0 |
| Rash | 6 (5%) | 1 (1%) |
| Vomiting | 6 (5%) | 0 |
| Rash, maculopapular | 5 (4%) | 0 |
| Alanine aminotransferase increased | 5 (4%) | 4 (3%) |
| Arthralgia | 5 (4%) | 0 |
| Aspartate aminotransferase increased | 4 (3%) | 3 (3%) |
| Blood alkaline phosphatase increased | 4 (3%) | 1 (1%) |
| Blood bilirubin increased | 4 (3%) | 2 (2%) |
| Dyspnoea | 4 (3%) | 0 |
| Infusion-related reaction | 4 (3%) | 0 |
| Lymphocyte count decreased | 4 (3%) | 0 |
| Asthenia | 3 (3%) | 0 |
| Back pain | 3 (3%) | 0 |
| Dermatitis acneiform | 3 (3%) | 0 |
| Dry mouth | 3 (3%) | 0 |
| Headache | 3 (3%) | 0 |
| Hypophosphataemia | 3 (3%) | 2 (2%) |
| Hypotension | 3 (3%) | 1 (1%) |
| Influenza-like illness | 3 (3%) | 0 |
| Muscle spasms | 3 (3%) | 0 |
| Thrombocytopenia | 3 (3%) | 0 |
| Renal failure | 2 (2%) | 2 (2%) |
| Autoimmune colitis | 1 (1%) | 1 (1%) |
| Liver disorder | 1 (1%) | 1 (1%) |
| Hypersensitivity | 1 (1%) | 1 (1%) |
| Multiple organ dysfunction syndrome | 1 (1%) | 1 (1%) |
| Portal vein thrombosis | 1 (1%) | 1 (1%) |
Overall, 41 patients (35%) had an adverse event leading to dose interruption, with no single adverse event predominating, and 9 patients (8%) had an event leading to treatment withdrawal. The majority of treatment discontinuations (77 of 102) and deaths (52 of 59) were due to progression. Immune-mediated all grade adverse events were reported in 14 patients (12%) and grade 3 or adverse events were reported in 8 patients (7%; supplementary table S7), most commonly rash (3% all grade [n=4]; 1% grade 3 or 4 [n=1]). No patients received systemic non-corticosteroid immunomodulatory agents (e.g., infliximab, tocilizumab) for immune-mediated events. 36 patients received corticosteroids.
Post-protocol therapies
Post-protocol therapy, defined as any therapy administered after progression on atezolizumab before study discontinuation, was reported for 25 patients (9 IC0, 12 IC1, 4 IC2/3) during follow-up. The most common therapy was gemcitabine-carboplatin (14 of 25 patients); other regimens given are listed in supplementary table S8.
Discussion
In this single-arm, phase 2 study, atezolizumab is the first anti–PD-L1/PD-1 agent to demonstrate durable responses with a tolerable safety profile in untreated cisplatin-ineligible mUC. Objective responses occurred across all PD-L1 subgroups and identified prognostic subgroups, with high complete response rates relative to previous chemotherapy trials.9 With 17·2 months of median follow-up, median response duration had not been reached in all patients or in any of these subgroups. Albeit a single arm study, the observed median overall survival of 15·9 months is still noteworthy when compared with first-line gemcitabine-carboplatin (9·3 months)9 or cisplatin-based regimens in eligible patients (15·2 to 15·8 months).20,21 Larger, randomised studies will be valuable in supporting these phase 2 findings.
Atezolizumab was well tolerated. Most treatment-related adverse events were of maximum grade 1 or 2, and immune-mediated events were manageable with systemic corticosteroids alone. The safety profile was consistent with previous atezolizumab trials across a range of cancers12–16 and compared favourably with cytotoxic chemotherapy; whereas 21% treatment discontinuation and high rates of haematologic toxicity (e.g., neutropenia)9 were reported with gemcitabine-carboplatin, the most appropriate comparator in this population, only 8% of patients in this study discontinued treatment due to an adverse event, and no neutropenia was observed. Furthermore, no decline in median glomerular filtration rate was observed in this cohort (mostly patients with baseline renal impairment) through ≥27 treatment cycles (data not shown)—a finding pertinent to patients with reduced kidney function or a solitary kidney common with upper-tract disease.
Evolution of responses over time was noteworthy in this study, suggesting response rates and other historical surrogates of efficacy in mUC chemotherapy trials (e.g., progression-free survival) assessed at early time points may not fully capture benefit of modern-day immunotherapy. Responses to immune checkpoint inhibitors can be delayed and display atypical kinetics. For example, in the primary analysis, response rates were numerically but not statistically higher than the pre-specified response rate in the PD-L1–selected subgroup; however, with longer follow-up, several patients experienced further tumour shrinkage, leading to new complete and partial responses and the lower bound of the objective response rate 95% CI to now exceed 10%. Furthermore, durable benefit was seen even in the absence of RECIST response (19·1-month median overall survival observed in the stable disease subgroup), an observation common to immunotherapy14,22 but not chemotherapy trials, which could have profound impacts on standards of mUC care. Cumulative toxicity often limits chemotherapy treatment to up to 6 to 8 cycles with platinum-based therapy,6, 8–10 even in responding patients, however discontinuing treatment may compromise benefit in patients receiving immunotherapy. Future trials will be challenged to identify appropriate surrogates of long-term benefit as well as optimal timing for alternative treatments.
IMvigor210 was designed to test the association of PD-L1 expression with atezolizumab efficacy. In contrast with previous reports,13,16 no statistically significant enrichment of response by PD-L1 expression was observed. Differences in baseline characteristics (e.g., tumour burden or nodal only vs visceral metastases) between populations or statistical assumptions underpowered to detect precise differences between IC subgroups for this initially exploratory cohort might have contributed to these findings. Such factors will be analysed in phase 3 studies IMvigor211 (platinum-treated patients) and IMvigor130 (treatment-naive patients; ClinicalTrials.gov numbers NCT02302807 and NCT02807636, respectively).
TGCA subtypes have previously been associated with prognostic differences in survival, with basal tumours tending to exhibit shorter survival durations.23,24 Still, outcomes observed in patients with luminal II samples are consistent with the IMvigor210 platinum-treated cohort,16 although, as for PD-L1 status, sample size was not sufficient to establish statistical significance in the current study cohort. The observation that patients with the highest tumour mutation load25 derived the longest survival from atezolizumab suggests that a threshold for tumour mutation load may need to be surpassed for generation of neo-antigens most suited for recognition by tumour-specific T cells. However, given the stochastic relationship between total mutation load and generation of neo-antigens, anti-tumour responses observed in some patients with lower mutation load are not unexpected—as seen in this study. These observations validate results from the platinum-treated population16 and additional cancer immunotherapy studies in other tumour types.26,27 As previously suggested, tumor mutation load, PD-L1 expression on immune cells and tumor TCGA subtypes may be independent predictors of response,16 and further analyses in larger mUC studies that incorporates multiple biomarkers may help patient selection for optimal efficacy.
Several populations enrolled in this trial warrant further study. Good outcomes were observed in patients with upper-tract disease—a group historically associated with a poor prognosis.28 Microsatellite instability, common in this population and associated with response to checkpoint inhibitors in some cancers,29 however, was found in only a few patients in our trial, precluding further study. Additionally, elderly patients tend to have poor outcomes30 and chemotherapy intolerance; the single-arm design of this trial may have attracted such patients who would otherwise not participate in trials with a chemotherapy control arm. Patients aged ≥80 years (21% of the study population) had outcomes similar to the intention-to-treat population with good tolerability.
Overall, atezolizumab demonstrated promising response durability and survival coupled with a low incidence of clinically relevant toxicities despite numerous comorbidities in this population. The observations in this phase 2 study are remarkable in this area of high unmet need and highlight the role of atezolizumab as an attractive first-line option for cisplatin-ineligible mUC. These results warrant further study in the phase 3 setting in this population (IMvigor130, ClinicalTrials.gov number NCT02807636), and suggest future potential for all patients in the first-line setting.
Supplementary Material
Consort Diagram
Supplementary Appendix
Research in context.
Evidence before this study
A PubMed search for phase 3 clinical trials published between 2005 and 2014 on advanced urothelial carcinoma and related MeSH terms yielded 17 articles. We examined the articles specific to treatment of patients in the first-line setting, along with international congress presentations during the time period. We identified an unmet clinical need for effective and tolerable approaches to treating patients with baseline characteristics that rendered them ineligible for cisplatin-based chemotherapy. No such treatments appeared to exist or be approved by the US Food and Drug Administration, European Medicines Agency, or related agencies, and the cytotoxic agents commonly employed in this population were consistently associated with toxicity and poor overall survival despite treatment.
Added value of this study
In this study, the humanised monoclonal anti-programmed death ligand 1 (PD-L1) antibody atezolizumab was evaluated in patients with previously untreated, locally advanced or metastatic urothelial cancer (mUC) who were ineligible for cisplatin-based chemotherapy. The original trial design for this study was focused on patients with disease progression during or following platinum-based chemotherapy and an exploratory cohort of first-line cisplatin ineligible patients; however, given the potential for benefit in the first-line setting, the exploratory cohort was expanded to approximately 100 patients, utilizing similar statistical assumptions. Objective responses by independent assessment according to Response Evaluation Criteria In Solid Tumors version 1.1 were durable, with 70% of patients continuing to respond after a median follow-up duration of almost 1·5 years. Overall survival also appeared to surpass historical rates, although differences in patient populations between studies, among other factors, complicate comparison. Atezolizumab also generally appeared to be safe and well tolerated in a patient population heavily dominated by renal insufficiency. Exploratory analyses to improve our understanding of the immune biology of atezolizumab efficacy identified correlates of response and survival including The Cancer Genome Atlas (TCGA) subtype and mutation load, which warrant further study as potential biomarkers for this agent in mUC.
Implications of all the available evidence
Cisplatin-based chemotherapy is the preferred first-line therapy for mUC and the only treatment shown to improve survival in patients with previously untreated disease. However, only a minority of patients with mUC receive first-line treatment with cisplatin-based chemotherapy. The population of patients who are ineligible for cisplatin has been underrepresented in recent clinical studies and as a result, these patients have poor outcomes. Atezolizumab shows potential as a first-line treatment option for these patients. Furthermore, biomarker data validates reports of this agent in the platinum-treated setting that linked intrinsic TCGA subtypes and mutation load with immunotherapy response.
Acknowledgments
The study was sponsored by F. Hoffmann-La Roche Ltd./Genentech, Inc., a member of the Roche Group. The authors acknowledge Fatema Legrand, Xiaodong Shen, Cathleen Ahearn, and Daniel Chen of Genentech, Inc., for their contributions to the study. Medical writing assistance for this report was provided by Ashley J Pratt PhD (Health Interactions, San Francisco, CA) and funded by F. Hoffmann-La Roche Ltd.
Declaration of Interests
AVB, MDG, JER, DPP, JB, YL, MSvdH, SL, EEKIII, ZB, RB, PH, SM, ACT, OOA, GDF, and DJB received personal fees from Roche/Genentech outside the submitted work. MDG reports personal fees from Merck, Astellas, BioMotiv, and Novartis, as well as grants from Novartis, BMS, and Celgene. JER received non-financial support and other support from Roche/Genentech during the conduct of the study, as well as personal fees from Agensys, Eli Lilly, Merck, Bayer, AstraZeneca, Sanofi, and Oncogenex outside the submitted work. TP received other support from Roche/Genentech, AstraZeneca, BMS, and Merck during the conduct of the study. DPP received grants from Roche/Genentech during the conduct of the study, as well as grants and personal fees from Merck, Astra Zeneca, Novartis, Pfizer, and Agenysis outside the submitted work. YL received grants and personal fees from Sanofi, personal fees from Astellas, Janssen, iPSEN, and BMS outside the submitted work. AN received personal fees from Roche/Genentech, and grants and personal fees from Merck Sharp & Dohme during the conduct of the study. JH-C received other support from Roche/Genentech, outside the submitted work. JLP-G and EYY received grants from Roche/Genentech during the conduct of the study. NAD, MR, and AD declare no competing interests. MSvdH received other support from Roche/Genentech as well as personal fees and grants from Astellas and personal fees from Astra Zeneca outside the submitted work. RD received personal fees from Roche/Genentech and Merck outside the submitted work. SS and SSS received personal fees from Roche/Genentech outside the submitted work. RWJ received personal fees from BMS, Merck, Nektar, Eisai, Novartis, and Cerulean outside the submitted work. UNV received other support from Roche/Genentech during the conduct of the study, as well as honoraria and Consulting fees from Roche/Genentech. DIQ received personal fees from BMS, Merck, AstraZeneca, Novartis, Roche/Genentech, Pfizer, and EMD Serono outside the submitted work. ID received personal fees from Janssen, Roche/Genentech, Amgen, Novartis, and Resrefabre, as well as other support from Astellas outside the submitted work. DRS received support for advisory board and speaker participation from Roche/Genentech. BJE received grants and personal fees from Roche/Genentech outside the submitted work. PDG received other support from Roche/Genentech during the conduct of the study; in addition, PDG received personal fees from Genentech, Dendreon, Bayer, and Myriad Genetics, as well as other support from Bayer, Merck, Mirati, and Oncogenex and grants from Genmab outside the submitted work. EYY received grants and personal fees from Eli Lilly, Roche/Genentech, Merck, Agensys, Janssen, Dendreon, Medivation, Astellas, Sanofi, and Bayer, as well as personal fees from Tolmar, Seattle Genetics, Tokai, Ferring, and AstraZeneca, outside the submitted work.
Footnotes
Contributors
ACT, OOA and GDF contributed to conception and design of the study. AVB, MDG, JER, TP, DPP, JB, YL, AN, JH-C, JLP-G, NAD, MSvdH, RD, SS, MMR, RWJ, AD, UNV, SSS, DIQ, ID, DRS, BJE, PDG, EYY, RB, SM and DFB contributed to data collection. EEK, RB, PSH and SM contributed to analysis of data related to exploratory gene expression and mutation load biomarkers. OOA was the medical monitor for the study and responsible for the database lock, data analysis, and interpretation. AVB, ACT, OOA, GDF, and DFB revised the manuscript critically for intellectual content and oversaw author review of the report. AVB, SL, RB, SB, and ACT contributed to the design of the figures. All authors were involved in data interpretation, drafting, review, and approval of the report, as well as the decision to submit for publication.
References
- 1.National cancer institute surveillance, epidemiology, and end results program. SEER cancer statistics factsheets: Bladder cancer. [accessed August 12, 2016]; http://seer.cancer.gov/statfacts/html/urinb.html.
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase no. 11. [accessed September 13, 2016]; http://globocan.iarc.fr/Pages/summary_table_site_sel.aspx.
- 3.Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol. 1992;10:1066–73. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 4.Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer "unfit" for cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–8. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- 5.Bellmunt J, Orsola A, Leow JJ, et al. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii40–8. doi: 10.1093/annonc/mdu223. [DOI] [PubMed] [Google Scholar]
- 6.National comprehensive cancer network. clinical practice guidelines in oncology: Bladder cancer. [accessed August 24, 2016];version 2.2016. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- 7.De Santis M, Wiechno PJ, Bellmunt J, et al. Vinflunine-gemcitabine versus vinflunine-carboplatin as first-line chemotherapy in cisplatin-unfit patients with advanced urothelial carcinoma: Results of an international randomized phase II trial (JASINT1) Ann Oncol. 2016;27:449–54. doi: 10.1093/annonc/mdv609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Necchi A, Pond GR, Raggi D, et al. Efficacy and safety of gemcitabine plus either taxane or carboplatin in the first-line setting of metastatic urothelial carcinoma: A systematic review and meta-analysis. Clin Genitourin Cancer. 2016 doi: 10.1016/j.clgc.2016.05.003. published online May 27. [DOI] [PubMed] [Google Scholar]
- 9.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–9. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galsky MD, Pal SK, Lin SW, et al. 2624 the effectiveness of chemotherapy in “real world” patients with metastatic bladder cancer. Eur J Cancer. 2015;51(suppl 3):S520–521. [Google Scholar]
- 11.Sonpavde G, Galsky MD, Latini D, Chen GJ. Cisplatin-ineligible and chemotherapy-ineligible patients should be the focus of new drug development in patients with advanced bladder cancer. Clin Genitourin Cancer. 2014;12:71–3. doi: 10.1016/j.clgc.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 14.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 15.McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: Long-term safety, clinical activity, and immune correlates from a phase ia study. J Clin Oncol. 2016;34:833–42. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galsky MD, Hahn NM, Rosenberg J, et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011;12:211–4. doi: 10.1016/S1470-2045(10)70275-8. [DOI] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–81. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 20.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 21.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC intergroup study 30987. J Clin Oncol. 2012;30:1107–13. doi: 10.1200/JCO.2011.38.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111:3110–5. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–65. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–11. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson R, Haddad A, Sagalowsky A, Margulis V. Upper tract urothelial carcinoma: Special considerations. Clin Adv Hematol Oncol. 2016;14:101–9. [PubMed] [Google Scholar]
- 29.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor JAI, Kuchel GA. Bladder cancer in the elderly: Clinical outcomes, basic mechanisms, and future research direction. Nat Clin Pract Urol. 2009;6:135–44. doi: 10.1038/ncpuro1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consort Diagram
Supplementary Appendix