Efficacy of a Carrageenan gel Against Transmission of Cervical HPV (CATCH): interim analysis of a randomized, double-blind, placebo-controlled, phase 2B trial (original) (raw)
. Author manuscript; available in PMC: 2021 Jul 12.
Published in final edited form as: Clin Microbiol Infect. 2018 Apr 21;25(2):210–216. doi: 10.1016/j.cmi.2018.04.012
Abstract
Objectives:
To evaluate the efficacy of a carrageenan-based lubricant gel in reducing the risk of genital human papillomavirus (HPV) infections in women.
Methods:
We conducted a planned interim analysis of a randomized, double-blind, placebo-controlled, phase 2B trial. Women aged 18 years and older were randomly assigned (1:1) to a carrageenan-based gel or a placebo gel to be self-applied every other day for the first month and before and after each intercourse during follow-up. Assessments were performed at 0.5, 1, 3, 6, 9 and 12 months. The primary outcome was incidence of a new infection by an HPV type that was not present at baseline. Intention-to-treat analyses were performed.
Results:
Between January 2013 and June 2017, a total of 280 participants were randomly assigned to the carrageenan (n = 141) or the placebo (n = 139) arm. All participants were included in safety analyses, but three (1%) were excluded from efficacy analyses (HPV results unavailable for two participants in the carrageenan and one participant in the placebo arm). The median follow-up time was 9.2 months (interquartile range, 1.9–13.2 months). A total of 59 (42%) of 139 participants in the carrageenan arm and 78 (57%) of 138 participants in the placebo arm became infected by at least one new HPV type (hazard ratio = 0.64, 95% confidence interval = 0.45–0.89, p 0.009). A total of 62 (44%) of 141 participants in the carrageenan arm versus 43 (31%) of 139 participants in the placebo arm reported an adverse event (p 0.02), none of which was deemed related to the gels.
Conclusions:
Our trial's interim analysis suggests that using a carrageenan-based lubricant gel can reduce the risk of genital HPV infections in women.
Keywords: Carrageenan, Gel, HPV, Human papillomavirus, Microbicide, Randomized controlled trial
Introduction
Cervical cancer is the fourth most common cancer and the fourth leading cause of cancer death in women worldwide, with 528 000 estimated new cases and 265700 estimated deaths in 2012 [1]. Virtually all cases of cervical cancer are caused by human papillomavirus (HPV) infection [2,3]. HPV is the most common sexually transmitted infection in the world, and most sexually active individuals will be infected by HPV at some point during their lifetime [4]. Although the majority of infections will clear within 2 years without any symptoms [5], others can lead to the development of clinical lesions such as warts or anogenital and oral cancers [6-8].
Universal vaccination of preteen girls is a highly effective intervention against HPV infection and its clinical consequences [9,10]. However, this intervention has important limitations: it only protects against specific HPV types, it is exclusively prophylactic (i.e. only effective in previously uninfected women) and it is too expensive for developing countries, which bear the heaviest burden of HPV and cervical cancer [11,12]. Aside from vaccination, there is currently no other effective intervention against HPV infection.
Carrageenan, an anionic polymer extracted from red algae, has been identified as a potent HPV infection inhibitor in preclinical studies [13-17]. This molecule is widely used as a thickener in many commercially available cosmetic and food products, including personal lubricants. Previous safety and acceptability trials of lubricants containing carrageenan reported that they were safe and well received [18-21]. These results supported clinical efficacy testing.
We conducted a phase 2B trial to assess the efficacy of a carrageenan-based lubricant gel in reducing genital HPV incidence and prevalence among sexually active women. We also aimed to evaluate safety and acceptability of the gel as well as adherence. We report here the planned interim analysis of the first primary outcome: incidence of a new HPV infection.
Methods
Study design
The Carrageenan gel Against Transmission of Cervical HPV (CATCH) study is a randomized, double-blind, placebo-controlled, phase 2B trial conducted in three outpatient clinics in the greater Montreal area, Canada. The study complied with the Guideline for Good Clinical Practice and regulatory requirements of Natural Health Product Directorate and Health Canada. Ethical approval was obtained from institutional review boards at participating centres (McGill University, Concordia University, Université de Montréal and CLSC Samuel-de-Champlain). This trial is registered with ISRCTN (http://www.isrctn.com/, ISRCTN96104919).
Participants
Women aged 18 years and older (the initial 40-year upper limit was abolished because of recruitment issues) were recruited through advertisements displayed in colleges, universities and venues frequented by students in Montreal and on social media. Participants were eligible if they (a) had an intact uterus, (b) had vaginal sex with a male partner in the last 3 months, (c) were expecting to have vaginal intercourses in the next 3 months with the same or different male partners and (d) were not currently in a relationship for longer than 6 months. Exclusion criteria were having a history of cervical lesions, cancer or genital warts; being HIV positive; being pregnant or currently breast-feeding; having had a pregnancy, abortion or genital surgery in the last 6 weeks; and having a known allergy or hypersensitivity to any lubricant or component of the study gels. All participants provided written informed consent.
Randomization and masking
Research nurses enrolled participants onto the trial. Participants were randomly assigned (1:1) to either the carrageenan-based or placebo gel as per a computer-generated concealed sequence using a block size of eight. Both gels were clear, odourless and tasteless, were of similar viscosity and were bottled in identical containers. To minimize the risk of unblinding, eight different product codes were used (four for each gel). Randomization and product code assignment was done via an online administration platform. Participants, nurses, laboratory staff and investigators were masked to group allocation.
Intervention
Participants were asked to self-apply the gel every other day for the first month and before each intercourse during the study period. Added in October 2015, they were also asked to apply the gel after each intercourse as per recent research findings suggesting that this might provide better protection [15]. They were instructed by a research nurse who followed a standardized protocol to apply the gel (at least 10 mL) inside their vagina and on their genital area using their fingers or an applicator. The carrageenan-based and placebo gels are manufactured in a US Food and Drug Administration good manufacturing processes–compliant facility (Carra-Shields Lab, St Petersburg, FL, USA).
Outcomes and procedures
The first primary outcome was the incidence of a newly detected infection by an HPV type that was not present at baseline. The second primary outcome was clearance of HPV types observed at baseline. Visits with a research nurse were scheduled at enrolment and 0.5, 1, 3, 6, 9 and 12 months. Sociodemographic, behavioural and sexual history data were collected using computer-assisted self-administered questionnaires. HPV testing was performed on vaginal samples that were self-collected as per a validated protocol [22]. HPV detection and genotyping of 36 genital HPV types was performed with Roche's linear array PCR assay (Roche, Basel, Switzerland). Types were analysed separately but also according to phylogenetic grouping based on tissue tropism and oncogenicity: alphapapillomavirus subgenus 1 (low oncogenic risk: HPVs 6, 11, 40, 42, 44, 54), subgenus 2 (high and intermediate oncogenic risk: HPVs 16, 18, 26, 31, 33, 34, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82) and subgenus 3 (commensal types: HPVs 61, 62, 71, 72, 81, 83, 84, 89) [23-25]. Participants were asked to abstain from oral sex, vaginal intercourse and gel use 48 hours before a visit. Previous investigations have shown that carrageenan does not interfere with HPV detection and genotyping [14].
Secondary outcomes were adherence to the intervention and safety/acceptability of the gels. In addition to self-administered questionnaires completed at each visit, participants were asked to fill in an electronic calendar on their daily sexual activities as well as condom and gel use. They also had the opportunity to report any adverse event at any time using this calendar. Research nurses were automatically notified via e-mail when a participant reported an adverse event, and an appointment with the study physician (PPT) was scheduled if needed. The Female Genital Grading Table for Use in Microbicide Studies [26] was used for the reporting and grading of adverse events.
To validate the vaccination history, we e-mailed all participants and asked for a proof of vaccination when applicable. This validation procedure was subsequently adopted for all women reporting having been vaccinated at the screening interview.
Statistical analysis
On the basis of previous studies involving similar populations [5,27], we assumed a 50% prevalence of HPV infections at baseline as well as a 20% cumulative incidence and a 40% clearance in the placebo group at 12 months. Using the method of Dupont and Plummer [28], we calculated that a total of 465 participants were required to detect a 50% difference in incidence (and 388 to detect a 50% difference in clearance) with 80% power using a two-sided significance level of 0.05 and assuming losses to follow-up of 10%. An interim analysis was planned for the time the study reached about 50% of the target sample size. A Data Safety and Monitoring Committee and a Steering Committee oversaw the study.
Only the first primary outcome (incidence) was analysed in this interim analysis. Analyses were done by intention to treat. We estimated hazard ratios (HR) and 95% confidence intervals (CIs) using univariate Cox models for the first occurrence of a new infection in each participant. We also computed two different models taking into account correlated data and considering all new HPV types acquired by each participant throughout follow-up. The first (model 1) was a Cox model stratified by HPV types and clustered by participants; the second (model 2) was a mixed-effect survival-time model considering an exponential distribution and a random effect for participants.
A priori subgroup analyses considering only the first occurrence of a new infection were performed according to the main baseline characteristics and to cumulative compliance to gel use before intercourse at the time of failure or censoring. A post hoc sensitivity analysis was conducted on the data of participants who validated their vaccination status by e-mail. All statistical analyses were performed using Stata 14.2 (StataCorp, College Station, TX, USA).
Results
Between 16 January 2013 and 9 June 2017, a total of 280 women were randomly assigned to the carrageenan (n = 141) or placebo (n = 139) arm (Fig. 1). All participants received the allocated intervention. All participants were included in the safety analysis, but two participants in the carrageenan arm and one in the placebo arm were excluded from the efficacy analysis because their HPV results were not available at the time of interim analysis.
Fig. 1.
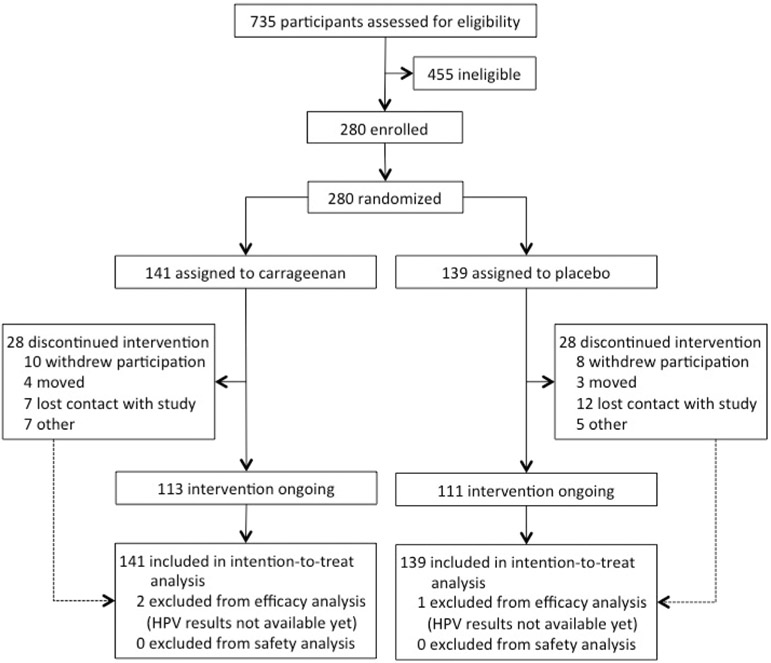
Trial profile design and participant allocation.
Participants' baseline and follow-up characteristics were similar between arms (Table 1 and Supplementary Fig. S1). The median follow-up time for the whole cohort was 9.2 months (interquartile range, 2.6–13.2 months). More individuals were infected by multiple HPV types (coinfection) in the placebo arm (n = 56, 40.3%) than in the carrageenan arm (n = 42, 29.8%), but this difference did not reach statistical significance (p 0.28). Detailed HPV prevalence data are shown in Supplementary Tables S1 and S2. Overall, 117 (41.8%) participants reported having been vaccinated: 80 (68.4%) with the quadrivalent vaccine, two (1.7%) with a mix of the quadrivalent and the nonavalent vaccines and 35 (29.9%) could not remember the vaccine name. Ninety-nine participants (35.4%) confirmed their vaccination status by e-mail. Of these, 15 changed the answer they gave in the enrolment questionnaire. At the interim analysis closing date, 98 (35.0%) of the 280 participants completed all seven visits: 51 in the carrageenan and 47 in the placebo arm (Supplementary Fig. S1).
Table 1.
Baseline and follow-up characteristics of participants
| Characteristic | Carrageenan (n = 141) | Placebo (n = 139) |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 24.6 (5.8) | 23.4 (4.7) |
| Median (IQR) | 23.0 (20.4–27.0) | 22.2 (19.9–25.2) |
| Missing, n (%) | 0 | 0 |
| Ethnicity, n (%) | ||
| French Canadian | 29 (20.6%) | 33 (23.7%) |
| English Canadian | 38 (27.0%) | 43 (30.9%) |
| Black Canadian | 11 (7.8%) | 9 (6.5%) |
| Latin American | 13 (9.2%) | 13 (9.4%) |
| East Asian | 9 (6.4%) | 14 (10.1%) |
| Other | 37 (26.2%) | 25 (18.0%) |
| Missing | 4 (2.8%) | 2 (1.4%) |
| Marital status, n (%) | ||
| Single | 91 (64.5%) | 97 (69.8%) |
| Living with a partner | 12 (8.5%) | 14 (10.1%) |
| Married | 3 (2.1%) | 1 (0.7%) |
| Divorced/separated/widowed | 35 (24.8%) | 27 (19.4%) |
| Missing | 0 | 0 |
| Smoking status, n (%) | ||
| Never | 96 (68.1%) | 78 (56.1%) |
| Past | 33 (23.4%) | 40 (28.8%) |
| Current | 12 (8.5%) | 20 (14.4%) |
| Missing | 0 | 1 (0.7%) |
| Age at first intercourse (years) | ||
| Mean (SD) | 17.5 (3.1) | 16.9 (2.5) |
| Median (IQR) | 17 (16–19) | 17 (16–18) |
| Missing, n (%) | 3 (2.1%) | 3 (2.2%) |
| No. of lifetime sex partners, n (%) | ||
| 1–3 | 32 (22.7%) | 30 (21.6%) |
| 4–6 | 32 (22.7%) | 19 (13.7%) |
| 7–10 | 26 (18.4%) | 32 (23.0%) |
| 11–20 | 25 (17.7%) | 31 (22.3%) |
| >20 | 26 (18.4%) | 27 (19.4%) |
| Missing | 0 | 0 |
| No. of sex partners in last month, n (%) | ||
| 0 | 29 (20.6%) | 24 (17.3%) |
| 1 | 87 (61.7%) | 85 (61.2%) |
| ≥2 | 25 (17.7%) | 30 (21.6%) |
| Missing | 0 | 0 |
| HPV DNA status, n (%) | ||
| Negative | 72 (51.1%) | 58 (41.7%) |
| Monoinfection | 25 (17.7%) | 24 (17.3%) |
| Coinfection | 42 (29.8%) | 56 (40.3%) |
| Missing PCR resultsa | 2 (1.4%) | 1 (0.7%) |
| Subgenus 1b | 16 (11.4%) | 29 (20.9%) |
| Subgenus 2c | 57 (40.4%) | 64 (46.0%) |
| Subgenus 3d | 29 (20.6%) | 45 (32.4%) |
| Vaccination status, n (%) | ||
| Yes | 54 (38.9%) | 63 (45.7%) |
| No | 80 (57.6%) | 74 (53.6%) |
| Missing | 5 (3.6%) | 1 (0.7%) |
| Validated vaccination status, n (%) | ||
| Yes | 15 (10.8%) | 16 (11.6%) |
| No | 35 (25.2%) | 33 (23.9%) |
| Missing | 89 (64.0%) | 89 (64.5%) |
| Follow-up time (months) | ||
| Mean (SD) | 8.5 (6.0) | 8.4 (5.8) |
| Median (IQR) | 8.8 (2.6–13.7) | 9.8 (2.6–13.0) |
| No. of visits per woman, n | ||
| Mean (SD) | 4.9 (2.0) | 5.1 (1.9) |
| Median (IQR) | 5 (3–7) | 6 (3–7) |
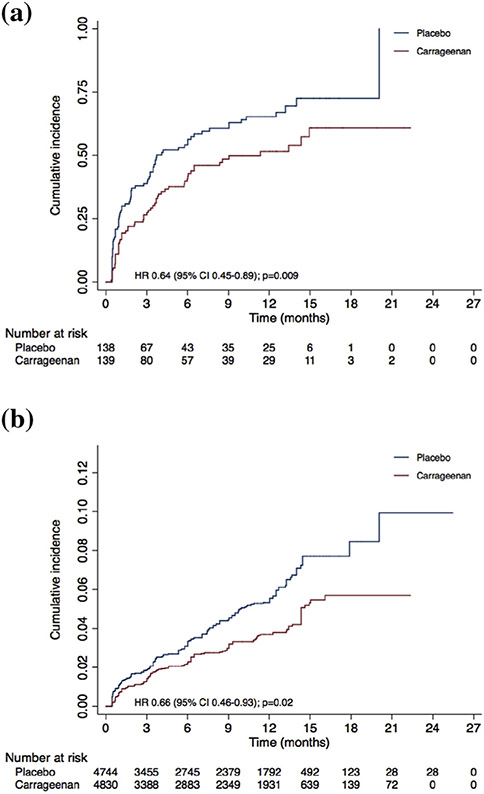
Fig. 2 shows the cumulative incidence of newly detected HPV infections for both arms. A total of 137 participants acquired at least one HPV type: 59 (41.8%) in the carrageenan and 78 (56.1%) in the placebo arm. The HR for the first occurrence of a new infection was 0.64 (95% CI = 0.45–0.89, p 0.009) (Fig. 2a). When considering all new HPV types acquired by each participant (not only the first infection), 139 infections occurred in the carrageenan versus 198 in the placebo arm (rate ratio = 0.68, 95% CI = 0.55–0.85, p 0.0007). Fig. 2b shows the cumulative incidence of all new HPV types by arm. When accounting for the correlated data structure and multiplicity of HPV types, the HRs were 0.66 (95% CI = 0.46–0.93, p 0.02) for model 1 and 0.60 (95% CI = 0.39–0.94, p 0.03) for model 2.
Fig. 2.
Cumulative incidence of new HPV infections by arm. Cumulative incidence of (a) first new HPV infection and (b) all new HPV infections. Numbers at risk for (b) correspond to number of HPV types that participants could have potentially acquired at each time point, i.e. 36 HPV types tested multiplied by number of participants at time point minus HPV types positive at baseline for these participants. HPV, human papillomavirus.
The actuarial mean time to a first infection was significantly greater in the carrageenan than in the placebo arm (Table 2). When restricting to the participants who acquired a first episode (arithmetic mean), the differences in time to acquisition were small and nonsignificant. A lower incidence of new HPV infection was consistently observed for all alphapapillomavirus subgenera. The reduction was significant for subgenus 1 (HR = 0.47, 95% CI = 0.24–0.89) and subgenus 2 (HR = 0.64, 95% CI = 0.44–0.94).
Table 2.
Incidence of and average time to new HPV infection by study arma
| HPV type | Carrageenan | Placebo | Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incidentcases | Actuarial mean(95% CI) | Arithmetic mean(95% CI) | Median(95% CI) | Incidentcases | Actuarial mean(95% CI) | Arithmetic mean(95% CI) | Median(95% CI) | Hazard ratio(95% CI) | p | |
| Any HPV type | 59 | 11.8b (10.0–13.7) | 3.5 (2.6–4.5) | 11.3 (6.0–)c | 78 | 8.3 (6.8–9.9) | 3.1 (2.3–4.0) | 3.7 (3.2–7.1) | 0.64 (0.45–0.89) | 0.009 |
| Subgenus 1d | 14 | 19.7b (18.4–21.0) | 5.1 (2.5–7.7) | NR | 27 | 15.3b (13.7–16.8) | 4.8 (3.0–6.6) | NR | 0.47 (0.24–0.89) | 0.02 |
| Subgenus 2e | 47 | 13.8b (12.0–15.7) | 4.0 (2.8–5.2) | 14.9 (9.3–)c | 64 | 10.4 (8.8–12.0) | 3.7 (2.6–4.7) | 9.0 (5.8–14.0) | 0.64 (0.44–0.94) | 0.02 |
| Subgenus 3f | 29 | 17.0b (15.3–18.6) | 4.2 (2.7–5.8) | NR | 39 | 13.7b (12.1–15.2) | 4.8 (3.4–6.2) | NR | 0.69 (0.42–1.11) | 0.15 |
The results favouring the intervention were consistent across all subgroups except for self-reported vaccination (Fig. 3). However, there was no indication of heterogeneity of effects when stratifying by validated vaccination status. There was no indication of a dose–response relationship with the estimated cumulative compliance.
Fig. 3.
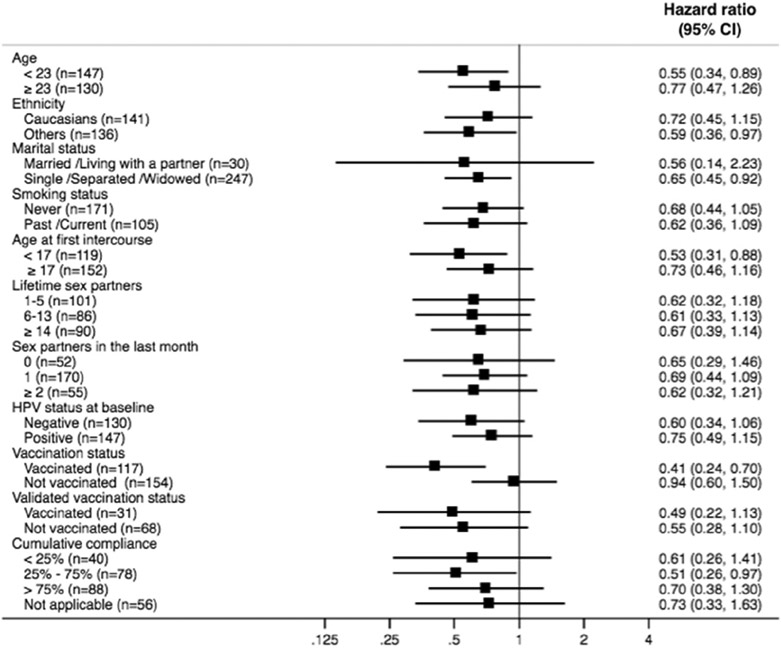
Subgroup analyses. Cumulative compliance to gel use is calculated from enrolment to time of failure or censoring. ‘Not applicable’ corresponds to participants who reported no intercourse between enrolment and time of failure or censoring.
Overall, 258 adverse events were reported by 105 (37.5%) of the 280 participants (Supplementary Table S3). More participants reported an adverse event in the carrageenan than in the placebo arm (Table 3). However, none of these adverse events was deemed related to the gels by the study physician, who was blinded to group allocation. No participant withdrew or was withdrawn from the study because of an adverse event.
Table 3.
Adverse events
| Adverse event | Carrageenan(n = 141) | Placebo(n = 139) | pa |
|---|---|---|---|
| Any | 62 (44.0%) | 43 (30.9%) | 0.02 |
| Unusually painful or heavy period | 0 | 3 (2.2%) | 0.08 |
| Vaginal bleeding in between menstrual periods | 3 (2.1%) | 4 (2.9%) | 0.69 |
| Pain during vaginal sex | 8 (5.7%) | 2 (1.4%) | 0.06 |
| Unusual vaginal discharge | 8 (5.7%) | 2 (1.4%) | 0.06 |
| Itching, burning or pain in genital area | 16 (11.3%) | 11 (7.9%) | 0.33 |
| Genital sore/ulcer | 1 (0.7%) | 2 (1.4%) | 0.55 |
| Needing to urinate more often than usual | 1 (0.7%) | 3 (2.2%) | 0.31 |
| Pain while urinating | 4 (2.8%) | 1 (0.7%) | 0.18 |
| Blood in urine | 3 (2.1%) | 1 (0.7%) | 0.32 |
| Lower abdominal pain | 2 (1.4%) | 0 | 0.16 |
| Lower back pain | 0 | 0 | — |
| Other | 16 (11.3%) | 14 (10.1%) | 0.73 |
Discussion
This is the first trial designed to evaluate the efficacy of a carrageenan-based gel in reducing the risk of genital HPV infections in women. In this interim analysis, the use of a carrageenan-based gel was associated with a 36% protective effect compared to the use of a placebo gel. The protective effect was observed for both low- and high-risk HPV types, and the gel appeared to be safe.
Our results for the incidence of new HPV infections are in accordance with initial observations in preclinical studies [13-17]. Carrageenan was first identified as a potent HPV infection inhibitor in an in vitro microbicide study [13]. Carrageenan is a sulphated polysaccharide that is widely used in the food and cosmetic industries for its thickening and stabilizing properties. Its structure is similar to heparan sulphate, an HPV cell-attachment factor, which allows it to bind directly to the HPV virus capsid. It thus prevents HPV infection by precluding the attachment of the virion to cell-surface receptors. In the aforementioned study [13], several commercially available lubricants containing carrageenan exhibited significant inhibition at dilutions up to 106-fold. These findings were later confirmed in two animal studies [16,17] and one ex vivo study [14]. In our study, carrageenan showed activity against all HPV types tested irrespective of taxonomic grouping, which is consistent with what has been observed in preclinical studies and with the proposed mechanism of action of carrageenan.
We chose to publish this interim analysis given the observed appreciable effect size of the intervention. Our decision was approved by the Data Safety and Monitoring Committee on the basis of the potential for these interim findings to prompt other investigations in different populations. Although results may fluctuate with more participants enrolled and a longer follow-up time, interim analyses tend to be confirmed by final analyses in most randomized controlled trials [29].
Although considerable, the effect size observed in our study was smaller than what we expected on the basis of preclinical studies. However, preclinical findings often translate into smaller effect sizes in clinical settings, which are much less controlled environments. In a clinical trial, participants' full compliance to the intervention is not guaranteed. Although by design CATCH study participants were at high risk of HPV exposure, we do not know the actual proportion of participants who were exposed to new HPV types during follow-up. Nevertheless, owing to randomization, exposure to HPV can be assumed to be comparable between arms. However, there may have been a difference in the risk of being infected by a new HPV type because there were more coinfections at baseline in the placebo than in the carrageenan arm, although this difference was not statistically significant. If more participants in the placebo arm were in fact not at risk of acquiring a new HPV infection because they were already infected by all the types to which they were exposed during follow-up, this could have biased the estimate towards the null.
Our results are partially in agreement with those of a post hoc substudy of a randomized, double-blind, placebo-controlled trial conducted in South Africa that was originally designed to assess the efficacy of a carrageenan-based gel in reducing the risk of HIV infection in women [30]. In this substudy, carrageenan was associated with a 38% protective effect, but only among the most compliant participants. In our study, a higher compliance was not associated with a higher protective effect. However, our exploratory analysis considered only compliance to gel use before intercourse. Other components such as gel use after intercourse and every other day for the first month as well as potential confounders of the relationship between compliance and incidence of HPV infections were not taken into account. A more thorough analysis of compliance will be reported in a subsequent publication.
The number of adverse events reported by participants was surprisingly high. However, none was attributed to the gels, and no participant withdrew from the study because of an adverse event. We believe that the high number of opportunities that participants had to report any noticeable change through the daily electronic calendar (257 days (mean follow-up) × 280 participants = 71960 opportunities) could partly explain the high number of events reported. There were also more adverse events in the carrageenan than in the placebo arm, which is in disagreement with previous safety studies that reported no association between carrageenan and any abnormal genital findings and/or change in vaginal flora [18-21]. In these studies, adverse events were mostly mild, not attributed to gel use and comparable between arms.
Considering the theoretical mechanism of action of carrageenan, we have no reason to believe that it would work differently depending on any physical, social or psychologic characteristic. However, our population (mainly composed of students) may not be representative of the general population, and generalization should be done with caution. Personal and/or social acceptability of the gel as well as compliance to its use could potentially limit its benefits.
Although our study is based on interim analysis, the findings to date suggest that the use of a carrageenan-based lubricant gel can reduce the risk of genital HPV infections of both low and high oncogenic risk in women. If confirmed upon final analysis, a carrageenan-based gel could be a useful adjunct to prophylactic HPV vaccines and a more affordable alternative for developing countries.
Supplementary Material
Supple fig tables
Acknowledgements
This study was funded by the Canadian Institute of Health Research (grants MOP-106610 and FDN-143347 to ELF). The CATCH Study Group includes the following: affiliated with the Division of Cancer Epidemiology, McGill University, Montréal, Canada: A. Rodrigues (study coordinator); N. Morykon and R. Rodrigues (management of subject participation and specimen collection); S. Bouten and S. Shapiro (data management); affiliated with the Déepartement de Microbiologie Méedicale et Infectiologie, Centre Hospitalier de l’Université de Montréal, Montréal, Québec, Canada: J. Guénoun (laboratory staff); and affiliated with the CLSC Samuel de Champlain, Longueil, Queébec, Canada: N. Slavetchva (collaborator).
We wish to thank the volunteering participants and the following employees of the CATCH trial: J. Selinger, M. Pastor and A. Chan for study promotion; and D. Bustillo-Dominguez and C. Nguyen-Huy for temporary management of subjects' participation and specimen collection. The authors also thank D. Edmond (Student Health Services Clinic, Concordia University) and the staff of the Student Health Services Clinics at McGill and Concordia universities for their collaboration.
Footnotes
Transparency declaration
Gel supplies were provided at no cost by CarraShield Labs Inc. (St Petersburg, FL). SM is a recipient of a FRQS/MSSS Resident Physician Health Research Career Training Scholarship from the Fonds de Recherche du Québec-Santé (FRQS). ELF reports grants and personal fees from Merck, grants, personal fees and nonfinancial support from Roche and personal fees from GSK outside the submitted work. AF reports personal fees from BD, Cepheid, Ventana, Roche and Merck outside the submitted work. FC reports having received a research grant through his institution from Becton Dickinson, personal fees for a participation in an expert group on HPV vaccination by Merck Sharp & Dohme and grants from CIHR and FRQS outside the submitted work. The other authors report no conflicts of interest relevant to this article. CIHR and CarraShield Labs Inc. had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
References
- [1].Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002;55:244–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518–27. [DOI] [PubMed] [Google Scholar]
- [4].Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006;24(Suppl. 1):S1–15. [DOI] [PubMed] [Google Scholar]
- [5].Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003;12:485–90. [PubMed] [Google Scholar]
- [6].Alemany L, Saulnier M, Alvarado-Cabrero I, Quiros B, Sameron J, Shin HR, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer 2015;136:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30(Suppl. 5):F12–23. [DOI] [PubMed] [Google Scholar]
- [8].Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsague X, Laporte L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 2014;15:1319–31. [DOI] [PubMed] [Google Scholar]
- [9].Brisson M, Van de Velde N, De Wals P, Boily MC. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine 2007;25:5399–408. [DOI] [PubMed] [Google Scholar]
- [10].Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008;359:821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goldie SJ, O’Shea M, Campos NG, Diaz M, Sweet S, Kim SY. Health and economic outcomes of HPV 16,18 vaccination in 72 GAVI-eligible countries. Vaccine 2008;26:4080–93. [DOI] [PubMed] [Google Scholar]
- [12].Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA 2007;298:743–53. [DOI] [PubMed] [Google Scholar]
- [13].Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog 2006;2:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Novetsky AP, Keller MJ, Gradissimo A, Chen Z, Morgan SL, Xue X, et al. In vitro inhibition of human papillomavirus following use of a carrageenan-containing vaginal gel. Gynecol Oncol 2016;143:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rodriguez A, Kleinbeck K, Mizenina O, Kizima L, Levendosky K, Jean-Pierre N, et al. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir Res 2014;108:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007;13:857–61. [DOI] [PubMed] [Google Scholar]
- [17].Roberts JN, Kines RC, Katki HA, Lowy DR, Schiller JT. Effect of Pap smear collection and carrageenan on cervicovaginal human papillomavirus-16 infection in a rhesus macaque model. J Natl Cancer Inst 2011;103:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kilmarx PH, van de Wijgert JH, Chaikummao S, Jones HE, Limpakarnjanarat K, Friedland BA, et al. Safety and acceptability of the candidate microbicide Carraguard in Thai women: findings from a phase II clinical trial. J Acquir Immune Defic Syndr 2006;43:327–34. [DOI] [PubMed] [Google Scholar]
- [19].Ramjee G, Morar NS, Braunstein S, Friedland B, Jones H, van de Wijgert J. Acceptability of Carraguard, a candidate microbicide and methyl cellulose placebo vaginal gels among HIV-positive women and men in Durban, South Africa. AIDS Res Ther 2007;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van de Wijgert JH, Braunstein SL, Morar NS, Jones HE, Madurai L, Strickfaden TT, et al. Carraguard vaginal gel safety in HIV-positive women and men in South Africa. J Acquir Immune Defic Syndr 2007;46:538–46. [DOI] [PubMed] [Google Scholar]
- [21].Kilmarx PH, Blanchard K, Chaikummao S, Friedland BA, Srivirojana N, Connolly C, et al. A randomized, placebo-controlled trial to assess the safety and acceptability of use of carraguard vaginal gel by heterosexual couples in Thailand. Sex Transm Dis 2008;35:226–32. [DOI] [PubMed] [Google Scholar]
- [22].Gravitt PE, Lacey JV Jr, Brinton LA, Branes WA, Kornegay JR, Greenberg MD, et al. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol Biomarkers Prev 2001;10:95–100. [PubMed] [Google Scholar]
- [23].Castle PE, Jeronimo J, Schiffman M, Herrero R, Rodriguez AC, Bratti MC, et al. Age-related changes of the cervix influence human papillomavirus type distribution. Cancer Res 2006;66:1218–24. [DOI] [PubMed] [Google Scholar]
- [24].Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst 2011;103:368–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shaw E, Ramanakumar AV, El-Zein M, Silva FR, Galan L, Baggio ML, et al. Reproductive and genital health and risk of cervical human papillomavirus infection: results from the Ludwig-McGill cohort study. BMC Infect Dis 2016;16:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].US Department of Health and Human Services; National Institutes of Health; National Institute of Allergy and Infectious Diseases; Division of AIDS. Division of AIDS table for grading the severity of adult and pediatric adverse events, addedum 1—female genital grading table for use in microbicide studies, version 1.0. November 2007. https://rsc.tech-res.com/docs/default-source/safety/addendum_1_female_genital_grading_table_v1_nov_2007.pdf?sfvrsn=8. [Google Scholar]
- [27].Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Influence of partner’s infection status on prevalent human papillomavirus among persons with a new sex partner. Sex Transm Dis 2010;37:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trial 1990;11:116–28. [DOI] [PubMed] [Google Scholar]
- [29].Woloshin S, Schwartz LM, Bagley PJ, Blunt HB, White B. Characteristics of interim publications of randomized clinical trials and comparison with final publications. JAMA 2018;319:404–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Marais D, Gawarecki D, Allan B, Ahmed K, Altini L, Cassim N, et al. The effectiveness of Carraguard, a vaginal microbicide, in protecting women against high-risk human papillomavirus infection. Antivir Ther 2011;16:1219–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supple fig tables