Effects of Structured Versus Unstructured Self-Monitoring of Blood Glucose on Glucose Control in Patients With Non-insulin-treated Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials (original) (raw)
Abstract
Background:
The use of self-monitoring of blood glucose (SMBG) in patients with non-insulin-treated type 2 diabetes is debated. Meta-analyses of randomized clinical trials (RCTs) suggest a small reduction of HbA1c in patients using SMBG, without considering potential confounders, such as SMBG regimen and use of SMBG data to adjust diabetes medications.
Methods:
A meta-analysis was performed including RCTs in patients with non-insulin-treated type 2 diabetes, with an intervention of ≥24 weeks and HbA1c as the primary endpoint, to verify the effect of SMBG (vs no monitoring), structured SMBG (vs unstructured), and of SMBG-driven therapy adjustments.
Results:
In RCTs (n = 8) comparing SMBG with no SMBG (1277 and 1072 patients, respectively), SMBG reduced HbA1c by −0.17% (95% CI −0.25 to −0.09%, P < .003). The reduction in HbA1c was greater in RCTs (n = 3) in which SMBG data were used to adjust diabetes medications (HbA1c decrease: −0.3% [95% CI −0.49 to −0.1%]) than in RCTs (n = 6) where SMBG data were not used for this purpose (HbA1c decrease: −0.1% [95% CI −0.2 to 0.0%]) (P < .005). In the RCTs comparing structured and unstructured SMBG (757 and 750 patients, respectively), in which structured SMBG data were also used to adjust diabetes medications, the HbA1c difference between groups at study end was −0.27% (95% CI −0.49 to −0.04%, P < .018).
Conclusions:
In RCTs performed in non-insulin-treated patients with type 2 diabetes, SMBG is associated with a significant, although small, reduction in HbA1c. HbA1c reduction was greater with structured SMBG and when structured SMBG data were used to adjust diabetes therapy.
Keywords: meta-analysis, non–insulin treated, type 2 diabetes, self-monitoring of blood glucose, treatment algorithms
Self-monitoring of blood glucose (SMBG) is an established component of diabetes management in patients with type 1 diabetes or insulin-treated type 2 diabetes.1,2 By contrast, patients on diabetes medications other than insulin do not usually monitor capillary glucose to modify the prescribed dose of diabetes medications in response to the glucose levels detected by SMBG. Furthermore, patients on oral diabetes medications other than sulfonylureas or glinides do not monitor capillary glucose to detect excessive lowering of glucose levels since their risk of hypoglycemia is generally very low. On the other hand, measurements of capillary glucose in patients with non-insulin-treated type 2 diabetes may increase the awareness of the effects of different type and amount of food and the benefit of physical exercise on glucose control, thus promoting positive and sustained lifestyle modifications. Documenting daily glucose patterns may also enable physicians to tailor an individualized diabetes treatment plan for each patient,2-4 through prescription of diabetes drugs having a preferential effect on fasting or postprandial glucose levels. Indeed, the International Diabetes Federation (IDF) recommends to evaluate postprandial glucose levels, which can be effectively assessed only through SMBG, among the key therapeutic targets in type 2 diabetes, regardless of insulin treatment prescription.5
Several meta-analyses and systematic reviews, exploring the effects of SMBG on glucose control in patients with non-insulin-treated type 2 diabetes, estimated a reduction of glycated hemoglobin A1c (HbA1c) levels ranging from 0.2% to 0.4% in patients using SMBG compared with control groups not using SMBG.6-12 The reported size of HbA1c reduction may be perceived as small and may be influenced by some characteristics of the study participants, including baseline HbA1c and type of prescribed diabetes medications. In a meta-analysis by the Cochrane Collaboration,10 it was concluded that the effect of SMBG on glycemic control in patients with type 2 diabetes who are not using insulin is small, especially after 12 months of follow-up, and that the evidence that SMBG affects patient satisfaction, general well-being or general health-related quality of life is modest. However, this study examined trials until 2011.
The SMBG regimen tested in a specific randomized clinical trial (RCT) may also affect the magnitude of HbA1c reduction. It has been reported that structured SMBG, that is, an SMBG regimen with clearly defined timing and frequency of glucose measurements, is more effective than unstructured SMBG, where timing and frequency of measurements are at the discretion of patients and/or health care providers.13 In the recent guidelines on SMBG use, the IDF supported structured SMBG as an integral component of diabetes care also in patients with non-insulin-treated type 2 diabetes.2 Moreover, the effect of SMBG on glycemic control may be greater when SMBG data are used by physicians to adjust the prescription of diabetes medications. The potential confounding effect of the SMBG regimen and use of SMBG data was not properly accounted for in previous meta-analyses.
The aim of the present study was to assess by a meta-analysis of published articles the effect of SMBG on HbA1c in patients with non-insulin-treated type 2 diabetes, documenting the impact of confounders, such as the predefined schedule for glucose measurements and use of SMBG data by physicians for the adjustment of diabetes medications.
Methods
This meta-analysis is reported according to the statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions.14
Data Sources and Searches
An extensive Medline, Embase, and Cochrane database search for “self-monitoring blood glucose [MeSH Terms] AND diabetes mellitus, Type 2 [MeSH Terms]” was performed to identify all published randomized clinical trials conducted in humans and published in the English language after January 1, 2000, and up to December 31, 2015. The www.clinicaltrials.gov website was also searched, using the same keywords, for unpublished trials. The identification of relevant abstracts, the selection of studies based on the criteria described above, and the subsequent data extraction were performed independently by two of the authors (EM and AA) and conflicts were resolved by discussion.
Study Selection
A meta-analysis was performed including all randomized clinical trials (RCTs) with a duration of at least 24 weeks, enrolling patients with non-insulin-treated type 2 diabetes, comparing SMBG with no SMBG, or structured SMBG with unstructured SMBG. RCTs enrolling patients with insulin-treated type 2 diabetes or patients with type 1 diabetes were excluded, unless separate data analyses for patients with non-insulin-treated type 2 diabetes were provided. The review protocol for these analyses was not published elsewhere.
Data Extraction and Quality Assessment
For all published RCTs (including the primary trial publications, and all subsequent reviews and/or pooled analyses reporting data on an individual RCT), the results reported in published manuscript(s) were the primary source of information. Information from the www.clinicaltrials.gov website was used to complete the results of published RCTs, when not reported in publications. Structured SMBG was defined as an SMBG regimen in which timing and frequency of capillary glucose measurements were specified in the study protocol, as opposed to unstructured SMBG in which timing and frequency of SMBG were at the discretion of patients and/or investigators. For all the RCTs included in our analysis, we also verified whether prescription of diabetes medications to participants was adjusted on the basis of SMBG data according to an algorithm defined in the study protocol.
The quality of the RCTs was assessed using some of the criteria proposed by Jadad et al.15 The derived score was not used as a criterion for including an RCT in this meta-analysis, and some items were used only for descriptive purposes.
Data Synthesis and Analysis
The principal outcome of this analysis was the effect of SMBG versus no SMBG, or of structured SMBG versus unstructured SMBG, on HbA1c. Subgroup analyses were performed for RCTs using structured SMBG or unstructured SMBG versus no SMBG, and for RCTs with or without adjustment of diabetes treatment according to an algorithm versus no SMBG, whenever more than one RCT was available. Heterogeneity was quantified by using I2 statistics and tested using the Cochran’s Q test. Even when a low heterogeneity was detected, a random-effects model was applied, because the validity of the tests for heterogeneity can be limited when the number of RCTs included in the analysis is small. To estimate possible publication/disclosure bias we used funnel plots and the Begg’s adjusted rank correlation test.16,17 Mean differences with 95% confidence intervals (95% CI) were calculated for HbA1c, generally on an intention-to-treat basis. All analyses were performed using the Meta package of the statistical software R, version 4.3.
Results
Retrieved Trials
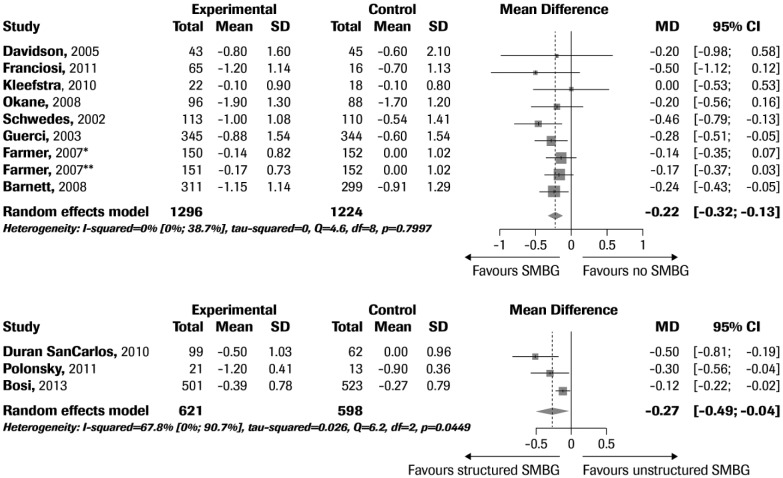
The RCTs flow diagram is reported in Figure 1. A total of 11 trials fulfilling the inclusion criteria were identified.18-29 Of these, 8 compared SMBG with no SMBG,18-25 and 3 structured with unstructured SMBG.26-29 The trial by Farmer et al21 compared SMBG with no SMBG and included two intervention groups, one trained to perform SMBG and to contact the doctor for interpretation of SMBG results (less intensive SMBG) and one trained to perform SMBG and self-interpret and apply the results to diet, physical activity, and drug adherence (more intensive SMBG). The two intervention groups were considered separately in this meta-analysis. The characteristics of the retrieved RCTs (including variables documenting trial quality) are reported in Table 1. The RCTs comparing SMBG with no SMBG included 1277 and 1072 patients, respectively; weighted mean duration of the intervention was 33.7 weeks. The mean age of participants was 59 years, baseline HbA1c was 8.3%, and baseline BMI was 30.8 kg/m2. The 3 RCTs comparing structured SMBG with unstructured SMBG included 856 and 812 patients, respectively; the duration of the intervention was 52 weeks in two RCTs and 26 weeks in one RCT. The mean age of participants was 59 years, baseline HbA1c was 7.9%, and BMI was 32.1 kg/m2.
Figure 1.
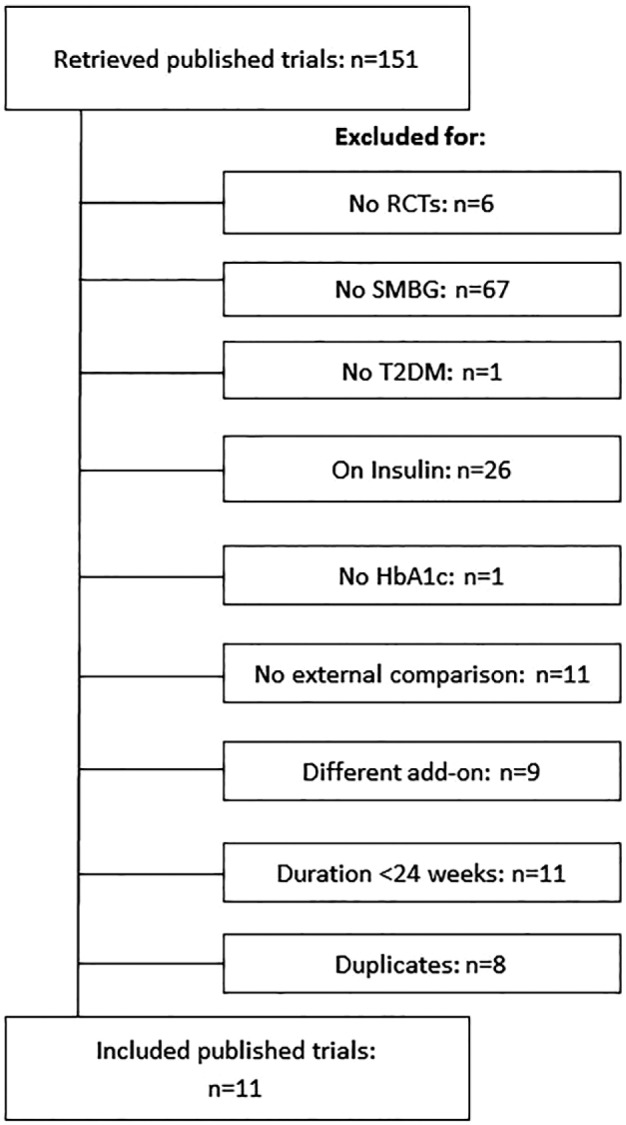
RCT flow diagram.
Table 1.
Characteristics of the Studies Included in the Meta-Analysis.
| Author, year | Structured SMBG | SMBG used for adjusting diabetes treatment | Duration of intervention (weeks) | Randomization | Allocation | Dropouts | Population analyzed | Number of patients | Baseline HbA1c control groupa | Baseline HbA1c intervention groupa |
|---|---|---|---|---|---|---|---|---|---|---|
| Comparisons of SMBG versus no SMBG | ||||||||||
| Schwedes, 200218 | Yes | No | 24 | A | NAD | NAD | PP | 223 | 8.4 (0.75) | 8.5 (0.86) |
| Guerci, 200319 | No | No | 24 | NAD | NAD | A | Practical ITT | 689 | 8.9 (1.3) | 9.0 (1.3) |
| Davidson, 200520 | Yes | No | 26 | NAD | NAD | A | ITT | 88 | 8.5 (2.2) | 8.4 (2.1) |
| Farmer1,b 200721 | Yes | No | 52 | A | A | A | ITT | 302 | 7.5 (1.09) | 7.4 (1.02) |
| Farmer2,b 200721 | Yes | Yes | 52 | A | A | A | ITT | 303 | 7.5 (1.09) | 7.5 (1.12) |
| O’Kane, 200822 | Yes | No | 52 | A | A | A | ITT | 184 | 8.6 (2.3) | 8.8 (2.1) |
| Barnett, 200823 | Yes | Yes | 27 | A | NAD | A | ITT | 610 | 8.1 (0.84) | 8.1 (0.89) |
| Kleefstra, 201024 | Yes | No | 52 | A | A | A | Practical ITT | 40 | 7.7 (0.4) | 7.5 (0.5) |
| Franciosi, 201125 | Yes | Yes | 26 | A | A | A | Practical ITT | 62 | 7.9 (0.6) | 7.9 (0.6) |
| Comparisons of structured versus unstructured SMBG | ||||||||||
| Duran, 201026 | Yes | Yes | 52 | A | NAD | A | Practical ITT | 161 | 6.6 (6.4-7.1) | 6.6 (5.8-7) |
| Polonsky, 201127 | Yes | Yes | 52 | A | A | A | Yes | 483 | 8.9 (1.2) | 8.9 (1.2) |
| Bosi, 201328,29 | Yes | Yes | 52 | A | A | A | Practical ITT, PP | 1024 | 7.3 (6.9-7.8) | 7.4 (6.9-7.8) |
Trials Comparing SMBG with no SMBG
In the RCTs comparing SMBG with no SMBG, I2 was 13.2% [0.0%, 55.1%], and the Cochran’s Q test was 9.22 (P = .3243). No evidence of publication bias was detected by funnel plot analysis (data not shown) or Begg’s adjusted rank correlation test (P = .6767).
Use of SMBG was associated with a significant reduction of HbA1c in comparison with no SMBG use (Figure 2). In all RCTs except one,19 SMBG was structured, that is, timing and frequency of measurements were defined in the study protocol; when this RCT using unstructured SMBG was excluded from the analysis, the results (HbA1c change: –0.2% [−0.3 to −0.1], P = .003) were similar to those obtained in the full dataset (HbA1c change: –0.17% [−0.25 to −0.09], P = .003). In 3 RCTs, SMBG data were used by physicians to adjust diabetes medications in the intervention group according to an algorithm detailed in the study protocol. When SMBG data were used to adjust diabetes medications, the effect of SMBG on HbA1c change was greater (–0.3% [–0.4 to 0.1], n = 3 RCTs) than that observed in RCTs in which diabetes therapy was not consistently modified on the basis of SMBG data (–0.1% [–0.2 to 0.0], n = 6 RCTs) (P = .005).
Figure 2.
Forest plot of mean HbA1c differences in studies comparing SMBG vs no SMBG (upper panel) or structured SMBG vs unstructured SMBG (lower panel).
Trials Comparing Structured SMBG with Unstructured SMBG
In the RCTs comparing structured with unstructured SMBG,26-29 the structured SMBG regimen produced a significantly greater reduction in HbA1c levels compared to the unstructured SMBG arm (–0.27% [−0.49 to −0.04], P = .018). In these RCTs, the results of SMBG were used by physicians to adjust diabetes treatment according to an algorithm only in the intervention arm of the study.
Discussion
In the RCTs included in this meta-analysis, SMBG produced a small but significant reduction in HbA1c, in line with previous meta-analyses or systematic reviews.6-12 Our meta-analysis is, however, the first to consider the SMBG regimen and use of SMBG data to adjust diabetes medications, which represent potential confounders of the effect of SMBG on glucose control.
It has been hypothesized that structured SMBG, in which timing and frequency of capillary glucose measurements are clearly defined, is more effective in reducing HbA1c than unstructured SMBG. The 3 RCTs aimed at testing this hypothesis have both confirmed the superiority of structured SMBG over unstructured SMBG,26-29 with an effect on glucose control of similar magnitude as that observed when comparing SMBG with no SMBG. On the other hand, while most of the RCTs comparing SMBG versus no SMBG were performed using structured SMBG regimens, the only RCT comparing unstructured SMBG with no SMBG19 reported a much smaller effect on HbA1c than the RCTs comparing structured SMBG with no SMBG.18,20-25
It is conceivable that the significant, albeit small, reduction of HbA1c associated with SMBG in RCTs conducted in non-insulin-treated patients with type 2 diabetes is due to modifications of either lifestyle or pharmacological therapy triggered by the SMBG results. Indeed, greater improvements in HbA1c were observed in those RCTs in which structured SMBG data were used by physicians to adjust the type and dose of diabetes medications according to a prespecified algorithm.21,23,25 Notably, in the 3 RCTs comparing structured SMBG and unstructured SMBG, and showing the superiority of the former, the study protocol also included algorithms with various pharmacological/lifestyle treatment strategies to be used in response to the specific glucose patterns identified through the SMBG.26-29 In the studies with structured SMBG in which an analysis was carried out to evaluate patients who adhered to the protocol (per-protocol analysis), greater HbA1c reductions were generally observed compared to the intention-to-treat dataset,21,27,29 underscoring the importance of adhering to the study procedures for achieving clinical outcomes.
Unlike earlier systematic reviews or meta-analysis,6-12 we did not include studies conducted prior to 2000, when glucose meters were less accurate and user-friendly than nowadays and lacked the ability to store capillary glucose results for downloading by health professionals during outpatient visits. As pointed out for insulin pumps, the progress of diabetes-related technologies should be considered when compiling studies for systematic reviews or meta-analysis of SMBG.30 Indeed, after 2013 no further studies have been published comparing SMBG versus no SMBG, suggesting that SMBG is beginning to be considered as standard care in the management of non-insulin-treated type 2 diabetes. The three more recent studies26-29 have indeed compared unstructured SMBG with structured SMBG, the latter being included in the management of non-insulin-treated type 2 diabetes in the IDF guidelines.2
We acknowledge that an undetected bias may be present in our analysis, because funnel plots and the Begg’s adjusted rank correlation test have a low statistical power when the number of RCTs included in the analysis is small. Therefore, our findings should be interpreted with caution, considering the small number of RCTs on this topic in patients with non-insulin-treated type 2 diabetes that met the criteria for being included in this meta-analysis. Furthermore, the potential for a publication bias favoring positive results cannot be excluded, although there is no evidence of such a bias in the specific analyses we conducted. Last, the between-group differences in HbA1c reductions are apparently of modest magnitude and may be may be perceived as clinically nonsignificant. However, in some studies,21,24-26,29 baseline HbA1c was not elevated (ie, <7.5%), and this may have explained the relatively modest changes in HbA1c levels upon intervention. Nevertheless, since any reduction in HbA1c reduces the risk of complications,1 treatment strategies in addition to medications that help patients achieve their glucose goals, such as structured SMBG, should be pursued.
Conclusions
In patients with non-insulin-treated type 2 diabetes, the benefits of using SMBG on glucose control are more evident with structured SMBG and when SMBG data are used by physicians to adjust the prescription of diabetes medications according to an algorithm. It should be considered that some of the most recent algorithms for the pharmacological treatment of type 2 diabetes, including those proposed by the IDF,2 recommend to prescribe different diabetes drugs on the basis of the glucose patterns documented using a structured SMBG regimen. Further studies are needed to verify whether diabetes treatment algorithms based on structured SMBG data are superior in terms of HbA1c reduction or risk of hypoglycemia to algorithms which do not use SMBG data.
Acknowledgments
The authors wish to thank Daniele Martelli for his invaluable help in the retrieval of articles and for his assistance in the preparation of the manuscript.
Footnotes
Abbreviations: IDF, International Diabetes Federation; RCT, randomized clinical trial; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: EM has received speaking fees from Roche Diagnostics, and consulting fees from Abbott and Lifescan. AA has no competing financial interest to disclose. FG has received speaking and consulting fees from Roche Diagnostics. MS has received consulting fees from Roche Diagnostics.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This meta-analysis was supported by an unconditioned educational grant by Roche Diagnostics.
References
- 1.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF guideline on self-monitoring of blood glucose in non-insulin treated type 2 diabetes. Available at: http://www.idf.org/guidelines/self-monitoring. Accessed May 2, 2014.
- 3.Ceriello A, Barkai L, Christiansen JS, et al. Diabetes as a case study of chronic disease management with a personalized approach: the role of a structured feedback loop. Diabetes Res Clin Pract. 2012;98:5-10. [DOI] [PubMed] [Google Scholar]
- 4.Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a diabetes care editors’ expert forum. Diabetes Care. 2013;36:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Diabetes Federation. 2011. guidelines for management of postmeal glucose in diabetes. http://www.idf.org/sites/default/files/postmeal%20glucose%20guidelines.pdf. Accessed May 2, 2014. [DOI] [PMC free article] [PubMed]
- 6.Clar C, Barnard K, Cummins E, et al. Self-monitoring of blood glucose in type 2 diabetes: systematic review. Health Technol Assess. 2010;14:1-140. [DOI] [PubMed] [Google Scholar]
- 7.Sarol JN, Jr, Nicodemus NA, Jr, Tan KM, Grava MB. Self-monitoring of blood glucose as part of a multi-component therapy among non-insulin requiring type 2 diabetes patients: a meta-analysis (1966-2004). Curr Med Res Opin. 2005:21:173-184. [DOI] [PubMed] [Google Scholar]
- 8.Towfigh A, Romanova M, Weinreb JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: a meta-analysis. Am J Manag Care. 2008;14:468-475. [PubMed] [Google Scholar]
- 9.Poolsup N, Suksomboon N, Rattanasookchit S. Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: an update. Diabetes Technol Ther. 2009;11:775-784. [DOI] [PubMed] [Google Scholar]
- 10.Malanda UL, Welschen LM, Riphagen, et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;1:CD005060. doi: 10.1002/14651858.CD005060.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Farmer AJ, Perera R, Ward A, et al. Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes. BMJ. 2012;344:e486. [DOI] [PubMed] [Google Scholar]
- 12.Zhu H, Zhu Y, Leung SW. Is self-monitoring of blood glucose effective in improving glycaemic control in type 2 diabetes without insulin treatment: a meta-analysis of randomised controlled trials. BMJ Open. 2016;6(9):e010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkin CG, Buskirk A, Hinnen DA, Axel-Schweitzer M. Results that matter: structured vs.unstructured self-monitoring of blood glucose in type 2 diabetes. Diabetes Res Clin Pract 2012;97:6-15. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-W94. [DOI] [PubMed] [Google Scholar]
- 15.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 2006;17:1-12. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] [Google Scholar]
- 18.Schwedes U, Siebolds M, Mertes G. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25:1928-1932. [DOI] [PubMed] [Google Scholar]
- 19.Guerci B, Drouin P, Grangé V, et al. Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab. 2003;29:587-594. [DOI] [PubMed] [Google Scholar]
- 20.Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial. Am J Med. 2005;118:422-425. [DOI] [PubMed] [Google Scholar]
- 21.Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Kane MJ, Bunting B, Copeland M, Coates VE. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336:1174-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnett AH, Krentz AJ, Strojek K, et al. The efficacy of self-monitoring of blood glucose in the management of patients with type 2 diabetes treated with a gliclazide modified release-based regimen. A multicentre, randomized, parallel-group, 6-month evaluation (DINAMIC 1 study). Diabetes Obes Metab. 2008;10:1239-1247. [DOI] [PubMed] [Google Scholar]
- 24.Kleefstra N, Hortensius J, Logtenberg SJJ, et al. Self-monitoring of blood glucose in tablet-treated type 2 diabetic patients (ZODIAC). Neth J Med. 2010;68:311-316. [PubMed] [Google Scholar]
- 25.Franciosi M, Lucisano G, Pellegrini F, et al. ROSES: role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabet Med. 2011;28:789-796. [DOI] [PubMed] [Google Scholar]
- 26.Duran A, Martin P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset type 2 diabetes mellitus: The St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2:203-211. [DOI] [PubMed] [Google Scholar]
- 27.Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34:262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scavini M, Bosi E, Ceriello A, et al. Prospective, randomized trial on intensive SMBG management added value in non-insulin-treated T2DM patients (PRISMA): a study to determine the effect of a structured SMBG intervention. Acta Diabetol. 2013;50:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosi E, Scavini M, Ceriello A, et al. Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA Randomized Trial. Diabetes Care. 2013;36:2887-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickup JC. The evidence base for diabetes technology: appropriate and inappropriate meta-analysis. J Diabetes Sci Technol. 2013;7:1567-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]