STEROID METABOLIC DISTURBANCES IN PRADER-WILLI SYNDROME (original) (raw)
. Author manuscript; available in PMC: 2019 Jul 24.
Published in final edited form as: Am J Med Genet. 1987 Dec;28(4):857–864. doi: 10.1002/ajmg.1320280410
Abstract
We have studied steroid sulfate conjugates in serum samples from 17 children with Prader-Willi syndrome (PWS) by extraction, enzymatic hydrolysis and chromatography of the hydrolysed, free steroids. The chromatograms in patients with PWS can be divided into 2 classes. Ten (4 with the deletion on chromosome 15 and 6 without) of 17 had a normal pattern with dehydroepiandrosterone (DHEA) as the only steroid detected. However, 7 out of 17 (3 with the deletion and 4 without) had a very different pattern. The chromatogram derived from these hydrolysates had 5 major peaks. One of these was DHEA; a second peak was tentatively identified as 16α-hydroxy-DHEA on the basis of column retention time and immunoreactivity. The remaining 3, more polar, compounds have not yet been identified. The presence of unusual steroid sulfo-conjugates in serum may correlate with other features of PWS and may be the basis for dividing PWS into two separate disease states: a) PWS-1 associated with DHEA-S as the only sulfo-conjugate and b) PWS-2 associated with unusual sulfo-conjugates. One interesting possiblitiy is that these sulfo-conjugates may have a hormonal function, even though no function has yet been recognized for DHEA-S. Then, PWS may be the common clinical manifestation of a variety of different defects in the sulfo-conjugate metabolic pathway.
Keywords: Prader-Willi syndrome, steroid sulfates, androstene sulfates, sulfo-conjugates
Recovery, estimated at each step by monitoring with [7α-3H]DHEA-S, was as follows: a) extraction step - 85%, b) application to the column - 90%, and c) pooled fractions - 80%. The calculated overall recovery was 61%. Other sulfo-conjugates may not have been recovered from the column fractions with equal efficiency.
The pooled sulfo-conjugate fraction was dissolved in 0.2 M acetate buffer, pH=4.0, and hydrolysed overnight at 37°C with Glusalase™ (DuPont, Garden City, NY) as previously described (Chasalow et al, 1985). The hydrolysis was done in the presence of benzene to prevent immediate oxidation of the hydrolysed products (Vanluchene et al, 1982). After hydrolysis, the free steroids were extracted with ethyl acetate and chromatographed on Sephadex LH-20 with iso-octane:ethyl acetate:methanol (4:1:1) as the eluant. The column (1 × 40 cm, SR-10J, Pharmacia, Piscataway, NJ) was eluted under low pressure in an upward-flow manner (Chasalow and Blethen, 1985). Fractions of 4 ml each were collected. Samples were applied via a 0.7 ml sample loop constructed from 2 4-way valves. The elution positions of some authentic steroids are: a) DHEA - fraction 9-10, b) androsterone - fraction 14-15, c) pregnenolone - fraction 6-7, d) 17-hydroxy-pregnenolone - fraction 14-15 and e) 16α-hydroxy-DHEA - fraction 20-25. DHEA and DHEA-like steroids were detected by RIA on dried column fractions with antibody prepared by immunization of rabbits with DHEA-7-CMO:BSA conjugate as the antigen (Chasalow and Blethen, 1985). The antigen had been purchased from Steraloids, Wilton, NH. [1, 2, 6, 7-3H]DHEA was purchased from Amersham, Chicago, IL. It was repurified by Sephadex LH-20 chromatography when nonspecific binding increased above 5% (Chasalow, 1979).
Serum was collected from subjects with PWS by MGB. At the same time serum was obtained from one parent to serve as an unaffected control (n=17). The labeling did not distinguish patients from control subjects. At the time of analysis (at LIJ) additional samples from obese subjects (n=5), children with premature adrenarche (n=10), and normal subjects (n=23) were added. Laboratory code numbers were assigned randomly without knowledge of which individuals had PWS. Only after the analyses were completed and the chromatograms scored as either DHEA only or as multi-peaked were the PWS subjects identified. All of the control samples (either from parents or from other individuals without PWS) only had cross-reacting material in the chromatogram that co-eluted with DHEA. None had a multi-peaked pattern.
RESULTS
Table I shows the results of measuring DHEA-S on serum samples from PWS individuals. Although in normal individuals the two methods of assaying for DHEA-S levels in serum yield approximately equal results (Chasalow et al, 1986), this was not true for serum from many individuals with PWS. Overall, Antibody A detected 50% more DHEA-S like material than was detected with Antibody B (unmatched assay results). The difference in ratio was statistically different from that observed in normal adults. Although infants also have unmatched results when the assay results are compared, they have higher levels of DHEA-S-like steroids when assayed with Antibody B when compared to Antibody A (Chasalow et al, 1985), which is the exact opposite of the results reported here.
Table 1.
Summary of Steroid Sulfo-Conjugate Results when Compared to sex and Occurrence of the Deletion on Chromosome 15.
| Case | Sex | Deletion | DHEA-S"A"(μg/dl) | DHEA-S"B"(μg/dl) | RatioA/B | Pattern |
|---|---|---|---|---|---|---|
| 1 | F | Yes | 172 | 111 | 1.55 | Multi-peaked |
| 2 | F | Yes | 454 | 185 | 1.72 | Multi-peaked |
| 3 | M | Yes | 466 | 354 | 1.32 | Multi-peaked |
| 4 | M | Yes | 735 | 295 | 2.49 | Multi-peaked |
| 5 | M | No | 423 | 246 | 1.72 | Multi-peaked |
| 6 | M | No | 740 | 381 | 1.94 | Multi-peaked |
| 7 | M | No | 471 | 241 | 1.95 | Multi-peaked |
| mean ± sd | 1.81 ± 0.37 | |||||
| 8 | F | Yes | 864 | 595 | 1.45 | DHEA only |
| 9 | F | Yes | 475 | 381 | 1.25 | DHEA only |
| 10 | F | Yes | 233 | 206 | 1.13 | DHEA only |
| 11 | M | Yes | 668 | 356 | 1.88 | DHEA only |
| 12 | M | Yes | 350 | 336 | 1.04 | DHEA only |
| 13 | M | Yes | 282 | 211 | 1.28 | DHEA only |
| 14 | F | No | 233 | 206 | 1.13 | DHEA only |
| 15 | M | No | 142 | 94 | 1.51 | DHEA only |
| 16 | M | No | 161 | 151 | 1.06 | DHEA only |
| 17 | M | 169 | 130 | 1.30 | DHEA only | |
| mean ± sd | 1.30 ± 0.26 | |||||
| Parents (n=17) | 1.19 ± 0.37 | |||||
| Premature Adrenarche (n=10) | 1.22 ± 0.26 | |||||
| Obesity (n=5) | 1.09 ± 0.05 |
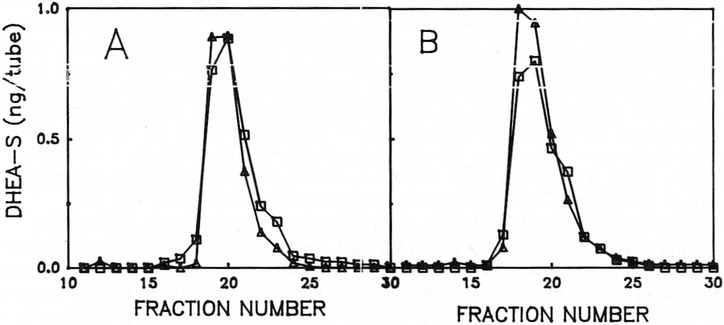
In order to determine the basis for the paradoxical increase in immunoreactive material detected with Antibody A, we extracted serum samples obtained from 17 PWS individuals. Each sample was extracted, chromatographed and representative chromatograms are shown in Figure 2. Panel 2A shows the chromatogram obtained from Case 14. Assays of individual fractions showed the presence of sulfo-conjugates in fractions 17-22 and both assays yielded approximately equal results, thus confirming the result obtained with the whole serum. Panel 2B shows the chromatogram obtained from Case 3, an individual with unmatched assay results. Assays of individuals fractions showed the presence of sulfo-conjugates in fractions 17-22, but both antibodies continued to yield unmatched results, thus confirming the presence of substantial amounts of steroid sulfo-conjugates other than DHEA-S.
Figure 2.
Sephasorb HP column chromatography of steroid sulfo-conjugates from children with PWS. Panel A - Chromatogram derived Case 14. Panel B - Chromatogram derived case 3.
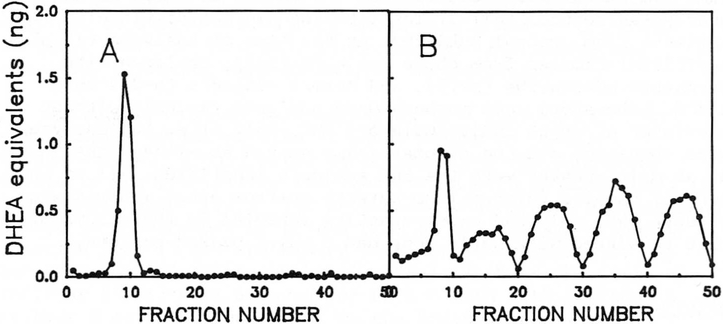
The sulfo-conjugate fractions were pooled, hydrolysed enzymatically with Glusalase, chromatographed and representative results are shown in Figure 3. In brief, 2 patterns of DHEA-like materials could be discerned in column eluates. Class 1 (Panel 3A) was a pattern similar to that observed with normal individuals and showed the presence of one peak of immunoreactive material that co-eluted with authentic DHEA and was assumed to be DHEA on the basis of co-elution and co-immunoreactivity with authentic material. Class 2 (Panel 3B) chromatograms were entirely different (multi-peak). Five immunoreactive peaks were detected with each DHEA-like peak having amounts of immunoreactive material approximately equal to that of DHEA itself. Since the antibody used was relatively specific for DHEA, each of these fractions probably contained much more material than was present in the DHEA fraction. Of the 17 serum samples studied, 10 had the Class 1 pattern and 7 had the Class 2 pattern (Table I). None of 40 samples obtained from individuals without PWS had the Class 2 pattern. Thus, the Class 2 pattern was unique to individuals with PWS but it was not a universal finding in all such individuals. The occurrence of the Class 2 pattern did not correlate with the presence or absence of the deletion on chromosome 15; nor did it correlate with the sex of the individuals (see Table I).
Figure 3.
Sephadex LH-20 column chromatography of steroid sulfo-conjugates from children with PWS after hydrolysis with Glusalase. Panel A - Chromatogram derived from Case 14. Panel B - Chromatogram derived from Case 3.
DISCUSSION
In order to explore the biochemical basis for the discordance in DHEA-S values observed in some patients with PWS, serum samples from affected individuals were extracted with methanol and chromatographed on Sephasorb HP. This column acts as a reverse phase column (the most polar compounds elute first) and characteristically separates steroids on the basis of the type and extent of conjugation. When serum samples from Cases 8-17 were chromatographed in this system, (Fig. 2A), all of the immunoreactive material detected with both antibodies co-eluted with DHEA-S. However, the extracts from Cases 1-7 showed unequal amounts of immunoreactive material in the silfo-conjugate fractions. This indicated that in addition to DHEA-S there were other steroid sulfo-conjugates present. In order to confirm the presence of other steroids, the pooled sulfo-conjugate fractions were subjected to hydrolysis and chromatography (Fig. 3). There were large amounts of steroids other than DHEA present in the hydrolysates from Cases 1-7 but not in those from Cases 8-17. Thus, we are left with the problem of rationalizing a unique steroid sulfo-conjugate pattern present in some, but not all, individuals with PWS. Although we have not yet accumulated enough material to permit the identification of each steroid, certain inferences can be made on the basis of their immunoreactivity and chromatographic mobility. Based on their detection by Antibody B (which has specificity for 3β-steroid sulfates) the native compounds must steroid sulfo-conjugates. The DHEA antibody is specific for δ5-3β hydroxy steroids and the identified compounds probably have that structure. In addition the compounds must be polyhydroxylated to account for their increased polarity. Some model compounds that fit these requirements are 16α-hydroxy-DHEA (probably the third peak), 7α-hydroxy-DHEA, 15β, 16α-dihydroxy-DHEA, 14, 16α-dihydroxy-DHEA, 17α-hydroxypregnenolone, 16α, 17α-dihydroxypregnenolone and other polyhydroxylated androstenes.
The observation of different biochemical parameters associated with characteristic clinical parameters has ample precedent in endocrinology. For example, children with the clinical triad of a) growth failure, b) severely delayed skeletal maturation and c) low somatomedin C levels may have growth hormone deficiency. Many of these children fail to secrete immunoreactive growth hormone from their pituitary even when stimulated by pharmacological agents. When treated with growth hormone, these children respond with increased growth. However, the same clinical triad is also present in children with Laron dwarfism. This group differs from the first group only in that they have high levels of growth hormone present in their serum. The defect in this group is believed to be the absence of growth hormone receptors. Due to the absence of receptors, these children do not respond to exogenous growth hormone with increased growth rates. Recently, some children with the same clinical findings including high levels of serum growth hormone who respond to exogenous growth hormone have been identified. The biochemical basis for this pattern may be the secretion of immunoreactive growth hormone without biological activity. In summary, the clinical picture of short stature, poor growth and low somatomedin levels can be caused by several different biochemical defects. It is only after extensive investigation that endocrinologists have learned to distinguish them and to treat them appropriately (Rosenfeld, 1987).
With the example of growth failure as a model, we can propose a general hypothesis to account for our observations that suggest there is a steroid metabolic disturbance in some children with PWS. First, there are other steroid sulfo-conjugates (in addition to DHEA-S) that are present in serum. These are usually present in small concentrations and are not detected because of the large amounts of DHEA-S in serum (small differences between large numbers). Second, these steroids may function as regulators of appetite and satiety. Third, one subset of PWS (PWS-1) fails to synthesize any of these compounds, the Class 1 pattern. Fourth, one subset of PWS (PWS-2) secretes large amounts of sulfo-conjugates, the Class 2 pattern. Depending on the exact mechanism of the biological function of the sulfo-conjugates, either subset could be caused by a receptor defect or by a defect in synthesis. For example, the absence of the compounds could indicate lack of synthesis, or alternatively, the high levels may be just biosynthetic intermediates accumulated by the attempt to synthesize sufficient amounts of the active material. An example of this latter phenomenon is the synthesis of huge excesses of 17-hydroxyprogesterone by individuals with 21-hydroxylase deficiency. Although at the present time we can not distinguish these possibilities, at least we now have a possible biochemical defect that may be related to the pathogenesis of the syndrome. In any circumstance, the occurrence of the large amounts of sulfo-conjugates in PWS certainly requires additional investigation.
Figure 1.
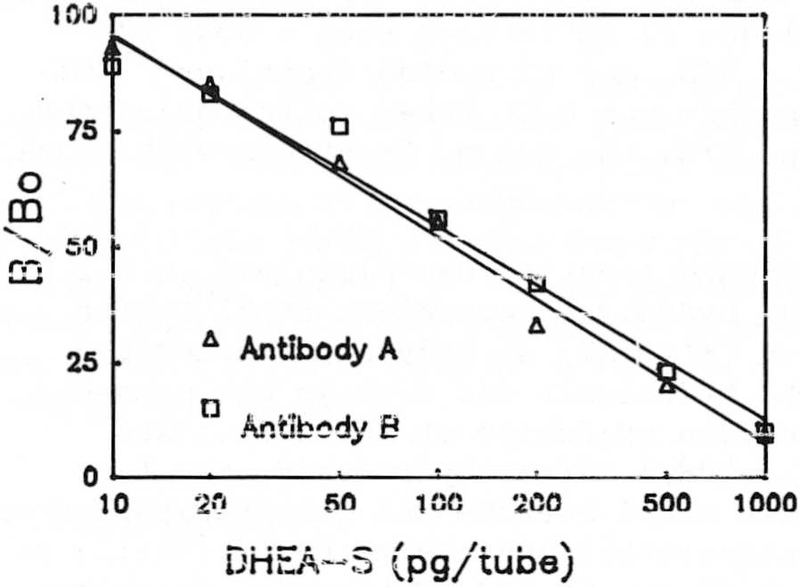
Displacement curves for antibodies A and B with authentic DHEA-S.
ACKNOWLEDGMENTS
This project supported in part by Long Island Jewish Medical Center Competitive Grant # 87-03 (FIC) and by Grant # CNRU (5P30 AM 26657) from Vanderbilt University (MGB).
Contributor Information
Fred I. Chasalow, Schneider Children's Hospital of Long Island Jewish Medical Center, New Hyde Park, NY
Sandra L. Blethen, Schneider Children's Hospital of Long Island Jewish Medical Center, New Hyde Park, NY
Judith G. Tobash, Schneider Children's Hospital of Long Island Jewish Medical Center, New Hyde Park, NY
Darlene Myles, Schneider Children's Hospital of Long Island Jewish Medical Center, New Hyde Park, NY.
Merlin G. Butler, Vanderbilt University Medical Center, Nashville, TN
REFERENCES
- Buster JE, Abraham GE (1972): Radioimmunoassay of plasma dehydroepiandrosterone sulfate. Analyt Lett 5:543–551. [Google Scholar]
- Butler MG, Meaney FJ (1987): An anthropometric study of 38 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet 26:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasalow FI (1979): Mechanism and control of testicular steroidogenesis. J Biol Chem 254:3000–3005. [PubMed] [Google Scholar]
- Chasalow FI, Blethen SL (1985): Dehydroepiandrosterone (DHEA) measurements in cord blood. Steroids 45:187–193. [DOI] [PubMed] [Google Scholar]
- Chasalow FI, Blethen SL, Taysi K (1985): Possible abnormalities of steroid secretion in children with Smith-Lemli-Opitz Syndrome and their parents. Steroids 46: 827–843. [DOI] [PubMed] [Google Scholar]
- Chasalow FI, Granoff AB, Tse TF, Blethen SL (1986): Adrenal steroid secretion in girls with pseudoprecocious puberty due to autonomous ovarian cysts. J Clin Endocrinol Metab 90:828–834. [DOI] [PubMed] [Google Scholar]
- Granoff AB, Chasalow FI, Blethen SL (1985): 17-Hydroxy-progesterone responses to adrenocorticotropin in children with premature adrenarche. J Clin Endocrinol Metab 60:409–415. [DOI] [PubMed] [Google Scholar]
- Rosenfeld RG (1987): Treatment of non-growth hormone deficient short stature In Hintz RL, Rosenfeld RG (eds): "Growth Abnormalites." New York: Churchill Livingstone, pp 109–128. [Google Scholar]
- Vanluchene E, Eechaute W, Vandekerckhove D (1982): Conversion of free 3-beta-hydroxy-5-ene-steroids by incubation with Helix Pomatia. J Steroid Biochem 16:701–705. [DOI] [PubMed] [Google Scholar]