Are Disagreements in Caregiver and Patient Assessment of Patient Health Associated with Increased Caregiver Burden in Caregivers of Older Adults with Cancer? (original) (raw)
Cancer‐related therapy is increasingly administered in the outpatient setting, resulting in increased dependence on caregivers suggest to provide physical and emotional support to patients. This article describes differences in patient versus caregiver assessments of patient health, considering caregiver perceptions of the patient's health and abilities compared to that reported by the patient.
Keywords: Aged, Caregivers, Neoplasms, Patient‐caregiver assessment
Abstract
Background.
As patients age, caregivers increasingly provide essential support and patient information. We sought to determine if patient‐caregiver assessments of patient health differ and if differences contribute to burden in caregivers of older adults with cancer.
Materials and Methods.
One hundred patients, aged ≥65, and their caregivers independently assessed patient function, comorbidity, nutrition, social activity, social support, and mental health. Caregivers completed the Caregiver Strain Index (CSI). Patient‐caregiver assessments were compared using the Wilcoxon signed rank test and paired t test. Association between caregiver burden and differences between patient‐caregiver assessments was examined using generalized linear regression.
Results.
Median patient age was 70 (range 65–91) and 70% had advanced disease. Sixty percent of patients reported requiring help with instrumental activities of daily living (IADLs); most had good social support (median Medical Outcomes Study [MOS]‐Social Support Survey score 92) and mental health (median Mental Health Inventory score 85).
Caregivers were a median age of 66 (range 28–85), 73% female, 68% spousal caregivers, and 79% lived with the patient. Caregivers rated patients as having poorer physical function (more IADLs dependency [p = .008], lower Karnofsky Performance Status [p = .02], lower MOS‐Physical Function [p < .0001]), poorer mental health (p = .0002), and having more social support (p = .03) than patients themselves. Three‐quarters of caregivers experienced some caregiver burden (mean CSI score 3.1). Only differences in patient‐caregiver assessment of the patient's need for help with IADLs were associated with increased caregiver burden (p = .03).
Conclusion.
Patient‐caregiver assessments of patient function, mental health, and social support differ. However, only differences in assessment of IADLs dependency were associated with increased caregiver burden.
Implications for Practice.
As patients age, there is a higher incidence of frailty and cognitive impairments. As a result, caregivers play an increasingly vital role in providing information about patient health to healthcare providers, which is used to help healthcare providers tailor treatments and optimize patient health. These findings highlight that caregiver reporting in older adults with cancer may not replace patient reporting in those older adults who are otherwise able to self‐report. Furthermore, clinicians should check for caregiver burden in caregivers who report providing more help with instrumental activities of daily living than patients themselves report and provide appropriate support as needed.
Introduction
Cancer‐related therapy is increasingly administered in the outpatient setting, resulting in increased dependence on caregivers to help with daily activities, such as dressing, bathing, and meal preparation, and to help with cancer‐related tasks such as transportation to appointments and managing treatment‐related side effects. Older adults in particular, who may have decreased physiologic reserve, may require increased assistance from caregivers during periods of stress, such as during treatment. This can place significant strain on caregivers [1].
Caregiver burden is a subjective feeling of stress that occurs when the demands of caregiving overwhelm caregiver resources to cope with those demands [2], [3]. Patient factors (such as advanced cancer stage [4], health status [5], increased symptom burden, and decreased quality of life [QOL] [6], [7], [8]), caregiver factors (such as female gender [6], [7], [9], [10], [11], age [5], [10], [12], [13], [14], comorbidity [5], relationship to patient [15], education [14], and employment status [10]), and caregiving characteristics (such as increased duration of caregiving [7] and more help required by the patient [6], [10], [14]) are associated with increased caregiver burden. This information is typically obtained from patients or their caregivers. Some of these factors are subjective and differences can exist between patient and caregiver perceptions.
A comprehensive geriatric assessment evaluates older patients on multiple domains (including physical, psychological, and social) and helps clinicians optimize patient health and tailor treatments appropriately [16], [17]. The accuracy of proxy‐provided information within the context of a geriatric assessment in older patients with cancer has not been studied. Because these patients are more likely to be frail and have geriatric syndromes, such as falls and incontinence, than older adults without cancer [18], this may represent a population in which proxy reporting is especially important. In the general population, differences in patient and proxy assessments of patient health, distress, and QOL have been associated with caregiver burden in some studies [19], [20], [21], [22], [23], but not in others [24], [25]. Most studies assessing patient‐proxy discrepancies and caregiver burden have been predominantly in patients with Alzheimer's disease or community‐dwelling older adults. However, several key factors are unique to older adults with cancer, including a higher likelihood of comorbidities, limitations in performing activities of daily living, and poor health, which may affect assessment results as well as caregiver burden [18]. Given the complexity of this patient population, assessment may be challenging and caregivers may be at particularly high risk of caregiver burden. Furthermore, differences in what the caregiver perceives the patient can do compared with what the patient can or is doing can potentially lead to caregiver strain due to the dissonance from the conflicting perceptions.
We hypothesized that the dissonance caused by a mismatch between caregiver and patient assessments may place these caregivers at higher risk of caregiver burden. We sought to describe if differences exist in patient and caregiver assessment of patient health and function, in the form of a geriatric assessment in older adults with cancer, and describe if this difference is associated with a higher risk of caregiver burden.
Methods
One hundred patient‐caregiver dyads were recruited from 134 eligible consecutive patients from the outpatient clinic (50 from the hematology‐oncology clinic and 50 from the solid tumor oncology clinic), representing a participation rate of 75%. Patients aged 65 and older with cancer, who had a primary caregiver who was also willing to participate, were eligible. Patients were asked to identify their primary caregiver, defined as the person who provided the most assistance to the patient in their health care and daily needs. This was confirmed by the caregivers. Caregivers who were <18 years were excluded. Patients and caregivers who did not speak English were excluded because some measures have only been validated in English. Approval from the institutional research and ethics board and the patient's oncologist was obtained. A trained member of the research team recruited and obtained informed consent from patients and caregivers.
Patient and caregiver sociodemographics and employment status were obtained via in‐person interviews by a member of the research team. In addition, the caregiver's relationship to the patient, cohabitation with the patient, and duration and time spent caring for the patient were obtained via interview with the caregiver. Patient and caregiver cognition were assessed using the Blessed‐Orientation‐Memory‐Concentration (BOMC) test (Table 1), but patients and caregivers were not excluded on the basis of their score. The patient's cancer diagnosis, stage, and treatments were gathered via chart review.
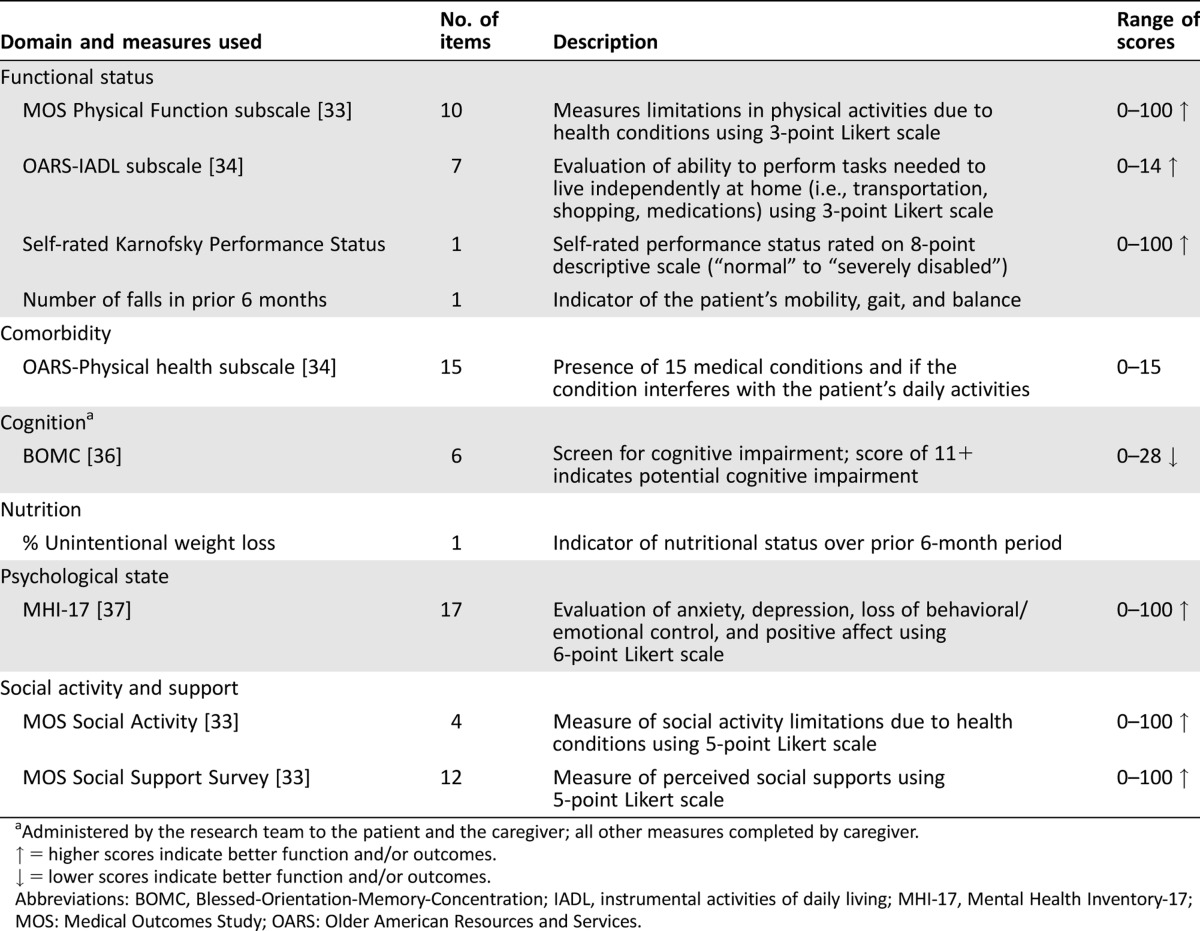
Table 1. Composition of geriatric assessment.
Geriatric Assessment Measures
Caregivers and patients completed a self‐administered geriatric assessment, which seeks to identify factors that place the patient at higher risk of morbidity and mortality and subsequently implement potential interventions to decrease this risk. The domains evaluated included patient functional status, comorbidities, psychological state, nutrition, social activity, and social support (Table 1). Caregivers were asked to complete the questionnaire based on their assessment of the patient. The measures utilized in this geriatric assessment were chosen based on the feasibility of obtaining this information through self‐report and their psychometric properties have been fully described previously [26]. The geriatric assessment was administered independently to patients and caregivers via a touchscreen interface.
Caregiver Burden
Caregivers completed the Caregiver Strain Index (CSI), a 13‐item measure of caregiver burden, via the touchscreen interface [28]. This instrument targets the following domains of caregiver burden: employment, time, financial, physical health, and social health. The caregiver's responses establish the presence of sleep disturbance; inconvenience; physical effort; confinement; family, personal, emotional, and employment disruption; time pressures; feeling upset from changes in patient behavior and personality; financial stress; and emotional and psychological overload. Answers to each question were dichotomized to yes = 1 and no = 0, and the sum of all the answers constitutes the CSI score. Scores range from 0–13, with scores ≥7 indicating high caregiver burden. This tool was chosen because it is short and easy to administer and is validated with good construct validity and a reliability coefficient alpha of 0.86.
Patient‐Caregiver Agreement
Each pair of patient and caregiver assessments were compared. There is no standard definition of what constitutes a significant difference in responses between patients and caregivers. Some studies have utilized exact agreement [29], whereas others use exact agreement ±25% of the scale [30], exact agreement half standard deviation (SD) [31], or agreement within one category of choice [29]. We utilized exact agreement for our analysis (defined as cases in which patient‐caregiver total scores on the assessment tools were identical) but also analyzed the results, defining differences as those whose scores differed by ± one SD of the measure.
Statistical Analysis
Patient and caregiver demographics, caregiver burden, and results of the geriatric assessment were summarized using descriptive statistics. Differences between patient and caregiver assessments were calculated as caregiver assessment minus patient assessment. All variables in the geriatric assessment, as assessed by patients and caregivers, were compared, except for patient cognition, which was objectively assessed through testing by the research team rather than by the caregiver. Wilcoxon signed rank test for geriatric assessments and paired t test for weight change were used to examine the significance of differences between patient‐caregiver assessments. Except for the BOMC tool, we did not utilize any cut‐points for evaluation of the tools used in the geriatric assessment because these have not been clearly identified and because normative values for these instruments were established in the general population and may not be applicable to patients with cancer.
The outcome variable, CSI, was analyzed as a continuous variable. For all the geriatric assessments, patient‐caregiver agreement variables were considered as categorical variables (agreement, caregivers rated patients as doing worse, or patients rated themselves as doing worse). Generalized linear model (GLM) was used to examine the association between patient‐caregiver agreement variables and CSI. The association between CSI and patient/caregiver demographics, cancer characteristics, and caregiver characteristics were also examined univariately to select for potential confounding factors for the multivariate GLM model. Variables with an association of p value <.05 with CSI on univariate analysis were considered to be included in the multivariate model. The final model only retained variables with p value <.05.
Statistical analyses were done using SAS 9.3 (SAS Institute, Cary, NC, https://www.sas.com/en_us/home.html).
Results
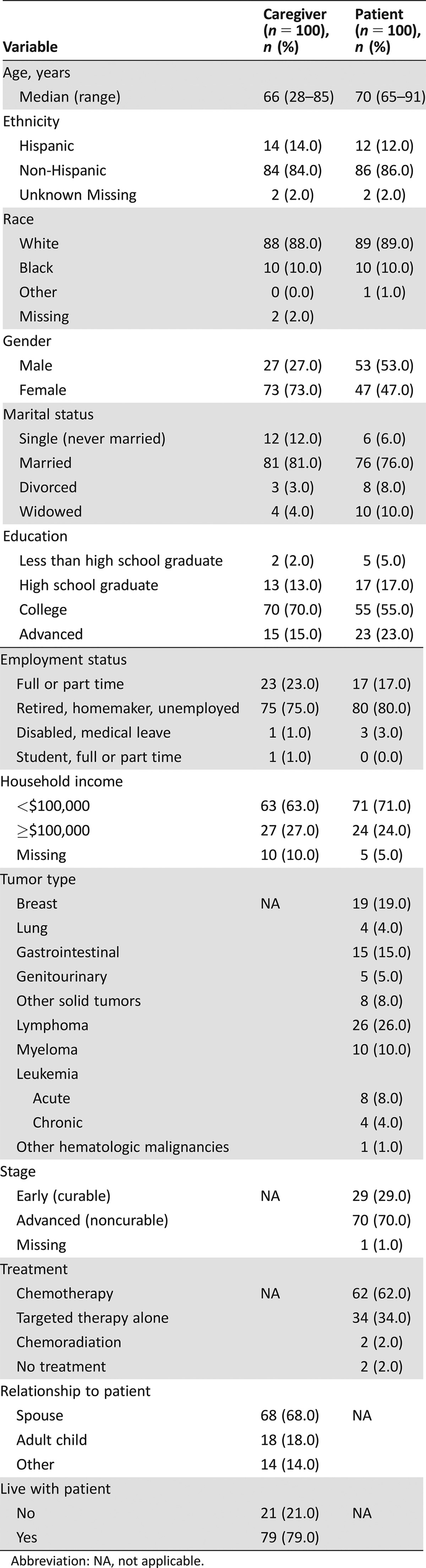
One hundred patient‐caregiver pairs were included (Fig. 1). Patient and caregiver characteristics are presented in Table 2. Median patient age was 70 (range 65–91) and median caregiver age was 66 (range 28–85). Most patients had lymphoma (26%), breast cancer (19%), or gastrointestinal cancers (15%). Most patients had advanced disease (70%) and were on treatment (62% chemotherapy). The majority of caregivers were female (73%), 79% lived with the patient, and 68% were spousal caregivers. Five percent of caregivers scored above the threshold for possible cognitive impairment (BOMC ≥11).
Figure 1.
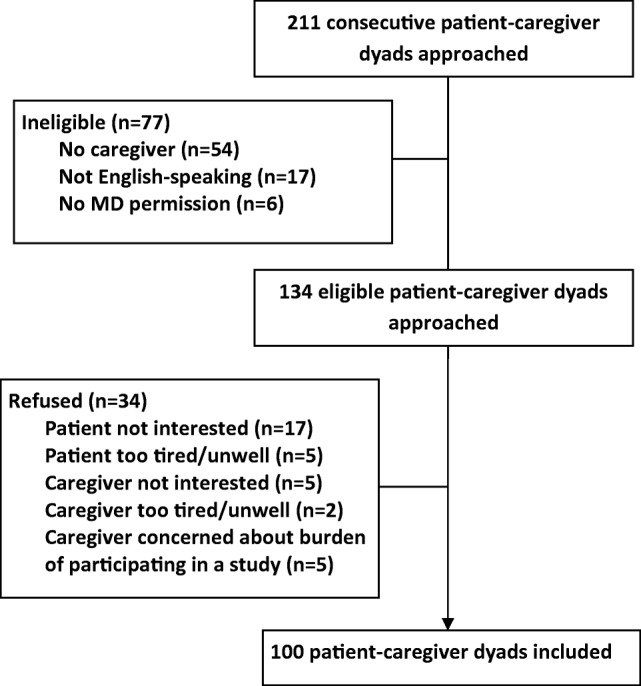
Enrollment of patients and caregivers.
Abbreviation: MD, medical doctor.
Table 2. Caregiver and patient demographics.
Geriatric Assessment—Patient‐reported Assessments
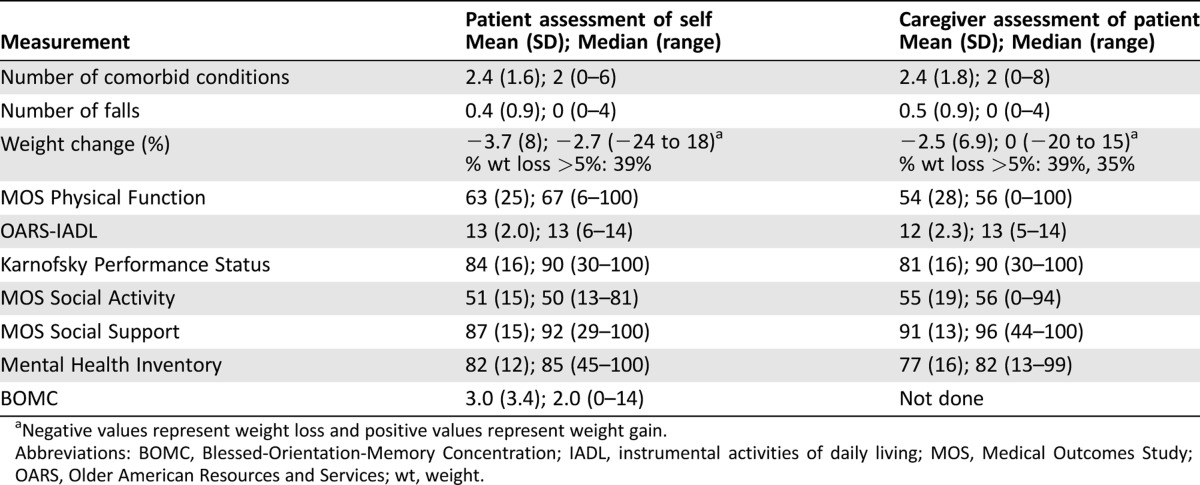
A summary of the results of the patients' geriatric assessment is shown in Table 3. These are the average scores of assessments done by all caregivers and all patients in the study. Patients reported a median of 2 comorbid conditions and 39% reported at least 5% unintentional weight loss over the preceding 6 months. Patients reported a median KPS of 90. Despite this, 60% of patients reported needing assistance with at least one of their instrumental activities of daily living (IADL). Patients reported that their social activity was affected by their health (mean Medical Outcomes Study [MOS]‐Social Activity score 50.8). Patients generally perceived themselves to be well supported and had high mean scores on assessments of social support and psychological health (mean MOS‐Social Support score of 87.1 and MHI of 82.0, respectively). Four percent of patients scored above the threshold for possible cognitive impairment (BOMC ≥11). This paper focuses on the results comparing patient and caregiver assessments.
Table 3. Geriatric assessment of patient as assessed by patient and caregiver.
Geriatric Assessment—Caregiver‐Patient Differences
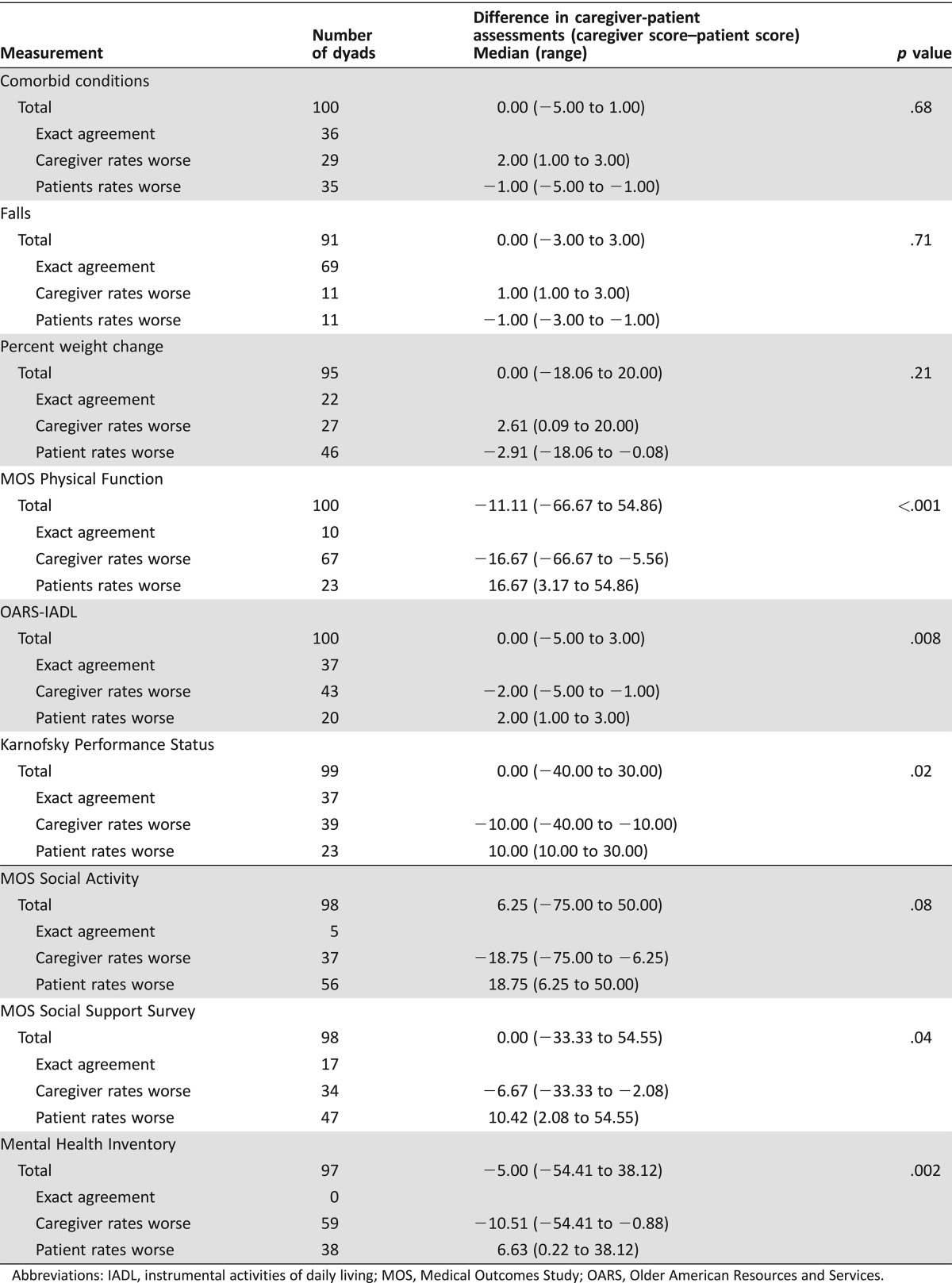
Patient and caregiver assessments were compared for each patient‐caregiver dyad (Table 4). Several differences were noted between caregiver and patient assessments of the patient. Similar results were seen whether “agreement” was defined as identical responses or as responses that differed by ± one SD, with the exception of social support, which was no longer significantly different (data not shown). Caregivers were more likely to rate patients as having poorer physical function (lower MOS‐Physical Function [p < .0001], requiring more help with IADLs [_p_ = .008], and lower KPS [_p_ = .02]). Caregivers also reported patients having more social support (MOS‐Social Support survey, _p_ = .03) and having poorer mental health (lower MHI, _p_ = .0002). There were no significant differences between the patient and caregiver assessments of the patient's comorbid conditions, falls, weight change, and social activity (_p_ > .05).
Table 4. Caregiver‐patient paired assessment differences.
Caregiver Burden
Most caregivers (75%) experienced some degree of caregiver burden (CSI ≥1), with a mean caregiver burden score as measured by the CSI of 3.1 (range 0–13). Fifteen percent of caregivers reported high caregiver burden (CSI ≥7; Table 5) [22]. Caregivers reported providing a median of 10 hours per week of care and most (61%) had been a caregiver for at least 1 year.
Table 5. Caregiver burden summary.
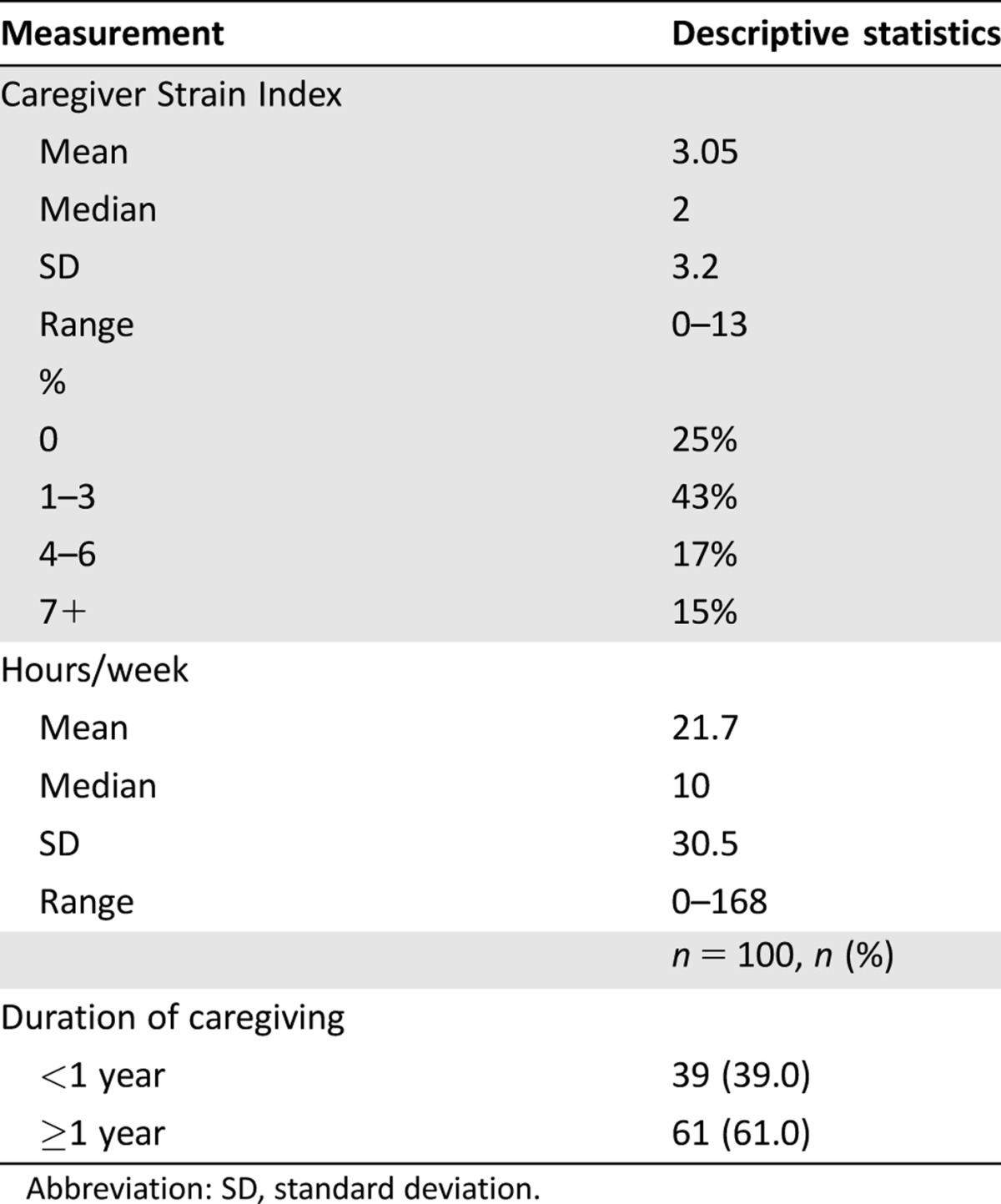
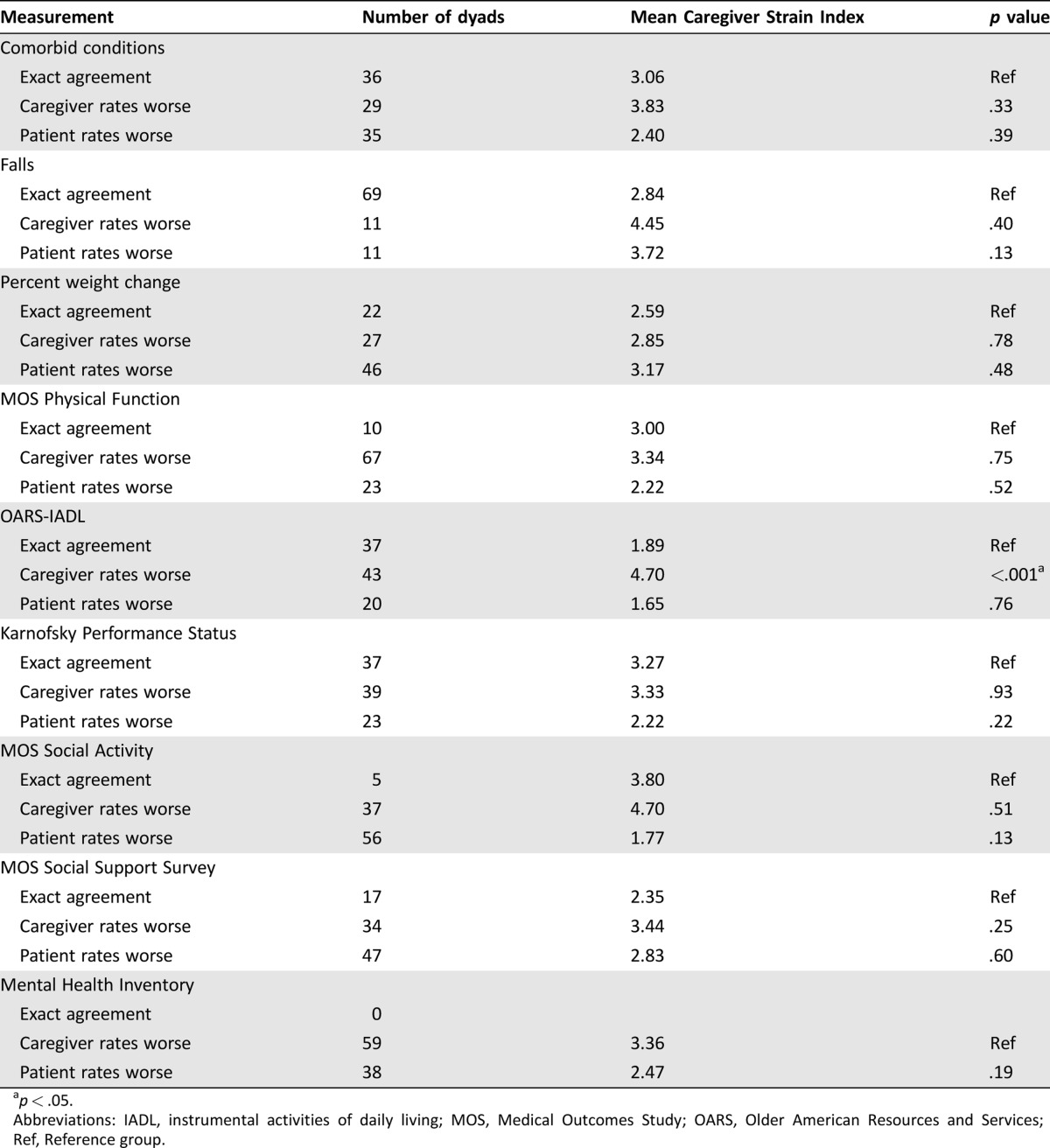
Mean CSI scores by whether patient‐caregiver assessments agreed or not are presented in Table 6. Those caregivers who assessed the patient to require more help with their IADLs (Older American Resources and Services [OARS]‐IADL) than reported by the patient reported higher caregiver burden (CSI 4.71 vs. 1.89, p < .001; Table 6). Similar results were found utilizing disagreement as differences ± one SD in assessments (CSI 5.73 vs. 2.23, p < .001). There was no significant association between caregiver burden and caregiver‐patient assessments for other geriatric assessment measures.
Table 6. Univariate analysis of association between Caregiver Strain Index and patient and caregiver assessments.
For patient‐related variables (demographics, cancer characteristics, and patient‐reported geriatric assessment results), patient‐reported KPS and weight change were negatively associated with CSI on univariate analysis (p < .05). Among caregiver‐related variables (demographics, caregiving characteristics, and results from the caregiver's assessment of the patient), caregiver age, race and/or ethnicity, employment status, and caregiver‐reported measures of patient physical function (OARS‐IADL, MOS‐Physical Function, and KPS) were negatively associated with CSI (p < .05). After adjustment for these variables, caregivers who reported patients as requiring more help with IADLs continued to experience increased caregiver burden (CSI 4.04 vs. 2.51, p = .026) compared with caregivers whose assessment agreed with the patient.
Discussion
Caregivers and older adults with cancer differed in their assessment of the patient's physical function, with caregivers reporting that patients needed more help with their IADLs and had poorer physical function than patients reported. As caregivers of older adults often provide information about patient health and function to clinicians, particularly as cognitive impairment and frailty are more common with increasing age, this is important to consider and may have significant implications as to how clinicians interpret patient performance status, which may influence treatments offered to the patient. Moreover, these differences in assessments between caregivers and patients were associated with an increased likelihood of greater caregiver burden, which has important implications given the growing number of caregivers caring for an aging cancer population.
We found that caregiver and patient assessments differ in some but not all domains of patient health and function. Patient and caregiver assessments were more likely to differ with respect to subjective health, such as the patient's mental health and social support, with no differences seen in more observable areas such as number of falls and weight loss, which has also been described in prior studies [20], [21], [24], [32], [33], [34], [35], [36], [37]. Unlike prior studies, however, caregiver‐patient assessments differed with respect to physical health and function. This may be because patients in this study were of intermediate physical health, as evidenced by the fact that although patients reported a median KPS of 90, 60% reported needing assistance with at least one of their IADLs, a finding consistent with prior studies [38]. These patients may be harder for caregivers to assess accurately compared with patients who were in either perfect health or very poor health (e.g., completely dependent) [24].
Most caregivers experienced some level of caregiver burden. However, caregivers who reported that patients required more help with their IADLs than patients themselves reported were more likely to experience higher levels of caregiver burden. This was true even after taking into account factors associated with caregiver burden in other studies, such as poor physical function and functional dependence [6], [10], [14]. It is unclear how much of a difference in patient‐caregiver assessment of IADLs is clinically significant. Further research is required to evaluate this. At this time, clinicians should consider screening for caregiver burden in those caregivers who report the patient as being more dependent than patients themselves do.
There are few other studies that have looked at differences in patient‐caregiver assessments in older adults with cancer and their association with caregiver burden, but similar findings have been seen in both the general cancer literature and geriatric studies. Several studies of cancer patients have suggested an association between caregiver burden and differences in caregiver‐patient assessments [32], [39], [40], [41], although none have looked specifically at patient function and health within the context of a geriatric assessment. However, the association between caregiver burden and differences in patient and caregiver assessments about the need for help with IADLs has been seen in a study of community‐dwelling adults aged 65 and over [21]. The patients' lack of awareness of impairments in their IADLs may be a source of stress for caregivers [42]. This may be due to concern about patient safety and/or conflict with the patient about their independence [43].
There are several limitations to our study. Because three‐quarters of caregivers were women and the majority of participants were non‐Hispanic, these results may not generalize to all caregivers. Additional research in males and patients of ethnic diversity is needed. Importantly, because all patients were fit enough to complete the geriatric assessment, it is unclear whether the results would be applicable to assessment of those patients who are too unwell to respond, a situation in which the accuracy of a caregiver response would be most relevant. Because we did not include an objective assessment of patient health or function, it is difficult to determine whether caregivers experience more burden because there is a discrepancy in assessments, or whether the presence of caregiver burden itself influences caregiver perception of patient function and health. Prior studies have posited that differences in assessments may be due to the tendency of patients to underestimate the level of support and help they require [44]. On the other hand, a study of patients with dementia found that although caregiver report was strongly associated with some aspects of patient function (walking, dressing), it correlated less strongly for others (toileting, shopping), suggesting that caregiver assessments are not always correct either [27]. It is likely that both are important factors to consider, with a bidirectional relationship as suggested by another study [39]. Lastly, due to the modest sample size, our analysis focused on the association between caregiver burden and patient‐caregiver assessments for each measure as a whole, rather than on differences in assessment for individual components of each measure.
Conclusion
Discrepancies were observed between patient and caregiver assessments in patient physical function, and these discrepancies were associated with increased caregiver burden. Caregivers who perceived patients to be more dependent for their IADLs than patients did were more likely to experience caregiver burden. Based on our results, we suggest that assessing both patients and caregivers is important when utilizing caregivers as proxies for understanding patient function and when assessing for burden in caregivers of older adults with cancer. Further research investigating caregiver burden and caregiver‐patient assessment, with the addition of an objective marker of patient health and function, would be helpful to better understand whether caregiver burden is the cause or consequence of differences in caregiver‐patient perception of patient health.
Acknowledgments
This work was supported by a National Institutes of Health R21 AGO41489 grant and a City of Hope Excellence Award.
Author Contributions
Conception/design: Matthew Loscalzo, Stephen Forman, Leslie Popplewell, Karen Clark, Tao Feng, David Smith, Arti Hurria
Provision of study material or patients: Tina Hsu, Stephen Forman, Leslie Popplewell, Rex Strowbridge, Redmond Rinehart, Dan Smith, Keith Matthews, Jeff Dillehunt, Arti Hurria
Collection and/or assembly of data: Tina Hsu, Rupal Ramani, Stephen Forman, Leslie Popplewell, Vani Katheria, Rex Strowbridge, Redmond Rinehart, Dan Smith, Keith Matthews, Jeff Dillehunt, Arti Hurria
Data analysis and interpretation: Tina Hsu, Matthew Loscalzo, Karen Clark, Tao Feng, David Smith, Canlan Sun, Arti Hurria
Manuscript writing: Tina Hsu, Matthew Loscalzo, Karen Clark, Tao Feng, David Smith, Canlan Sun, Arti Hurria
Final approval of manuscript: Tina Hsu, Matthew Loscalzo, Rupal Ramani, Stephen Forman, Leslie Popplewell, Karen Clark, Vani Katheria, Rex Strowbridge, Redmond Rinehart, Dan Smith, Keith Matthews, Jeff Dillehunt, Tao Feng, David Smith, Canlan Sun, Arti Hurria
Disclosures
Matthew Loscalzo: Novartis, Lilly (H); Karen Clark: Novartis, Lilly (H), SupportScreen (IP); Arti Hurria: Celegene, GlaxoSmithKline, Novartis (RF), GTx, Boehringer Ingelheim Pharmaceuticals, Carevive, Sanofi (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Given BA, Given CW, Kozachik S. Family support in advanced cancer. CA Cancer J Clin 2001;51:213–231. [DOI] [PubMed] [Google Scholar]
- 2.Sherwood PR, Given BA, Doorenbos AZ et al. Forgotten voices: Lessons from bereaved caregivers of persons with a brain tumour. Int J Palliat Nurs 2004;10:67–75. [DOI] [PubMed] [Google Scholar]
- 3.Scherbring M. Effect of caregiver perception of preparedness on burden in an oncology population. Oncol Nurs Forum 2002;29:E70–E76. [DOI] [PubMed] [Google Scholar]
- 4.Weitzner MA, McMillan SC, Jacobsen PB. Family caregiver quality of life: Differences between curative and palliative cancer treatment settings. J Pain Symptom Manage 1999;17:418–428. [DOI] [PubMed] [Google Scholar]
- 5.Maguire R, Hanly P, Hyland P et al. Understanding burden in caregivers of colorectal cancer survivors: What role do patient and caregiver factors play? Eur J Cancer Care (Engl) 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Garlo K, O'Leary JR, Van Ness PH et al. Burden in caregivers of older adults with advanced illness. J Am Geriatr Soc 2010;58:2315–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadhwa D, Burman D, Swami N et al. Quality of life and mental health in caregivers of outpatients with advanced cancer. Psychooncology 2013;22:403–410. [DOI] [PubMed] [Google Scholar]
- 8.Borges EL, Franceschini J, Costa LH et al. Family caregiver burden: The burden of caring for lung cancer patients according to the cancer stage and patient quality of life. J Bras Pneumol 2017;43:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrank B, Ebert‐Vogel A, Amering M et al. Gender differences in caregiver burden and its determinants in family members of terminally ill cancer patients. Psychooncology 2016;25:808–814. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Spillers RL. Quality of life of family caregivers at 2 years after a relative's cancer diagnosis. Psychooncology 2010;19:431–440. [DOI] [PubMed] [Google Scholar]
- 11.Pinquart M, Sorensen S. Gender differences in caregiver stressors, social resources, and health: An updated meta‐analysis. J Gerontol B Psychol Sci Soc Sci 2006;61:P33–P45. [DOI] [PubMed] [Google Scholar]
- 12.Gilbar O. Gender as a predictor of burden and psychological distress of elderly husbands and wives of cancer patients. Psychooncology 1999;8:287–294. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein NE, Concato J, Fried TR et al. Factors associated with caregiver burden among caregivers of terminally ill patients with cancer. J Palliat Care 2004;20:38–43. [PubMed] [Google Scholar]
- 14.Vahidi M, Mahdavi N, Asghari E et al. Other side of breast cancer: Factors associated with caregiver burden. Asian Nurs Res (Korean Soc Nurs Sci) 2016;10:201–206. [DOI] [PubMed] [Google Scholar]
- 15.Papastavrou E, Charalambous A, Tsangari H et al. The burdensome and depressive experience of caring: What cancer, schizophrenia, and Alzheimer's disease caregivers have in common. Cancer Nurs 2012;35:187–194. [DOI] [PubMed] [Google Scholar]
- 16.Hurria A, Browner IS, Cohen HJ et al. Senior adult oncology. J Natl Compr Canc Netw 2012;10:162–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Extermann M, Aapro M, Bernabei R et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55:241–252. [DOI] [PubMed] [Google Scholar]
- 18.Mohile SG, Xian Y, Dale W et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst 2009;101:1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothman ML, Hedrick SC, Bulcroft KA et al. The validity of proxy‐generated scores as measures of patient health status. Med Care 1991;29:115–124. [DOI] [PubMed] [Google Scholar]
- 20.Schulz R, Cook TB, Beach SR et al. Magnitude and causes of bias among family caregivers rating Alzheimer disease patients. Am J Geriatr Psychiatry 2013;21:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long K, Sudha S, Mutran EJ. Elder‐proxy agreement concerning the functional status and medical history of the older person: The impact of caregiver burden and depressive symptomatology. J Am Geriatr Soc 1998;46:1103–1111. [DOI] [PubMed] [Google Scholar]
- 22.Neumann PJ, Araki SS, Gutterman EM. The use of proxy respondents in studies of older adults: Lessons, challenges, and opportunities. J Am Geriatr Soc 2000;48:1646–1654. [DOI] [PubMed] [Google Scholar]
- 23.Leon‐Salas B, Olazaran J, Muniz R et al. Caregivers' estimation of patients' quality of life (QoL) in Alzheimer's disease (AD): An approach using the ADRQL. Arch Gerontol Geriatr 2011;53:13–18. [DOI] [PubMed] [Google Scholar]
- 24.Sneeuw KC, Aaronson NK, Sprangers MA et al. Comparison of patient and proxy EORTC QLQ‐C30 ratings in assessing the quality of life of cancer patients. J Clin Epidemiol 1998;51:617–631. [DOI] [PubMed] [Google Scholar]
- 25.Arguelles S, Loewenstein DA, Eisdorfer C et al. Caregivers' judgments of the functional abilities of the Alzheimer's disease patient: Impact of caregivers' depression and perceived burden. J Geriatr Psychiatry Neurol 2001;14:91–98. [DOI] [PubMed] [Google Scholar]
- 26.Hurria A, Gupta S, Zauderer M et al. Developing a cancer‐specific geriatric assessment: A feasibility study. Cancer 2005;104:1998–2005. [DOI] [PubMed] [Google Scholar]
- 27.Robinson BC. Validation of a Caregiver Strain Index. J Gerontol 1983;38:344–348. [DOI] [PubMed] [Google Scholar]
- 28.Sneeuw KC, Aaronson NK, Osoba D et al. The use of significant others as proxy raters of the quality of life of patients with brain cancer. Med Care 1997;35:490–506. [DOI] [PubMed] [Google Scholar]
- 29.Milne DJ, Mulder LL, Beelen HC et al. Patients' self‐report and family caregivers' perception of quality of life in patients with advanced cancer: How do they compare? Eur J Cancer Care (Engl) 2006;15:125–132. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs DI, Kumthekar P, Stell BV et al. Concordance of patient and caregiver reports in evaluating quality of life in patients with malignant gliomas and an assessment of caregiver burden. Neurooncol Pract 2014;1:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higginson IJ, Gao W. Caregiver assessment of patients with advanced cancer: Concordance with patients, effect of burden and positivity. Health Qual Life Outcomes 2008;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones JM, McPherson CJ, Zimmermann C et al. Assessing agreement between terminally ill cancer patients' reports of their quality of life and family caregiver and palliative care physician proxy ratings. J Pain Symptom Manage 2011;42:354–365. [DOI] [PubMed] [Google Scholar]
- 33.Lobchuk MM, Degner LF. Symptom experiences: Perceptual accuracy between advanced‐stage cancer patients and family caregivers in the home care setting. J Clin Oncol 2002;20:3495–3507. [DOI] [PubMed] [Google Scholar]
- 34.Magaziner J, Bassett SS, Hebel JR et al. Use of proxies to measure health and functional status in epidemiologic studies of community‐dwelling women aged 65 years and older. Am J Epidemiol 1996;143:283–292. [DOI] [PubMed] [Google Scholar]
- 35.Magaziner J, Zimmerman SI, Gruber‐Baldini AL et al. Proxy reporting in five areas of functional status. Comparison with self‐reports and observations of performance. Am J Epidemiol 1997;146:418–428. [DOI] [PubMed] [Google Scholar]
- 36.Sneeuw KC, Sprangers MA, Aaronson NK. The role of health care providers and significant others in evaluating the quality of life of patients with chronic disease. J Clin Epidemiol 2002;55:1130–1143. [DOI] [PubMed] [Google Scholar]
- 37.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824–1831. [DOI] [PubMed] [Google Scholar]
- 38.Sharpe L, Butow P, Smith C et al. The relationship between available support, unmet needs and caregiver burden in patients with advanced cancer and their carers. Psychooncology 2005;14:102–114. [DOI] [PubMed] [Google Scholar]
- 39.Porter LS, Keefe FJ, McBride CM et al. Perceptions of patients' self‐efficacy for managing pain and lung cancer symptoms: Correspondence between patients and family caregivers. Pain 2002;98:169–178. [DOI] [PubMed] [Google Scholar]
- 40.Leroy T, Fournier E, Penel N et al. Crossed views of burden and emotional distress of cancer patients and family caregivers during palliative care. Psychooncology 2016;25:1278–1285. [DOI] [PubMed] [Google Scholar]
- 41.DeBettignies BH, Mahurin RK, Pirozzolo FJ. Insight for impairment in independent living skills in Alzheimer's disease and multi‐infarct dementia. J Clin Exp Neuropsychol 1990;12:355–363. [DOI] [PubMed] [Google Scholar]
- 42.Tang ST, McCorkle R. Use of family proxies in quality of life research for cancer patients at the end of life: A literature review. Cancer Invest 2002;20:1086–1104. [DOI] [PubMed] [Google Scholar]
- 43.Krishnasamy M, Wilkie E, Haviland J. Lung cancer health care needs assessment: Patients' and informal carers' responses to a national mail questionnaire survey. Palliat Med 2001;15:213–227. [DOI] [PubMed] [Google Scholar]
- 44.Zanetti O, Geroldi C, Frisoni GB et al. Contrasting results between caregiver's report and direct assessment of activities of daily living in patients affected by mild and very mild dementia: The contribution of the caregiver's personal characteristics. J Am Geriatr Soc 1999;47:196–202. [DOI] [PubMed] [Google Scholar]