Structural basis of G-quadruplex unfolding by the DEAH/RHA helicase DHX36 (original) (raw)
. Author manuscript; available in PMC: 2019 Jun 13.
Published in final edited form as: Nature. 2018 Jun 13;558(7710):465–469. doi: 10.1038/s41586-018-0209-9
Abstract
Guanine-rich nucleic acid sequences challenge the replication, transcription, and translation machinery by spontaneously folding into G-quadruplexes, the unfolding of which requires forces greater than what most polymerases can exert1,2. Eukaryotic cells host numerous helicases capable of unfolding G-quadruplexes3. The molecular basis for helicase recognition and unfolding of G-quadruplexes remains poorly understood. DHX36 (RHAU, G4R1), a member of the DEAH/RHA family of helicases, binds both DNA and RNA G-quadruplexes with uniquely high affinity4,5,6, is consistently found bound to G-quadruplexes in cells7,8, and is a major source of G-quadruplex unfolding activity in HeLa cell lysates6. DHX36 is a multi-functional helicase implicated in G-quadruplex-mediated transcriptional and post-transcriptional regulation, and is essential for heart development, hematopoiesis, and embryogenesis in mice9,10,11,12. Here, we report the co-crystal structure of bovine DHX36 bound to a DNA with a G-quadruplex and a 3’ single-stranded (ssDNA) segment. We show that the N-terminal DHX36-specific motif folds into a DNA-binding-induced α-helix that together with the OB-fold-like subdomain selectively binds parallel G-quadruplexes. Comparison with our unliganded and ATP-analog-bound DHX36 structures, together with single-molecule FRET analysis, suggests that G-quadruplex binding alone induces rearrangements of the helicase core, which by pulling on its ssDNA tail, drive G-quadruplex unfolding by one residue at a time.
DEAH/RHA helicases share a structural core13,14,15,16,17,18 consisting of two RecA-like domains followed by a C-terminal domain (itself comprised of degenerate-winged-helix, ratchet-like and oligonucleotide and oligosaccharide-binding-fold-like subdomains; hereafter, WH, RL, and OB). At its N-terminus, DHX36 augments the DEAH/RHA core with a glycine-rich element followed by the DHX36-specific motif (DSM; Fig. 3a, Extended Data Fig. 1). The DSM is essential for DHX36 binding to G-quadruplexes19. We co-crystallized a DHX36 construct missing the glycine-rich element but containing the full DSM (hereafter DHX36-DSM; this construct has G-quadruplex binding and repetitive unfolding activity comparable to wild-type bovine and human DHX36; Extended Data Fig. 2) with a 24-nt DNA (hereafter, DNA_Myc_) comprised of a c-_Myc_promoter-derived G-quadruplex-forming sequence20 followed by a 3’ single-stranded extension of 7 thymidines. We also crystallized a truncated DHX36 without the glycine-rich and DSM elements (hereafter, DHX36-Core). Structures were solved through the single-wavelength anomalous dispersion (SAD) and molecular replacement methods (Extended Data Tables 1–2; Extended Data Fig. 3; Methods).
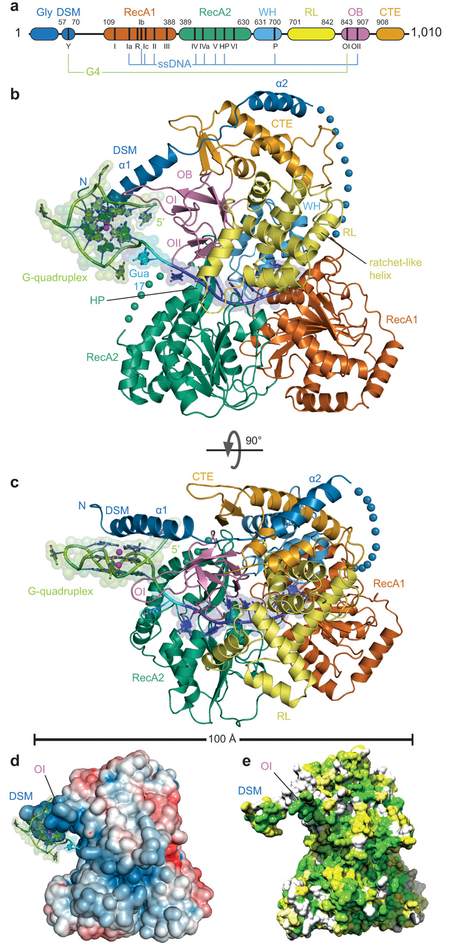
Figure 1. Overall structure of the DHX36 – G-quadruplex DNA complex.
(a) Domain organization; G-quadruplex (G4) and ssDNA-interacting regions indicated. (b) Cartoon representation of the co-crystal structure of DHX36 bound to DNAMyc, color-coded as ina. Spheres denote two disordered segments (blue, 20 and 53 residues in the crystallization constructand wild-type, respectively; and green 13 residues). OB loops I and II (OI and OII) contact DNA. (c) 90° rotation. (d) Electrostatic potential calculated omitting DNA from the co-crystal structure (blue to red, ±5_k_B_T_). (e) Phylogenetic conservation among 250 DHX36 orthologs (white to green, least to most conserved).
In the DHX36-DSM-DNAMyc complex, the RecA1, RecA2, and C-terminal domains are arranged as a trefoil (Fig. 3b). Connected to RecA1 by a disordered linker, the N-terminal extension folds into two α-helices, the first of which contains the DSM. This DSM helix projects away from the body of the helicase and contacts the 5’ (top) face of the bound G-quadruplex (Fig. 3c). OB contacts both the G-quadruplex and the adjacent single-stranded segment of DNA_Myc_, the 3’-side of the latter being held in a nucleic acid binding channel formed by the RecA1, RecA2, and C-terminal domains. The amphipathic DSM helix is overall cationic, and the path of the single-stranded DNA follows a positively-charged groove between the RecA2 and C-terminal domains (Fig. 3d). This groove is too narrow to accommodate double-stranded DNA, consistent with the requirement6,21,22,23 for a 3’ single-stranded extension for DHX36 activity. Phylogenetic conservation largely follows this groove and extends to the non-polar face of the DSM helix (Fig. 3e).
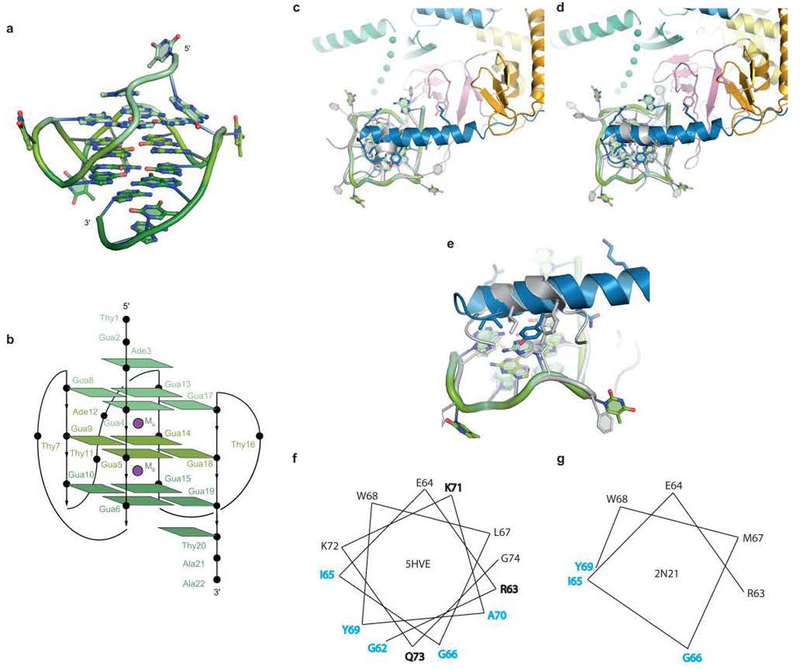
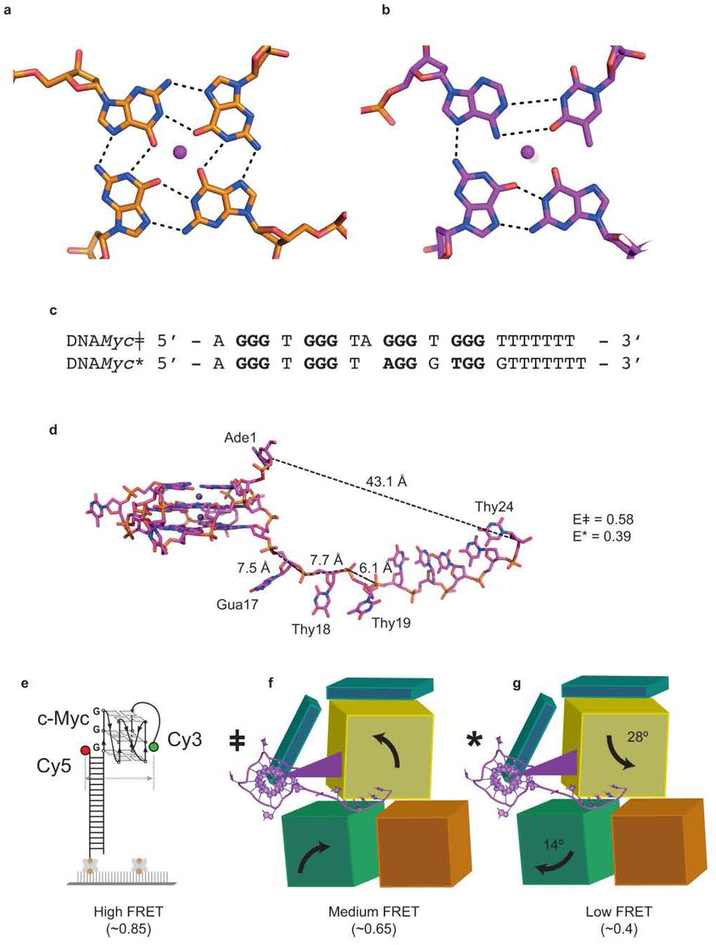
Solution NMR demonstrated20,24 that residues 1–17 of DNA_Myc_ fold into a stable, parallel, 3-tiered G-quadruplex (Extended Data Fig. 4a,b). Our co-crystal structure reveals that association with the helicase reorganizes the DNA. Instead of three canonical G-quartets, the DHX36-bound DNA contains two G-quartets stacked underneath a non-canonical A•T•G•G quartet. The top G-quartet is absent because Gua17, which was the 3’-most guanine of the bottom G-quartet20, has been pulled by the helicase into the 3’ single-stranded region. Shifting the DNA sequence by one residue while maintaining an overall three-tiered quadruplex structure with minimal propeller loops forces a new Ade10•Thy14 Watson-Crick pair to form the top quartet together with Gua2 and Gua6 (Fig. 4a,Extended Data Fig. 4b). The DHX36-bound rearranged quadruplex is considerably less stable than the free, canonical c-Myc G-quadruplex (Extended Data Fig. 5), indicative of the degree of destabilization caused by DHX36 binding alone.
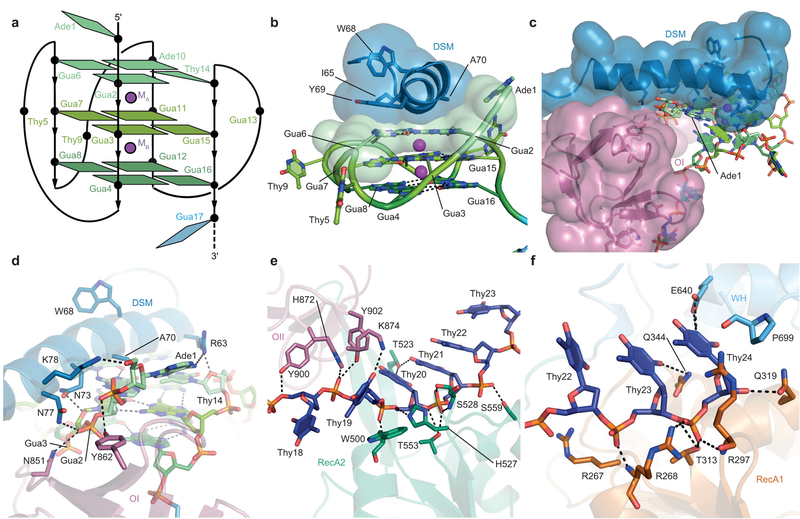
Figure 2. DHX36-DNA interaction.
(a) Schematic of the DHX36-bound all-parallel quadruplex. (b) The DHX36-specific motif (DSM) stacks on the 5’ (top) non-canonical quartet. Transparent spheres, van der Waals radii. (c) The DSM and the OI loop of OB flank Ade1. (d) Interaction of the DSM and loop OI of DHX36 with the DNA backbone near the 5’ of DNA_Myc_. (e) Interaction of the OB loop OII and the RecA2 domain with Thy18–Thy 22 of the 3’ single-stranded region of DNA_Myc_. (f) Interaction of the RecA1 domain and WH of DHX36 with Thy23–Thy24 of the 3’ single-stranded region of DNA_Myc_.
Previously, the DSM was shown19to be necessary but not sufficient for high-affinity G-quadruplex binding by DHX36; the full-length helicase and an isolated DSM peptide bind to G-quadruplexes with dissociation constants of <10 pM and 310 nM, respectively4,12. This is consistent with the helicase core contributing to the DHX36-DNA interface (Fig. 3b,c). In the co-crystal structure, a hydrophobic core is formed by the α-helical DSM residues I65, W68, Y69, and A70, producing a flat non-polar surface that stacks on the nucleobases of the top quartet of the bound G-quadruplex (Fig. 4b), reminiscent of the mode of G-quadruplex recognition by planar small molecules25. The single-stranded Ade1, which is 5’ to the G-quadruplex in our structure, packs between the α1 DSM helix and OB (Fig. 4c). The C-terminal side of the α1 DSM helix and the OI loop of OB hydrogen-bond extensively with the sugar-phosphate backbone of 5’ leader (Ade1) and the G-quadruplex residues immediately following it (Fig. 4d). Formation of a composite G-quadruplex-binding surface between the DSM and OB explains why in a previously reported NMR structure of a low-affinity complex between an 18-amino acid DSM-derived peptide and a G-quadruplex26, the DNA-binding-induced α-helix is out of register with that seen in our co-crystal structure (Extended Data Fig. 4c-g). In addition, DHX36 does not markedly discriminate between DNA and RNA substrates4,27, and it recognizes the 3’ single-stranded region of DNA_Myc_primarily by contacts with phosphates of its backbone (Fig. 4e,f). A second loop from OB (hereafter OII) contacts the backbones of Thy18 and Thy19 (Fig. 4e), while WH and the RecA1 domain interact with Thy23 and Thy24 (Fig. 4f).
While other helicases can resolve both antiparallel and parallel G-quadruplexes,23, DHX36 has a strong preference for the latter, being inactive on fully antiparallel G-quadruplexes, and exhibiting reduced activity on G-quadruplexes with mixed parallel and antiparallel connectivity21,22,26. Bound to DHX36, DNA_Myc_ has three single-nucleotide double-chain-reversal loops (Thy5, Thy9, and Gua13) that do not sterically interfere with DSM recognition of the top quartet. Our structure suggests that the preference of DHX36 for parallel G-quadruplexes likely arises from the steric interference of diagonal and lateral loops with DSM binding. In addition, a 5’ G-tract with the opposite polarity would interfere with binding to the OI loop.
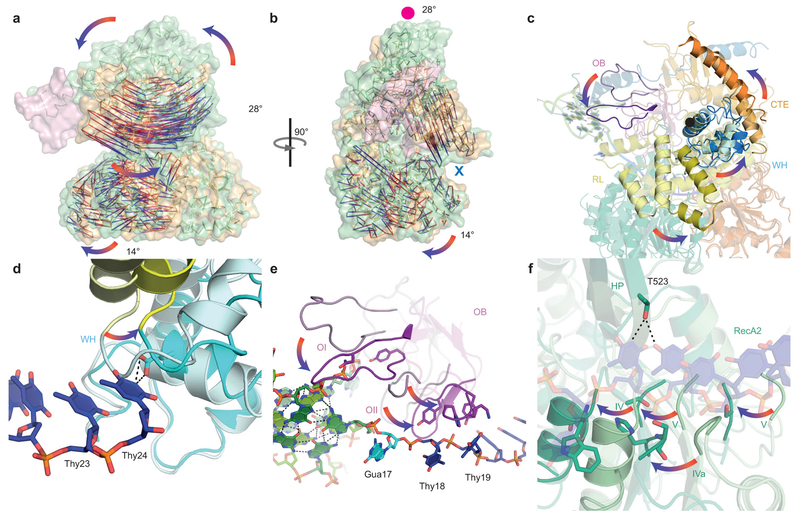
Our DHX36-DSM-DNA_Myc_ co-crystal structure provides an unprecedented view of the open, ATP-independent conformation adopted by a nucleic-acid bound DEAH/RHA helicase. Superposition of the RecA1 domains of our DHX36-Core and DHX36-DSM-DNA_Myc_ structures shows that DNA binding alone induces 28° and 14° rotations of the C-terminal and RecA2 domains, respectively (Fig. 5a,b,c). This conformation accommodates five stacked single-stranded DNA residues between the 5’-β-hairpin (hereafter HP) and the constriction formed by R297, Q319, and P699 (Fig. 4e,f). Compared to the ATP analog-bound and unliganded states, the nucleic acid-interacting elements of RecA2 (motifs IV, IVa, and V; Fig. 3a) shift away from RecA1 by 6 Å, approximately the distance between successive nucleotides (Fig. 5f). HP acts as a fulcrum upon core opening, unstacking Thy18 and Thy19 on one side, and stabilizing the 3’ stack of nucleotides by hydrogen bonding with T523 on the other (Fig. 5e,f). The opening motion may allow G-quadruplex unfolding by one residue, and is consistent with the one-nucleotide displacement of the DHX36-bound DNA_Myc_ structure relative to its free solution conformation (Extended Data Fig. 4a,b). Together with published structures of DEAH/RHA helicases in the ground16,17, transition15, and post-hydrolysis13,14 states, our DHX36 structures support the hypothesis17 that DEAH/RHA helicases cycle between 4- and 5-nucleotide stack states enforced by the HP and 3’-constriction site to unwind their substrates (Extended Data Figs. 6,7).
Figure 3. DNA-binding-induced structural transitions of DHX36.
(a) Superposition of DHX36-DSM-DNA_Myc_ and DHX36-Core structures (green and orange, respectively, Cα vectors from red to blue) through RecA1. DNA_Myc_, pink. (b) 90° view. Red circle and blue cross denote C-terminal domain rotation out of and into the plane, respectively. (c) C-terminal sub-domains unliganded and DNA-bound, pastel and solid colors, respectively. Black circle, approximate axis of rotation. (d) The WH in unliganded and DNA-bound states. Thy24 of DNA_Myc_ impinges on the loop linking WH and RL. (e) The OI and OII loops of OB in unliganded and DNA-bound states. (f) RecA2 in unliganded and DNA-bound states.
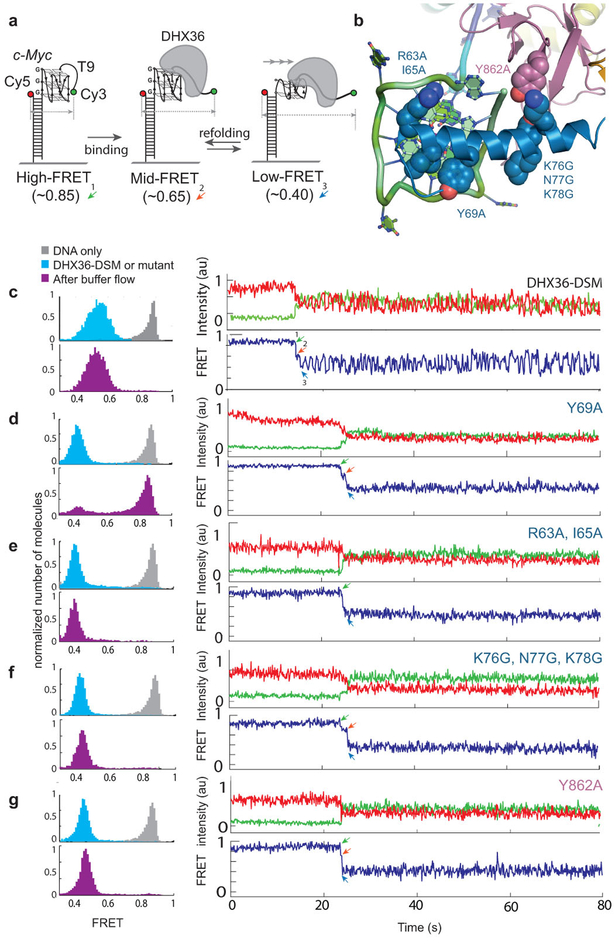
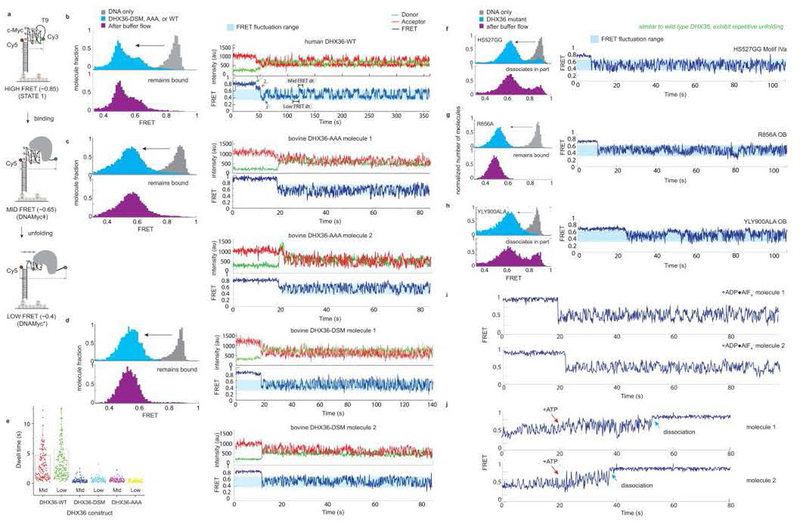
We examined structure-guided mutants of DHX36 using a single-molecule FRET (smFRET) assay previously developed to characterize the repetitive, ATP-independent G-quadruplex unfolding activity of the wild-type helicase22 (Fig. 6a–c; Extended Data Fig. 2). The c-Myc promoter-derived parallel G-quadruplex DNA employed exhibits high FRET (Fig. 6a). DHX36 binding induces conformational changes, in which oscillations in FRET efficiency between medium and low (~0.6 and ~0.4, respectively) reflect repetitive unfolding between the canonical (with three complete G-quartets) and reorganized (pulled by one nucleotide) DNA_Myc_ G-quadruplex (Extended Data Fig. 8), respectively28. The repetitive unfolding activity is ATP-independent, as the absence of ATP or presence of non-hydrolyzable ATP analogs do not affect it (Extended Data Fig. 2i,j). We hypothesize that the repetitive unfolding activity stems from ATP-independent helicase core opening and reciprocating rotation of the C-terminal domain. ATP is likely only required for release of DNA from the helicase as rapid dissociation occurs upon ATP addition (Extended Data Fig. 2i,j).
Figure 4. Single-molecule FRET analysis of DHX36-DSM mutants.
. (a) Reporter with a G-quadruplex of the DNA_Myc_ sequence, and a 9-thimidine single-stranded 3’ tail. DHX36 shifts FRET from high (~0.8) to medium–low oscillation (~0.6, canonical DNA_Myc_, ~0.4, reorganized DNA_Myc_, Extended Data Figs. 4,10). (b) Structure-guided mutations. (c) The DHX36-DSM crystallization construct remains DNA-bound upon flow and exhibits repetitive unfolding, similar to wild-type (Extended Data Fig. 2). (d) The Y69A mutant lacks repetitive unfolding and dissociates from G-quadruplex upon flow. (e)-(g) Three mutants remain bound following low, but lack repetitive unfolding. Each experiment was highly reproducible and in triplicate (data from >10,000 molecules/experiment).
Solution NMR of a DSM-derived peptide26 (Extended Data Fig. 4c-g), as well as proteolytic susceptibility of the DHX36-DSM N-terminus indicate that α1 is intrinsically disordered29, becoming fully α-helical upon interaction with the substrate G-quadruplex. Our mutagenesis shows that, as in other examples29 of ligand-induced protein structure, binding free energy is distributed non-uniformly across the DSM. The R63A•I65A and KNK76GGG mutations of residues anchoring α1 on the quadruplex backbone are less deleterious than mutation of Y69 (Fig. 6b, d-g). Mutation of Y69, which directly stacks on the top quartet (Fig. 4a), weakens the helicase-DNA association to such an extent that, uniquely among the mutants examined, this protein dissociates from DNA upon buffer flow (Fig. 6d). Ligand-induced folding of α1 may allow DHX36 to mold to G-quadruplexes of different local structure, and even to antiparallel substrates with lower efficiency, during its mechanochemical cycle.
Our DHX36 co-crystal structure shows how a protein that evolved to recognize G-quadruplex-containing nucleic acids combines binding to the face and backbone of the G-quadruplex with recognition of both 5’ and 3’ single-stranded extensions. DHX36 unfolding activity was previously shown to be highly sensitive to the stability of its G-quadruplex substrates5,22. This sensitivity is consistent with our demonstration that nucleic acid binding energy is transduced by DHX36 into a discrete, directed pulling force arising from C-terminal domain rotation and helicase core opening. These ATP-independent structural changes remodel the G-quadruplex, resulting in a substrate unwound by a single nucleotide. Our analysis thus highlights the importance of ATP-independent structural changes for nucleic acid remodeling by a canonical DEAH/RHA helicase and constitutes a starting point for further structural analysis of the mechanochemical cycle of these important enzymes.
METHODS
Protein expression and purification.
Bos taurus DHX36-Core, DHX36-Core-SeMet, and DHX36-DSM were expressed in E. coli LOBSTR (DE3) (ref. 30). All proteins have C-terminal 8xHis-tags and the latter also an N-terminal GST-tag. Starter cultures were grown at 37 °C in MDAG-135 media31. Production cultures in Terrific Broth were induced with 1 mM IPTG at 20 °C and grown overnight. Lysis was in 50 mM HEPES-KOH (pH 7.5), 0.5 M KCl, 10 mM β-mercaptoethanol, 0.1% (v/v) Tween-20, 10% (v/v) glycerol, and Sigmafast EDTA-Free protease inhibitor cocktail. Lysate supernatant was treated with polyethyleneimine (0.05%, v/v) and loaded on a Ni-NTA Superflow (Qiagen) column. The proteins were eluted with 500 mM imidazole. DHX36-Core was further purified on a Superdex 200 PG column (GE Healthcare) in 20 mM HEPES-KOH pH 7.5, 150 mM KCl, 10% (v/v) glycerol, 0.5 mM TCEP, and 2.5 mM MgCl2. For DHX36-DSM, the eluate from the Ni-NTA column was loaded onto GSTrap 4B (GE Healthcare) column, washed with 20 mM HEPES-KOH (pH 7.5), 150 mM KCl, 10% (v/v) glycerol, and 1 mM TCEP (pH 7.0), and eluted with 25 mM reduced glutathione. The eluted DHX36-DSM was incubated at 20 °C with TEV protease (1:10 mass ratio) for 1 h. Then, DNA_Myc_ (5’-AGG GTG GGT AGG GTG GGT TTT TTT-3’) was added (2:1 DHX36-DSM:DNA_Myc_ molar ratio) and the mixture was incubated for 30 min at 21 °C. The mixture was dialyzed (50 kDa MWCO membrane) against 50 mM HEPES-KOH (pH 7.5), 150 mM KCl, and 10% (v/v) glycerol overnight at 4 °C and then incubated with Amintra GST resin (Expedeon) for 1 h at 4 °C. For crystallization, the complex was reductively methylated as described32. ESI-MS indicated a mass of 109,098 ± 2 Da, which corresponds to the dimethylation of 63 out of a total of 66 lysines in DHX36-DSM. After methylation, the DHX36-DSM-DNA_Myc_complex was further purified by size-exclusion chromatography as DHX36-Core. For expression of DHX36-Core-SeMet, PASM-5052 autoinduction expression media31 was inoculated with a starter culture grown in MDAG-135 media. Cultures were grown at 20 °C for ~ 6 days. Purification was as described above. The mass of DHX36-Core-Semet by ESI-MS was 101,011 ± 2 Da, corresponding to a methionine labeling efficiency of 95.8%. All proteins were purified to >98% homogeneity, with the exception of DHX36-Core-SeMet, which was purified to >80% homogeneity (as judged by Coomassie blue staining of serial dilutions analyzed by SDS-PAGE). All DHX36 constructs used in this study contain a deletion of residues 1–54, which encompasses the Gly-rich region. DHX36-core contains a deletion of residues 1–149. DHX36-AAA contains a KKK192AAA mutation to prevent spontaneous proteolysis. DHX36-DSM contains a deletion of residues 111–159 and surface entropy reduction mutations EEK435YYY and KDTK752AATA. All mutations used to generate the various constructs (DHX36-Core, DHX36-AAA, DHX36-DSM, and structure-guided mutants) were generated using the QuikChange Lighting kit (Agilent). DHX36-DSM mutants for smFRET were purified essentially as above, but after elution from the GSTrap 4B column, the GST tag was cleaved by TEV protease in buffer with 400 mM KCl and removed by a second passage through the GSTrap 4B resin. The mutant proteins were then dialyzed against 50 mM HEPES-KOH (pH 7.5), 600 mM KCl, 10% (v/v) glycerol and 1 mM TCEP (pH 7.0) overnight at 4 °C and purified by size-exclusion chromatography (Superdex 200 PG, GE Healthcare) in the same buffer.
Crystallization and diffraction data collection.
Hanging drops were prepared by mixing 1 μl each of DHX36-Core (5 g/l) and reservoir [0.2 M ammonium citrate (pH 7.0) and 20% (w/v) PEG3350], and were equilibrated by vapor diffusion at 21 °C. DHX36-Core-AlF4− was crystallized under the same conditions in the presence of ADP•AlF4−(1 mM). ADP•AlF4− was prepared by mixing in order the following molar ratio: 1 part Na-ADP (100 mM), 1 part AlCl3(1 M), and 5 parts NaF (500 mM). DHX36-Core-BeF3−was crystallized by vapor diffusion at 21 °C using a reservoir consisting of 0.2 M potassium sodium tartrate (pH 7.4) and 20% (w/v) PEG 3350. ADP•BeF3− was prepared using the same protocol as above, except replacing AlCl3 with BeSO4. DHX36-Core-SeMet crystals were grown by vapor diffusion at 21 °C by combining 1.5 μl protein solution (5 g/l), 1 μl reservoir [50 mM sodium cacodylate (pH 7.1), 150 mM ammonium carbonate (pH 6.9), and 13.8% (v/v) 2-propanol], and 0.5 μl microseed stock. The stock was made by crushing crystals of DHX36-Core. All DHX36-Core crystals were soaked in their respective reservoir solutions supplemented with 30% (v/v) glycerol prior to flash-freezing in liquid nitrogen. All DHX36-Core crystals grew as rhombohedra to maximum dimensions of 500 × 300 × 300 μm3 in 1–2 weeks. DHX36-DSM complexed with DNA_Myc_ was crystallized at 21 °C by hanging-drop vapor diffusion. Drops were prepared by combining complex solution (1.5 μl, 3 g/l), reservoir (1.0 μl) and microseed stock (0.5 μl). The reservoir consisted of 200 mM sodium malonate (pH 7.0) and 25% (w/v) PEG 3350. The seed stock was from crystals of unmethylated DHX36-DSM in complex with DNA_Myc_ [grown in 45 mM MES-monohydrate (pH 5.7), 180 mM KCl, 29 mM MgCl2, 4.5% (w/v) PEG 8000, 10 mM HEPES-NaOH (pH 7.5), and 3% (v/v) 2-propanol]. Methylated DHX36-DSM-DNA_Myc_ crystals grew as plates to maximum dimensions of 500 × 100 × 5 μm3 in 2–8 weeks. After growth, the reservoir solution was changed successively to 30% and 40% (w/v) PEG 3350 (other components unchanged) for a week each. Crystals, mounted on 90° bent loops (Mitegen), were flash-frozen in liquid nitrogen without further cryoprotection. Diffraction data were collected at 100 K in rotation mode with 0.9792 Å X-radiation at beamlines 5.0.1 and 5.0.2 of the Advanced Light Source (ALS), Lawrence Berkeley National Laboratory, and beamline 17-ID-B of the Advanced Photon Source (APS), Argonne National Laboratory. Data were indexed, integrated, and scaled using HKL2000 (ref. 33) (Extended Data Tables 1 and 2).
Structure determination and refinement.
Datasets from five DHX36-Core-SeMet crystals were scaled and merged together in HKL2000 (Extended Data Table 2). A heavy-atom substructure comprised of 18 selenium atoms was identified in this high-redundancy dataset by HySS (ref. 34) implemented in PHENIX AutoSol (ref. 35). The resulting experimental SAD phases (mean overall figure of merit = 0.56) were density-modified with RESOLVE (ref.36) to produce an electron density map in which manual model building using COOT (ref. 37) could begin (Extended Data Fig. 3). Iterative rounds of manual model building interspersed with rigid-body, simulated annealing, energy minimization, and individual isotropic _B_-factor refinement in PHENIX produced a near-complete model (R_free = 35.2) that could be placed (TFZ = 20.2) into the DHX36-Core dataset using PHASER (ref. 38). Further rounds of manual model building interspersed with refinement produced the current DHX36-Core, DHX36-Core-AlF4−, and DHX36-Core-BeF3− models (Extended Data Table 1). Refined coordinates of the RecA1 domain from the 2.2 Å-resolution DHX36-Core structure were used as a search model against the DHX36-DSM-DNA_Myc dataset, yielding38 a solution with TFZ = 13.1. Subsequently, the RecA2 and C-terminal domains were successively placed (TFZs = 25.1 and 22.4, respectively). Rigid-body refinement followed by simulated annealing and restrained individual isotropic_B_-factor refinement was in turn followed by manual model building and further refinement to yield the current model of the DNA-protein complex (Extended Data Table 1). Coordinate precision estimates are from PHENIX. Structure figures were prepared with PyMol and Chimera (refs39,40).
Differential scanning calorimetry.
Three DNAMyc sequences (IDT, Extended Data Fig. 5a) at 0.1 mM concentration in 20 mM cacodylic acid-KOH (pH 7.2) and 20 mM or 150 mM KCl were heated at 95 °C for 2.5 min, placed on ice for 10 min, and warmed to 21 °C over 20 min. The DNAs were analyzed by size-exclusion chromatography (Superdex 75 Increase, GE Healthcare) in 20 mM cacodylic acid-KOH (pH 7.2) and either 20 mM or 150 mM KCl(Extended Data Fig. 5b). For DSC, DNA samples prepared in 20 mM cacodylic acid-KOH (pH 7.2) and 20 mM KCl were degassed for 5–7 min prior to measurements (MicroCal VP-DSC differential scanning calorimeter). Thermograms were acquired between 20–105 °C at a scan rate of 0.5 °C min−1 and at a constant pressure of 24 p.s.i. Three to five heating and cooling cycles were collected at least in duplicate for two independent preparations of each DNA sequence. Thermograms were highly reproducible (Extended Data Fig. 5c), and were analyzed with Origin software (OriginLab). The reference ‘buffer versus buffer’ (20 mM cacodylic acid-KOH (pH 7.2) and 20 mM KCl) was subtracted from the sample data prior to curve-fitting (Levenberg-Marquardt non-linear least-squares method) to determine_T_m and Δ_H_.
Single-molecule FRET analyses.
smFRET analyses of DHX36-DSM and site-directed mutants were performed as described22,28.
Extended Data
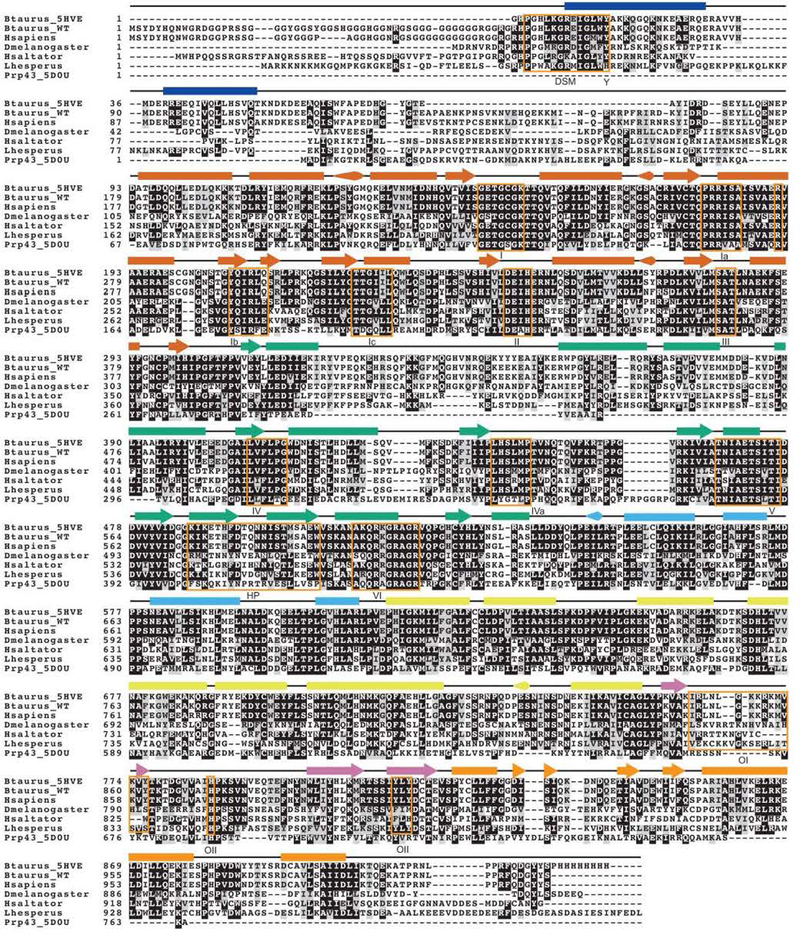
Extended Data Figure 1. Sequence alignment of DHX36 orthologs.
The Bos taurus DHX36 construct used to solve the DHX36-DSM-DNA_Myc_ co-crystal structure (PDB ID: 5HVE), wild-type Bos taurus DHX36, Homo sapiens DHX36, Drosophila melanogaster DHX36,Herpegnathos saltator DHX36, Latrodectus hesperus DHX36, and the Chaetomium thermophilum Prp43 crystallization construct41 (PDB ID: 5D0U) are aligned with a 0.5 threshold for percent similarity (gray shading). The glycine-rich region is responsible for DHX36 recruitment to stress granules, but it is not necessary for DHX36 binding or resolution of G-quadruplexes. Identical residues are shaded in black. Secondary structure from the DHX36-DSM-DNA_Myc_ co-crystal structure is indicated above each alignment section, with arrow, rectangle and cone denoting α-helix, β-strand, and 310-helix, respectively. Secondary structure is color-coded by domain or subdomain as in Fig. 1. Alignment was performed with Clustal Omega (ref. 42) and depicted using BoxShade (http://sourceforge.net/projects/boxshade/).
Extended Data Figure 2. Single-molecule FRET analysis of wild-type human DHX36 and bovine DHX36 constructs.
(a) Schematic of the smFRET assay22,28. See Extended Data Fig. 10 for FRET state assignments. (b) Binding of wild-type human DHX36 (ref. 22; DHX36-WT) to the G-quadruplex substrate, induces a shift from a high to medium and low FRET states (gray and cyan histograms, respectively). The shift is interpreted as the binding of DHX36 to the G-quadruplex substrate. Upon buffer flow, dissociation is not observed (purple histogram). Wild-type human DHX36 displays repetitive unfolding activity (ref. 22), as indicated by the oscillation between medium and low FRET states after binding to the G-quadruplex substrate (blue trace). (c) Binding of wild-type bovine DHX36 (incorporating a KKK192AAA mutation to prevent spontaneous proteolysis; DHX36-AAA) to the G-quadruplex substrate, induces a shift from a high to medium and low FRET states (gray and cyan histograms, respectively). The shift is interpreted as the binding of DHX36 to the G-quadruplex substrate. Upon buffer flow, dissociation is not observed (purple histogram). Wild-type bovine DHX36 (DHX36-AAA) displays repetitive unfolding activity, as indicated by the oscillation between low and medium FRET states after binding to the G-quadruplex substrate (blue trace). FRET traces are shown for two molecules. (d) Deletion of residues 111–159, mutation EEK435YYY, and mutation KDTK752AATA to generate DHX36-DSM does not impair G-quadruplex binding or repetitive unfolding activity. FRET traces are shown for two molecules. (e) Dwell time comparison between human DHX36-WT (gray bars), bovine wild-type DHX36 (DHX36-AAA, cyan bars) and bovine DHX36-DSM (orange bars). All three proteins show a comparable FRET range, and the two bovine constructs exhibit similar dwell times between the medium and low FRET states. Dwell times between the bovine constructs and the human construct are different, which likely arises from interspecies differences. Each experiment was performed three times. Data are reported as box dot plots, with the data center as the median ± standard error of 1000 dwell times from 200 representative molecules. Mutation of (f) motif IVa (“hook loop”), (g) the OB subdomain residue R856, and (h) OII does not result in impaired repetitive unfolding activity. However, partial dissociation following washing is observed with the (f) motif IVa and (h) OII mutation. (i) Pre-incubation of bovine DHX36-AAA with the non-hydrolyzable ATP γ-phosphate hydrolysis transition state mimic ADP•AlF4− does not affect repetitive unfolding activity on G-quadruplex substrates. (j) Addition of ATP (red arrow) while DHX36-AAA is displaying repetitive unfolding activity on G-quadruplex substrates results in DHX36 dissociation (blue arrow) on the seconds timescale. Each experiment was repeated three times producing highly similar results. Each measurement yields data from at least 10,000 molecules.
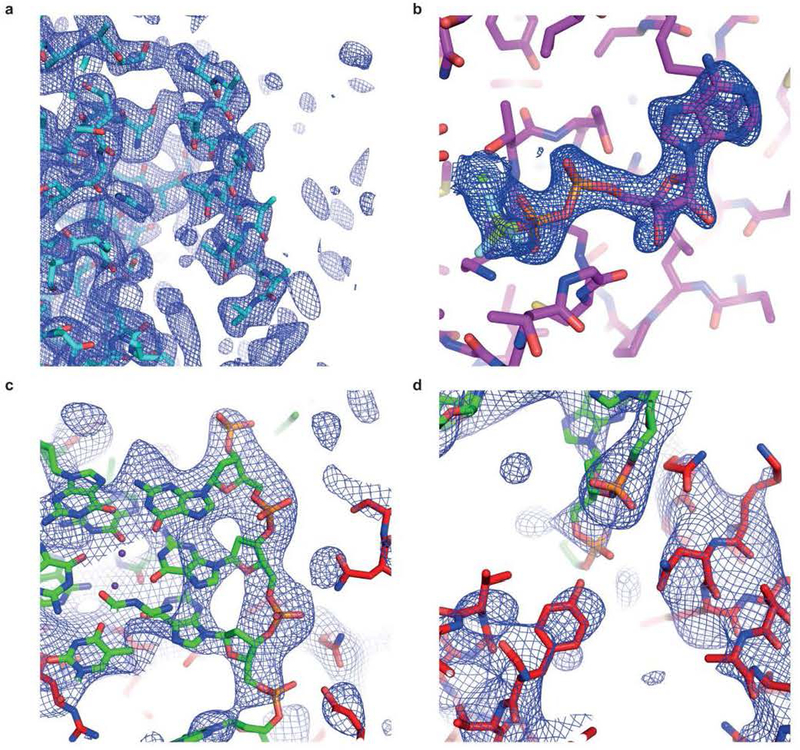
Extended Data Figure 3. Electron density maps superimposed on refined structures.
(a) Portion of the density-modified 3.1 Å-resolution experimental SAD electron density map of selenomethionyl DHX36-core contoured at 1 s.d. above mean peak height, superimposed on a partially refined atomic model (Methods). (b) Portion of the 2.5 Å-resolution simulated-annealing omit 2|_F_o|-|_F_c| electron density map of DHX36-Core in complex with ADP•BeF3− (PDB ID: 5HVC) contoured at 1.5. (c) Portion of a simulated annealing-omit 2|_F_o|-|_F_c| electron density map of the DHX36-DSM-DNA_Myc_complex corresponding to the G-quadruplex, contoured at 1 s.d.. (d) Portion of the electron density map (c) corresponding to the OI loop and the DSM helix (lower left and right, respectively). A portion of the DNA is in the upper center.
Extended Data Figure 4. Comparison of DNAMyc and DSM with solution structures of a_c_-Myc promoter sequence-derived parallel DNA G-quadruplex and DSM bound to a parallel DNA G-quadruplex.
(a) Cartoon representation of the c-Myc_G-quadruplex structure20,24 adopted by the DNA of sequence 5’ – TGA GGG TGGG TA GGG TGGG TAA – 3 (PDB ID: 1XAV). (b) Schematic of the c-Myc G-quadruplex (PDB ID: 1XAV); compare withFig. 2a. The DHX36-DSM-DNA_Myc co-crystal structure (PDB ID 5HVE; colored as in Fig. 1) was superimposed through the G-quadruplex with the solution structure26 (PDB ID: 2N21; gray) of a DSM-derived peptide bound to a G-quadruplex. (c) If the superposition is performed so that the 5' and 3' G-tracts of the G-quadruplexes from the two structures align, the α-helix of the solution structure of the DSM-derived peptide is oriented approximately 90° with respect to the DSM α-helix from the DHX36-DSM-DNA_Myc_co-crystal structure. (d) If arbitrarily rotated along the quadruplex 4-fold axis, the DSM α-helices from both structures approximately align. (e) Even with this rotation, the two structures differ in the DSM side chains presented to the DNA. (f),(g) Helical wheel representations of the DSM α-helices from the DHX36-DSM-DNA_Myc_co-crystal structure and the solution structure of the DSM-derived peptide bound to a G-quadruplex, respectively. Residues in cyan and bold make van der Waals contacts with the quadruplex face and hydrogen bond with the DNA backbone, respectively. Residue numbers correspond to the DHX36-DSM-DNA_Myc_ co-crystal structure.
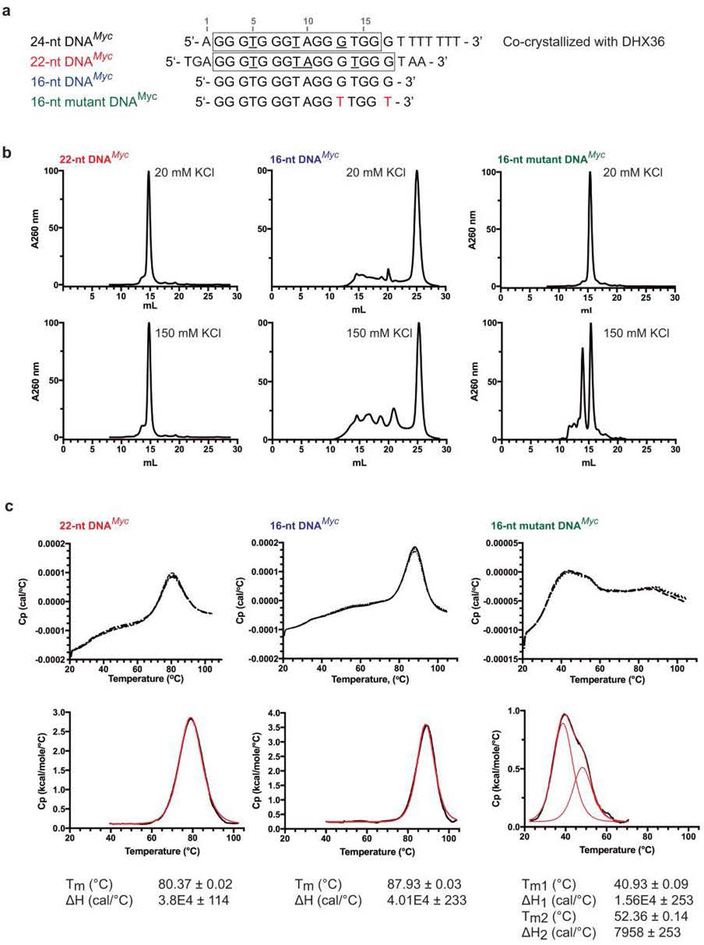
Extended Data Figure 5. Analysis of DNA_Myc_ conformers by differential scanning calorimetry (DSC).
(a) DNA constructs used in the analysis. DNA_Myc_, DNA used for co-crystallization with DHX36-DSM (Methods). Residues that form a three-tiered G-quadruplex and those that form propeller loops in the free DNA are boxed and underlined, respectively. 16-nt DNA_Myc_, DNA minimized to eliminate 5' and 3' single-stranded extensions to the G-quadruplex. 16-nt mutant DNA_Myc_, variant of the former with two mutations (red) to enforce the three quartets observed in the DHX36-DSM-DNA_Myc_ co-crystal structure. (b) Size-exclusion chromatograms (Methods) of 22-nt DNA_Myc_, 16-nt DNA_Myc_ and 16-nt mutant DNA_Myc_ in the presence of either 150 mM or 20 mM KCl, demonstrating greater conformational homogeneity of the DNAs at lower KCl concentration. (c) DSC thermograms (prior to buffer correction) for the three DNAs, in 20 mM KCl. Three independent experiments are plotted for each DNA. (d) Triplicate non-linear least-squares analyses of thermograms for the three DNAs. Black and red curves, buffer-corrected DSC data and curve-fits, respectively.T_m (melting temperature) and Δ_H (enthalpy change) are reported as means ± standard deviations. Each experiment was repeated three times with two sets of identical DNA preparations.
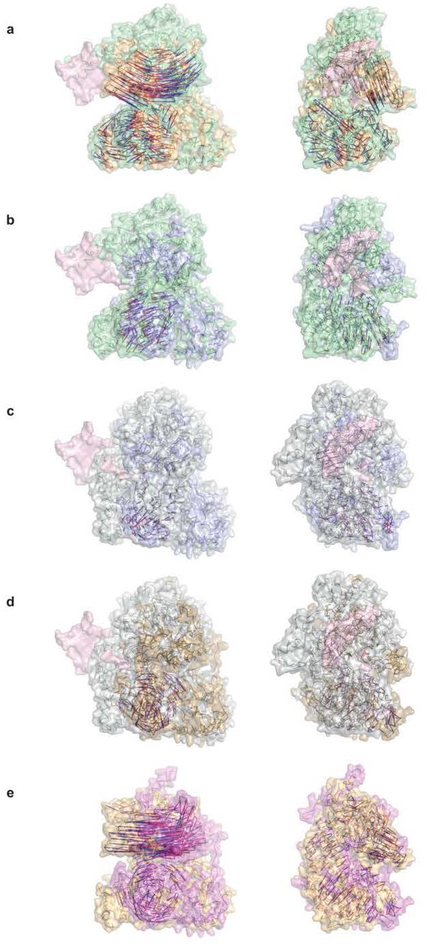
Extended Data Figure 6. Alignments of the structures of DHX36, MLE, and Prp43.
RecA1 domains were superimposed. Vectors from red to blue denote Cα displacement between identical or structurally homologous residues. (a) Superposition of DHX36-DSM-DNA_Myc_and unliganded DHX36-Core (5HVA) structures (green and orange, respectively). DNAMyc is pink. (b) Superposition of DHX36-DSM-DNA_Myc_ (green) and Prp43 (ref.16) bound to rU16 and ADP•BeF3− (5LTA; blue; “ground”). DNA_Myc_ from the DHX36-DSM-DNA_Myc_ structure is in pink. (c) Superposition of Prp43 bound to rU8 and ADP•BeF3− (5LTA; blue; “ground”) to MLE (ref. 15) bound to rU15 and ADP•AlF4− (5AOR; silver; “transition”). DNA_Myc_ from the DHX36-DSM-DNA_Myc_ structure is in pink. (d) Superposition of MLE bound to rU15 and ADP•AlF4− (5AOR; silver; “transition”) and Prp43 bound13,14 to ADP (3KX2/2XAU; gold; “post-hydrolysis). DNA_Myc_ from the DHX36-DSM-DNA_Myc_ structure is in pink. (e) Superposition of Prp43 bound to ADP (3KX2/2XAU; gold; “post-hydrolysis) to unliganded DHX36-Core (5HVA; magenta; “apo”).).
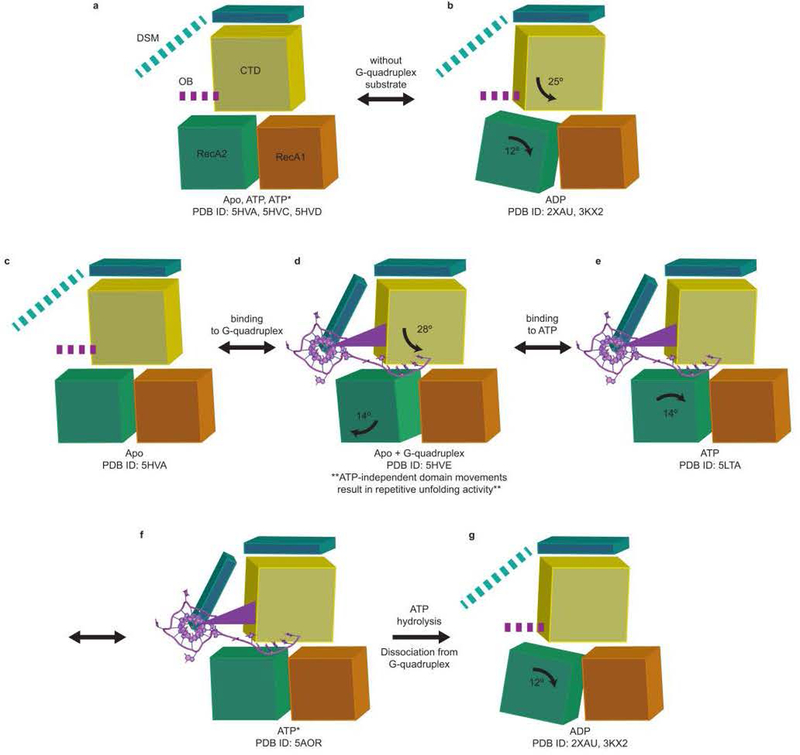
Extended Data Figure 7. Model of the mechanochemical cycle of the DEAH/RHA helicase DHX36.
The domain motions are based on the superpositions in Extended Data Fig. 6. The orange, green, yellow, and blue blocks represent RecA1, RecA2, C-terminal domain, and N-terminal extension, respectively. The purple wedge represents OB. Bold dotted lines represent likely intrinsically disordered protein motifs that fold upon G-quadruplex binding. (a,b) In the absence of a G-quadruplex nucleic acid substrate, DHX36 cycles between an apo (or structurally indistinguishable ATP-bound) and post-hydrolysis states. (c,d) DHX36 binds the G-quadruplex substrate and pulls on it in the 3’-direction through concerted and opposite rotations of RecA2 and C-terminal domains. Oscillation of the RecA2 and C-terminal domains is likely responsible for the ATP-independent repetitive unfolding activity detected by smFRET (ref. 22, Extended Data Fig. 2and Fig. 4). (d,e) Binding of ATP induces domain closure., (f,g) ATP hydrolysis yields a post-hydrolysis state that is incompatible with nucleic acid binding. ADP dissociates, and DHX36 is reset back to its apo state, (c). In addition to the rearrangement of motif Va (ref. 17), ATP hydrolysis is stimulated by nucleic acid binding likely because nucleic acid binding results in the opening of the helicase core. Diffusion into the NTP binding pocket is thus increased. Model (e) is based on the superposition in Extended Data Fig. 6b. Model (f) is based on the superposition in Extended Data Fig. 6c. Model (g) is based on the superposition in Extended Data Fig. 6d. Model (h) is based on the superposition in Extended Data Fig. 6e.).
Extended Data Figure 8. Comparison of canonical and reorganized DNA_Myc_ G-quadruplex.
DNA_Myc_ǂ denotes the canonical DNA_Myc_ structure20,24 whereas DNA_Myc_* represents the reorganized DNA_Myc_ found in the DHX36-DSM-DNA_Myc_ co-crystal structure. (a) Structure of the DNA_Myc_ǂ top G-quartet (PDB ID: 2N21). (b) Structure of the DNA_Myc_* top G-quartet. (c) Primary sequence alignment of the canonical and reorganized DNA_Myc_ G-quadruplex. Bold residues participate in formation of a quartet. (d) The structure of DNA_Myc_ G-quadruplex found in our co-crystal structure, represented here by DNA_Myc_*. Distances between Ade1-Thy24 as well as Gua16-Gua17, Gua17-Thy18, and Thy18-Thy19 are indicated. Theoretical FRET efficiencies (E) for DNA_Myc_ǂ and DNA_Myc_* were calculated using_E_ = 1/[1 + (r/R_0)6] where_R_0 = 53 Å for the Cy3-Cy5 pair and_r is the distance between Cy3-Cy5. Since smFRET experiments were performed with a DNA_Myc_G-quadruplex containing a 3' ssDNA extension of 9 thymines, we added the distance between two thymines to the theoretical FRET efficiency model assuming an average internucleotide distance of 7.1 Å. Since the difference between the hypothetical DNA_Myc_ǂ previously solved by NMR and DNA_Myc_* found in our co-crystal structure is 1 nucleotide, we modeled r_ǂ and_r* as 50.2 Å and 57.3 Å. From these parameters, we obtained predicted FRET efficiencies of 0.58 and 0.39 for DNA_Myc_ǂ and DNA_Myc_*, respectively. These predicted FRET efficiencies closely match the experimental oscillating FRET efficiencies of ~0.6 and ~0.4. (e) The high FRET state of ~0.85 is observed prior to DHX36 binding to the DNA_Myc_ G-quadruplex. (f) DHX36 initially binds to DNA_Myc_ǂ (~0.6). (g) Likely due to ATP-independent C-terminal domain rotations also observed16 with Prp43p, the DNA_Myc_, G-quadruplex is partially unwound to DNA_Myc_* (~0.4). DHX36 then oscillates between DNA_Myc_* and DNA_Myc_ǂ in an ATP-independent repetitive unfolding activity.
Extended Data Table 1.
Data collection and refinement statistics
| DHX36-DSM-DNA_Myc_ (PDB: 5HVE) | DHX36-Core (PDB: 5HVA) | DHX36-Core-BeF3– (PDB: 5HVC) | DHX36-Core-AlF4– (PDB: 5HVD) | |
|---|---|---|---|---|
| Data collection | ||||
| Space group | _P_2121 21 | _P_21 | _P_21 | _P_21 |
| Cell dimensions | ||||
| a,b, c (Å) | 72.5, 79.3, 212.1 | 61.3, 109.2, 62.4 | 61.9, 111.5, 63.0 | 62.0, 112.5, 63.2 |
| α, β, γ (°) | 90, 90, 90 | 90, 112.7, 90 | 90, 110.7, 90 | 90, 110.3, 90 |
| Resolution (Å) | 39.6−3.8 (3.9−3.8)a | 39.6−2.2 (2.3−2.2) | 37.8−2.5 (2.6−2.5) | 46.7−2.6 (2.7−2.6) |
| _R_merge(%) | 27.5 (168) | 8.66 (46.7) | 7.52 (25.9) | 15.0 (80.0) |
| <_I_>/<σ(_I_)> | 7.8 (1.3) | 18.3 (3.0) | 16.9 (3.6) | 9.9 (1.2) |
| _CC_1/2 | 0.994 (0.486) | 0.997 (0.883) | 0.996 (0.949) | 0.994 (0.646) |
| Completeness (%) | 99.2 (94.0) | 99.8 (98.1) | 91.5 (87.8) | 98.8 (98.2) |
| Redundancy | 9.6 (7.6) | 6.5 (6.2) | 3.5 (3.4) | 5.1 (3.6) |
| Refinement | ||||
| Resolution (Å) | 39.6−3.8 (3.9−3.8) | 39.6−2.2 (2.3−2.2) | 37.8−2.5 (2.6−2.5) | 46.7−2.6 (2.6−2.6) |
| No. reflections | 12606 (1153) | 37004 (3636) | 25665 (2446) | 26260 (2612) |
| _R_work/_R_free(%) | 23.8/28.0 | 17.7/21.7 | 19.4/23.3 | 17.3/21.3 |
| No. atoms | 7202 | 6477 | 6318 | 6266 |
| Protein | 6697 | 6286 | 6189 | 6126 |
| DNA | 503 | 0 | 0 | 0 |
| Ligand/ion | 2 | 0 | 32 | 32 |
| Water | 0 | 180 | 97 | 108 |
| Mean _B_-factors (Å2) | 120.9 | 53.6 | 60.9 | 50.1 |
| Protein | 118.4 | 53.8 | 61.8 | 50.0 |
| DNA | 155.2 | N/A | N/A | N/A |
| Ligand/ion | 117.5 | N/A | 47.2 | 78.8 |
| Water | N/A | 47.3 | 53.3 | 45.5 |
| R.m.s. deviations | ||||
| Bond lengths (Å) | 0.002 | 0.004 | 0.002 | 0.002 |
| Bond angles (°) | 0.50 | 0.75 | 0.52 | 0.56 |
| Ramachandran analysis (%) | ||||
| Favored | 91.2 | 96.3 | 97.2 | 96.8 |
| Allowed | 7.5 | 3.2 | 2.8 | 3.2 |
| Disallowed | 1.3 | 0.5 | 0.0 | 0.0 |
| Mean coordinate precision (Å) | 0.47 | 0.30 | 0.32 | 0.21 |
Extended Data Table 2.
Data collection statistics for DHX36-core-SeMet crystals
| DHX36-Core-SeMet 1 | DHX36-Core-SeMet 2 | DHX36-Core-SeMet 3 | DHX36-Core-SeMet 4 | DHX36-Core-SeMet 5 | DHX36-Core-SeMet <1,2,3,4,5> | |
|---|---|---|---|---|---|---|
| Data collection | ||||||
| Space group | _P_21 | _P_21 | _P_21 | _P_21 | _P_21 | _P_21 |
| Cell dimensions | ||||||
| a,b, c (Å) | 62.6, 115.7, 64.0 | 62.5, 113.6, 63.8 | 62.5, 113.4, 63.8 | 62.4, 113.5, 64.0 | 62.6, 114.7, 63.9 | 62.6, 114.7, 63.9 |
| α,β,γ (°) | 90, 107.3, 90 | 90, 108.0, 90 | 90, 108.0, 90 | 90, 107.8, 90 | 90, 107.8, 90 | 90, 107.8, 90 |
| Resolution (Å) | 46.7−3.2 (3.3−3.2)a | 46.6−3.3 (3.4−3.3) | 46.6−3.0 (3.1−3.0) | 46.6−2.9 (3.0−2.9) | 46.7−3.1 (3.2−3.1) | 46.7−3.1 (3.2−3.1) |
| _R_merge(%) | 17.4 (87.6) | 17.4 (94.1) | 14.5 (84.8) | 14.8 (74.8) | 14.4 (81.5) | 22.4 (102) |
| <_I_>/<σ(_I_)> | 10.5 (1.3) | 10.8 (1.5) | 11.6 (1.2) | 11.0 (1.4) | 12.1 (1.4) | 22.7 (2.1) |
| _CC_1/2 | 0.995 (0.643) | 0.996 (0.646) | 0.997 (0.743) | 0.997 (0.745) | 0.997 (0.798) | 0.999 (0.753) |
| Completeness (%) | 97.5 (80.5) | 98.6 (88.6) | 92.5 (60.8) | 90.6 (51.2) | 97.9 (12.1) | 98.9 (90.1) |
| Redundancy | 7.0 (5.0) | 7.0 (4.9) | 6.7 (4.8) | 6.7 (4.3) | 7.0 (5.0) | 31.1 (15.5) |
ACKNOWLEDGEMENTS
We thank the staff of sector 5 of ALS and beamline 17-ID-B of APS for crystallographic data collection; Y. He, National Heart, Lung and Blood Institute (NHLBI) for protein production; G. Piszczek (NHLBI) for DSC; R. Levine and D.-Y. Lee (NHLBI) for mass spectrometry; C. Fagan, C. Jones, T. Numata, R. Trachman III, K. Warner, and J. Zhang for discussions. This work was partly conducted at the ALS on the Berkeley Center for Structural Biology beamlines, which are supported by the US National Insitutes of Health (NIH). Use of ALS and APS was supported by the US Department of Energy. This work was supported in part by the NIH (GM105453, S.M.), American Chemical Society (RSG-12–066-01-DMC, S.M.), National Science Foundation Physics Frontiers Center Program (0822613, S.M.), Wellcome Trust (099232/z/12/z, S.B.), European Research Council (339778, S.B.), Cancer Research UK (C12303/A17197 and C9681/A18618, S.B.), NIH-Oxford-Cambridge Scholars Program (M.C.C.), Cambridge Trust (M.C.C.), and the intramural program of the NHLBI, NIH.
REFERENCES
- 1.Hänsel-Hertsch R, Di Antonio M & Balasubramanian S DNA G-quadruplexes in the human genome: detection, functions and therapeutic potential. Nat Rev Mol Cell Biol 18, 279–284 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Rhodes D & Lipps HJ G-quadruplexes and their regulatory roles in biology. Nuclec Acids Res 43, 8627–8637 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendoza O, Bourdoncle A, Boulé JB, Brosh RMJ & Mergny JL G-quadruplexes and helicases. Nuclec Acids Res 44, 1989–2006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giri B et al. G4 resolvase 1 tightly binds and unwinds unimolecular G4-DNA. Nucleic Acids Res 39, 7161–7168 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MC, Murat P, Abecassis K, Ferré-D’Amaré AR & Balasubramanian S Insights into the mechanism of a G-quadruplex-unwinding DEAH-box helicase. Nucleic Acids Res 43, 2223–2231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughn JP et al. The DEXH protein product of the DHX36 gene is the major source of tetramolecular quadruplex G4-DNA resolving activity in HeLa cell lysates. J Biol Chem 280, 38117–38120 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Lattmann S, Stadler MB, Vaughn JP, Akman SA & Nagamine Y The DEAH-box RNA helicase RHAU binds an intramolecular RNA G-quadruplex in TERC and associates with telomerase holoenzyme. Nucleic Acids Res 39, 9390–9404 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McRae EKS et al. Human DDX21 binds and unwinds RNA guanine quadruplexes. Nucleic Acids Res 45, 6656–6668 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nie J et al. Post-transcriptional regulation of Nkx2–5 by RHAU in heart development. Cell Reports 13, 723–732 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Lai JC et al. The DEAH-box helicase RHAU is an essential gene and critical for mouse hematopoiesis. Blood 119, 4291–4300 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Sexton AN & Collins K The 5’ guanosine tracts of human telomerase RNA are recognized by the G-quadruplex binding domain of the RNA helicase DHX36 and function to increase RNA accumulation. Mol Cell Biol 31, 736–743 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booy EP et al. The RNA helicase RHAU (DHX36) unwinds a G4-quadruplex in human telomerase RNA and promotes the formation of the P1 helix template boundary. Nucleic Acids Res 40, 4110–4124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walbott H et al. Prp43p contains a processive helicase structural architecture with a specific regulatory domain. EMBO J 29, 2194–2204 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Andersen G & Nielsen K Structural basis for the function of DEAH helicases. EMBO Rep 11, 180–186 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prabu JR et al. Structure of the RNA helicase MLE reveals the molecular mechanisms for uridine specificity and RNA-ATP coupling. Mol Cell 60, 487–499 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Tauchert MJ, Fourmann JB, Lührmann R & Ficner R Structural insights into the mechanism of the DEAH-box RNA helicase Prp43. eLife 6, 762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Staley J, Andersen G & Nielsen K Structure of the DEAH/RHA ATPase Prp43p bound to RNA implicates a pair of hairpins and motif Va in translocation along RNA. RNA 23, 1110–1124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen MC & Ferré-D’Amaré AR Structural basis of DEAH/RHA helicase activity. Crystals 7, 253 (2017). [Google Scholar]
- 19.Lattmann S, Giri B, Vaughn J, Akman S & Nagamine Y Role of the amino terminal RHAU-specific motif in the recognition and resolution of guanine quadruplex-RNA by the DEAH-box RNA helicase RHAU. Nucleic Acids Res 38, 6219–6233 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambrus A, Chen D, Dai J, Jones RA & Yang D Solution structure of the biologically relevant G-quadruplex element in the human c-Myc promoter. Implications for G-quadruplex stabilization. Biochemistry 44, 2048–2058 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Smaldino PJ et al. Mutational dissection of telomeric DNA binding requirements of G4 resolvase 1 shows that G4-structure and certain 3’-tail sequences are suffcient for tight and complete biniding. PLos One 10, e0132668 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tippana R, Hwang H, Opresko PL, Bohr VA & Myong S Single-molecule imaging reveals a common mechanism shared by G-quadruplex-resolving helicases. Proc Natl Acad Sci USA 113, 8448–8453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yangyuoru P, Bradburn D, Liu Z, Xiao T & Russell R The G-quadruplex (G4) resolvase DHX36 efficiently and specifically disrupts DNA G4s via a translocation-based helicase mechanism. J Biol Chem 293, 1924–1932 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan AT, Modi YS & Patel DJ Propeller-type parallel-stranded G-quadruplexes in the human_c-myc_ promoter. J Am Chem Soc 126, 8710–8716 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnmacht SA & Neidle S Small-molecle quadruplex-targeted drug discovery. Bioorg Med Chem Lett 24, 2602–2612 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Heddi B, Cheong VV, Martadinata H & Phan AT Insights into G-quadruplex specific recognition by the DEAH-box helicase RHAU: solution structure of a peptide–quadruplex complex. Proc Natl Acad Sci USA 112, 9608–9613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creacy SD, Routh ED, Iwamoto F & Nagamine Y G4 resolvase 1 binds both DNA and RNA tetramolecular quadruplex with high affinity and is the major source of tetramolecular quadruplex G4-DNA and G4-RNA resolving activity in HeLa cell lysates. J Biol Chem 283, 34626–34634 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tippana R, Xiao W & Myong S G-quadruplex conformation and dynamics are determined by loop length and sequence. Nucleic Acids Res 42, 8106–8114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright PE & Dyson HJ Intrinsically disordered proteins in cellular signalling and regulation. Nature Rev Mol Cell Biol 16, 18–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen KR, Leksa NC & Schwartz TU Optimized E. coli expression strain LOBSTR eliminates common contaminants from His-tag purification. Proteins 81, 1857–1861 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Studier FW Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41, 207–234 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Rayment I et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261, 50–58 (1993). [DOI] [PubMed] [Google Scholar]
- 33.Otwinowski Z & Minor W Processing of diffraction data collected in oscillation mode. Meth. Enzymol. 276, 307–326 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Grosse-Kunstleve RW & Adams PD Substructure search procedures for macromolecular structures. Acta Crystallogr D 59, 1966–1973 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Adams PD et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terwilliger TC Maximum-likelihood density modification. Acta Crystallogr D 56, 965–972 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emsley P & Cowtan K Coot: model-building tools for molecular graphics. Acta Crystallogr D 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 38.McCoy A et al. Phaser crystallographic software. J Appl Cryst 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLano WL The PyMOL Molecular Graphics System. (DeLano Scientific, 2002).
- 40.Pettersen EF et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Tauchert MJ, Fourmann J-B, Christian H, Lührmann R & Ficner R Structural and functional analysis of the RNA helicase Prp43 from the thermophilic eukaryote Chaetomium thermophilum. Acta Crystallogr F 72, 112–120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sievers F et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7, 539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]