Dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis of safety data from phase IIb/III clinical trials (original) (raw)
Abstract
Aim
To evaluate the safety and tolerability of dapagliflozin, a highly selective sodium‐glucose co‐transporter‐2 inhibitor, in patients with type 2 diabetes mellitus (T2DM).
Methods
Data were pooled from 13 placebo‐controlled trials of up to 24 weeks’ duration (dapagliflozin, n = 2360; placebo, n = 2295). Larger placebo‐/comparator‐controlled pools of 21 (≤208 weeks; dapagliflozin, n = 5936; control, n = 3403) and 30 trials (≥12 weeks; dapagliflozin, n = 9195; control, n = 4629) assessed the rare adverse events (AEs) of diabetic ketoacidosis (DKA) and lower limb amputation, respectively.
Results
Over 24 weeks, the overall incidence of AEs and serious AEs (SAEs) was similar for dapagliflozin and placebo: 60.0% vs 55.7% and 5.1% vs 5.4%, respectively. Rates of hypoglycaemia, volume depletion AEs, urinary tract infections (UTIs) and fractures were balanced between the groups. Genital infections were more frequent with dapagliflozin (5.5%) vs placebo (0.6%) and renal function AEs occurred in 3.2% vs 1.8% of patients (the most common renal AE was decreased creatinine clearance: 1.1% vs 0.7%). In the 21‐study pool, 1 SAE of DKA and 3 AEs of ketonuria/metabolic acidosis occurred with dapagliflozin vs none with control; estimated combined incidence for these events was 0.03% (95% confidence interval 0.010‐0.089). In the 30‐study pool, lower limb amputation occurred in 8 (0.1%) and 7 (0.2%) patients receiving dapagliflozin and control, respectively.
Conclusion
The overall incidence rates of AEs and SAEs were similar in the dapagliflozin and placebo/control groups, including the incidence of hypoglycaemia, volume depletion, fractures, UTIs, amputations and DKA. Genital infections were more frequent with dapagliflozin than placebo.
Keywords: antidiabetic drug, dapagliflozin, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors are a newer class of antihyperglycaemic agents that not only lower blood glucose levels, body weight and systolic blood pressure, but have also recently demonstrated cardiovascular (CV) safety.1, 2 The safety of these agents is of keen interest to physicians treating patients with type 2 diabetes mellitus (T2DM). Common adverse events (AEs) have been associated with the use of SGLT2 inhibitors such as genital infection, urinary tract infection (UTI) and polyuria.3
Dapagliflozin is a highly selective SGLT2 inhibitor that lowers blood glucose levels in an insulin‐independent manner by suppressing renal glucose reabsorption and increasing urinary glucose excretion.4, 5, 6 In phase III trials, dapagliflozin was efficacious and well tolerated in the early and late stages of T2DM, with no major imbalances in safety events observed between the dapagliflozin and control arms.7, 8, 9, 10, 11
Although the CV safety of SGLT2 inhibitors has been confirmed in recent outcome trials, pooled analyses of these agents have previously shown differing overall safety profiles. Empagliflozin was well tolerated with no increase in the risk of hypoglycaemia (except in patients on background sulphonylureas) and UTI and volume depletion (except in patients aged ≥75 years, where a higher incidence was observed with both empagliflozin 10 and 25 mg) compared with placebo.12, 13 Low rates of bone fractures, AEs consistent with decreased renal function and diabetic ketoacidosis (DKA) were observed in the empagliflozin and placebo groups.12, 13 Small increases in serum creatinine and small decreases in estimated glomerular filtration rate (eGFR) were noted across the overall pool of patients, with larger changes in the empagliflozin groups compared with placebo. Genital infections were more frequent with empagliflozin vs placebo. Canagliflozin was associated with a slightly higher incidence of hypoglycaemia vs the comparator. Also, higher incidences of genital infections, UTI and AEs related to volume depletion were observed with canagliflozin vs comparator.14 The incidence rates of fractures and DKA were low in the canagliflozin and comparator groups.14
Over the past couple of years, drug safety communications regarding the risk of DKA, fracture and serious UTIs of pyelonephritis, urosepsis and acute kidney injury related to the use of SGLT2 inhibitors have been issued by the US Food and Drug Administration (FDA).15, 16 Another drug safety communication was also issued more recently regarding the potential for an increased risk of lower limb amputation (mostly affecting the toes) in patients receiving canagliflozin,17 prompted by an increase in lower limb amputation reported in an interim analysis of the CANVAS trial in patients with T2DM with a history/risk of CV disease.18 This was further confirmed in the final analysis of the CANVAS programme, which showed increased risk of lower limb amputation with canagliflozin vs placebo (6.3 vs 3.4 per 1000 patient‐years; hazard ratio 1.97; 95% confidence interval [CI] 1.41 to 2.75).1
The present analysis builds on a previous publication on the safety of dapagliflozin19 by evaluating the safety and tolerability of dapagliflozin in a larger pool of studies and reporting the incidence of amputation and DKA, which were not included in the previous analyses.
2. METHODS
2.1. Patient population
Three pooled patient populations were included in this analysis. The majority of AEs were evaluated using pooled data from 13 placebo‐controlled, double‐blind, phase IIb/III studies of up to 24 weeks’ duration (Figure S1), in which patients with T2DM were randomized to receive dapagliflozin 10 mg (N = 2360) or placebo (N = 2295). This included 3 phase IIb studies of 12 weeks’ duration, and 10 phase III studies of 24 weeks’ duration. The placebo‐controlled pool was analysed to better enable identification of any difference in the frequency of AEs with dapagliflozin vs placebo. (ClinicalTrials.gov identifiers: NCT00263276, NCT00357370, NCT00528372, NCT00528879, NCT00643851, NCT00660907, NCT00663260, NCT00831779, NCT00673231, NCT00680745, NCT00683878, NCT00736879, NCT00855166, NCT00859898, NCT00972244, NCT00976495, NCT00984867, NCT01031680, NCT01042977, NCT01095653, NCT01095666, NCT01137474, NCT01195662, NCT01217892, NCT01294423, NCT01294436, NCT01392677, NCT01606007, NCT01619059, NCT01646320)
Two larger and longer‐term pools were used to evaluate the incidence of DKA and amputation, which are both rare events. The greater number of patient‐years included in these pools increased the odds of observing these events. A pool of 21 placebo‐/active comparator‐controlled trials (≤208 weeks; dapagliflozin [N = 5936] and placebo/active comparator [N = 3403]) was used to assess DKA (Figure S2) and a pool of 30 placebo‐/active comparator‐controlled trials (≥12 weeks; dapagliflozin [N = 9195] and placebo/active comparator [N = 4629]) was used to assess lower limb amputation (Figure S3). The 21‐study pool had the same data cut as for the 13‐study pool. Additional studies were analysed for amputations that were not part of the dapagliflozin clinical programme (such as those with a study group receiving saxagliptin plus dapagliflozin). In these two pools, the doses of dapagliflozin given across all studies were 2.5, 5, 10, 20 and 50 mg; however, dapagliflozin 5 and 10 mg were the most frequently used doses.
2.2. Outcomes
The outcomes analysed in the 13‐study pool were overall AEs and serious AEs (SAEs), AEs occurring in ≥3% of the pooled population, and AEs of hypoglycaemia, renal function, volume depletion, UTI, genital infection and fracture. Change in the eGFR (calculated using the Modification in Diet and Renal Disease formula) over time and changes in the plasma lipid profile were also evaluated in the 13‐study pool. DKA and lower limb amputation were evaluated in the 21‐ and 30‐study pools, respectively. Although AEs of special interest were not independently adjudicated, they were identified based on prespecified lists of Medical Dictionary for Regulatory Activities (MedDRA version 15.1) predefined terms. Amputation events were identified by free‐text searches in individual study databases and clinical study reports.
Events of hypoglycaemia were categorized into major, minor or other. Major episodes were defined as symptomatic episodes requiring third‐party assistance because of severe impairment in consciousness or behaviour, with a capillary or plasma glucose value <3 mmol/L and a prompt recovery after glucose or glucagon administration. Minor episodes were defined as either symptomatic, with a capillary or plasma glucose measurement <3.5 mmol/L regardless of need for external assistance, or asymptomatic, with a capillary or plasma glucose value <3.5 mmol/L and not qualifying as a major episode. Other episodes of hypoglycaemia were defined as reported suggestive episodes but did not meet the criteria for major or minor episodes.
2.3. Statistical analysis
All patients who received at least 1 dose of study treatment were included in the analyses. The analyses were post hoc and no statistical hypothesis testing was performed. Descriptive statistics were used to describe safety parameters and all analyses were performed using all available data regardless of rescue during the specified treatment period.
2.4. Ethical approval
All studies were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All enrolled patients provided written informed consent before entering the trial.
3. RESULTS
3.1. Patient disposition
Patient baseline characteristics were generally similar in the dapagliflozin and control groups in the studies included in the 3 study pools (data for the 13‐ and 21‐study pools are shown in Table S1). As data for the 30 studies were not formally pooled, baseline data tables are not available for this pool. In the 13‐study pool, at baseline, the mean duration since T2DM diagnosis was 8.9 years, the mean glycated haemoglobin (HbA1c) value was 8.2% (66 mmol/mol), the mean systolic blood pressure was 132 mm Hg and the majority of patients (>85%) had an eGFR ≥60 mL/min/1.73 m2. Over 90% of patients had a body mass index ≥25 kg/m2. Overall, 10.0% and 11.6% of dapagliflozin‐ and placebo‐treated patients, respectively, were treated with loop diuretics.
3.2. Overall AEs: 13‐study pool
The overall incidence of AEs is shown in Table 1. The proportions of patients reporting ≥1 AE were 60.0% and 55.7% in the dapagliflozin and placebo groups, respectively. Most events were not serious, were considered not related to study drug, and did not lead to study discontinuation. SAEs occurred in 5.1% and 5.4% of patients, respectively, and death was an infrequent event with similar occurrence in each group.
Table 1.
Summary of overall AEs (13‐study pool)
| Placebo group (N = 2295; 957.9 patient‐years) n (%) | Dapagliflozin 10 mg group (N = 2360; 997.6 patient‐years) n (%) | |
|---|---|---|
| ≥1 AE | 1279 (55.7) | 1416 (60.0) |
| AE leading to discontinuation | 82 (3.6) | 102 (4.3) |
| ≥1 SAE | 123 (5.4) | 120 (5.1) |
| SAE leading to discontinuation | 24 (1.0) | 16 (0.7) |
| Deaths | 4 (0.2) | 7 (0.3) |
| Most common adverse events (≥3% in either treatment group) | ||
| Nasopharyngitis | 133 (5.8) | 126 (5.3) |
| Diarrhoea | 87 (3.8) | 79 (3.3) |
| Headache | 83 (3.6) | 81 (3.4) |
| Upper respiratory tract infection | 91 (4.0) | 72 (3.1) |
| UTI | 61 (2.7) | 91 (3.9) |
| Back pain | 56 (2.4) | 83 (3.5) |
3.3. AEs of special interest: 13‐study pool
3.3.1. Hypoglycaemia
The overall incidence of hypoglycaemia was 13.7% and 12.4% with dapagliflozin and placebo, respectively (Table 2). Major hypoglycaemia occurred infrequently, with 3 and 2 events reported with dapagliflozin and placebo, respectively. Most of the events of major hypoglycaemia occurred in patients receiving insulin as background therapy. Only 1 patient in the dapagliflozin group discontinued treatment because of hypoglycaemia. This patient was receiving dapagliflozin added to insulin and metformin, and discontinued treatment after reporting major hypoglycaemia on day 107.
Table 2.
Summary of AEs of special interest (13‐, 21‐ and 30‐study pools)
| Placebo‐controlled 13‐study poola | ||
|---|---|---|
| Placebo group (N = 2295; 957.9 patient‐years) | Dapagliflozin 10 mg group (N = 2360; 997.6 patient‐years) | |
| Hypoglycaemia, n (%) | 284 (12.4) | 324 (13.7) |
| Major episode | 2 (0.1) | 3 (0.1) |
| Leading to discontinuation | 0 | 1 (<0.1) |
| AEs of renal function b , n (%) | 42 (1.8) | 76 (3.2) |
| Most common renal function AEs (>1 patient in either treatment group), n (%) | ||
| Creatinine renal clearance decreased | 16 (0.7) | 27 (1.1) |
| Renal impairment | 12 (0.5) | 20 (0.8) |
| Blood creatinine increased | 9 (0.4) | 15 (0.6) |
| eGFR decreased | 3 (0.1) | 7 (0.3) |
| Renal failure | 2 (0.1) | 4 (0.2) |
| Renal failure acute | 1 (<0.1) | 3 (0.1) |
| Volume depletion AEs c , n (%) | 17 (0.7) | 27 (1.1) |
| Most common volume depletion AEs (>1 patient in either treatment group), n (%) | ||
| Hypotension | 5 (0.2) | 15 (0.6) |
| Syncope | 3 (0.1) | 6 (0.3) |
| Orthostatic hypotension | 6 (0.3) | 2 (0.1) |
| Dehydration | 0 | 2 (0.1) |
| UTI, n (%) | 81 (3.5) | 110 (4.7) |
| Most common UTI AEs (>1 patient in either treatment group), n (%) | ||
| UTI | 61 (2.7) | 91 (3.9) |
| Cystitis | 15 (0.7) | 16 (0.7) |
| Prostatitis | 3 (0.1) | 0 |
| Genital infection, n (%) | 14 (0.6) | 130 (5.5) |
| Most common genital infection AEs (>1 patient in either treatment group), n (%) | ||
| Vulvovaginal mycotic infection | 7 (0.3) | 34 (1.4) |
| Balanitis | 0 | 29 (1.2) |
| Vaginal infection | 1 (<0.1) | 18 (0.8) |
| Genital infection fungal | 2 (0.1) | 12 (0.5) |
| Genital infection | 1 (<0.1) | 11 (0.5) |
| Vulvovaginal candidiasis | 1 (<0.1) | 8 (0.3) |
| Balanitis candida | 0 | 6 (0.3) |
| Vulvovaginitis | 0 | 5 (0.2) |
| Genital candidiasis | 0 | 3 (0.1) |
| Vulvitis | 0 | 2 (0.1) |
| Fractures, n (%) | 17 (0.7) | 8 (0.3) |
| Potential events of DKA | Placebo/comparator‐controlled 21‐study pool | |
|---|---|---|
| Control (N = 3403; 3637.6 patient‐years) | Dapagliflozin total (N = 5936; 6247.2 patient‐years) | |
| SAE of DKA, n | 0 | 1 |
| AE of ketonuria, n | 0 | 2 |
| AE of metabolic acidosis, n | 0 | 1 |
| Estimated incidence of DKA, % (95% CI) | 0 | 0.02 (0.004, 0.059) |
| Estimated incidence of DKA/metabolic acidosis, % (95% CI) | 0 | 0.03 (0.010, 0.089) |
| Amputation | Placebo/comparator‐controlled 30‐study pool | |
|---|---|---|
| Control (N = 4629; 4177 patient‐years) | Dapagliflozin total (N = 9195; 8059 patient‐years) | |
| Patients with lower‐limb amputation, n (%) | 7 (0.2) | 8 (0.1) |
3.3.2. AEs of renal function
Overall, AEs of renal function were reported in 76 (3.2%) and 42 (1.8%) patients receiving dapagliflozin and placebo, respectively. A greater number of events of decreased renal creatinine clearance or renal impairment were observed with dapagliflozin (Table 2; Table S2); however, most events were transient, of mild/moderate intensity, and not accompanied by marked abnormalities of renal function (data not shown). The proportion of patients reporting AEs of renal function was larger in the subgroup with baseline eGFR of 30 to <60 mL/min/1.73 m2 than in the subgroup with eGFR ≥60 mL/min/1.73 m2 (Table S2). When stratified by age, AEs of renal function were more frequent in patients aged ≥65 years than in those aged <65 years (Table S2).
In patients receiving dapagliflozin, there was an initial decline in eGFR at week 1 (Figure 1), followed by a return towards baseline levels over time (mean change from baseline at week 24: −1.45 and −0.67 mL/min/1.73 m2 with dapagliflozin and placebo, respectively). The change from baseline over time was consistent in the overall study population and in patients with baseline eGFR ≥60 mL/min/1.73 m2; however, compared with patients with a higher baseline eGFR, dapagliflozin‐treated patients with an eGFR of 30 to <60 mL/min/1.73 m2 had a smaller reduction in eGFR at week 1, which returned to baseline by week 4 (Figure S4).
Figure 1.
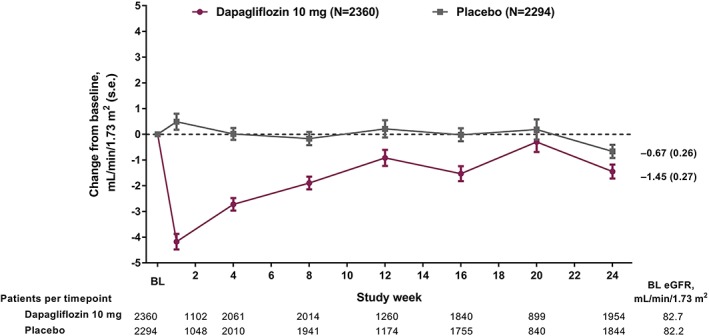
Values for eGFR over time (13‐study pool). eGFR was calculated using the Modification in Diet and Renal Disease (MDRD) formula. BL, baseline; s.e., standard error. Patients at baseline denote the number of treated patients with non‐missing baseline values
3.3.3. Volume depletion
The incidence of AEs of volume depletion (hypotension/hypovolemia/dehydration) was 1.1% and 0.7% with dapagliflozin and placebo, respectively (Table 2). In both treatment groups, half of the AEs of volume depletion occurred by week 8, with 18.5% (5/27) and 17.6% (3/17) occurring during the first 2 weeks of treatment in the dapagliflozin and placebo groups, respectively. When stratified by age, the frequency of AEs of volume depletion was similar in patients aged <65 and ≥65 years in the placebo group, whereas in the dapagliflozin group, patients aged ≥65 years were more likely to have an AE of volume depletion (Table S3). The frequency of AEs of volume depletion in patients using loop diuretics was 2.5 times higher than in patients not using them in both treatment groups (Table S3). In addition, AEs of volume depletion occurred less frequently in patients with baseline eGFR ≥60 mL/min/1.73 m2 than in those with baseline eGFR 30 to <60 mL/min/1.73 m2 regardless of the treatment group (Table S3).
Hypotension was the most common AE of volume depletion in both treatment groups; however, most events were considered unrelated to study drug, were of mild/moderate intensity and did not require drug interruption/discontinuation. Syncope was the second most frequently reported AE of volume depletion, with episodes of syncope distributed throughout the 24‐week treatment period (Table S4).
3.3.4. Urinary tract infection
The occurrence of UTI was 4.7% and 3.5% with dapagliflozin and placebo, respectively (Table 2), and UTIs were more frequent in women than men in both treatment groups (Table S5). The majority of UTIs were mild/moderate in intensity, and UTI‐related discontinuations were infrequent and similar to dapagliflozin and placebo (5 [0.2%] and 2 [0.1%], respectively). Most patients in both treatment groups responded to initial antimicrobial treatment without requiring any additional treatment. Most UTIs had flora consistent with those found in patients with T2DM and were not kidney infections. Furthermore, of the patients who reported UTIs, 26 (23.6%) and 24 (29.6%) patients in the dapagliflozin and placebo groups, respectively, had a medical history of UTI (Table S5).
3.3.5. Genital infection
Genital infections were more frequent with dapagliflozin vs placebo (5.5% vs 0.6%). More women than men experienced genital infections in both treatment groups (Table S5). All events were mild/moderate in intensity and discontinuations because of genital infections were reported for 5 (0.2%) patients treated with dapagliflozin (vulvovaginal mycotic infection [3 patients], balanitis [1 patient] and vulvovaginal candidiasis [1 patient]) and no patient receiving placebo. In both treatment groups, most patients with genital infections (≥80%) responded to initial antimicrobial treatment and did not need additional treatment. Of the patients who reported genital infections, 8 (6.2%) and 2 (14.3%) patients in the dapagliflozin and placebo groups, respectively, had a medical history of recurrent yeast infection (Table S5).
3.3.6. Fracture
The proportion of patients reporting fractures was small in both the dapagliflozin (8 [0.3%]) and placebo (17 [0.7%]) groups (Table 2). The most common fracture locations were the foot, ankle, femoral neck, hand, radius, upper limb and spine (Table S6). There was no consistent pattern to fracture location in either group.
3.3.7. Lipids
Small mean increases from baseline in HDL cholesterol (0.07 mmol/L), LDL cholesterol (0.07 mmol/L) and total cholesterol (0.11 mmol/L) were observed, whereas a small decrease in triglycerides (−0.07 mmol/L) was noted (Table S7).
3.4. DKA: 21‐study pool
One SAE of DKA, 2 AEs of ketonuria and 1 AE of metabolic acidosis were reported in the dapagliflozin group and none in the control group (Table 2). The estimated incidence of DKA/metabolic acidosis with dapagliflozin was 0.03% (95% CI 0.010‐0.089) and the estimated incidence of DKA alone was 0.02% (95% CI 0.004‐0.059). The SAE of DKA occurred in a patient who was receiving dapagliflozin 10 mg added to metformin and insulin. An event of gastroenteritis was also recorded in this patient on the same day (day 214), and both DKA and gastroenteritis were considered unrelated to the study drug. Treatment was continued after the event resolved.
3.5. Amputation: 30‐study pool
Lower limb amputation (ie, leg and foot amputation, mostly affecting the toes) was reported for 8 (0.1%) and 7 (0.2%) dapagliflozin‐ and control‐treated patients, respectively (Table 2). There were no apparent between‐group differences in the baseline characteristics of patients who had an amputation. In both groups, patients who had an amputation had a high prevalence of risk factors for amputation, including neuropathy, CV disease, dyslipidaemia and nephropathy. Time to amputation was similar for dapagliflozin‐ and control‐treated patients. In the dapagliflozin group, the onset of events occurred >150 days after initiation of study treatment in the majority (5/8) of patients.
4. DISCUSSION
This pooled analysis reports the safety findings for dapagliflozin, including the newer emergent safety issues of ketoacidosis and lower limb amputation, thereby adding to the knowledge of the safety of this agent in patients with T2DM. This analysis identified no new safety signals for dapagliflozin.
The risk of hypoglycaemia associated with SGLT2 inhibitors is low, as suggested by their mechanism of action and clinical experience to date, and a similar incidence of hypoglycaemia was observed between dapagliflozin and placebo in the analysis reported here. In a pooled analysis of empagliflozin trials, 23.1%, 22.7% and 22.7% of patients receiving empagliflozin 10 and 25 mg and placebo, respectively, reported AEs of hypoglycaemia.12 In a pooled analysis of canagliflozin trials, AEs of hypoglycaemia were reported for 6.9%, 4.1% and 2.9% of patients receiving canagliflozin 100 and 300 mg, and placebo, respectively, as monotherapy, and for 44.4%, 49.4%, and 34.0% of patients, respectively, who were also receiving background antihyperglycaemic drugs.14
Renal function AEs were observed with dapagliflozin in the present analysis. AEs consistent with decreased renal function have also been shown with other SGLT2 inhibitors (1.8, 1.8 and 2.2 per 100 patient‐years with empagliflozin 10 and 25 mg and placebo, respectively [events consistent with decreased renal function, based on narrow standardised MedDRA queries acute renal failure],13 and 2.1, 2.5 and 2.6 per 100 patient‐years with canagliflozin 100 and 300 mg and control,14 respectively). It is worth noting that there are differences in the clinical trial programmes for each SGLT2 inhibitor regarding the definition of AEs of renal function, the requirements for laboratory testing, and the criteria for discontinuation and reporting of AEs based on laboratory values.
The AEs of renal function observed with dapagliflozin were primarily associated with transient changes in serum creatinine or renal creatinine clearance, and were rarely associated with SAEs of renal failure or marked abnormalities of serum creatinine >2.5 g/dL (data not shown). Notably, some of the dapagliflozin studies had protocol‐specific discontinuation requirements for patients with confirmed calculated creatinine clearance that decreased below a specific level (this level varied across studies) and/or increases in serum creatinine. The AEs of renal function were recorded cumulatively over the 24‐week treatment period; it can be speculated that the initial decline in eGFR may have been recorded as an AE although eGFR levels returned towards baseline over time. The early, transient changes were not indicative of progressive renal dysfunction, however, as demonstrated by the gradual increase towards baseline in eGFR over time. It has been postulated that the transient decreases in eGFR relate to the haemodynamic changes resulting from diuresis and a reduction in blood pressure.20 Furthermore, SGLT2 inhibition restricts proximal tubular reabsorption of glucose and sodium, leading to increased sodium delivery to the macula densa that alters tubuloglomerular feedback causing renal glomerular afferent vasoconstriction. This may have also contributed to the initial decline in eGFR, which subsequently returns to baseline once the initial transient natriuresis observed with dapagliflozin resolved.21, 22
In our pooled analysis, eGFR did not return to baseline in the overall population treated with dapagliflozin, possibly because of the relatively short duration of treatment; however, in longer‐term dapagliflozin studies, the initial decline in eGFR was observed to return to near‐ or above‐baseline levels by week 24 that remained stable to week 102.23 This finding correlates with the findings noted in the empagliflozin and canagliflozin trials. In the EMPA‐REG OUTCOME trial, a short‐term eGFR decrease followed by stabilization over time was observed for empagliflozin (median treatment duration 2.6 years, median observation time 3.1 years).24 Likewise, an initial decrease in eGFR (∼3‐6 weeks) that attenuated over time has also been observed for canagliflozin.25
Osmotic diuresis caused by SGLT2 inhibition may potentially lead to volume depletion in susceptible patients; however, the incidence of volume depletion was low in our analysis and analyses of the currently marketed SGLT2 inhibitors have demonstrated that the risk of these events lessens over time.14, 26 The incidence of events of volume depletion was observed to be low with empagliflozin 10 and 25 mg and placebo (1.8, 1.9 and 1.7 per 100 patient‐years, respectively), with an increased incidence of events observed in patients aged ≥75 years (3.2, 3.0 and 2.3 per 100 patient‐years, respectively) and those receiving loop diuretics (5.0, 4.4 and 3.5 per 100 patient‐years, respectively).13 The incidence of events of volume depletion over 104 weeks was 2.9%, 3.6% and 2.1% with canagliflozin 100 and 300 mg and control, respectively.14 In the CANVAS trial, the incidence of events of volume depletion was 26.0 vs 18.5 per 1000 patient‐years with canagliflozin vs placebo, respectively.1 While volume depletion was low with dapagliflozin and empagliflozin, there is need for caution when prescribing an SGLT2 inhibitor to the elderly and those receiving loop diuretics.
Clinical trials show that SGLT2 inhibitors are associated with an increased risk of genital infections; however, clinical data on the associated risk of UTIs are less consistent. In our analysis, no increase in the risk of UTIs was observed with dapagliflozin vs placebo. The proportions of patients reporting UTIs were 15.1%, 14.5% and 15.0% with empagliflozin 10 and 25 mg and placebo, respectively.13 The incidence rates of UTIs were 7.7, 6.8 and 6.4 per 100 patient‐years with canagliflozin 100 and 300 mg and placebo, respectively.14
The incidence of genital infection was higher with dapagliflozin vs control in the present analysis, and this is consistent with other analyses of dapagliflozin, empagliflozin and canagliflozin.2, 12, 13, 14, 19, 27 A meta‐analysis comparing all SGLT2 inhibitors with placebo observed a 4 to 6 times increased risk of genital infection with SGLT2 inhibitor use.28
There is an increased risk of fracture in patients with T2DM, and some anti‐hyperglycaemic therapies (eg, thiazolidinediones) may further exacerbate this.29 SGLT2 inhibition has the potential to increase serum phosphate levels, which has been hypothesized to exert adverse effects on bone30; however, in this pooled analysis, dapagliflozin did not increase fracture risk vs control. Furthermore, no clinically meaningful changes in bone minerals including calcium, inorganic phosphorus and 25‐hydroxyvitamin D have been reported with dapagliflozin vs placebo.31
No increased incidence of fracture was observed in pooled analyses comparing empagliflozin 10 and 25 mg and placebo (2.8, 2.5 and 2.9 per 100 patient‐years, respectively)13 or canagliflozin 100 and 300 mg and control (1.2, 1.4 and 1.1 per 100 patient‐years, respectively)14; however, the FDA has previously strengthened the drug safety warnings for canagliflozin relating to fracture owing to a higher incidence of fracture observed with canagliflozin vs placebo in an interim analysis of the CANVAS trial.18 This was confirmed by the final analysis of the CANVAS trial in patients with a history of CV disease or at high CV risk, which showed higher fracture rates with canagliflozin vs placebo (16.9 vs 10.9 per 1000 patient‐years; hazard ratio 1.55; 95% CI 1.21‐1.97)1; however, the CANVAS‐R (CANVAS‐Renal) trial in patients with a history of CV disease or at high CV risk1 and other canagliflozin studies that enrolled a general diabetes population have not shown any differences in fracture risk with canagliflozin vs control.32
Small mean changes in lipid variables were observed in the present analysis; however, these changes were not considered to be clinically meaningful. Likewise, small changes in lipids were noted in the pooled analysis of empagliflozin trials.13 Canagliflozin showed increases in LDL cholesterol, non‐HDL cholesterol and HDL cholesterol compared with control14; however, CV safety of canagliflozin has been confirmed in the recent CV outcomes trial (CANVAS) in patients with T2DM and high CV risk.1
Post‐marketing reports of SGLT2 inhibitor‐associated ketoacidosis33 resulted in the FDA issuing a safety communication concerning euglycaemic ketoacidosis in patients receiving SGLT2 inhibitors. The American Association of Clinical Endocrinologists and American College of Endocrinology subsequently released a position statement34 that highlighted the need to fully understand the mechanisms of the metabolic effect of SGLT2 inhibitors, with a focus on analysing the potential problem of DKA with lower‐than‐anticipated glucose levels.
In the present analysis, there was only 1 reported SAE of DKA. It may have occurred in response to insulin dose reduction, which is considered as a precipitant of DKA.34 In a pooled analysis of empagliflozin studies (n = 12 620), incidence rates of DKA were 0.1 (n = 5), <0.1 (n = 1) and 0.1 (n = 5) per 100 patient‐years with empagliflozin 10 and 25 mg and placebo, respectively.13 In clinical trials of canagliflozin (n = 17 596), incidence rates of DKA and related events were 0.52 (n = 4), 0.76 (n = 6), and 0.24 (n = 2) per 1000 patient‐years with canagliflozin 100 and 300 mg and comparator, respectively.34 The majority of canagliflozin‐treated patients reporting serious DKA had associated precipitating factors such as non‐compliance with insulin therapy, and 6 of the 10 canagliflozin‐treated patients indicated evidence of autoimmune diabetes (ie, type 1 diabetes, etc.).35
A low frequency of amputation was observed in dapagliflozin‐ and placebo‐/active comparator‐treated patients; however, the number of patients with amputation events was too low to draw any conclusions based on the differences observed in baseline characteristics. Although drug safety communications have been issued regarding amputations observed with canagliflozin in the CANVAS trial1; possible mechanisms are unclear.
Bladder cancer is another safety issue that has been previously explored, owing to a numerical imbalance observed between dapagliflozin‐ and control‐treated patients in the new drug application submitted to the US FDA in 2011.36 In an updated analysis of >9000 patients in the phase IIb/III dapagliflozin trials, 9/5936 and 1/3403 dapagliflozin‐ and comparator‐treated patients, respectively, reported bladder cancer, with an incident rate ratio of 5.168 (95% CI 0.68‐233.55).37 The totality of the data, with a small number of bladder cancer cases and the lack of carcinogenic potential of dapagliflozin in preclinical studies, does not support a causal relationship between dapagliflozin and increased bladder cancer risk. There was however, the possibility of ascertainment bias possibly because of a thorough investigation of microhaematuria from the time of randomization. Bladder cancer is being further assessed in a post‐authorization safety study and the DECLARE‐TIMI 58 (NCT01730534) trial.
This pooled analysis included treatment‐naïve patients and those receiving multiple background therapies including insulin. All analyses included data after the initiation of rescue therapy. It is possible that background therapies may have contributed to the incidence of some AEs, in particular hypoglycaemia. Another limitation was that the data were collected from clinical trials and because real‐world patients do not always fit the profile of those included in clinical trials, findings from this analysis should be applied to the real world with caution.
In conclusion, overall, the proportion of patients experiencing AEs, including hypoglycaemia, volume depletion, UTI and fracture, was generally balanced in the dapagliflozin and placebo/active comparator treatment groups. AEs of renal function with dapagliflozin were related to transient changes in renal laboratory values. Genital infections were more frequent with dapagliflozin vs placebo, and this finding is consistent with the previously reported data for SGLT2 inhibitors. A very low frequency of lower limb amputations was observed in the patients in the dapagliflozin and control groups. AEs of DKA, ketonuria or metabolic acidosis were rare, with 1 event of DKA reported in the dapagliflozin group. No new safety signals for dapagliflozin were identified in this pooled analysis of a large number of patients.
Supporting information
Figure S1. Flow chart of Phase 2b/3 studies included in the 13‐study pool.
Figure S2. Studies included in the 21‐study pool for the assessment of diabetic ketoacidosis.
Figure S3. Studies included in the 30‐study pool for the assessment of amputations.
Figure S4. eGFR over time by baseline eGFR subgroups (13‐ study pool).
Table S1. a, Baseline demographics and clinical characteristics (13‐study pool). b, Baseline demographics and clinical characteristics (21‐study pool).
Table S2. Adverse events of renal functiona by subgroups (13‐study pool).
Table S3. Adverse events of volume depletion by subgroups (13‐study pool).
Table S4. Patient‐level data on serious and non‐serious AEs of hypotension and syncope.
Table S5. Recurrence, history and severity of genital infections and urinary tract infections (13‐study pool).
Table S6. Incidence of fractures (13‐study pool).
Table S7. Mean changes from baseline in fasting lipids (13‐study pool).
ACKNOWLEDGMENTS
This analysis was supported by AstraZeneca. Editorial support was provided by Shelley Narula of inScience Communications, Springer Healthcare Ltd, London, UK, and was funded by AstraZeneca.
Conflict of interest
S. J. is a consultant for AstraZeneca, Janssen and Eli Lilly. J. S. has attended advisory boards and/or speaker's bureaus for Takeda, Bayer, Novartis, Merck Sharp & Dohme, Amgen, AstraZeneca, Bristol‐Myers Squibb, Novo Nordisk, Sanofi, Berlin‐Chemie, Eli Lilly, Boehringer Ingelheim, Merck, Roche, Ipsen, Pfizer, Janssen and LifeScan, and has received project‐specific research support from AstraZeneca, Takeda, Novartis, Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Novo Nordisk, Sanofi, Ipsen, Pfizer, Janssen, Servier, Eli Lilly, Apitope, Intarcia and Roche. A.S. has attended advisory boards and/or speaker's bureaus for AstraZeneca, Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim and Janssen, and has received research support from Novo Nordisk. C. J. B. received grants and personal fees from AstraZeneca, Bristol‐Myers Squibb, Sanofi and personal fees from Boehringer Ingelheim, Eli Lilly, Johnson & Johnson, Merck Sharp & Dohme, Novo Nordisk, Elcelyx and Poxel, outside of the submitted work. C. K. and A. M. L. are employees and stockholders of AstraZeneca.
Author contributions
All authors made substantial contributions to analysis and interpretation of data. All authors contributed to drafting the article or revising it critically for important intellectual content, and provided final approval of the version to be published.
Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20:620–628. https://doi.org/10.1111/dom.13124
†Presentations The data in this manuscript were presented at the 77th Scientific Sessions of the American Diabetes Association, San Diego, California, June 9–13, 2017, and at the 53rd Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, September 11–15, 2017.
Funding information This study was funded by AstraZeneca.
REFERENCES
- 1.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 3.Mosley JF II, Smith L, Everton E, Fellner C. Sodium‐glucose linked transporter 2 (SGLT2) inhibitors in the management of Type‐2 diabetes: a drug class overview. P T. 2015;40:451–462. [PMC free article] [PubMed] [Google Scholar]
- 4.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513–519. [DOI] [PubMed] [Google Scholar]
- 5.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium‐glucose co‐transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. [DOI] [PubMed] [Google Scholar]
- 6.Scheen AJ. SGLT2 inhibitors: benefit/risk balance. Curr Diab Rep. 2016;16:92. [DOI] [PubMed] [Google Scholar]
- 7.Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: a randomized double‐blind placebo‐controlled 102‐week trial. Diabet Med. 2015;32:531–541. [DOI] [PubMed] [Google Scholar]
- 8.Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add‐on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled 102‐week trial. BMC Med. 2013;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauck MA, Del Prato S, Duran‐Garcia S, et al. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add‐on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes Metab. 2014;16:1111–1120. [DOI] [PubMed] [Google Scholar]
- 10.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–136. [DOI] [PubMed] [Google Scholar]
- 11.Jabbour SA, Hardy E, Sugg J, Parikh S. Dapagliflozin is effective as add‐on therapy to sitagliptin with or without metformin: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study. Diabetes Care. 2014;37:740–750. [DOI] [PubMed] [Google Scholar]
- 12.Kohler S, Salsali A, Hantel S, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016;38:1299–1313. [DOI] [PubMed] [Google Scholar]
- 13.Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of Empagliflozin in patients with type 2 diabetes: pooled analysis of phase I‐III clinical trials. Adv Ther. 2017;34:1707–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu R, Balis D, Xie J, Davies MJ, Desai M, Meininger G. Longer‐term safety and tolerability of canagliflozin in patients with type 2 diabetes: a pooled analysis. Curr Med Res Opin. 2017;33:553–562. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration . FDA drug safety communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. https://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 4, 2016.
- 16.Food and Drug Administration . FDA drug safety communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. 2015. http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Accessed October 11, 2017.
- 17.Food and Drug Administration . FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin. 2017. https://www.fda.gov/Drugs/DrugSafety/ucm557507.htm. Accessed May 16, 2017.
- 18.Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf. 2014;37:815–829. [DOI] [PubMed] [Google Scholar]
- 20.Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh P, Thomson SC. Renal homeostasis and tubuloglomerular feedback. Curr Opin Nephrol Hypertens. 2010;19:59–64. [DOI] [PubMed] [Google Scholar]
- 22.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. [DOI] [PubMed] [Google Scholar]
- 23.Kohan DE, Fioretto P, Johnsson K, Parikh S, Ptaszynska A, Ying L. The effect of dapagliflozin on renal function in patients with type 2 diabetes. J Nephrol. 2016;29:391–400. [DOI] [PubMed] [Google Scholar]
- 24.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. [DOI] [PubMed] [Google Scholar]
- 25.Perkovic V, Jardine M, Vijapurkar U, Meininger G. Renal effects of canagliflozin in type 2 diabetes mellitus. Curr Med Res Opin. 2015;31:2219–2231. [DOI] [PubMed] [Google Scholar]
- 26.Johnsson K, Johnsson E, Mansfield TA, Yavin Y, Ptaszynska A, Parikh SJ. Osmotic diuresis with SGLT2 inhibition: analysis of events related to volume reduction in dapagliflozin clinical trials. Postgrad Med. 2016;128:346–355. [DOI] [PubMed] [Google Scholar]
- 27.Fulcher G, Matthews DR, Perkovic V, et al. Efficacy and safety of Canagliflozin used in conjunction with sulfonylurea in patients with type 2 diabetes mellitus: a randomized, controlled trial. Diabetes Ther. 2015;6:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium‐glucose co‐transporter‐2 inhibitors in type 2 diabetes mellitus: systematic review and network meta‐analysis. Diabetes Obes Metab. 2016;18:783–794. [DOI] [PubMed] [Google Scholar]
- 29.Loke YK, Singh S, Furberg CD. Long‐term use of thiazolidinediones and fractures in type 2 diabetes: a meta‐analysis. CMAJ. 2009;180:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol. 2015;3:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ljunggren O, Bolinder J, Johansson L, et al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab. 2012;14:990–999. [DOI] [PubMed] [Google Scholar]
- 32.European Medicines Agency . Summary of product characteristics: Canagliflozin. 2013.
- 33.Candelario N, Wykretowicz J. The DKA that wasn't: a case of euglycemic diabetic ketoacidosis due to empagliflozin. Oxf Med Case Reports. 2016;2016:144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handelsman Y, Henry RR, Bloomgarden ZT, et al. American Association of Clinical Endocrinologists and American College of endocrinology position statement on the association of Sglt‐2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22:753–762. [DOI] [PubMed] [Google Scholar]
- 35.Peters AL, Henry RR, Thakkar P, Tong C, Alba M. Diabetic ketoacidosis with canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care. 2016;39:532–538. [DOI] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration . Dapagliflozin. Background document. 2011. https://wayback.archive‐it.org/7993/20170405220514/https:/www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM262996.pdf. Accessed October 11, 2017.
- 37.Ptaszynska A, Cohen SM, Messing EM, Reilly TP, Johnsson E, Johnsson K. Assessing bladder cancer risk in type 2 diabetes clinical trials: the Dapagliflozin drug development program as a 'Case Study'. Diabetes Ther. 2015;6:357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of Phase 2b/3 studies included in the 13‐study pool.
Figure S2. Studies included in the 21‐study pool for the assessment of diabetic ketoacidosis.
Figure S3. Studies included in the 30‐study pool for the assessment of amputations.
Figure S4. eGFR over time by baseline eGFR subgroups (13‐ study pool).
Table S1. a, Baseline demographics and clinical characteristics (13‐study pool). b, Baseline demographics and clinical characteristics (21‐study pool).
Table S2. Adverse events of renal functiona by subgroups (13‐study pool).
Table S3. Adverse events of volume depletion by subgroups (13‐study pool).
Table S4. Patient‐level data on serious and non‐serious AEs of hypotension and syncope.
Table S5. Recurrence, history and severity of genital infections and urinary tract infections (13‐study pool).
Table S6. Incidence of fractures (13‐study pool).
Table S7. Mean changes from baseline in fasting lipids (13‐study pool).