Olson's Extinction and the latitudinal biodiversity gradient of tetrapods in the Permian (original) (raw)
Abstract
The terrestrial vertebrate fauna underwent a substantial change in composition between the lower and middle Permian. The lower Permian fauna was characterized by diverse and abundant amphibians and pelycosaurian-grade synapsids. During the middle Permian, a therapsid-dominated fauna, containing a diverse array of parareptiles and a considerably reduced richness of amphibians, replaced this. However, it is debated whether the transition is a genuine event, accompanied by a mass extinction, or whether it is merely an artefact of the shift in sampling from the palaeoequatorial latitudes to the palaeotemperate latitudes. Here we use an up-to-date biostratigraphy and incorporate recent discoveries to thoroughly review the Permian tetrapod fossil record. We suggest that the faunal transition represents a genuine event; the lower Permian temperate faunas are more similar to lower Permian equatorial faunas than middle Permian temperate faunas. The transition was not consistent across latitudes; the turnover occurred more rapidly in Russia, but was delayed in North America. The argument that the mass extinction is an artefact of a latitudinal biodiversity gradient and a shift in sampling localities is rejected: sampling correction demonstrates an inverse latitudinal biodiversity gradient was prevalent during the Permian, with peak diversity in the temperate latitudes.
Keywords: Tetrapoda, Olson's Extinction, Permian, latitudinal biodiversity gradient, sampling bias
1. Introduction
It has long been recognized that the terrestrial tetrapod fauna of the early Permian differs substantially from that of the middle and late Permian. The early Permian fauna is dominated by pelycosaurian-grade synapsids, with the carnivorous Sphenacodontidae and herbivorous Edaphosauridae being the most abundant and diverse large amniotes [1–4]. The eureptile clade Captorhinidae is also prevalent at this time, and there is a diverse array of amphibians [5]. During the middle and late Permian, this fauna is replaced by a therapsid-dominated fauna with therapsid synapsids representing the most common large carnivores (dinocephalians, therocephalians, gorgonopsians) and herbivores (dinocephalians, dicynodonts) [6–9]. Parareptile diversity increases [10] while the amphibian fauna is noticeably reduced [5,11]. These faunal changes were accompanied by a shift towards more complex ecosystems with more trophic levels [2].
A detailed understanding of this transition has been hindered so far by the geographical patchiness of the fossil record. The lower Permian terrestrial record is dominated almost entirely by palaeoequatorial records from North America and Western Europe, whereas the middle and upper Permian record is known primarily from the palaeotemperate localities of Russia and South Africa [12,13]. There is very little overlap between the North American and Russian faunas [14], making it difficult to establish over what time scale the transition took place. It might even be argued that the apparent change in faunas in reality represents the geographical shift in the record; thus, the lower Permian fauna would represent the equatorial fauna while the middle Permian fauna would represent the temperate fauna.
Alternatively, it has also been suggested in the past that the faunal transition was accompanied and possibly driven by a mass extinction event dubbed Olson's Extinction [3,15]. Olson's Extinction has been demonstrated in global diversity estimates using sampling correction [4,5,10,16], but even so there is still disagreement. Benson & Upchurch [11] suggested that the apparent mass extinction could be an artefact of the geographical shift in sampling localities, arguing that the sampling of more species-rich low-latitude localities in the Kungurian, and more species-poor higher-latitude localities in the Roadian, could have produced the apparent drop in diversity across the boundary.
Fortunately, these two conflicting hypotheses make explicit predictions that can be tested against the fossil record. For the (I) artefact hypothesis (due to sampling of different latitudes) one would expect (i) substantially different faunas in different latitudes even in the early Permian, with faunal composition in high latitude localities more similar to middle Permian localities; and (ii) a latitudinal biodiversity gradient with higher species richness in low latitude localities. For the (II) transition and mass extinction hypothesis, one would expect (i) the lower Permian faunal composition of high latitudes to be more similar to contemporary low latitude faunas than to high latitude middle Permian faunas; and (ii) the decrease in species richness across the early–middle Permian boundary should be apparent even in a single latitudinal bin.
Here, using sampling correction techniques [17,18], recent discoveries and up-to-date knowledge of the taxa and biostratigraphy, we can address the following questions. (i) Were there differences between the faunas at different latitudes during the early Permian? (ii) When did the transition between the lower and middle Permian faunas occur, and did it occur simultaneously at different latitudes? (iii) Were there substantial differences in the patterns and changes in species richness at different latitudes?
2. Results and discussion
(a). Analysis of faunal similarity
Fortunately, there is temporal overlap between the palaeoequatorial and palaeotemperate formations across the Kungurian–Roadian boundaries, allowing a comparison of the faunas, at least in Laurasia. The Kungurian-aged Inta Assemblage from the Timano-Pechora region of Russia (palaeotemperate Laurasia) can be compared with the considerably better-sampled palaeoequatorial Laurasian fauna of the USA. The Inta fauna has not been extensively discussed in connection with the faunal turnover, despite being the only example available of a lower Permian terrestrial fauna from Russia, as it is an extremely poorly sampled fauna with only 14 specimens representing eight species, and some of these have been lost [19]. Recent examinations of the stratigraphy have supported a correlation between Inta and the faunas of the Vale and Choza formations of Texas and the Hennessey Formation of Oklahoma [14], representing the Laurasian equatorial faunas.
The Roadian data also come from Russia and the USA. Although there has been substantial debate surrounding the biostratigraphy of the terrestrial faunas across the lower–middle Permian transition [1,12,14,19–23], it is now widely accepted that the San Angelo Formation of Texas, the Chickasha Formation of Oklahoma and the faunas of the Kazanian series from Russia (the Golyusherma Subassemblage) are contemporaneous and of Roadian age (but see discussion in the electronic supplementary material text). The Cala del Vino Formation of Sardinia is also included as an equatorial Laurasian locality (see electronic supplementary material text), despite only having produced a single tetrapod species: the caseid Alierasaurus ronchii [24,25].
These faunas were used in a quantitative comparison between faunas across the lower–middle Permian boundary in both palaeoequatorial and palaeotemperate latitudes, at least in Laurasia. Unfortunately, there is no information from Gondwana during the Roadian. Recent discoveries in the Pedra de Fogo Formation of northeastern Brazil [26] provide data on a lower Permian palaeoequatorial fauna of Gondwana, but there is no post-transition data until the late Permian (see the electronic supplementary material text for a more detailed discussion of the Pedra de Fogo Fauna).
A recent modification of the Forbes similarity index [27], hereafter referred to as Forbes* index, was used to compare the faunas in the four latitudinal bins: palaeotemperate and equatorial in both the Kungurian and Roadian. The Inta fauna (Kungurian palaeotemperate) is found to show greater similarity to the Kungurian equatorial formation than to the Roadian palaeotemperate fauna from Golyurshma (table 1). The difference is not found to be significant, probably due to the small sample size from Inta. Meanwhile Golyusherma group shows greatest similarity with the palaeoequatorial Roadian fauna.
Table 1.
Pairwise Forbes* similarity values and associated _p_-values illustrating the faunal similarity between the latitudinal and time bins in Laurasia. A Forbes* value of 1 indicates an identical set of taxa in the two assemblages or one assemblage being a subset of the other; a value of 0 indicates no taxa shared.
| Kungurian equatorial | Kungurian temperate | Roadian equatorial | Roadian temperate | |
|---|---|---|---|---|
| Kungurian equatorial | n.a. | 0.6536555 (p = 0.285) | 0.8800886 (p = 0.026) | 0.5356452 (p = 0.909) |
| Kungurian temperate | 0.6536555 (p = 0.285) | n.a. | 0.2570298 (p = 0.047) | 0.4798522 (p = 0.201) |
| Roadian equatorial | 0.8800886 (p = 0.026) | 0.2570298 (p = 0.047) | n.a. | 0.6265310 (p = 0.366) |
| Roadian temperate | 0.5356452 (p = 0.909) | 0.4798522 (p = 0.201) | 0.6265310 (p = 0.366) | n.a. |
These results suggest that Inta is best interpreted as a pre-transition fauna rather than there being a distinct temperate fauna prior to the Kungurian–Roadian boundary, a result largely driven by the amphibians present. Intasuchus silvicola and Syndyodosuchus tetricus are both archegosauroid temnospondyls [28], and although this clade contains taxa from the USA, Russia and South America throughout the Permian, Ruta et al.'s [28] supertree found both species most closely related to lower Permian taxa from the USA_._ The other temnospondyl amphibian from Inta, Clamorosaurus nocturnes, is an eryopid, a clade abundant in contemporary formations of the USA (see below) and completely unknown from the middle Permian. Amniotes from Inta are mostly uninformative, the most abundant of which are captorhinids, previously referred to Riabininus uralensis but now recognized as undifferentiated from better-known American forms [29]. Captorhinids are found globally throughout the Permian in both temperate and equatorial regions, so this taxon does not provide a useful point of comparison. Riabininus is a single tooth-rowed captorhinid [29], a group most commonly found in the early Permian of the USA, but not unknown from the upper Permian palaeotemperate latitudes [30]. The only other amniote worth mentioning is an indeterminate form previously assigned to Gnorhimosuchus satpaevi, which has been considered variously a bolosaurid and captorhinid [31,32]. Like captorhinids, bolosaurids are known from both the early and middle Permian of the USA and Russia [33–35], so are also not helpful in this comparison.
The amphibians of Inta indicate a fauna similar to that of the equatorial early Permian (i.e. a pre-transition fauna). Unfortunately, there are no fossils of large amniote taxa known (whether due to poor sampling or taphonomy is unclear), but the ichnotaxa Dromopus and Dimetropus are known from the area [36], with the latter traditionally associated with sphenacodontid synapsids [37–39]. Although recent examination shows Dimetropus to be more diverse than previously thought [40], it is still considered representative of large pelycosaurian-grade synapsids, and both Dimetropus and Dromopus are both highly characteristic of the early Permian.
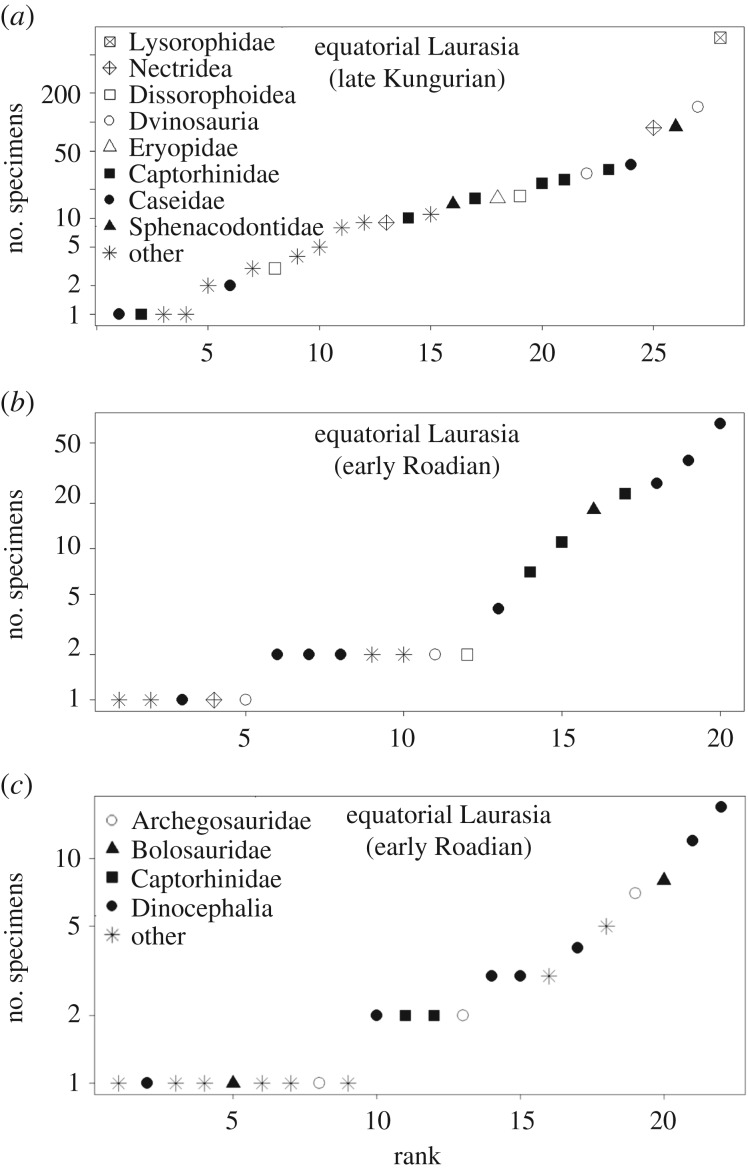
While the Kungurian of Russia appears to have contained a pre-transition fauna, the Kazanian (Roadian) aged palaeotemperate fauna of Russia is decidedly middle Permian in character. The most abundant taxa are dinocephalian therapsids (figure 1c), which continued to dominate the terrestrial realm both in South Africa and Russia until the end of the middle Permian [9]. The most abundant taxon in the Golyusherma subassemblage is the herbivorous dinocephalian Parabradysaurus sileantjevi, but also present is the anteosaur Microsyodon orlovi [41] and the enigmatic Kamagorgon ulanovi (of uncertain affinity but considered a dinocephalian by Ivakhnenko [42]). There is a large amount of Kazanian material assigned either to Phthinosaurus borissiaki or to the clade to which it supposedly belongs, the Phthinosuchia [41,43]. This clade is of untested affinity and monophyly, but the most recent opinion on Phthinosuchia assigns it to Dinocephalia [42]. Also abundant in the Kazanian of Russia are the bolosaurid Belebey and the melosaurine archegosaurids Koinia silantjevi and Melosaurus kamaensis (figure 1c). As mentioned above, bolosaurids are known throughout the early and middle Permian in palaeoequatorial and palaeotemperate regions, but Melosaurinae is known only from the middle Permian of Russia [32].
Figure 1.
Rank-abundance distributions of the tetrapods from (a) late Kungurian equatorial Laurasian bin, (b) early Roadian equatorial Laurasian bin and (c) early Roadian temperate Laurasian bin. Legend in (a) also applies to (b).
Interestingly, the Roadian marks the separation between the equatorial fauna of the USA and the temperate fauna of Russia. The Roadian palaeotemperate bin might exhibit greater similarity with the contemporary equatorial bin than the earlier temperate bin, but the Roadian palaeoequatorial bin shows the greatest similarity to the Kungurian equatorial bin (table 1).
The faunas of the Hennessey, Vale and Choza formations (Oklahoma and Texas) represent the best-sampled assemblages of the late Kungurian. Their fauna is largely similar to that found in the earlier Cisuralian, except for a replacement in the large herbivore guild of Edaphosauridae by another pelycosaur family, Caseidae [1,8,44–46], the most abundant of which is Cotylorhynchus romeri (figure 1a). Edaphosaurus was still present, but is represented only by some neural spine fragments [47] and is clearly not a substantial part of the fauna. As in other lower Permian formations in the USA, the largest terrestrial carnivores are pelycosaurian-grade synapsids from the family Sphenacodontidae (figure 1a), most of which have been assigned to Dimetrodon giganhomogenes. Other abundant, though smaller, terrestrial taxa include the dissorophoid temnospondyl Tersomius and several taxa from the eureptile family Captorhinidae (figure 1a). By far the most common tetrapod taxa in these formations are aquatic amphibians (figure 1a). The most abundant of these are small bodied—the lysorophid Lysorophus tricarinatus, the dvinosaur Trimerorhachis and the nectridean _Diplocaulus magnicornis_—but a large aquatic carnivore also occurs with high frequency: the eryopid temnospondyl Eryops.
The Roadian-aged San Angelo and Chickasha formations represent similar environments to the Kungurian formations from Oklahoma and Texas: fluvial/deltaic [48,49]. Nevertheless, there are differences between the Kungurian and Roadian faunas in the USA. One example is the substantially reduced presence of amphibians (figure 1); a decrease is observed in both abundance and diversity (a fall from 14 species to 5). The few amphibians that are present are from clades characteristic of the early Permian, including a microsaur (Cymatorhiza kittsi), a nectridean (Diplocaulus parvus) and two dissorophoids (Fayalla chicashaensis and Slaugenhopia texensis).
It has previously been suggested that the Roadian ecosystems contained a novel food chain, in which the dominant carnivores were varanopid synapsids while the previous top predators, the sphenacodontids, declined to extinction [4,14]. While it is true that varanopids increase in diversity (species richness and morphological diversity) across the Kungurian–Roadian boundary [4], the data presented here show that sphenacodontids remain the most abundant large carnivores in the Roadian of the USA. There are two varanopid species present in the USA at this time, Varanodon agilis and Watongia meieri, with lengths of 1.5 m or more [48,50], but each is represented only by a single specimen. On the other hand, 18 specimens are assigned to Sphenacodontidae (figure 1b), although only one species has been named (Dimetrodon angelensis). The primary consumers in this ecosystem are caseids and captorhinids (figure 1b), again similar to the lower Permian fauna. The only taxon known from Sardinia is a caseid.
In summary, the faunal transition observed across the Kungurian–Roadian boundary does not appear to be an artefact of the latitudinal shift in sampling. Quantitative and qualitative examination of the tetrapod fauna of Inta indicates it is more similar to the contemporary equatorial fauna than the later fauna of the same latitude. In Russia, a transition to the therapsid fauna already appears to have occurred by the Roadian (see electronic supplementary material for discussion of Mezen). However, aside from the much-reduced diversity of amphibians, the Roadian of the USA contained much the same set of clades as was present in the Kungurian; it is a pre-transition fauna. Hence, it seems that the transition between the faunas was not consistent in the different latitudinal bins. It is unfortunate that there are no middle Permian equatorial formations aged later than the Roadian, preventing further study of the progress of the transition. It is not until the late Permian that equatorial data are again available, by which time the amniotes present were very much characteristic of the post-transition fauna. The equatorial amphibian fauna, however, retained many characteristics of the Cisuralian fauna as late as the Changhsingian (see electronic supplementary material for a more detailed discussion of the late Permian faunas in different latitudinal bins). The similarity of the amphibians of Moradi and Ikakern to the taxa present in the early Permian has been noted previously, as has their unusual morphology [51], both of which suggest the long isolation of these localities leading to a highly autapomorphic set of species. Sidor [51] suggested that the extreme aridity of the equatorial environments prevented the migration of amphibian taxa into or out of Moradi and Ikakern. The amniotes, not limited by a tie to water, were better able to disperse, leading to more homogeneous amniote faunas.
(b). The latitudinal biodiversity gradient
A ubiquitous pattern governing the distribution of modern species is the decreased diversity in higher latitudes relative to lower latitudes, a pattern termed the latitudinal biodiversity gradient (LBG) [52,53]. However, this pattern appears to have only been pervasive since the Neogene, and to have varied greatly in deep time [54–57]. Mannion et al. [58] recently suggested that the modern LBG was only found in periods of glaciation. During hothouse periods when there were no polar icecaps, peak biodiversity was concentrated in temperate latitudes.
The early–middle Permian is an important data point when examining the LBG. During this period, the last period of glaciation prior to the Neogene was ending, and the extensive southern icecap present during the Carboniferous and Cisuralian disappeared [59]. The southern tundra appears to have been present until the middle Permian, at which point it was replaced by a cold temperate climate from 30–60°S [60]. The transition between the lower and middle Permian tetrapod faunas coincided with a trend towards warmer and dryer climate [60], providing a perfect opportunity to examine the impact of changing climate on the LBG.
A modern LBG was posited by Benson & Upchurch [11] as an explanation for the apparent decrease in global tetrapod biodiversity across the Kungurian–Roadian boundary, dubbed Olson's Extinction (artefact hypothesis, above). They suggested that the observed drop in species richness was an artefact of the increased sampling of the temperate latitudes and reduced sampling of equatorial latitudes; if a modern LBG was present in the Permian, it would be a lower-diversity fauna sampled in the Roadian.
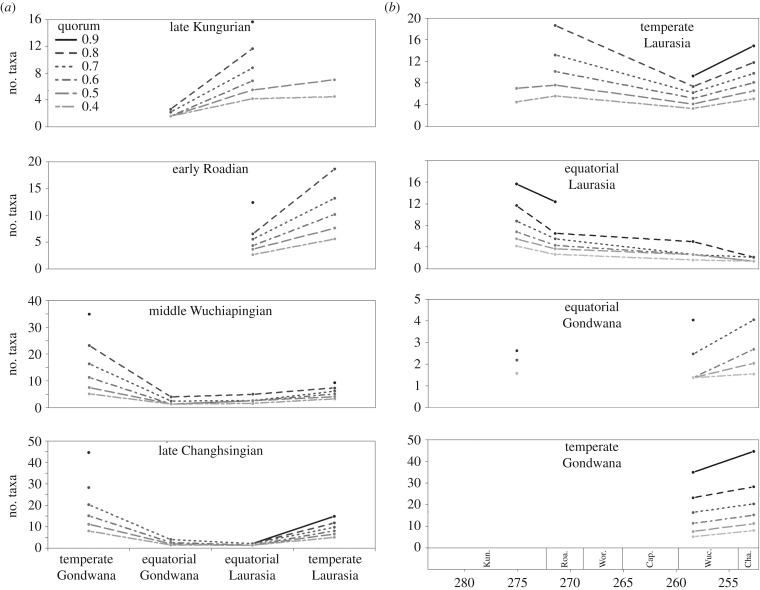
However, in all time bins tested, both subsampled diversity (figure 2a) and the diversity calculated using TRiPS (electronic supplementary material, data S16) show a reduced diversity in equatorial latitudes relative to temperate latitudes. The gradient is weak during the Kungurian but strengthens during the middle and late Permian. In Laurasia during the Roadian and Wuchiapingian, the diversity in the temperate latitudes was more than double that of the equatorial latitudes. During the Changhsingian the gradient seems to strengthen still further. Given the continual trend towards increased temperature throughout the Permian [61], the data presented here support the theory of Mannion et al. [58]: the trend towards a hothouse climate shifts the diversity peak towards temperate latitudes, leading to the establishment of an inverted LBG already in the early Permian. This aspect of the artefact hypothesis proposed by Benson & Upchurch [11]—that Olson's Extinction was an artefact of different sampling of the different latitudes—requires a modern LBG, whereas it is shown here that an inverse gradient was prevalent throughout the Permian. The shift in sampling from palaeoequatorial to palaeotemperate localities was a shift to more species-rich localities, and therefore cannot be used to explain the observed drop in diversity.
Figure 2.
Subsampled diversity curves. (a) Comparing latitudinal bins and (b) comparison through time.
Nevertheless, our detailed examination of individual faunas shows that Olson's Extinction was a more complicated event than has been previously suggested. The strengthening of the LBG across the Kungurian–Roadian boundary appears to have been caused by a sharp drop in species richness in the palaeoequatorial latitudes; that of the temperate faunas shows no decrease (figure 2; electronic supplementary material, data S16). The equatorial and temperate faunas appear to have responded differently to the climatic changes occurring across the Kungurian–Roadian boundary. The low-latitude extinctions may be due to the disappearance of the everwet biome prevalent since the Carboniferous [13]. The formations in the temperate latitude exhibit a complete faunal turnover across this boundary, but the species richness of the pre- and post-transition faunas was largely unchanged. On the other hand, the equatorial fauna remained unchanged in composition until later in the Permian, but the species richness dropped noticeably (figure 2). While the overall decline in global tetrapod biodiversity across the Kungurian–Roadian boundary has been borne out by sampling correction [4,5,10,11,16], in temperate latitudes the turnover appears to have been rapid, and a new equally diverse fauna replaced the old by the early Roadian. In the equatorial latitudes, replacement was slow; during the early Roadian the tetrapod fauna was merely a depauperate version of the Kungurian fauna. Equatorial species richness does not appear to have recovered even by the late Permian.
3. Conclusion
With an up-to-date biostratigraphy and modern quantitative methods, it is possible to empirically test the two hypotheses explaining the apparent faunal shift during the middle Permian: (i) the artefact hypothesis and (ii) the transition and mass extinction hypothesis. As discussed in the introduction, each hypothesis makes two predictions, one regarding the relative similarity of the faunas in different latitudinal bins (tested using the Forbes* similarity metric) and one regarding the relative species richness in the different latitudinal bins (tested using sampling corrected diversity estimates). In the case of the artefact hypothesis, neither prediction is borne out: (i) the lower Permian faunas at different latitudes are not substantially different, and in fact the Kungurian temperate faunas are more similar to contemporary equatorial assemblages than the Roadian temperate faunas; and (ii) the diversity estimates do not show higher species richness at low palaeolatitudes, but instead in the palaeotemperate latitudes.
While it therefore appears that the faunal transition is not an artefact of sampling biases, the event does appear to have been more complex than previously considered. The faunal transition was rapid at higher latitudes, with the middle Permian fauna established by the Roadian, while conversely at lower latitudes the transition appears to have been slower, with equatorial Roadian faunas still containing a similar assemblage of taxa to that present in the Kungurian, albeit less diverse. A post-transition equatorial fauna is not seen until the late Permian (although the lack of equatorial samples from the Wordian and Capitanian should be noted).
Olson's Extinction has been a controversial event since it was first described, but detailed examination of the faunas shows it was an important episode in the early evolution of terrestrial ecosystems. Although hindered by a geographically patchy record, the data are available to reveal the pattern of the mass extinction, including taxonomic selectivity, geographical variations and the establishment of the post-extinction fauna.
4. Material and methods
This study focuses on the four time bins indicated in electronic supplementary material, table S1, selected as the only time periods when terrestrial specimens are known from localities from both temporal and equatorial palaeolatitudes. The localities in each time bin were assigned to one of four latitudinal bins: equatorial Laurasia, equatorial Gondwana, temperate Laurasia and temperate Gondwana. The boundary between equatorial and temperate is set at palaeolatitude 25° north and south. The formations present in each time and latitudinal bin are shown in electronic supplementary material, table S1.
A database of the specimens found in each latitudinal and time bin and their taxonomic assignments was collated from a variety of sources, including the published literature, the Paleobiology Database [62] downloaded via the Fossilworks website (http://fossilworks.org/), museum catalogues and direct observation. The data itself and full details on the data's provenance and treatment are provided in the electronic supplementary material text and data.
These data were used to calculate a diversity (species richness) estimate for each latitudinal and time bin. Due to the high heterogeneity of sampling during the Permian [4,6,7,11,63], sampling correction was implemented using the Shareholder Quorum Subsampling method (SQS) [17] and TRiPS [18]. The former method was implemented in R version 3.1.2 [64] using version 3.3 of the script available on the website of John Alroy (http://bio.mq.edu.au/~jalroy/SQS.html). In each bin, diversity was calculated at six quorum levels, from 0.4 to 0.9 at intervals of 0.1 (note that diversity cannot be calculated at all quorum levels in all samples; the maximum quorum possible is the Good's U-value of the sample). 10 000 subsampling trials were carried out at each quorum level. Following recommendations of Alroy [17], the most abundant species in each sample was not included when calculating the proportion of shares contributing to the quorum, although it was included in diversity counts if drawn. TRiPS is an approach that estimates richness and confidence intervals by modelling sampling within each time interval as a homogeneous Poisson process [18]. This analysis was performed in R using the script provided by Starrfeltt & Liow [18]. The results shown in the paper are using SQS, while those of TRiPS are presented in the electronic supplementary material, data S16.
Pairwise analyses of faunal similarity were carried out on the Laurasian latitudinal bins across the Kungurian–Roadian boundary using the recently published Forbes* similarity measure [27]. This is based on an older metric [65] that ranges from 0 to 1, with 0 meaning no taxa are shared between two assemblages and 1 meaning the assemblages are identical or one is a subset of the other. Alroy [27] modified this metric to make it suitable for palaeontological data, where sampling is incomplete and the total number of taxa present was unknown. Since, with very few exceptions, the species present are endemic to each formation, the similarity of the faunas must be assessed based on sharing taxa with close phylogenetic relationships. Unfortunately, the available distance/similarity metrics do not yet incorporate phylogenetic information, and many of the Russian taxa have yet to be incorporated into a phylogenetic analysis. Until these issues are resolved, we follow the recent practice of Sidor [51] of performing the similarity analysis at the family level (or, where family-level taxonomy is not so well established, e.g. within Dinocephalia, at higher taxonomic levels). The Forbes* metric was calculated using a custom R script (see electronic supplementary material) written from the equations presented by Alroy [27]. To provide a measure of the statistical significance of the similarity/distance between the assemblages, 1000 simulated datasets were constructed, where assemblages with the same sample size as the observed data had their taxa randomly assigned to families. These simulated datasets were compared pairwise with the Forbes* metric and the results were compared with the observed data.
Supplementary Material
Supplementary methods and discussion
Supplementary Material
Supplementary Data
Supplementary Material
Supplementary Figure
Acknowledgements
We would like to thank Roger Benson and Roger Close, as well as the Fröbisch working group, for helpful comments and discussion. Alexander Dunhill and an anonymous reviewer provided many helpful suggestions on how to improve an early draft of the manuscript. This is Paleobiology Database Official Publication number 278. We are grateful to John Alroy and Johannes Müller for uploading the relevant data to the database via the Fossilworks platform.
Data accessibility
Fossil occurrence data are uploaded as online electronic supplementary material.
Authors' contributions
N.B., M.O.D. and B.S.R collected data. N.B. analysed data. N.B., M.O.D., B.S.R. and J.F. wrote the paper.
Competing interests
The authors declare no competing interests.
Funding
This study was funded by a Deutsche Forschungsgemeinschaft grant (number FR 2457/5-1), awarded to J.F., the DST/NRF Centre of Excellence in Palaeosciences, the NRF African Origins Platform, and the Scatterlings of Africa programmes of the Palaeontological Scientific Trust (PAST).
References
- 1.Olson EC. 1962. Late Permian terrestrial vertebrates, USA and USSR. Trans. Am. Philos. Soc. 52, 1–224. ( 10.2307/1005904) [DOI] [Google Scholar]
- 2.Olson EC. 1966. Community evolution and the origin of mammals. Ecology 47, 291–302. ( 10.2307/1933776) [DOI] [Google Scholar]
- 3.Sahney S, Benton MJ. 2008. Recovery from the most profound mass extinction of all time. Proc. R. Soc. B 275, 759–765. ( 10.1098/rspb.2007.1370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brocklehurst N, Kammerer CF, Fröbisch J. 2013. The early evolution of synapsids and the influence of sampling on their fossil record. Paleobiology 39, 470–490. ( 10.1666/12049) [DOI] [Google Scholar]
- 5.Ruta M, Benton MJ. 2008. Calibrated diversity, tree topology and the mother of mass extinctions: the lesson of temnospondyls. Palaeontology 51, 1261–1288. ( 10.1111/j.1475-4983.2008.00808.x) [DOI] [Google Scholar]
- 6.Fröbisch J. 2013. Vertebrate diversity across the end-Permian mass extinction—separating biological and geological signals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 372, 50–61. ( 10.1016/j.palaeo.2012.10.036) [DOI] [Google Scholar]
- 7.Fröbisch J. 2014. Synapsid diversity and the rock record in the Permian–Triassic Beaufort Group (Karoo Supergroup), South Africa. In Early evolutionary history of the Synapsida (eds Kammerer CF, Angielczyk KD, Fröbisch J), pp. 305–320. Dordrecht, Netherlands: Springer. [Google Scholar]
- 8.Pearson MR, Benson RBJ, Upchurch P, Fröbisch J, Kammerer CF. 2013. Reconstructing the diversity of early terrestrial herbivorous tetrapods. Palaeogeogr. Palaeoclimatol. Palaeoecol. 372, 42–49. ( 10.1016/j.palaeo.2012.11.008) [DOI] [Google Scholar]
- 9.Day MO, Ramezani J, Bowring SA, Sadler PM, Erwin DH, Abdala F, Rubidge B. 2015. When and how did the terrestrial mid-Permian mass extinction occur? Evidence from the tetrapod record of the Karoo Basin, South Africa. Proc. R. Soc. B 282, 20150834 ( 10.1098/rspb.2015.0834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruta M, Cisneros JC, Liebrecht T, Tsuji LA, Müller J. 2011. Amniotes through major biological crises: faunal turnover among parareptiles and the end-Permian mass extinction. Palaeontology 54, 1117–1137. ( 10.1111/j.1475-4983.2011.01051.x) [DOI] [Google Scholar]
- 11.Benson RBJ, Upchurch P. 2013. Diversity trends in the establishment of terrestrial vertebrate ecosystems: interactions between spatial and temporal sampling biases. Geology 41, 43–46. ( 10.1130/G33543.1) [DOI] [Google Scholar]
- 12.Lucas SG. 2004. A global hiatus in the Middle Permian tetrapod fossil record. Stratigraphy 1, 47–64. [Google Scholar]
- 13.Kemp TS. 2006. The origin and early radiation of the therapsid mammal-like reptiles: a palaeobiological hypothesis. J. Evol. Biol. 19, 1231–1247. ( 10.1111/j.1420-9101.2005.01076.x) [DOI] [PubMed] [Google Scholar]
- 14.Benton MJ. 2012. No gap in the Middle Permian record of terrestrial vertebrates. Geology 40, 339–342. ( 10.1130/G32669.1) [DOI] [Google Scholar]
- 15.Olson EC. 1982. Extinctions of Permian and Triassic nonmarine vertebrates. GSA Special Papers 190, 501–512. [Google Scholar]
- 16.Brocklehurst N, Ruta M, Müller J, Fröbisch J. 2015. Elevated extinction rates as a trigger for diversification rate shifts: early amniotes as a case study. Sci. Rep. 5, 17104 ( 10.1038/srep17104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alroy J. 2010. Geographical, environmental and intrinsic biotic controls on Phanerozoic marine diversification. Palaeontology 53, 1211–1235. ( 10.1111/j.1475-4983.2010.01011.x) [DOI] [Google Scholar]
- 18.Starrfeltt J, Liow LH. 2016. How many dinosaur species were there? Fossil bias and true richness estimated using a Poisson sampling model. Phil. Trans. R. Soc. B 371, 20150219 ( 10.1098/rstb.2015.0219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozovsky VR. 2005. Olson's gap or Olson's bridge, that is the question. In The nonmarine Permian, New Mexico Museum of Natural History and Science Bulletin no. 30 (eds Lucas SG, Zeigler KE), pp. 179–184. Albuquerque, New Mexico: New Mexico Museum of Natural History. [Google Scholar]
- 20.Lucas SG, Heckert SB. 2001. A global hiatus in the record of Middle Permian tetrapods. J. Vertebr. Paleontol. 21, 75 ( 10.1671/0272-4634%282001%29021%5B0397%3AMLTFTU%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 21.Lozovsky VR. 2003. Correlation of the continental Permian of norther Pangea: a review. B. Soc. Paleontol. Ital. Volume Especiale 2, 239–244. [Google Scholar]
- 22.Reisz RR, Laurin M. 2001. The reptile Macroleter: first vertebrate evidence for correlation of Upper Permian continental strata of North America and Russia. Geol. Soc. Am. Bull. 113, 1229–1233. ( 10.1130/0016-7606%282001%29113%3C1229%3ATRMFVE%3E2.0.CO%3B2) [DOI] [Google Scholar]
- 23.Reisz RR, Laurin M. 2002. The reptile Macroleter: first vertebrate evidence for correlation of Upper Permian continental strata of North America and Russia: discussion and reply. Geol. Soc. Am. Bull. 114, 1174–1175. [Google Scholar]
- 24.Romano M, Nicosia U. 2014. Alierasaurus ronchii, gen. et sp. nov.: a caseid from the Permian of Sardinia, Italy. J. Vertebr. Paleontol. 34, 900–913. ( 10.1080/02724634.2014.837056) [DOI] [Google Scholar]
- 25.Ronchi A, Sacchi E, Romano M, Nicosia U. 2011. A huge caseid pelycosaur from north-western Sardinia and its bearing on European Permian stratigraphy and palaeobiogeography. Acta Palaeontol. Pol. 56, 723–738. ( 10.4202/app.2010.0087) [DOI] [Google Scholar]
- 26.Cisneros JC, et al. 2015. New Permian fauna from tropical Gondwana. Nat. Commun. 6, 8676 ( 10.1038/ncomms9676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alroy J. 2015. A new twist on a very old binary similarity coefficient. Ecology 96, 575–586. ( 10.1890/14-0471.1) [DOI] [PubMed] [Google Scholar]
- 28.Ruta M, Pisani D, Lloyd GT, Benton MJ. 2007. A supertree of Temnospondyli: cladogenetic patterns in the most species-rich group of early tetrapods. Proc. R. Soc. B 274, 3087–3095. ( 10.1098/rspb.2007.1250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modesto S, Rybczynski N. 2000. The amniote faunas of the Russian Permian: implications for Late Permian terrestrial vertebrate biogeography. In The age of the dinosaurs in Russia and Mongolia (eds Benton MJ, Shishkin MA, Unwin DM, Kurochkin EN), pp. 17–34. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 30.Modesto S, Smith RMH. 2001. A new Late Permian captorhinid reptile: a first record from the South African Karoo. J. Vertebr. Paleontol. 21, 405–409. ( 10.1671/0272-4634%282001%29021%5B0405%3AANLPCR%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 31.Efremov IA. 1954. The fauna of terrestrial vertebrates in the Permian copper sandstones of the western Cis-Urals. T. Paleontol. Inst. 56, 146. [Google Scholar]
- 32.Golubev VK. 2005. Permian tetrapod stratigraphy. N. M. Mus. Nat. Hist. Sci. Bull. 30, 95–99. [Google Scholar]
- 33.Reisz RR, Barkas V, Scott D. 2002. A new Early Permian bolosaurid reptile from the Richards Spur Dolese Brothers Quarry, near Fort Sill, Oklahoma. J. Vertebr. Paleontol. 22, 23–28. ( 10.1671/0272-4634%282002%29022%5B0023%3AANEPBR%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 34.Reisz RR, Müller J, Tsuji LA, Scott D. 2007. The cranial osteology of Belebey vegrandis (Parareptilia; Bolosauridae) from the Middle Permian of Russia, and its bearing on reptilian evolution. Zool. J. Linn. Soc. 151, 191–214. ( 10.1111/j.1096-3642.2007.00312.x) [DOI] [Google Scholar]
- 35.Müller J, Li J, Reisz RR. 2008. A new bolosaurid parareptile, Belebey chengi sp. nov, from the Middle Permian of China and its paleogeographic significance. Naturwissenschaften 95, 1169–1174. ( 10.1007/s00114-008-0438-0) [DOI] [PubMed] [Google Scholar]
- 36.Lucas SG, Lozovsky VR, Shishkin MA. 1999. Tetrapod footprints from Early Permian redbeds of the Northern Caucasus, Russia. Ichnos 6, 277–281. ( 10.1080/10420949909386459) [DOI] [Google Scholar]
- 37.Haubold H. 1971. Ichnia amphibiorum et reptiliorum fossilium. Handbuch der Paläoherpetologie. Stuttgart, Germany: Fischer-Verlag. [Google Scholar]
- 38.Haubold H. 1973. Die Tetrapodenfahrten aus dem Perm Europas. Freiberg Forsch H. 285, 5–55. [Google Scholar]
- 39.Haubold H. 2000. Tetrapodenfährten aus de dem Perm—Kenntnisstand und Progress 2000. Hallesches Jahrb Geowiss 22, 1–16. [Google Scholar]
- 40.Sacchi E, Cifelli R, Citton P, Nicosia U, Romano M. 2014. Dimetropus osageorum n. isp. from the Early Permian of Oklahoma (USA): a trace and its trackmaker. Ichnos 21, 175 ( 10.1080/10420940.2014.933070) [DOI] [Google Scholar]
- 41.Ivakhnenko MF. 1995. New primitive therapsids from the Permian of Eastern Europe. Paleontol. Z. 29, 110–119. [Google Scholar]
- 42.Ivakhnenko MF. 2008. Cranial morphology and evolution of Permian Dinomorpha (Eotherapsida) of Eastern Europe. Paleontol. J. 42, 859–995. ( 10.1134/S0031030108090013) [DOI] [Google Scholar]
- 43.Efremov IA. 1940. Preliminary description of new Permian and Triassic tetrapods of the USSR. Trudy Paleontol. Inst. 10, 1–140. [Google Scholar]
- 44.Olson EC. 1958. Fauna of the Vale and Choza: 14—Summary, review and integration of the geology and the faunas. Fieldiana Geol. 10, 397–448. [Google Scholar]
- 45.Olson EC. 1968. The family Caseidae. Fieldiana Geol. 17, 225–349. [Google Scholar]
- 46.Reisz RR. 2005. Oromycter, a new caseid from the Lower Permian of Oklahoma. J. Vertebr. Paleontol. 25, 905–910. ( 10.1671/0272-4634%282005%29025%5B0905%3AOANCFT%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 47.Daly E. 1973. A Lower Permian vertebrate fauna from southern Oklahoma. J. Paleontol. 47, 562–589. [Google Scholar]
- 48.Olson EC. 1965. New Permian vertebrates from the Chickasha Formation in Oklahoma. Okla. Geol. Surv. Circ. 70, 1–70. [Google Scholar]
- 49.Olson EC, Beerbower JR. 1953. The San Angelo formation, Permian of Texas, and its vertebrates. J. Geol. 61, 389–423. ( 10.1086/626109) [DOI] [Google Scholar]
- 50.Reisz RR, Laurin M. 2004. A reevaluation of the enigmatic Permian synapsid Watongia and of its stratigraphic significance. Can. J. Earth Sci. 41, 377–386. ( 10.1139/e04-016) [DOI] [Google Scholar]
- 51.Sidor CA. 2013. The vertebrate fauna of the Upper Permian of Niger—VIII. Nigerpeton ricqlesi (Temnospondyli: Cochleosauridae) and tetrapod biogeographic provinces. C. R. Palevol. 12, 463–472. ( 10.1016/j.crpv.2013.05.005) [DOI] [Google Scholar]
- 52.Hillebrand H. 2004. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211. ( 10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 53.Willig MR, Kaufman DM, Stevens RD. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309. ( 10.1146/annurev.ecolsys.34.012103.144032) [DOI] [Google Scholar]
- 54.Archibal SB, Bossert WH, Greenwood DR, Farrell BD. 2010. Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology 36, 374–398. ( 10.1666/09021.1) [DOI] [Google Scholar]
- 55.Rose PJ, Fox DL, Marcot J, Badgley C. 2011. Flat latitudinal gradient in Paleocene mammal richness suggests decoupling of climate and biodiversity. Geology 39, 163–166. ( 10.1130/G31099.1) [DOI] [Google Scholar]
- 56.Mannion PD, Benson RBJ, Upchurch P, Butler RJ, Carrano WT, Barrett PM. 2012. A temperate palaeodiversity peak in Mesozoic dinosaurs and evidence for Late Cretaceous geographical partitioning. Glob. Ecol. Biogeogr. Lett. 21, 898–908. ( 10.1111/j.1466-8238.2011.00735.x) [DOI] [Google Scholar]
- 57.Yasuhara M, Hunt G, Dowsett HJ, Robinson MM, Stoll DK. 2012. Latitudinal species diversity gradient of marine zooplankton for the last three million years. Ecol. Lett. 15, 1174–1179. ( 10.1111/j.1461-0248.2012.01828.x) [DOI] [PubMed] [Google Scholar]
- 58.Mannion PD, Upchurch P, Benson RBJ, Goswami A. 2014. The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. 29, 42–50. ( 10.1016/j.tree.2013.09.012) [DOI] [PubMed] [Google Scholar]
- 59.Montanez IP, et al. 2007. CO2-forced climate and vegetation instability during Late Paleozoic deglaciation. Science 315, 87–91. ( 10.1126/science.1134207) [DOI] [PubMed] [Google Scholar]
- 60.Rees PM, Ziegler AM, Gibbs MT, Kutzbach JE, Behling PJ, Rowley DB. 2002. Permian phytogeographic patterns and climate data/model comparisons. J. Geol. 110, 1–31. ( 10.1086/324203) [DOI] [Google Scholar]
- 61.Royer DL, Berner RA, Montanez IP, Tarbor N, Beerling DJ. 2004. CO2 as a primary driver of Phanerozoic climate change. GSA Today 14, 4–10. ( 10.1130/1052-5173%282004%29014%3C4%3ACAAPDO%3E2.0.CO%3B2) [DOI] [Google Scholar]
- 62.Müller J, Alroy J. 2015. Taxonomic occurrences of Tetrapoda recorded in the Paleobiology Database. Fossilworks. See http://fossilworks.org.
- 63.Brocklehurst N, Fröbisch J. 2014. Current and historical perspectives on the completeness of the fossil record of pelycosaurian-grade synapsids. Palaeogeogr. Palaeoclimatol. Palaeoecol. 399, 114–126. ( 10.1016/j.palaeo.2014.02.006) [DOI] [Google Scholar]
- 64.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 65.Forbes SA. 1907. On the local distribution of certain Illinois fishes: an essay in statistical ecology. Bull. Illinois State Lab. Nat. Hist. 7, 2722. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods and discussion
Supplementary Data
Supplementary Figure
Data Availability Statement
Fossil occurrence data are uploaded as online electronic supplementary material.