Delayed conception in women with low-urinary iodine concentrations: a population-based prospective cohort study (original) (raw)
Abstract
STUDY QUESTION
Is iodine deficiency associated with decreased fecundability?
SUMMARY ANSWER
Moderate to severe iodine deficiency is associated with a 46% decrease in fecundability.
WHAT IS KNOWN ALREADY
Iodine deficiency is common in women of childbearing age but its effect on fecundability has not been investigated.
STUDY DESIGN, SIZE, DURATION
The LIFE Study, a population-based prospective cohort study, enrolled 501 women who had discontinued contraception within 2 months to become pregnant between 2005 and 2009.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women reported on risk factors for infertility by interview then kept daily journals of relevant information. Women used fertility monitors to time intercourse relative to ovulation then used home digital pregnancy tests to identify pregnancies on the day of expected menstruation. Urine samples for iodine analysis were collected on enrollment.
MAIN RESULTS AND THE ROLE OF CHANCE
Samples were in the deficiency range in 44.3% of participants. The group whose iodine–creatinine ratios were below 50 μg/g (moderate to severe deficiency) had a 46% reduction in fecundity (P = 0.028) compared with the group whose iodine–creatinine ratios were in the adequate range: adjusted fecundability odds ratio of becoming pregnant per cycle, 0.54 (95% confidence interval 0.31–0.94).
LIMITATIONS, REASONS FOR CAUTION
Iodine concentrations vary within individuals over time, so the data must be interpreted by group as we have done; residual confounding is possible.
WIDER IMPLICATIONS OF THE FINDINGS
Significant delays in becoming pregnant occur at iodine concentrations that are common in women in the USA and parts of Europe. Replicating these findings will be important to determine whether improving iodine status could be beneficial in improving fecundability.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, USA. Contracts N01-HD-3-3355; N01-HD-3-3356; N01-HD-3-3358 and HHSN275201100001l/HHSN27500007. None of the authors has any conflict of interest to declare.
Keywords: conception, fecundability, fecundity, fertility, iodine
Introduction
Despite the fact that iodine deficiency is a global problem, the effect of mild to moderate iodine deficiency on a woman’s ability to become pregnant has received virtually no attention (Hetzel and Dunn, 1989; Zimmermann et al., 2008; Zimmermann, 2011). One-third of European school-aged children have insufficient iodine intakes (Zimmermann et al., 2015). In the USA, iodine deficiency has become a concern for both pregnant women and women of childbearing age (Caldwell et al., 2013). The percentage of women of childbearing age with urinary iodine sample concentrations below 50 μg/l (deficient) increased 3.8 times between 1971–1974 and 1988–1994 (Hollowell et al., 1998, Pan et al., 2013). More than 30% of urinary iodine concentrations in non-pregnant women of childbearing age are below the target of 100 μg/l (Pan et al., 2013, Stagnaro-Green et al., 2015). To our knowledge, however, no studies have examined the effects of iodine deficiency on fecundity. This investigation was conducted to determine whether urinary iodine status in women attempting to become pregnant was associated with time required to become pregnant.
Materials and Methods
Ethical approval
Full human subjects’ approval was obtained from all participating institutions and all couples gave informed consent before any data collection.
Study cohort and design
The LIFE Study was a prospective cohort study that enrolled 501 couples who were discontinuing contraception to become pregnant or were within 2 months of having done so. The study methods have been reported in detail previously (Buck Louis et al., 2011). In brief, couples were recruited from a defined area of 16 counties in Michigan and Texas. Couples planning a pregnancy were initially contacted by letter, then by a telephone screening interview. After obtaining full and informed consent, couples who wished to participate were seen at their homes for in depth interviews and training. Blood and urine samples were obtained.
The protocol called for keeping daily journals of lifestyle, sexual intercourse, menstruation and pregnancy test results. Women used Clearblue Easy™ Fertility Monitor (SPD Development Company Ltd., formerly Unipath, UK) to help time intercourse to ovulation. A menstrual cycle was determined using fertility monitor data and daily journal information. Pregnancies were identified promptly by the Clearblue Easy™ digital home pregnancy test (Behre et al., 2000). Pregnancy was defined as a positive hCG test on the day of expected menstruation.
Spot urine samples used for iodine and creatinine determinations were collected at the time of enrollment. Factors potentially affecting fecundity were documented by trained interviewers. Height and weight were measured by research assistants using a standard protocol to calculate body mass index. Based on the interview data, measurements and the literature, covariates identified as risk factors and adjusted for in the analysis were: female age (years), body mass index (normal—less than 25, overweight—25–29.9 or obese—30 or greater), preconception serum cotinine concentration categorized as non-smokers (<3 ng/ml), passive (3 to <10 ng/ml), and active smokers (at least 10 ng/ml), (Wall et al., 1988) study site, difference in age between male and female partners, frequency of sexual intercourse during the fertile period (5 days prior to ovulation to 1 day after) and history of thyroid disease.
Eligibility criteria
Women were eligible if they were planning a pregnancy; were married or in a committed relationship; were between 18 and 40 years of age; had self-reported menstrual cycles ranging from 21 to 42 days; had not used injectable hormonal contraception in the past 12 months; were not off contraception for more than 2 months; were able to communicate in English or Spanish; and had a partner aged 18 or older.
Statistical analyses
Iodine status was characterized using World Health Organization (WHO) (WHO, 2007) cut-offs: normal (100 or greater μg/l), mild deficiency (50–99 μg/l), moderate deficiency (20–49 μg/l) and severe deficiency (<20 μg/l). The moderate and severe deficiency groups were combined due to the limited number of samples in the severe group. The analysis was performed using iodine to creatinine concentration ratios (below 50, 50 to 99 and 100 μg/g and above). Thyroid disease (yes/no) was first analyzed as a covariate in all participants because hypothyroidism may be due to severe iodine deficiency. Then thyroid disease was treated as an exclusion because hypothyroidism treatment might affect iodine measures.
Descriptive characteristics were compared using Student’s _t_-test or Wilcoxon nonparametric test for continuous data and chi-squared test or Fisher’s exact test for categorical data. Iodine concentrations were compared by pregnancy status (pregnant, not pregnant or withdrew) using the Kruskal–Wallis test. Cox models for discrete survival time were used to estimate the 95% confidence Interval (CI) and fecundability odds ratio (FOR) which estimates the adjusted odds of becoming pregnant in each cycle conditional on not achieving pregnancy in the previous cycle. FORs <1 indicate a lower probability of becoming pregnant, a reduction in fecundity or a longer time to pregnancy compared with the reference group in that cycle (Rothman et al., 2008).
Couples who withdrew before pregnancy, or who were not pregnant after 12 months of follow-up, were censored at the last observed cycle. Additional analyses were conducted to assess the association between fecundability and iodine–creatinine concentrations using splines, as well as examining the effect of body mass index and cotinine using splines to model nonlinear effects. We also conducted analyses using the iodine concentration while adjusting for creatinine concentration as a covariate.
Laboratory methods
Each urine sample (100 μl) was mixed with 1μl of acetic acid and 1μl of ascorbic acid (Mishra et al., 2000, Minakata et al., 2010) and incubated at room temperature for 10 min. After tetramethylammonium hydroxide digestion was performed at 90°C (Blazewicz et al., 2014, Huynh et al., 2015), 1.87 ml of water and 1.5μl of acetic acid were added and centrifuged for 15 min at 4000 × g. The supernatant was injected into high-performance liquid chromatography–tandem mass spectrometry (Quattro LC, Micromass, Waters Corporation, Milford, MA, USA) with a 250 mm × 2 mm IonPac AS-21 anion exchange column (Dionex, Sunnyvale, CA, USA). An isocratic mobile phase of 20 mM aqueous methylamine was used at a flow rate of 300 μl/min. Iodide was monitored by the mass transition of m/z 127→m/z 127 for I−. The cone voltage and the collision energy were 40 and 22 V, respectively. The limit of quantitation for urinary iodide was 5 ng/ml. Procedural blank, duplicate and matrix spike samples were included in each batch of 50–75 samples analyzed. Iodide was not detected in procedural blanks. Reference urine samples from the Centers for Disease Control and Prevention (CDC) of known iodide concentration (from EQUIP, Ensuring the Quality of Iodine Procedures) were also analyzed with each batch of samples. Laboratory personnel were masked to all other study data.
Results
There were 501 women initially enrolled. 348 (69.5%) entered the study in the woman’s first cycle after beginning unprotected intercourse; 61 (12.2%) entered in the second cycle and 92 (18.4%) entered in their third menstrual cycle. Of the 501 women enrolled, 467 (93%) had sufficient urine available for iodine analysis. Over the 12-month study period, 332 (71%) became pregnant; 47 (10%) did not become pregnant and 88 (19%) withdrew or were lost to follow-up.
Women ranged in age from 18 to 40 years (Table I). Women who completed the study differed significantly from those who withdrew on body mass index (BMI), education, race/ethnicity and income. Women who became pregnant were younger, more likely to have at least a college education, and had a higher income than women who did not become pregnant. Ninety percent of the women in the LIFE cohort took vitamins during the study. The percentage of women who had a history of hypothyroidism or hyperthyroidism did not differ significantly by group. Caffeine and alcohol use were not significantly different across groups.
Table I.
Characteristics of participants by study outcome.
| Variablea | Became pregnant (n = 332) | Did not become pregnant (n = 47) | Withdrew/lost to follow-up (n = 88) | Total (n = 467) |
|---|---|---|---|---|
| Women’s age (years) n (%)* | ||||
| 29 or less | 180 (54.2) | 23 (48.9) | 39 (44.3) | 242 (51.8) |
| 30–34 | 114 (34.3) | 12 (25.5) | 31 (35.2) | 157 (33.6) |
| 35 or more | 38 (11.4) | 12 (25.5) | 18 (20.5) | 68 (14.6) |
| Women’s age in years (median and IQR) | 29.0 (27.0 to 33.0) | 30.0 (28.0 to 35.0) | 30.5 (26.5 to 34.0) | 29.0 (27.0 to 33.0) |
| Women’s race/ethnicity n (%)** | ||||
| Non-Hispanic White | 277 (84.2) | 36 (76.6) | 57 (64.8) | 370 (79.7) |
| Non-Hispanic Black | 6 (1.8) | 3 (6.4) | 13 (14.8) | 22 (4.7) |
| Hispanic | 26 (7.9) | 7 (14.9) | 12 (13.6) | 45 (9.7) |
| Other | 20 (6.1) | 1 (2.1) | 6 (6.8) | 27 (5.8) |
| Women’s education n (%)** | ||||
| Less than high school graduate | 0 (0.0) | 1 (2.1) | 2 (2.3) | 3 (0.6) |
| High school graduate or equivalent | 14 (4.3) | 1 (2.1) | 8 (9.1) | 23 (5.0) |
| Some college or technical school | 48 (14.6) | 9 (19.1) | 28 (31.8) | 85 (18.4) |
| College graduate or higher | 266 (81.1) | 36 (76.6) | 50 (56.8) | 352 (76.0) |
| Women’s caffeinated drinks per menstrual cycle n (%) | ||||
| None | 7 (2.1) | 1 (2.1) | 3 (3.7) | 11 (2.4) |
| 1 to less than 14 | 66 (19.9) | 12 (25.5) | 16 (19.8) | 94 (20.5) |
| 14 to less than 41 | 133 (40.2) | 20 (42.6) | 23 (28.4) | 176 (38.3) |
| 41 or more | 125 (37.8) | 14 (29.8) | 39 (48.1) | 178 (38.8) |
| Women’s alcoholic drinks per menstrual cycle n (%) | ||||
| None | 52 (15.7) | 3 (6.4) | 8 (9.9) | 63 (13.7) |
| 1 to less than 4 | 94 (28.4) | 14 (29.8) | 23 (28.4) | 131 (28.5) |
| 4 to less than 21 | 123 (37.2) | 24 (51.1) | 32 (39.5) | 179 (39.0) |
| 21 or more | 62 (18.7) | 6 (12.8) | 18 (22.2) | 86 (18.7) |
| Household income ($) n (%)** | ||||
| Less than 50 000 | 41 (12.7) | 15 (31.9) | 27 (31.0) | 83 (18.1) |
| 50 000–99 999 | 158 (48.8) | 21 (44.7) | 41 (47.1) | 220 (48.0) |
| More than 100 000 | 125 (38.6) | 11 (23.4) | 19 (21.8) | 155 (33.8) |
| Women’s body mass index n (%)* | ||||
| Less than 25 | 167 (50.3) | 21 (44.7) | 27 (31.0) | 215 (46.1) |
| 25–29.9 | 86 (25.9) | 13 (27.7) | 28 (32.2) | 127 (27.3) |
| 30 or greater | 79 (23.8) | 13 (27.7) | 32 (36.8) | 124 (26.6) |
| Women’s body mass index (median and IQR)** | 25.0 (22.3 to 29.6) | 25.6 (22.3 to 30.7) | 27.9 (24.2 to 34.7) | 25.6 (22.4 to 30.4) |
| Men’s age (years) n (%) | ||||
| 29 or less | 118 (35.5%) | 18 (38.3%) | 29 (33.0%) | 165 (35.3%) |
| 30–34 | 131 (39.5%) | 14 (29.8%) | 31 (35.2%) | 176 (37.7%) |
| 35 or more | 83 (25.0%) | 15 (31.9%) | 28 (31.8%) | 126 (27.0%) |
| Men’s age in years (median and IQR) | 31.0 (28.0 to 34.5) | 32.0 (28.0 to 36.0) | 32.0 (28.0 to 35.0) | 31.0 (28.0 to 35.0) |
| Men’s body mass index n (%) | ||||
| Less than 25 | 60 (18.1%) | 5 (10.9%) | 14 (16.3%) | 79 (17.1%) |
| 25–29.9 | 132 (39.9%) | 19 (41.3%) | 38 (44.2%) | 189 (40.8%) |
| 30 or greater | 139 (42.0%) | 22 (47.8%) | 34 (39.5%) | 195 (42.1%) |
| Men’s body mass index (median and IQR) | 28.8 (26.1 to 32.3) | 29.8 (26.5 to 32.9) | 29.0 (26.3 to 32.7) | 29.0 (26.2 to 32.4) |
Urinary iodine concentrations were sufficient (100 or greater μg/l) in 260 (55.7%), mildly deficient (50–99 μg/l) in 102 (21.8%), moderately deficient (20–49 μg/l) in 97 (20.8%) and severely deficient (<20 μg/l) in 8 (1.7%) samples. Iodine excretion category was not significantly related to age, education, income, race/ethnicity, BMI or history of hypo- or hyperthyroidism (Table II). Any history of hypothyroidism was reported by 36 women (7.7%), of whom 30 (6.4%) were being treated during the study. Any history of hyperthyroidism was reported by 5 women (1.1%), of whom 3 (0.6%) were being treated during the study. There was no significant difference in the probability of becoming pregnant in women who had thyroid disease compared with women who did not.
Table II.
Medical and socio-demographic factors by WHO categories of urinary iodine concentration (μg/l)a.
| Characteristicb | Sufficient ≥ 100 μg/l (n = 260) | Mild deficiency 50–99 μg/l (n = 102) | Moderate or severe deficiency 0–49 μg/l (n = 105) | Total (n = 467) |
|---|---|---|---|---|
| Women’s age in years (median and IQR) | 29.0 (27.0 to 32.0) | 30.0 (27.0 to 33.0) | 30.0 (27.0 to 34.0) | 29.0 (27.0 to 32.0) |
| Women’s race/ethnicity, n (%) | ||||
| Non-Hispanic White | 196 (76.0) | 83 (82.4) | 90 (86.5) | 370 (79.7) |
| Non-Hispanic Black | 11 (4.3) | 7 (6.9) | 4 (3.8) | 22 (4.7) |
| Hispanic | 32 (12.4) | 7 (6.9) | 6 (5.8) | 45 (9.7) |
| Other | 19 (7.4) | 4 (3.9) | 4 (3.8) | 27 (5.8) |
| Women’s education, n (%) | ||||
| Less than high school graduate | 2 (0.8) | 1 (1.0) | 0 (0.0) | 3 (0.6) |
| High school graduate or equivalent (GED) | 11 (4.3) | 5 (5.0) | 7 (6.7) | 23 (5.0) |
| Some college or technical school | 52 (20.2) | 16 (15.8) | 17 (16.3) | 85 (18.4) |
| College graduate or higher | 193 (74.8) | 79 (78.2) | 80 (76.9) | 352 (76.0) |
| Household income ($), n (%) | ||||
| Less than 50 000 | 51 (20.1) | 16 (15.8) | 16 (15.5) | 83 (18.1) |
| 50 000–99 999 | 123 (48.4) | 51 (50.5) | 46 (44.7) | 220 (48.4) |
| More than 100 000 | 80 (31.5) | 34 (33.7) | 41 (39.8) | 155 (33.8) |
| Women’s body mass index, n (%) | ||||
| Less than 25 | 110 (42.5) | 55 (53.9) | 50 (47.6) | 215(46.1) |
| 25–29.9 | 68 (26.3) | 28 (27.5) | 31 (29.5) | 127 (27.3) |
| 30 or greater | 81 (31.3) | 19 (18.6) | 24 (22.9) | 124 (26.6) |
| Women’s body mass index (median and IQR) | 26.2 (22.4 to 32.1) | 24.3 (22.4 to 28.7) | 25.4 (22.5 to 29.1) | 25.6 (22.4 to 30.4) |
The median iodine concentrations and interquartile ranges (IQR) were 112.8 μg/l (53.6 to 216.9) in the entire population, 114.1 μg/l (103.1 to 126.3) in those who became pregnant, 97.2 μg/l (73.5 to 128.5) in those who did not become pregnant, and 113.6 μg/l (92.9 to 138.9) in those who withdrew. Median iodine concentrations were lower in the group that did not become pregnant versus the group that did, but the difference did not reach statistical significance (P = 0.27). Iodine concentrations in the moderate or severely deficient range were more common in the women who did not become pregnant (29.8%) compared with the women who did become pregnant (21.4%) (P = 0.23). Iodine concentrations in the mild deficiency range were present in approximately equal percentages of women who did (22.3%) and did not (21.3%) become pregnant (P = 0.88).
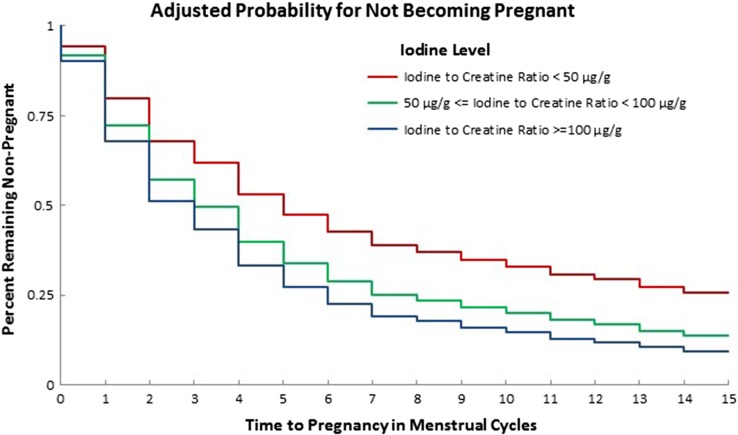
Time to pregnancy provided a powerful tool for examining iodine effects because the time under observation in those who left the study could be included in the analysis. For example, if a woman participated for several months without becoming pregnant, we were able to include that information in the analysis. Using iodine–creatinine ratio adjusting for thyroid disease, we found that the group with iodine–creatinine ratios <50 μg/g took significantly longer to get pregnant (Fig. 1) than those with iodine–creatinine ratios ≥100 μg/g (P = 0.028). In fact, the FOR was reduced by 46% (FOR 0.54, 95% CI 0.31–0.94) over each cycle (Table III). Excluding all women with any past or current thyroid disease, the FOR was 0.56 (0.32, 0.98) P = 0.041. Alternatively, using WHO criteria, adjusting for urine volume by a creatinine term in the regression analysis, the group of women within the moderately or severely iodine-deficient range took significantly longer (P = 0.021) to become pregnant than those whose samples were in the iodine sufficient range, FOR 0.63 (0.43, 0.93). Thus, moderate to severe deficiency was associated with an important increase in time to pregnancy reflecting decreased fecundity after accounting for thyroid disease, censoring and other risk factors by both iodine–creatinine ratio and WHO criteria.
Figure 1.
Percentage of women not achieving pregnancy by number of observed menstrual cycles. Group iodine excretion is reported as iodine–creatinine ratio. Women with any history of thyroid disease are excluded. Data are adjusted for time-off birth control, female age (in years), BMI (categorized as normal, overweight or obese), preconception cotinine concentration categorized as non-smokers (<3 ng/ml), passive (3 ng/ml to <10 ng/ml) and active (at least 10 ng/ml) and study site. ‘Observed events’ is defined as the number of pregnancies occurring in the interval.
Table III.
Fecundability odds ratioa for becoming pregnant by iodine to creatinine ratio.
| Study population | All | All | Exclusions: any history of thyroid disease | Exclusions: any history of hypothyroidism | Exclusions: current treatment for any thyroid disease | Exclusions: current treatment for hypothyroidism |
|---|---|---|---|---|---|---|
| Crude FOR (95% CI) | Adjusted FORb (95%CI) | Adjusted FORb (95%CI) | Adjusted FORb (95%CI) | Adjusted FORb (95%CI) | Adjusted FORb (95%CI) | |
| Iodine to creatinine ratio: (≥100 μg/g) | Reference | Reference | Reference | Reference | Reference | Reference |
| Iodine to creatinine ratio: (<50 μg/g) | 0.530 (0.31, 0.906) | 0.539 (0.310, 0.936) | 0.559 (0.319, 0.977) | 0.567 (0.324, 0.991) | 0.567 (0.325, 0.99) | 0.566 (0.324, 0.988) |
| Iodine to creatinine ratio: (50–99 μg/g) | 0.798 (0.582, 1.094) | 0.806 (0.583, 1.113) | 0.851 (0.607, 1.192) | 0.863 (0.617, 1.208) | 0.823 (0.592, 1.146) | 0.821 (0.590, 1.142) |
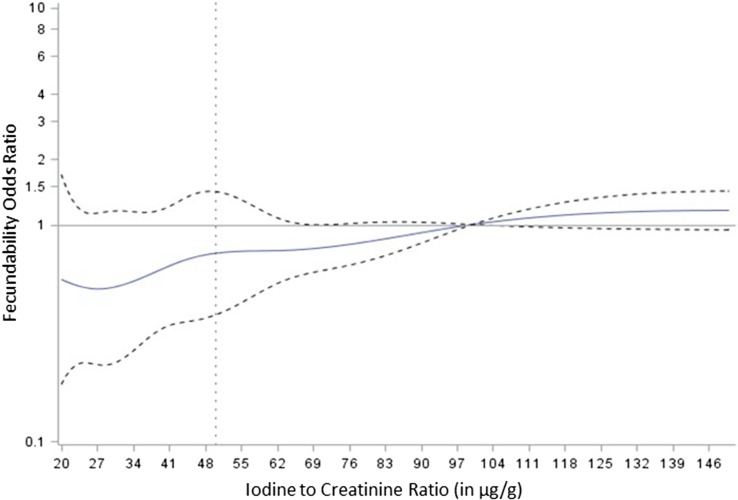
Additional sensitivity analyses dropping women who were being treated for hypo/hyperthyroidism (P = 0.046), dropping all women who ever had hypothyroidism (P = 0.046), and adjusting for BMI and cotinine using splines (P = 0.023) (data not shown) all confirmed that iodine concentrations in the moderate to severe deficiency range were associated with significant delay in becoming pregnant. In contrast, the group whose iodine–creatinine ratios were 50 to 99 μg/g did not have a significantly longer time to pregnancy than women whose ratios were above 100 μg/g (in the sufficient range) after adjustment (FOR 0.81, 95% CI 0.58–1.12) (Table III). Using splines to examine the relationship between iodine and fecundability (Fig. 2) showed that fecundity was decreased when iodine–creatinine ratio was in the deficient range.
Figure 2.
Fecundability odds ratio by iodine concentration spline analysis. The vertical dotted line marks the level at which iodine was associated with a statistically significant reduction in fecundability in the primary analysis. The horizontal solid line is the estimated FOR and the horizontal dashed lines are their 95% CI at each level of iodine.
Discussion
Women who had iodine samples in the moderate to severe deficiency range took significantly longer to become pregnant, experiencing a 46% decrease in probability of becoming pregnant over each cycle compared to the iodine sufficient group. The mildly deficient range group had a smaller, non-significant increase in time to conception suggesting that the risk, if any, is modest in this group. In our study, 44.3% of the participants had sample concentrations below the sufficiency concentration, 22.5% in the moderate to severe deficiency range. The significant delay in time to pregnancy in that group raises serious concerns given the high prevalence of iodine deficiency in women of childbearing age.
There is good biological support for our findings. Mild to moderate iodine deficiency during pregnancy has demonstrable effects on thyroid function (Glinoer, 2001). Thyroid-stimulating hormone concentrations rise; thyroglobulin concentrations are above the normal range in two-thirds of women; and goiter is more common. Treating iodine-deficient sheep increased pregnancy rates from 37 to 100% (Ferri et al., 2003). Despite the lack of studies of iodine deficiency and fecundity in humans, it is well known that insufficient iodine causes hypothyroidism, and there is evidence for a number of mechanisms by which hypothyroidism could cause infertility. Low thyroid hormone concentrations are associated with thyrotropin-releasing hormone elevations that stimulate prolactin, which in turn interferes with GnRH pulsatility (Poppe et al., 2007, Chang and Auchus, 2014). They also cause decreased granulosa cell steroid production (Trokoudes et al., 2006, Chang and Auchus, 2014) and alterations in androgen and estrogen concentrations (Trokoudes et al., 2006, Chang and Auchus, 2014). In addition, thyroid hormone receptors are expressed in oocytes, granulosa cells and cumulus cells (Zhang et al., 1997) and thyroid hormone in conjunction with follicle stimulating hormone regulates ovarian follicle development in rats (Kobayashi et al., 2009). Thus, there are a number of biological mechanisms by which iodine deficiency can interfere with fertility.
This study has numerous strengths. A large number of women were identified shortly after they discontinued using contraception (avoiding the problem that clinically ascertained populations who volunteer for these studies may have chronic infertility). The use of sensitive hCG pregnancy tests ensured accurate diagnosis of pregnancies. Daily journals allowed time to pregnancy to be calculated accurately. Actual urine iodine measures were available at entry, avoiding the problem that dietary reports of iodine intake are inaccurate (Gahche et al., 2013).
Some limitations should be noted. Iodine concentrations may vary considerably within individuals over time (Rasmussen et al., 1999) so data must be interpreted by group as we have done, not by individual. The study population was largely white, so the findings may not be generalizable to other racial/ethnic groups. Iodine was measured when women enrolled. Iodine excretion might have changed over the study period. We were unable to measure thyroid hormones. Some women withdrew during the study; however, we were able to show that their iodine concentrations did not differ significantly from those who completed the study and they did not differ from those who remained on risk factors for delayed conception. Furthermore, many women contributed to the time to pregnancy analysis until they withdrew. Although women who did not become pregnant were much more likely to have samples in the moderate to severe iodine deficiency range than women who became pregnant (30 vs 21%) this difference did not reach statistical significance. We were, however, able to confirm the finding that moderate to severe deficiency decreases fecundity in multiple analyses taking into account urine concentration, treated and untreated thyroid disease and other potential confounding factors and it remained statistically significant in all these analyses.
Iodine deficiency is not rare, occurring in over two-thirds of British school girls. (Vanderpump et al., 2011) Approximately one-third of American women of childbearing age have urinary iodine concentrations below 100 μg/l (Stagnaro-Green et al., 2011; Pan et al., 2013). Although it seems incongruous that deficiency would be common in a population with high sodium intake, the likely explanation is that most sodium in the diet comes from processed food, and it appears that most salt in processed food is not iodized. Almost half the women in our study had iodine samples in the deficient range suggesting that many women trying to become pregnant could be at risk for fecundity problems.
Little information has been available on the impact of mild or moderate iodine deficiency, despite its frequency (Zimmermann et al., 2008, Bath et al., 2013, Rayman and Bath, 2015). Fecundity has not, to our knowledge, been investigated. Therefore, our data provide important guidance for establishing target iodine concentrations in women of childbearing age. The population of women who have low-urinary iodine status (<50 μg/g or 50 μg/l) are clearly at risk for problems should they attempt to become pregnant. The group between 50 and 100 μg/g or μg/l may be at risk, but if they are, the risk is much smaller. The demand for iodine increases substantially during pregnancy, which is why the lower limit of normal is increased to 150 μg/l (WHO, 2007). Therefore, it is reasonable to recommend that concentrations of at least 100 μg/l are desirable in populations of women of childbearing age if they may become pregnant.
In summary, our data show that groups of women with iodine concentrations in the moderate to severely deficient range experience a significantly longer time to pregnancy and diminished fecundity. These findings should be replicated to determine whether iodine deficiency might be added to the list of considerations when evaluating women with fecundity problems. The US and European countries where iodine deficiency is common should evaluate the need for programs to increase iodine intake for women of childbearing age, particularly those trying to become pregnant.
Authors’ roles
G.M.B.L. and J.L.M. designed the study. G.M.B.L., J.L.M., J.W., K.K., Y.W. and Q.W. were involved in data collection. All authors were involved in data analysis and preparation of the manuscript and approved the final version.
Funding
This study was funded by the Intramural Research Program; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Institutes of Health, USA (contracts N01-HD-3-3355; N01-HD-3-3356; N01-HD-3-3358 and HHSN275201100001l/HHSN27500007).
Conflict of interest
None of the authors has any conflict of interest to declare.
References
- Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013;382:331–337. [DOI] [PubMed] [Google Scholar]
- Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, Nieschlag E. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod 2000;15:2478–2482. [DOI] [PubMed] [Google Scholar]
- Blazewicz A, Klatka M, Dolliver W, Kocjan R. Determination of total iodine in serum and urine samples by ion chromatography with pulsed amperometric detection - studies on analyte loss, optimization of sample preparation procedures, and validation of analytical method. J Chromatogr B Analyt Technol Biomed Life Sci 2014;962:141–146. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, Boyd Barr D, Schrader SM, Kim S, Chen Z et al. . Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE Study. Paediatr Perinat Epidemiol 2011;25:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children’s Study and in U.S. women (15-44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013;23:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Auchus R. Chapter 25: endocrine disturbances affecting reproduction In: Strauss JF, Barbieri RL (eds). Yen and Jaffe’s Reproductive Endocrinology, 7th edn Philadelphia: Elsevier, 2014, 551–564. [Google Scholar]
- Ferri N, Ulisse S, Aghini-Lombardi F, Graziano FM, Di Mattia T, Russo FP, Arizzi M, Baldini E, Trimboli P, Attanasio D et al. . Iodine supplementation restores fertility of sheep exposed to iodine deficiency. J Endocrinol Invest 2003;26:1081–1087. [DOI] [PubMed] [Google Scholar]
- Gahche JJ, Bailey RL, Mirel LB, Dwyer JT. The prevalence of using iodine-containing supplements is low among reproductive-age women, NHANES 1999–2006. J Nutr 2013;143:872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinoer D. Pregnancy and iodine. Thyroid 2001;11:471–481. [DOI] [PubMed] [Google Scholar]
- Hetzel BS, Dunn JT. The iodine deficiency disorders: their nature and prevention. Annu Rev Nutr 1989;9:21–38. [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Hannon WH, Flanders DW, Gunter EW, Maberly GF, Braverman LE, Pino S, Miller DT, Garbe PL et al. . Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J Clin Endocrinol Metab 1998;83:3401–3408. [DOI] [PubMed] [Google Scholar]
- Huynh D, Zhou SJ, Gibson R, Palmer L, Muhlhausler B. Validation of an optimized method for the determination of iodine in human breast milk by inductively coupled plasma mass spectrometry (ICPMS) after tetramethylammonium hydroxide extraction. J Trace Elem Med Biol 2015;29:75–82. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Orisaka M, Cao M, Kotsuji F, Leader A, Sakuragi N, Tsang BK. Growth differentiation factor-9 mediates follicle-stimulating hormone-thyroid hormone interaction in the regulation of rat preantral follicular development. Endocrinology 2009;150:5566–5574. [DOI] [PubMed] [Google Scholar]
- Minakata K, Yamagishi I, Kanno S, Nozawa H, Suzuki M, Suzuki O. Determination of iodide in urine using electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:1683–1686. [DOI] [PubMed] [Google Scholar]
- Mishra S, Singh V, Jain A, Verma KK. Determination of iodide by derivatization to 4-iodo-N,N-dimethylaniline and gas chromatography-mass spectrometry. Analyst (Lond) 2000;125:459–464. [DOI] [PubMed] [Google Scholar]
- Pan Y, Caldwell KL, Li Y, Caudill SP, Mortensen ME, Makhmudov A, Jones RL. Smoothed urinary iodine percentiles for the US population and pregnant women: National Health and Nutrition Examination Survey, 2001–2010. Eur Thyroid J 2013;2:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction. Clin Endocrinol (Oxf) 2007;66:309–321. [DOI] [PubMed] [Google Scholar]
- Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr 1999;53:401–407. [DOI] [PubMed] [Google Scholar]
- Rayman MP, Bath SC. The new emergence of iodine deficiency in the UK: consequences for child neurodevelopment. Ann Clin Biochem 2015;52:705–708. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd edn Philadelphia: Lippincott Williams and Wilkins, 2008, 640. [Google Scholar]
- Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S et al. . Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011;21:1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagnaro-Green A, Dogo-Isonaige E, Pearce EN, Spencer C, Gaba ND. Marginal iodine status and high rate of subclinical hypothyroidism in Washington DC women planning conception. Thyroid 2015;25:1151–1154. [DOI] [PubMed] [Google Scholar]
- Trokoudes KM, Skordis N, Picolos MK. Infertility and thyroid disorders. Curr Opin Obstet Gynecol 2006;18:446–451. [DOI] [PubMed] [Google Scholar]
- Vanderpump MP, Lazarus JH, Smyth PP, Laurberg P, Holder RL, Boelaert K, Franklyn JA. Iodine status of UK schoolgirls: a cross-sectional survey. Lancet 2011;377:2007–2012. [DOI] [PubMed] [Google Scholar]
- Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health 1988;78:699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd edn Geneva: WHO press, 2007. [Google Scholar]
- Zhang SS, Carrillo AJ, Darling DS. Expression of multiple thyroid hormone receptor mRNAs in human oocytes, cumulus cells, and granulosa cells. Mol Hum Reprod 1997;3:555–562. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB. The role of iodine in human growth and development. Semin Cell Dev Biol 2011;22:645–652. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Gizak M, Abbott K, Andersson M, Lazarus JH. Iodine deficiency in pregnant women in Europe. Lancet Diabetes Endocrinol 2015;3:672–674. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet 2008;372:1251–1262. [DOI] [PubMed] [Google Scholar]