Improved Cognition While Cycling in Parkinson’s Disease Patients and Healthy Adults (original) (raw)
. Author manuscript; available in PMC: 2018 Apr 1.
Abstract
Persons with Parkinson’s disease (PD) are typically more susceptible than healthy adults to impaired performance when two tasks (dual task interference) are performed simultaneously. This limitation has by many experts been attributed to limitations in cognitive resources. Nearly all studies of dual task performance in PD employ walking or balance-based motor tasks, which are commonly impaired in PD. These tasks can be performed using a combination of one or two executive function tasks. The current study examined whether persons with PD would demonstrate greater dual task effects on cognition compared to healthy older adults (HOAs) during a concurrent cycling task. Participants with and without PD completed a battery of 12 cognitive tasks assessing visual and verbal processing in the following cognitive domains: speed of processing, controlled processing, working memory and executive function. Persons with PD exhibited impairments compared to healthy participants in select tasks (i.e., 0-Back, 2-Back and operation span). Further, both groups unexpectedly exhibited dual task facilitation of response times in visual tasks across cognitive domains, and improved verbal recall during an executive function task. Only one measure, 2-back, showed a speed-accuracy trade-off in the dual task. These results demonstrate that, when paired with a motor task in which they are not impaired, people with PD exhibit similar dual task effects on cognitive tasks as HOAs, even when these dual task effects are facilitative. More generally, these findings demonstrate that pairing cognitive tasks with cycling may actually improve cognitive performance which may have therapeutic relevance to cognitive decline associated with aging and PD pathology.
Keywords: Parkinson’s disease, cognition, dual task, visual processing, cognitive-motor interference
1. Introduction
There is an extensive literature examining the effects of performing simultaneous cognitive and motor tasks in both healthy and pathological aging. This research consistently documents decrements in performance across one or both tasks under dual task conditions compared to baseline, single task conditions (see reviews in McDowd & Shaw, 2000; Verhaeghen, Sliwinski & Cerella, 2003). Furthermore, relative to young adults, HOAs experience increased interference between cognitive and motor tasks, as evidenced by decreased accuracy and/or increased response times in cognitive tasks (e.g., Li, Lindenberger, Freund & Baltes, 2001), and these effects are exacerbated in adults with cognitive impairment (Holtzer, Burright, & Donovick, 2004, Al-Yahya et al., 2011). Performance interference on one task by another simultaneously-performed task is broadly termed the dual task effect (DTE) or cognitive-motor interference in the specific case of concurrently performed cognitive and motor tasks (Woollacott & Shumway-Cook, 2002; Yogev-Seligmann, Hausdorff, Giladi, 2008; Al-Yahya et al., 2011). The most commonly used motor tasks in this literature are walking and postural control. The dominant hypothesis to account for dual task effects specifies a capacity sharing model in which cognitive and motor tasks contend for limited attentional resources; thus, task performance in one or both tasks suffers when the combined task demands surpass the amount of available resources (Pashler, 1994; Yogev-Seligmann et al., 2008).
Persons with Parkinson’s disease (PD) are known to experience greater dual tasks effects than their healthy peers (e.g., Benecke, Rothwell, Dick, Day & Marsden, 1986; Ho, Iansek, & Bradshaw, 2002). The disease leads to early degenerative changes that occur not only in subcortical structures, such as the substantia nigra, but also in limbic structures that serve as channels for bidirectional information exchange between posterior temporal and parietal association cortices and prefrontal cortex (Alexander, DeLong & Strick, 1986; Braak & Braak, 2000; Braak, Ghebrremedhin, Rub, Bratzke, & del Tredici, 2004). Changes to these pathways may be responsible for early cognitive decline in PD (Cropley, Fujita, Innis, & Nathan, 2006; Zgaljardic et al., 2006). In addition, subsequent degeneration resulting from the spread of Lewy body inclusions into prefrontal and posterior association areas (Braak & Braak, 2000; Braak et al., 2004) further impairs cortical function. As a result, cognitive impairment develops in 25 to 80 percent of persons with PD over the course of the disease (e.g., Cummings et al., 1988; Aarsland, Andersen, Larsen, & Lolk, 2003) with deficits reported across a wide variety of cognitive domains, including speed of processing, working memory, and executive functioning. Moreover, deficits may be more severe within the visual domain (Cooper et al., 2009; Uc et al., 2005).
The greater susceptibility of persons with PD to dual task interference effects has been demonstrated within the context of both cognitive-motor and motor-motor dual task paradigms (e.g., Benecke et al., 1986; Ho, Iansek, & Bradshaw, 2002). For instance, cognitive-motor dual task studies report exaggerated differences in motor performance (i.e., walking) between HOAs and those with PD when cognitive distracter tasks are cognitively-demanding (Yogev et al., 2005). Similar, findings have been reported for concurrent cognitive tasks (Kemps, Szmalec, Vandierendonck, & Crevits, 2005). Considering the extent to which they have been documented within the literature, the exaggerated interference effects in dual task in persons with PD have been recognized as a defining feature of the disease (O’Shea, Morris, & Iansek, 2002). With a few exceptions, studies have primarily focused on changes in motor performance in gait and postural stability tasks, when combined with a limited range of cognitive tasks, chosen largely to emphasize executive functions (Woollacott & Shumway-Cook, 2002; Kelly, Eusterbrock, & Shumway-Cook, 2012). Since control of gait and postural stability are both impaired in PD, we reasoned that the endemic dual task effects during these motor tasks may result primarily from the increased attention required for these tasks by people with PD. Cycling is largely preserved in persons with PD (Snijders & Bloem, 2010; Snijders, van Kesteren, & Bloem, 2011); thus, we postulated that dual task impairments in cognition might be minimized during cycling in people with PD compared to healthy adults. Further, very few studies have examined the effects of dual task cycling on simultaneous cognitive tasks in any population, a gap this study begins to address.
Cycling, in general, impacts performance differently than other motor tasks, like walking or postural stability, as evidenced by research within the acute exercise literature which reports improved performance in some cognitive abilities during a cycling motor task. Concurrent cycling has led to improvements in information processing, working memory and executive functions (e.g., Tomporowski, 2003; Audiffren, Tomporowski, & Zagrodnik, 2008; Audiffren, Tomporowski, & Zagrodnik, 2009; Lambourne & Tomporowski, 2010; Lucas et al., 2012). Acute exercise methodology requires participants to exercise, usually cycling, walking or jogging, at a steady, moderate to high intensity as measured by heart rate, and to perform a secondary cognitive task either during the exercise or immediately afterward. Outcome measures focus on differences in cognitive performance between single and dual task conditions. In a meta-analysis of acute exercise studies, Lambourne and Tomporowski (2010) note that the choice of motor task influences performance on secondary cognitive tasks. In particular, enhancements in cognitive performance are consistently noted during and after a cycling task, while performance impairments are noted during treadmill walking with only slight improvements following the walking task. Differences in task demands may contribute to the differential effects of various motor tasks on secondary task completion. For instance, it has been suggested that cycling consumes a lower amount of attentional (e.g., Lambourne & Tomorowski, 2010) and postural control resources (Yogev-Seligmann et al., 2013) than other motor tasks. The facilitative effects of cycling on cognition in healthy adults have been attributed to increased exercise-induced arousal affecting functioning of prefrontal brain regions (e.g., Audiffren et al., 2008). Thus, while some motor tasks may impair secondary cognitive task performance, others, such as cycling, may facilitate performance.
We have recently reported unexpected dual task benefits on cycling during a range of cognitive tasks in both HOAs and people with PD (Altmann et al., 2015). In that study, participants performed a large battery of cognitive tasks while sitting in a quiet room or while pedaling a stationary bicycle. In the dual task, both groups pedaled significantly faster during the six (in the PD group) or seven (in the HOA group) easiest tasks, which tap speed of processing and controlled processing. Notably, cycling speeds during more difficult cognitive tasks were also, for the most part, somewhat faster than in the single task, and there were no significant dual task costs on cycling speed in either group. The cognitive scores reported in that publication include group level differences showing impaired performance of the PD group in both accuracy and response times in a variety of tasks. Only one task showed a significant dual task cost across both groups, accuracy on the 2-back task, and one task showed a significant dual task benefit across groups, response times on the digit symbol substitution task, although there were many marginal effects unreported in that paper. The current study presents data from the same study with a larger group of PD participants, many of whom had been excluded from the previous paper due to missing cycling data.
1.1 Purpose of the Study
The purpose of the current study was to investigate the effects of performing a concurrent cycling task on cognitive performance in persons with PD compared to healthy controls. A second aim was to examine the effects of cycling on cognitive performance across a wider range of cognitive tasks than has been previously reported. As such, we systematically assessed performance across a broad range of cognitive domains including processing speed, controlled processing, working memory and executive functioning. Finally, cognitive tasks from both visual and verbal modalities were assessed to examine possible modality specific differences in dual task effects between groups, since people with PD have been reported to experience particular difficulty with visual tasks (Cooper et al., 2009; Uc et al., 2005). The study purposefully chose to compare cognition in the PD group to a group of HOAs, since it has been reported that people with PD have cognitive impairments relative to age-matched peers (Aarsland, Andersen, Larsen, Lolk, & Kragh-Sorensen, 2003; Dirnberger & Jahanshahi, 2013; Taylor & Saint-Cyr, 1995). We hypothesized that the PD group would have poorer performance than HOAs in most tasks. More specifically, considering previous findings of greater visual-spatial than verbal impairments in PD (Cooper et al., 2009; Uc et al., 2005), we predicted that group differences in performance would be disproportionately found in visual-spatial tasks. Further, based on our previous findings (Altmann et al., 2015), we predicted that any dual task benefits experienced by the HOA group would be reduced in the PD group.
2. Material and Methods
2.1 Participants
Thirty-nine individuals with Parkinson’s disease (PD) and 21 healthy older adults (HOAs) participated in this study. There was a significant age difference between groups such that HOAs (M = 72.86, SD = 8.95) were significantly older than Parkinson’s participants (M = 66.23, SD = 8.54), t(1,58) = 2.82, p = .007. There was, however, no significant difference in years of education between HOAs (M = 18.10, SD = 2.98) and persons with PD (M = 17.41, SD = 4.01), t(1,58) = .685, p =.495. Participant demographics by group are displayed in Table 1. Persons with PD were recruited from the Center for Movement Disorders and Neurorestoration at the University of Florida, in Gainesville, Florida. Healthy older adults were recruited from the Speech, Language, and Hearing Sciences participant pool at the University of Florida, through contact lists provided by the Department of Applied Physiology and Kinesiology, and through word of mouth. All participants signed an Informed Consent approved by the University’s Institutional Review Board and were provided with monetary compensation in exchange for their participation.
Table 1.
Demographics of the two groups
| Measure | PD Mean (SD) | HOA Mean (SD) | p |
|---|---|---|---|
| N | 39 | 21 | |
| Age | 66.23 (8.54) | 72.86 (8.95) | .007 |
| Education (yrs.) | 17.41 (4.01) | 18.10 (2.98) | .495 |
| Dementia Rating Scale-2 (max = 144) | 140.18 (3.53) | 141.63 (1.77) | .098 |
| Schwab & England1 (max = 100) | 86.55 (13.07) | ||
| Maximum | 100 | ||
| Minimum | 60 | ||
| UPDRS2 Total Score | 34.97 (10.91) | ||
| Maximum | 53 | ||
| Minimum | 15 |
Inclusion criteria for Parkinson’s participants included the following: 1) A clinical diagnosis of idiopathic PD using the UK Brain Bank criteria made by a neurologist specializing in Movement Disorders; 2) a modified Hoehn and Yahr scale score between 1 and 3 in the “on” medication state (Hoehn & Yahr, 1967); 3) A stable regimen of antiparkinsonian and psychotropic medications for 30 days prior to participation; and 4) age between 30–80 years. Exclusion criteria included the following: 1) evidence of secondary or atypical parkinsonism as suggested by the presence of any of the following: a) history of stroke(s), b) significant exposure to toxins or neuroleptics, c) history of encephalitis, d) neurological signs of upper motor neuron disease, cerebellar involvement, supranuclear gaze palsy, or significant orthostatic hypotension, e) MRI scan with significant evidence of brain atrophy or other abnormalities (e.g., lacunar infarcts or iron deposits in the putamen); 2) significant cognitive impairment (e.g., Mini-Mental State Exam; MMSE score <25) (Folstein, Folstein, & McHugh, 1975) or major psychiatric disorder (e.g., major depression, generalized anxiety) that would cause difficulty in study participation; 3) Presence of significant motor fluctuations (i.e., unpredictable/painful/severe “on” and “off” fluctuations); 4) history of significant falls (i.e., a score of >1 in the fall item of the UPDRS Part II); 5) taking moderate to high doses of beta-blockers with a resting heart rate below 60/beats per min; or 6) taking cognition altering drugs (i.e., cognition-enhancing or cognition-impairing) (e.g., donepezil, rivastigmine, galantamine and benztropine).
Inclusion criteria for HOAs included the following: 1) neurologically healthy (e.g., MMSE > 24); and 2) between the ages of 30–80 years. Exclusion criteria were as follows: 1) presence of major psychiatric disorder (e.g., major depression, generalized anxiety) that would cause difficulty in study participation; 2) history of significant falls; 3) taking cognition altering drugs (i.e., cognition-enhancing or cognition-impairing medications). All participants had normal or corrected to normal vision.
In addition to those measures utilized for initial screening criteria, persons with PD were administered the revised Unified Parkinson’s Disease Rating Scale (UPDRS; Goetz et al., 2008) as a measure of disease severity and the Schwab and England Activities of Daily Living Scale (Schwab & England, 1969) as a measure of the ability of individuals with PD to cope with everyday tasks. Furthermore, all participants were administered the Dementia Rating Scale-2 (DRS-2; Mattis, Jurica & Leitten, 2001) as an additional measure of cognitive impairment. Scores on these measures by group are also displayed in the demographics summary in Table 1.
2.2 Apparatus
During single task testing, the experiment was conducted using a Dell Latitude E6510 series laptop with a 15.6 inch widescreen display and a 1280 × 800 resolution. Tasks were run using MediaLab version 2006.2.40 (Jarvis, 2006a) software running DirectRT software version 2006.2.0.28 (Jarvis, 2006b). During the dual task, cognitive tasks were projected from the laptop onto a back-lit projection screen located within four meters of the participant who was seated on a stationary cycle.
2.3 Cognitive Tasks
All participants were administered the same twelve cognitive tasks during single and dual task sessions. As cognitive tasks varied in their degree of difficulty, tasks were administered in the same order in both sessions. Simpler tasks were interspersed among difficult tasks in order to avoid possible cognitive fatigue. For a breakdown of dependent variables by cognitive task see Table 2 (See also Table 2, Altmann et al., 2015).
Table 2.
Performance of the two groups in single and dual task conditions.
| Cognitive Domain | Outcome Measure/s | PD | HOA | ||
|---|---|---|---|---|---|
| Single Task (SD) | Dual Task (SD) | Single Task (SD) | Dual Task (SD) | ||
| Processing Speed | |||||
| Speed of Articulation (verbal) | Number of Syllables | 53.92 (9.91) | 54.44 (10.46) | 57.76 (8.22) | 58.69 (7.05) |
| Simple Visual Attention (visual) | Response Times | 465 (169) | 458 (134) | 414 (55) | 423 (63) |
| Digit Symbol Substitution (visual) | Response Times ⋄ | 2800 (734) | 2383 (573) | 2498 (477) | 2371 (439) |
| Controlled Processing | |||||
| Stroop Colors (verbal) | Response Times | 612 (87) | 660 (133) | 612 (95) | 608 (74) |
| Stroop Color-Word (verbal) | Response Times | 961 (234) | 959 (239) | 886 (113) | 857 (95) |
| 0-Back (visual) | Response Times *⋄ | 646 (144) | 596 (92) | 581 (100) | 554 (93) |
| Working Memory | |||||
| Digit Span Forward (verbal) | Number Lists Correct | 8.38 (2.09) | 8.16 (2.47) | 8.84 (2.65) | 9.05 (2.92) |
| Digit Span Backward (verbal) | Number Lists Correct | 6.32 (1.93) | 6.27 (2.33) | 7.05 (2.39) | 6.95 (2.17) |
| 1-Back (visual) | Response Times | 780 (263) | 743 (178) | 746 (160) | 739 (347) |
| 2-Back (visual) | Response Times *⋄ | 1311 (689) | 1046 (386) | 990 (320) | 795 (186) |
| Accuracy (%) ‡ | 83.90 (15.73) | 77.02 (16.82) | 89.14 (13.46) | 81.05 (7.48) | |
| Executive Function | |||||
| Operation Span (verbal) | Response Times | 1550 (564) | 1554 (786) | 1328 (369) | 1232 (374) |
| Letters Recalled (#) *⋄ | 3.36 (.87) | 3.84 (.96) | 4.46 (1.15) | 4.74 (1.12) | |
| Visual Memory Updating (visual) | Response Times⋄ | 2646 (1298) | 2358 (854) | 2547 (1166) | 2006 (768) |
| Accuracy (%) | 68.83 (13.97) | 70.19 (18.15) | 78.29 (13.42) | 75.00 (18.38) |
Cognitive tasks measured the domains of basic processing speed, controlled processing, working memory and executive function (Colcombe & Kramer, 2003). For all tasks response times were measured from the onset of the stimulus, which was centered in the middle of the screen, until a voice response was made, and stimuli remained visible until a response was detected. Tasks had 20 trials unless otherwise specified. In all tasks except the digit span tasks, stimuli were randomly presented. Participants saw different lists of stimuli in the single and dual task conditions.
Three tasks assessed basic processing speed. During a speed of articulation task, participants repeated the syllable “pa” as many times as possible in 10 seconds. The dependent variable for this task was number of times the participant said “pa” within the given time-frame. A simple visual attention task required that participants verbally respond “GO” every time a large blue star appeared on the screen. The star appeared centered on a white background at variable inter-stimulus intervals. Mean response time across trials was the dependent variable. An adapted version of the digit symbol substitution test (Wechsler, 1958) also assessed processing speed. In this visual search/speed of processing task, participants saw an array of nine symbols (i.e., letters of the Korean alphabet) at the top of the screen paired with the digits 1 through 9. Participants said the number from the array which corresponded to the symbol shown (Hansch et al., 1982; Lezak, Howieson, Loring, Hannay, & Fischer, 2004). Arrays varied across trials. The dependent variable of interest was mean response time across trials.
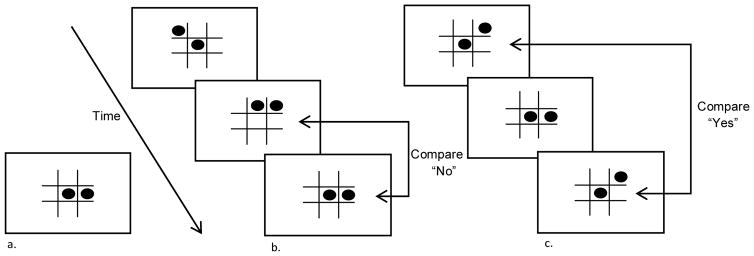
Controlled Processing was assessed using three tasks; Stroop colors, a Stroop color-word task (both verbal tasks) and a visual 0-Back task. During the Stroop colors task (Lezak et al., 2004; Spreen & Strauss, 1998) a set of four bolded Xs appeared on a black screen in a red, blue, or green font. Participants named the color of the font. During the Stroop color-word task (Lezak et al., 2004; Spreen & Strauss, 1998), a series of color words displayed in incongruent color fonts appeared on a black screen (e.g., the word RED presented in green font). Participants named the color font in which the word appeared. The dependent variable of interest for both Stroop tasks was response time. A visual 0-Back task (Braver et al., 1997) provided a measure of controlled processing within the visual domain. In this task, participants viewed a continuous series of tic-tac-toe figures one at a time on the screen. Participants said “yes” if the current figure matched a pre-specified target figure and “no” if it did not match the target stimulus. There were a total of 40 trials of which 25 percent were critical “yes” trials. The dependent variable was mean response time for critical trials. See Figure 1 for an example of stimuli for the 0-, 1- and 2-Back tasks (the latter 2 tasks are described below).
Figure 1.
Stimuli and Task Samples.
Sample stimuli and task examples for 0-Back, 1-Back and 2-Back Tasks. a. 0-back reference stimuli. All other stimuli were compared to this reference. b. 1-Back task example. Participants compared the current stimulus on the screen to the stimulus one screen prior (both comparisons are No Match trials). c. 2-Back task example. Participants compared the current stimulus to the stimulus seen two screens prior.
There were four tasks employed to assess working memory. Digit Span Forward (DSF) and Digit Span Backward (DSB) tasks (Wechsler, 1987) were both completed as measures of verbal memory. During these tasks, participants repeated increasingly long lists of digits in verbatim or reverse order, respectively. Stimuli for these tasks were presented orally, while stimuli for all other tasks were presented on the screen. There were two lists at each of seven levels for a maximum score of 14 in each task, and the task was terminated when a participant erred on both trials at a level. The dependent measure of interest for each task was total number of correct lists recalled. Visual memory was assessed using visual 1-Back and 2-Back tasks (Braver et al., 1997). During the 1-Back, participants saw a continuous series of tic-tac-toe figures one at a time on a screen. Participants said “yes” if the current figure matched the one shown immediately before it, and “no” if it did not match the previous stimulus. There were a total of 40 trials of which 25 percent were critical “yes” trials. The dependent variable of interest was mean response time for correct, critical trials. During the 2-Back task, much like the 1-Back, participants saw a continuous series of figures one at a time on the screen. Participants said “yes” if the current figure matched the figure shown 2 screens prior, and “no” if it did not match the target stimulus. There were a total of 40 trials of which 25 percent were critical “yes” trials. The dependent variables of interest were mean proportion of correct critical trials and mean response time for critical trials.
Executive function was measured via two tasks; a verbal operation span task, and a novel visual memory updating task. In the operation span task (Conway et al., 2005), participants repeated aloud six letters shown on the screen for 6000ms, then verified 0–4 single-step arithmetic solutions as a distracter task (e.g., 3 + 2 = 5 for a “yes” response and 3 + 1 = 2 for a “no” response). Following the distracter arithmetic problems, participants were prompted to recall the letters. There were four trials at each distracter length. The dependent variable was the mean number of letters recalled in sequence. Executive function in the visual domain was assessed via a novel visual memory updating task based on those described in Fougnie and Marois (2006). Participants saw a series of 1–4 tic-tac-toe stimuli presented individually for 500ms each, followed by an array showing the same number of stimuli in a row on the screen. Participants verified (i.e., with “yes” or “no” responses) whether the array matched the stimuli just presented in the same order. There were four trials at each array length for a total of 16 trials; half with “yes” answers. The 1-figure condition was excluded from analysis due to its similarity with the 1-Back task. As this was a novel task and the most appropriate, relevant dependent variable is yet undetermined, the dependent variables were the total proportion of correct responses and response times for correct trials.
2.4 Cycling Task
To obtain single task cycling rates, participants were told to peddle at a comfortable pace for two minutes before beginning the dual task testing. During the dual task session, participants performed the cognitive tasks while simultaneously pedaling on a stationary bicycle located approximately four meters from a projection screen. Participants were instructed to cycle at a comfortable, self-paced cadence during the tasks but not to cycle between tasks, while the experimenter was explaining task instructions or during practice items. After instructing participants to begin pedaling at the start of a given task, no additional instructions were provided regarding cycling. Participants rested between tasks as needed, and heart rates were monitored at all times. The cycling task provided minimal resistance to cycling movement. Cycling rate was captured using a 10-camera motion capture system (Vicon Motion Capture System, Los Angeles, CA).
2.5 General Procedure
In an attempt to control for practice effects associated with task demands, each participant was randomly assigned to complete either the single task session first followed by the dual task or vice versa. Single and dual task sessions were completed within seven days of the other. Single task sessions were conducted in a quiet room with participants seated at a desk in front of a laptop screen. Dual task sessions were completed with participants simultaneously pedaling a stationary cycle while completing the cognitive tasks under the conditions described above. In order to collect voice responses, participants wore a wireless headset microphone during both single and dual task sessions. The microphone recording sensitivity was calibrated for each participant individually prior to commencement of testing. All responses were recorded by the computer for later scoring. Responses were scored for accuracy and/or response times as indicated above by trained research assistants using Audacity 1.2.6 (Mazzoni, 2006).
2.6 Data Analysis
Response times for correct responses only were analyzed. Prior to computation of mean response times, for each task, z-scores were computed for each participant. Individual trial responses that fell 3 standard deviations above or below a participant’s mean for a given task were excluded from further analysis (less than 5 percent of data). Based on group affiliation, z-scores were also calculated for mean participant scores (i.e., response times and/or accuracy) for each task. Participants whose mean scores fell above or below 3.5 SDs of their participant group for a given task were also excluded from further analysis for that specific task (less than 2 participants per task).
2.7 Statistical Analyses
A 2 × 2 repeated measures ANOVA was performed for each cognitive task separately for accuracy and/or response time dependent variables. Session was a within-subject variable (i.e., single, dual) and group a between subject variable (i.e., PD, HOA). Statistical significance was set at a critical α ≤ 0.05, and comparisons with p < .07 are reported as showing a trend toward significance. Explorations of significant interactions were adjusted for multiple comparisons using Bonferroni correction. All statistical analyses were conducted using IBM SPSS Statistics for Windows 21 version 21.0 (Armonk, NY: IBM Corp). Group performance effects by task are displayed in Table 2.
3. Results
3.1 Processing Speed
The analysis of speed of articulation revealed that neither the main effect of dual task nor interaction between dual task and group reached significance. Similarly, the simple visual attention task analyses revealed no significant effects. Analysis of response times for the digit symbol substitution task indicated a main effect of dual task, F(1,58) = 14.45, p = .001, ηp2= .20, such that participants responded faster in the dual task (M = 2371, SD = 527) than during the single task session (M = 2694, SD = 677). The main effect of group and interaction were not significant.
3.2 Controlled Processing
Response times for the Stroop colors task evidenced no significant effects. Similarly, response times for the Stroop color-word test did not reveal any significant results for group, dual task or interaction. Response times for the 0-back indicated a significant main effect of dual task, F(1, 56) = 5.00, p = .03, ηp2= .08, such that participants exhibited faster response times during the dual task (M = 581, SD = 93) than during the single task session (M = 624, SD = 13). Analysis also revealed a significant main effect of group, F(1, 56) = 3.96, p = .05, ηp2= .07. Healthy older adults (M = 564, SD = 88) responded more quickly than PD participants (M = 616, SD = 101). The interaction effect was not significant.
3.3 Working Memory
Analyses of Digit Span Forward and Digit Span Backward tasks revealed no significant effects for either task. Regarding the 1-back, analysis of response times also yielded non-significant effects. Accuracy on the 1-back was at or near ceiling, so no analyses are reported. Analyses of the 2-back accuracy on critical trials revealed a significant effect of dual task, _F(_1, 52) = 6.77, p = .012, ηp2= .12. Participants were more accurate in the 2-back in the single task condition (M = 85.85, SD = 14.95) than they were in the dual task condition (M = 78.78, SD = 14.47). There was no significant effect of group or interaction. Concerning 2-back response times for critical trials, there was a significant main effect of dual task, F(1, 52) = 11.11, p = .002, ηp2= .18, such that participants responded more quickly during the dual task (M = 953, SD = 347) than the single task (M = 1201, SD = 605). Furthermore, there was a significant main effect of group, F(1, 52) = 5.24, p = .026, ηp2= .09, such that HOAs (M = 892, SD = 226) were significantly faster than persons with PD (M = 1145, SD = 460). The interaction effect was not significant.
3.4 Executive Function
Concerning recall rates for the verbal executive function task, operation span, there was a significant main effect of dual task, F(1, 55) = 22.30, p < .001, ηp2= .29. Participants recalled more letters in sequence in the dual task (M = 4.17, SD = 1.10) than in the single task (M = 3.76, SD = 1.11). The main effect of group was also significant, F(1, 55) = 14.55, p < .001, ηp2= .21. The HOA group recalled more letters (M = 4.60, SD = 1.21) than the PD group (M = 3.60, SD = .95). The interaction effect was non-significant. Regarding response times on the operation span task, there were no significant effects. Lastly, regarding the visual memory updating task, analysis of accuracy scores revealed a non-significant main effect of session and interaction, but a strong trend toward a main effect of group, F(1,56) = 3.60, p = .06, ηp2= .06. Overall, HOAs were more accurate (M = 76.64, SD = 13.96) than persons with PD (M = 69.51, SD = 13.65). Regarding response times, results revealed a significant main effect of dual task, F(1,57) = 8.61, p = .005, ηp2= .13, such that across groups, participants responded more quickly during the dual task session (M = 2233, SD = 835) than the single (M = 2611, SD = 1243). The main effect of group and the interaction effect were not significant.
3.5 Cycling Task
Cycling data for a subset of the participants in the current study has been reported in Altmann et al. (2015). Due to task demands (e.g., participants requiring spotting) cycling data was missing for occasional tasks, so there was complete cycling data from only 29 persons with PD. To summarize those findings, Altmann and colleagues reported that HOAs cycled faster than persons with PD, but both groups showed similar patterns in DTEs across tasks. Specifically, cycling speeds during six tasks (speed of articulation, simple visual attention, 0-back, Stroop colors, Stroop color-word, and 1-back) were significantly faster than single task, baseline cycling speeds for both groups. In fact, cycling speeds were fastest during the easiest tasks and declined as the cognitive tasks increased in difficulty, revealing a significant inverse relationship between cognitive task difficulty and motor performance during dual tasks. However, cycling speed never fell significantly below baseline cycling speeds even during the most difficult working memory and executive function tasks.
4. Discussion
The current dual task study examined the effects of concurrent cycling on cognitive performance in persons with PD compared to HOAs across an array of tasks varying in difficulty and domain (i.e., visual vs. verbal). The results demonstrated that participants with PD performed more poorly than healthy adults on some tasks even though those adults were significantly older. These group differences were confined to a few select tasks which tapped both working memory and executive functions. Both groups, however, demonstrated dual task benefits on performance which were almost exclusively limited to timed tasks within the visual domain. Unexpectedly, participants with PD experienced dual task benefits of similar magnitude as HOAs in these same tasks. There were no significant interaction effects between group and session across any of the cognitive tasks. These findings are discussed separately below.
4.1 Group Differences in Cognitive Performance
Considering that the PD group was significantly younger than the HOA participants, it is somewhat surprising that differences between groups were found on any cognitive tasks. Increased age is associated with declines in cognitive performance, with age-related declines consistently documented in various domains such as working memory, cognitive speed and executive functioning (e.g., Verhaeghen & Salthouse, 1997; Van Hooren et al., 2007; Salthouse, 2009). Furthermore, differences in cognitive function have been documented between healthy adult groups in their 60s and 70s (e.g., Van Hooren et al., 2007). Therefore, age-related cognitive decline associated with the older age of our HOA group could have masked even greater declines in the PD group than those reported here. Persons with PD have well documented deficits in speed of processing, working memory and executive functioning (e.g., Cooper et al., 2009; Uc et al., 2005); thus, the current findings emphasize the severity of cognitive impairment in some domains. Consistent with our expectations, the PD group performed more poorly than HOAs in the 0-Back (controlled processing) and 2-Back (working memory) tasks as evidenced by slower response times. Furthermore, persons with PD exhibited worse recall on the operation span (executive function) task than HOAs. Two of these effects can be attributed to task difficulty as the 2-back and operation span tasks were amongst the most difficult tasks in the battery. Moreover, a strong trend toward poorer performance by persons with PD in the accuracy of the visual memory updating task (executive function), which also ranks amongst the most difficult tasks, was noted. These tasks all involve elements of both working memory and executive function. For instance, each new trial of the 2-back requires encoding a new figure into memory, comparison to the stored contents of memory, and then switching to the next item and updating the current items in working memory. The operation span requires maintenance of items in memory while performing a distracting task, and thus, qualifies as a divided attention task. The visual memory updating task requires updating the sequence of figures to be recalled with each additional item, and then comparison to an array of figures that differ only in sequence or in the position of dots on one figure. Updating, comparing, switching, and divided attention tasks all require executive function (Suchy, 2009). Thus, these findings are consistent with previous reports that people with PD tend to be impaired in both working memory and executive function (Dirnberger & Jahanshahi, 2013; Taylor & Saint-Cyr, 1995). However, there were also group differences in the 0-back task, which is a relatively easy task requiring comparison of the current stimulus to a stored representation in memory. Notably, all of these tasks require maintenance of an item or items in memory during distracting conditions, which may be the underlying deficit, especially since in three of these tasks, stimuli to be recalled were purposefully not verbally encodable. In support of this, there was no significant group difference on the 1-back, which did not require retrieving information from memory, only comparison to a representation in the short-term visual memory of the visual sketchpad (Baddeley, 2003).
While we expected group differences to emerge in both verbal and visual tasks, we anticipated disproportionate impairment in visual tasks in persons with PD, as it has been documented that patients present with greater deficits in the visual domain (Cooper et al., 2009; Uc et al., 2005). This hypothesis was somewhat supported, as significant deficits were noted in the visual 0-Back and 2-Back tasks, and there was a trend in the visual memory updating task, but there were deficits in only one verbal task (i.e., operation span). Two possible explanations for the disparity are that: the neural systems underlying visual processing are affected earlier in the time course of the disease than those underlying verbal processing, or impairments affect both systems simultaneously but the verbal system has more interconnectivity due to its central role in organizing, analyzing and planning behavior, and so is more resilient to decline in the face of damage than the visual system. However, it is clear that, even if visual processing deficits are influencing performance on some tasks (e.g., the 0-back), task requirements and difficulty play a critical, over-arching role in determining the performance of the PD group.
The relative lack of differences in performance between PD participants and healthy controls can be explained by a number of group features. First, Dementia Rating Scale (DRS-2; Mattis et al., 2001) scores were well within normal limits for both groups, and the PD patients did not significantly differ from healthy controls on the DRS-2, suggesting little to no cognitive impairment in the PD group. Additionally, education levels of both groups were extremely high, with mean years of education at 17.35 and 18.73 for the Parkinson’s and control groups respectively, indicating that a majority of both groups attended some graduate school. Additionally, as this study was part of a larger exercise intervention, the PD group had to be willing to participate in a 5 month long exercise study, a requirement that may have selected for only the most aware, cognitively intact individuals. Finally, the healthy control group was significantly older than the patient group. Thus, group differences would likely have been more apparent if the control group were more similarly matched in age to the patient group. However, despite these group characteristics and differences, it is notable that there were still some cognitive differences between groups.
4.2 Cycling Effects on Cognitive Performance
The results of the dual task analyses here are more consistent with the acute exercise literature rather than traditional dual task research. Both Parkinson’s participants and healthy controls exhibited dual task facilitation during select cognitive tasks rather than decrements in performance. There was dual task improvement in accuracy rates of only one task, the operation span (executive function), which impacted both groups. Furthermore, both groups exhibited faster response times during dual task cycling compared to the single task in tasks that span the four cognitive domains assessed: digit symbol substitution (processing speed), 0-Back (controlled processing), 2-Back (working memory), and visual memory updating (executive function). Importantly, excluding the 2-back task which declined in accuracy, facilitative effects on response times were not associated with declines in accuracy or in cycling speed relative to single task performance. Indeed, participants maintained or increased their cycling speed during these cognitive tasks compared to single task cycling speed (Altmann et al., 2015). It is notable that the majority of the facilitative effects are in response times rather than accuracy. This is consistent with the findings of McMorris, Sproule, Turner and Hale (2011) who, in a meta-analysis of the effects of acute exercise on cognition, reported that speed rather than accuracy accounted for most of the effect on cognition. Facilitation of response times may have relevant clinical implications as patients with PD often exhibit cognitive slowing (i.e., bradyphrenia) (Park & Stacy, 2009). While bradyphrenia may partially respond to dopaminergic therapies, these therapies may also have detrimental effects on cognition (e.g., Park & Stacy, 2009). Thus, while it is unknown if the facilitative effects on response time noted here may translate into long-term benefits, cycling could be investigated as an intervention for improved information processing in this patient population.
Interestingly, although the results better reflect acute exercise findings of improvement than dual task interference, we did not find improvements in tasks in which prior literature has documented cycling facilitation, such as the Stroop (e.g., Hogervorst, Riedel, Jeukendrup, Jolles, 1996; Lucas et al., 2012) and simple reaction time (e.g., Hogervorst et al., 1996; Davranche, Burle, Audiffren, Hasbroucq, 2006) tasks, suggesting that effects across these tasks may be dependent on task administration. Furthermore, facilitative cycling effects noted here were in tasks (i.e., 0-back, 2-Back, and visual-working memory) that, to our knowledge, have not been implemented in previous acute exercise cycling studies. Given these findings, it is important to note differences between our cycling task and those typical of the acute exercise literature. Our participants were instructed to cycle at a self-selected and comfortable pace, so rate was controlled by the participant, while acute exercise studies have implemented either a steady-state (constant) or increasing intensity methodology in which motor performance was carefully regulated to maintain a target measure of physiological arousal (e.g., percent of maximum volume of oxygen uptake, for reviews See Tomporowski, 2003 and Lambourne & Tomorowski, 2010). Thus, while performance in both cognitive and motor domains were allowed to vary in the current study, as with traditional dual task research, only cognitive performance is typically allowed to vary in traditional acute exercise studies. Further, our cycling paradigm assessed cognitive performance during short bouts of cycling (no task lasting longer than approximately 5 minutes) while acute exercise studies often assess performance during and following much longer bouts of exercise. Additionally, Lambourne and Tomporoswki (2010) note in their meta-analytic review that cognitive test performance generally declines during the initial 10 minutes of exercise and subsequent 10 minute interval. In contrast, we noted a trend toward dual task facilitation in the visual working memory task, which was the first task to be administered to participants, as well as in subsequent tasks spaced throughout the battery. Taken together, differences between the acute exercise and our methodology suggest that a different facilitative mechanism may underlie our cycling dual task effects than those noted in previous literature.
Other than the improvement in accuracy in the operation span task, facilitation effects were confined to visual tasks. Any kind of dual task improvement in cognition is particularly puzzling, especially considering that there were no dual task declines in cycling performance relative to baseline. Indeed, as previously reported in Altmann et al. (2015), cycling speed improved the most in the easiest tasks and percent improvement in cycling speed declined as cognitive task difficulty increased, that is, the amount of facilitation of cycling speed was directly related to the difficulty of the cognitive task. Consequently, during the cognitive tasks that improved, participants cycled equivalently or faster than baseline, but never showed a significant dual task cost on cycling. To explain dual task benefits on cycling, Altmann and colleagues (2015) suggested the Arousal and Attentional Demands (AAD) model. This model specifies that in cognitive-motor dual tasks there is both an increase in physiological state arousal associated with the motor task (Audiffren, 2009; Lambourne & Tomporowski, 2010), and cognitive arousal associated with the perceived difficulty of the cognitive tasks (Horvitz, 2000; Puglisi-Allegra & Ventura, 2012). This arousal increases attentional resources that can be applied to either or both of the concurrent tasks. When the amount of increased attentional resources matches the demands of the concurrent tasks, baseline single task performance is maintained in both tasks. When participants overestimate the difficulty of the task, more attentional resources are allocated and performance improves in one or both tasks, leading to dual task benefits. Only when the amount of attentional resources is less than required to perform the two tasks, due to limitations in resources or underestimation of the difficulty of the two tasks, does performance suffer. Based on this reasoning, one possible explanation for the improved response times in visual tasks is that participants over-estimated the difficulty of these tasks, leading to greater cognitive arousal (Horvitz, 2000; Puglisi-Allegra & Ventura, 2012), so that there were enough attentional resources to facilitate both response times on cognitive tasks and increase cycling speed. A similar argument can be made for the more challenging verbal operation span task, during which facilitation was limited to task accuracy, while response times and cycling speed did not differ from single task levels. The over-estimation of difficulty in the visual tasks likely arose because these were novel tasks with novel stimuli (Korean letters and the tic-tac-toe figures), which are more likely to induce cognitive arousal (Horvitz, 2000). In contrast, it may have been easier to estimate the difficulty of the verbal tasks, as the Stroop and Digit Spans are very common neuropsychological tests which the PD group would have experienced as part of their clinical assessments, and the HOA group, most of whom had participated in at least one study previous to this, would have encountered in a research setting.
An alternative to this explanation is that the visual tasks were easier overall than the verbal tasks. If it were the case that increased arousal levels in the dual task increased processing resources, the verbal tasks may have been difficult enough to nullify the facilitative effects, while performance on the easier visual tasks benefited from the arousal effects. This account, however, would not explain the improvement in operation span accuracy, or the lack of findings in the easiest of visual tasks (e.g., simple visual attention).
4.3 Limitations and Future Directions
A potential limitation of this study is that PD participants were only tested in the “on medication” state. Thus, future studies including a measure of performance in the “off-medication” state would provide greater insight into the effects of cycling on cognition in PD. Furthermore, this study included PD participants that were mildly to moderately impaired by the disease pathology with little to no evidence of cognitive impairment. Consequently, it remains unknown how a cycling task may affect cognitive performance in persons with greater levels of motor and cognitive impairment. Given that both patient and control groups displayed facilitative effects largely limited to visual tasks, future research may further examine the possible differential effects of cycling on visual and verbal tasks. Finally, our participants cycled at a self-selected pace while a fixed cycling speed is implemented in the majority of the acute exercise literature. Thus, future studies could compare the effects of fixed and self-paced cycling speeds on cognitive performance in both healthy and patient populations.
5. Conclusion
In summary, we documented differences in performance between PD and control groups in select tasks assessing controlled processing, working memory and executive functioning in the absence of cognitive impairment in our persons with PD. We conclude that non-demented participants with PD display similar facilitative effects of a cycling dual task on cognition as healthy controls. However, although they performed more poorly on some tasks, the participants with PD did not show greater dual task effects than healthy adults. Rather, both groups improved their performance, particularly their response times, on an array of cognitive tasks tapping visual processing, and also improved recall in a complex executive function task requiring working memory while cycling. These findings are not consistent with most dual task studies; however, they are somewhat consistent with the acute exercise literature which has documented facilitative effects of cycling on cognition, although important differences in methodology and findings suggest that a different or additional mechanism may underlie these results.
Highlights.
- Dual task effects on cognition may be facilitative rather than detrimental during concurrent cycling.
- Persons with Parkinson’s disease exhibit similar dual-task benefits as healthy adults.
- Facilitation arises primarily in response times in visual tasks across cognitive domains.
- Differences in methodology and findings suggest a different or additional mechanism than described in the acute exercise literature.
Acknowledgments
This research was supported by grant R21AG0033284-1A2 from the National Institute on Aging at the National Institutes of Health to Drs. Altmann and Hass. We would like thank the 2010-2014 members of the Language over the Lifespan lab, the Neuromechanics lab and Center for Neurorestoration and Movement Disorders at the University of Florida for their help and support with this project. This project was also supported by the National Parkinson Foundation Center of Excellence located at the University of Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, Andersen K, Larsen JP, Lolk A. Prevalence and Characteristics of Dementia in Parkinson Disease: An 8-Year Prospective Study. Archives of Neurology. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9(1):357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Altmann LJP, Stegemöller E, Hazamy AA, Wilson JP, Okun MS, McFarland NR, … Hass CJ. Unexpected Dual Task Benefits on Cycling in Parkinson Disease and Healthy Adults: A Neuro-Behavioral Model. PLoS One. 2015:1–13. doi: 10.1371/journal.pone.0125470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(3):715–28. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Audiffren M. Acute exercise and psychological functions: A cognitive-energetic approach. In: McMorris T, Tomporowski P, Audiffren M, editors. Exercise and Cognitive Functions. Oxford, UK: Wiley-Blackwell; 2009. pp. 3–40. [Google Scholar]
- Audiffren M, Tomporowski PD, Zagrodnik J. Acute aerobic exercise and information processing: Energizing motor processes during a choice reaction time task. Acta Psychologica. 2008;129(3):410–419. doi: 10.1016/j.actpsy.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Audiffren M, Tomporowski PD, Zagrodnik J. Acute aerobic exercise and information processing: modulation of executive control in a random number generation task. Acta Psychologica. 2009;132(1):85–95. doi: 10.1016/j.actpsy.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature Reviews Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JPR, Day BL, Marsden CD. Performance of simultaneous movements in patients with Parkinson’s disease. Brain. 1986;109(4):739–757. doi: 10.1093/brain/109.4.739. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Pathoanatomy of Parkinson’s Disease. Journal of Neurology. 2000;247(Supplement 2):11/13–11/10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebrremedhin E, Rub U, Bratzke H, del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell and Tissue Research. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12(5):769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Cooper CA, Mikos AE, Wood MF, Kirsch-Darrow L, Jacobson CE, Okun MS, … Fernandez HH. Does laterality of motor impairment tell us something about cognition in Parkinson disease? Parkinsonism & Related Disorders. 2009;15(4):315–317. doi: 10.1016/j.parkreldis.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Innis RB, Nathan PJ. Molecular Imaging of the Dopaminergic System and its Association with Human Cognitive Function. Biological Psychiatry. 2006;59(10):898–907. doi: 10.1016/j.biopsych.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Darkins A, Mendez M, Hill MA. Alzheimer’s disease and Parkinson’s disease: Comparison of speech and language alterations. Neurology. 1988;38(5):680–684. doi: 10.1212/wnl.38.5.680. [DOI] [PubMed] [Google Scholar]
- Davranche K, Burle B, Audiffren M, Hasbroucq T. Physical exercise facilitates motor processes in simple reaction time performance: an electromyographic analysis. Neuroscience Letters. 2006;396(1):54–56. doi: 10.1016/j.neulet.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. Journal of Neuropsychology. 2013;7(2):193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fougnie D, Marois R. Distinct Capacity Limits for Attention and Working Memory: Evidence From Attentive Tracking and Visual Working Memory Paradigms. Psychological Science. 2006;17(6):526–534. doi: 10.1111/j.1467-9280.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, … Dubois B. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Hansch EC, Syndulko K, Cohen SN, Goldberg ZI, Potvin AR, Tourtellotte WW. Cognition in Parkinson disease: an event-related potential perspective. Annals of Neurology. 1982;11(6):599–607. doi: 10.1002/ana.410110608. [DOI] [PubMed] [Google Scholar]
- Ho AK, Iansek R, Bradshaw JL. The effect of a concurrent task on Parkinsonian speech. Journal of Clinical and Experimental Neuropsychology. 2002;24(1):36–47. doi: 10.1076/jcen.24.1.36.972. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Riedel W, Jeukendrup A, Jolles J. Cognitive performance after strenuous physical exercise. Perceptual and Motor Skills. 1996;83:479–488. doi: 10.2466/pms.1996.83.2.479. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Burright RG, Donovick PJ. The sensitivity of dual-task performance to cognitive status in aging. Journal of the International Neuropsychological Society. 2004;10(2):230–238. doi: 10.1017/S1355617704102099. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbic and nigrastriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp; 2012. Released. [Google Scholar]
- Jarvis BG. DirectRT (Version 2006.2.028) New York, NY: Empirisoft; 2006a. [Google Scholar]
- Jarvis BG. MediaLab (Version 2006.2.40) New York, NY: Empirisoft Corporation; 2006b. [Google Scholar]
- Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinson’s Disease. 2012;2012 doi: 10.1155/2012/918719. Article ID 918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemps E, Szmalec A, Vandierendonck A, Crevits L. Visuo-spatial processing in Parkinson’s disease: evidence for diminished visuo-spatial sketch pad and central executive resources. Parkinsonism & Related Disorders. 2005;11(3):181–186. doi: 10.1016/j.parkreldis.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Lambourne K, Tomporowski P. The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Research. 2010;1341:12–24. doi: 10.1016/j.brainres.2010.03.091. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Li KZH, Lindenberger U, Freund AM, Baltes PB. Walking While Memorizing: Age Related Differences in Compensatory Behavior. Psychological Science. 2001;12(3):230–237. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- Lucas SJ, Ainslie PN, Murrell CJ, Thomas KN, Franz EA, Cotter JD. Effect of age on exercise-induced alterations in cognitive executive function: Relationship to cerebral perfusion. Experimental Gerontology. 2012;47(8):541–551. doi: 10.1016/j.exger.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Mattis S, Jurica PJ, Leitten CL. Dementia Rating Scale-2: Professional Manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Mazzoni D. Audacity (Version 1.2.6) [Computer Software] 2006 Retrieved January 20, 2011 6) [Computer Software]: Retrieved January 20, 2011, from http://audacity.sourceforge.net/
- McDowd JM, Shaw RJ. Attention and aging: A functional perspective. In: Craik FI, Salthouse TA, editors. The handbook of aging and cognition. Mahwah, NJ: Psychology Press; 2000. pp. 221–292. [Google Scholar]
- McMorris T, Sproule J, Turner A, Hale BJ. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: a meta-analytical comparison of effects. Physiology & Behavior. 2011;102(3):421–428. doi: 10.1016/j.physbeh.2010.12.007. [DOI] [PubMed] [Google Scholar]
- O’Shea S, Morris ME, Iansek R. Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Physical herapy. 2002;82(9):888–897. [PubMed] [Google Scholar]
- Park A, Stacy M. Non-motor symptoms in Parkinson’s disease. Journal of Neurology. 2009;256(3):293–298. doi: 10.1007/s00415-009-5240-1. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychological Bulletin; Psychological Bulletin. 1994;116(2):220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Ventura R. Prefrontal/accumbal catecholamine system processes high motivational salience. Frontiers in Behavioral Neuroscience. 2012;6:31. doi: 10.3389/fnbeh.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30(4):507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab RS, England AC. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham FJ, Donaldson IML, editors. Third Symposium on Surgery in Parkinson’s Disease. Edinburgh: Livingstone; 1969. pp. 152–7. [Google Scholar]
- Snijders AH, Bloem BR. Cycling for freezing of gait. New England Journal of Medicine. 2010;362(13) doi: 10.1056/NEJMicm0810287. [DOI] [PubMed] [Google Scholar]
- Snijders AH, van Kesteren M, Bloem BR. Cycling is less affected than walking in freezers of gait. Journal of Neurology, Neurosurgery & Psychiatry. 2011;83(5):575–576. doi: 10.1136/jnnp-2011-300375. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms and commentary. 2. New York, NY: Oxford University Press; 1998. [Google Scholar]
- Suchy Y. Executive functioning: Overview, assessment and research issues for non-neuropsychologists. Annals of Behavioral Medicine. 2009;37:106–116. doi: 10.1007/s12160-009-9097-4. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA. The neuropsychology of Parkinson’s disease. Brain and Cognition. 1995;28(3):281–296. doi: 10.1006/brcg.1995.1258. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychologica. 2003;112(3):297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005;65(12):1907–1913. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- Van Hooren SAH, Valentijn AM, Bosma H, Ponds RWHM, Van Boxtel MPJ, Jolles J. Cognitive functioning in healthy older adults aged 64–81: a cohort study into the effects of age, sex, and education. Aging, Neuropsychology, and Cognition. 2007;14(1):40–54. doi: 10.1080/138255890969483. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age–cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological bulletin. 1997;122(3):231. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J. Aging and dual-task performance: a meta-analysis. Psychology and Aging. 2003;18(3):443. doi: 10.1037/0882-7974.18.3.443. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The measurement and appraisal of adult intelligence. Baltimore, MD: Williams and Wilkins; 1958. [Google Scholar]
- Wechsler D. Wechsler Memory Scale Revised. New York, NY: Psychological Corporation; 1987. [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait & Posture. 2002;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? European Journal of Neuroscience. 2005;22(5):1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Movement Disorders. 2008;23(3):329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Feigin A, Eidelberg D. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology. 2006;28(7):1127–1144. doi: 10.1080/13803390500246910. [DOI] [PMC free article] [PubMed] [Google Scholar]