Response to Fungal Dysbiosis by Gut Resident CX3CR1+ Mononuclear Phagocytes Aggravates Allergic Airway Disease (original) (raw)
. Author manuscript; available in PMC: 2019 Dec 12.
Published in final edited form as: Cell Host Microbe. 2018 Nov 29;24(6):847–856.e4. doi: 10.1016/j.chom.2018.11.003
Summary
Sensing of the gut microbiota, including fungi, regulates mucosal immunity. Whether fungal sensing in the gut can influence immunity at other body sites is unknown. Here we show that fluconazole-induced gut fungal dysbiosis has persistent effects on allergic airway disease in a house dust mite challenge model. Mice with a defined community of bacteria, but lacking intestinal fungi were not susceptible to fluconazole-induced dysbiosis, while colonization with a fungal mixture recapitulated the detrimental effects. Gut resident mononuclear phagocytes (MNPs) expressing the fractalkine receptor CX3CR1 were essential for the effect of gut fungal dysbiosis on peripheral immunity. Depletion of CX3CR1+ MNPs or selective inhibition of Syk signaling downstream of fungal sensing in these cells ameliorated lung allergy. These results indicate that disruption of intestinal fungal communities can have persistent effects on peripheral immunity and aggravate disease severity through fungal sensing by gut resident CX3CR1+ MNPs.
Keywords: Mycobiota dysbiosis, Mycobiome, Fungi, Gut-lung axis, CX3CR1+ mononuclear phagocytes
Graphical Abstract

eTOC blurb
How mycobiota influences immunity in gut-distal sites is not well understood. Li et al. developed protocols for gut-targeted depletion of phagocytes and mycobiota-free mice to investigate the influence of fungi on gut-lung crosstalk. They reveal that intestinal phagocytes sense fungal dysbiosis and mediate gut-lung-directed immunity to aggravate allergic airway disease.
Introduction
The intestinal microbiota is a dynamic community of bacteria, viruses, fungi and protozoa (Blander et al., 2017). These organisms often share the similar niches in the gut and play an important role in host immunity (Blander et al., 2017). “Dysbiosis” can be both a consequence and a cause of many metabolic, neurological and inflammatory diseases. Numerous studies have shown that antibiotic-induced gut bacterial dysbiosis can result in major immunological outcomes in the host and affect the development of several inflammatory and allergic diseases (Arrieta et al., 2015; Huffnagle, 2010; Wypych and Marsland, 2018). Several recent studies indicate that fungal dysbiosis occurs in the gut, and might modulate host immunity and influence the development and outcome of intestinal and lung disease (Arrieta et al., 2015; Iliev and Leonardi, 2017; Liguori et al., 2016; Wheeler et al., 2016).
Fungal dysbiosis can occur under two major conditions. First, antibiotic treatment eliminates bacterial species that promote the resistance against fungal colonization during homeostasis. This loss ultimately leads to yeast overgrowth and fungal dysbiosis (Fan et al., 2015; Noverr et al., 2004). Several groups have shown that Candida albicans or C. parapsilosis overgrowth under such a condition can promote lung allergy (Kim et al., 2014; Noverr et al., 2004). Second, treatment with antifungal drugs promotes the overgrowth of drug-resistant filamentous fungi. This phenomenon can impact systemic immunity, contributing to the development of allergic airway disease (AAD) (Wheeler et al., 2016). However, the mechanisms by which fungi modulate gut-lung axis-directed immune activation are not well understood.
Recent advances in transplantation medicine, HIV management and cancer therapies have led to a massive increase in patients living with a compromised immunity who are often treated with antifungal drugs for extended periods of time (Brown et al., 2012; Hamad, 2010). Additionally, less severe but highly prevalent conditions such as mucosal and vulvovaginal candidiasis (affecting 50 to 75% of women in their childbearing years) are often treated with oral fluconazole, the most widely prescribed antifungal drug (Brown et al., 2012; Hamad, 2010). Despite this widespread use of antifungal drugs, little is currently known about their effect on host immunity and disease. In contrast, new studies on antibiotic drugs have highlighted the persistent effects of early-life antibiotic exposure on the development of AAD (Arrieta et al., 2015; Wypych and Marsland, 2018). Although antifungal drugs were recently shown to affect gut fungal and bacterial communities (Wheeler et al., 2016), it is unclear whether fungal dysbiosis has a persistent consequence on systemic immunity and how dysbiotic fungi are sensed in the gut to affect immunity at distal sites, such as the lung.
As a central hub of mucosal immunity, the gastrointestinal tract is naturally equipped with a cellular machinery to sense and interact with the microbiota (Mowat and Agace, 2014). The intestines harbor several subsets of phagocytes, which are known to respond to infections or to fluctuations in the commensal bacteria and fungi (Diehl et al., 2013; Leonardi et al., 2018; Mowat and Agace, 2014; Panea et al., 2015). We have recently shown that among these a population of CX3CR1+ gut resident mononuclear phagocytes (MNPs) expresses several antifungal receptors and is crucial for the induction of innate and adaptive immune responses to intestinal fungi (Leonardi et al., 2018). Whether fungal sensing in the gut can influence immunity at other body sites is currently unknown.
Here we provide evidence for the persistent effect of gut fungal dysbiosis on immunity at distal body sites. Mice that carry a minimal bacterial community of functionally diverse anaerobic and aerobic bacteria, but are “mycobiota-free” (MyF-ASF) were resistant to fluconazole-induced dysbiosis and exacerbated AAD. Intestinal inoculation with “dysbiotic” fungal community recapitulated the detrimental effect of fluconazole-induced gut dysbiosis on lung immunity during house dust mite (HDM) challenge, suggesting that intestinal fungi activate gut-lung axis directed mechanisms to affect systemic immunity targeting distal body sites. CX3CR1+ MNPs that are involved in the uptake of intestinal fungi, were essential for mediating the effect of gut fungal dysbiosis on peripheral immunity. Depletion of CX3CR1+ gut resident MNPs or selective inhibition of spleen tyrosine kinase (Syk) signaling within these intestinal phagocytes ameliorated the symptoms of HDM-induced lung allergy. These results suggest that perturbation of healthy intestinal fungal communities can lead to the expansion of specific fungi that can affect systemic immunity and AAD through an interaction with CX3CR1+ MNPs in the gut.
Results
Fluconazole-induced gut fungal dysbiosis has persistent immune effects on AAD.
We have previously shown that fluconazole-induced gut fungal dysbiosis exacerbates the development of HDM-induced AAD (Wheeler et al., 2016). To further investigate the underlying cause of fluconazole-induced gut fungal dysbiosis we developed a protocol where HDM immunization occurred after withdrawal of the drug (Post-Fluc group, Figure 1A). We determined that fluconazole was absent from the serum of mice soon after its withdrawal (Figure S1A and Figure 1B). This provided an opportunity to study the effect of fungal dysbiosis on AAD without the potentially confounding factor of fluconazole co-administration. Specifically, we treated wild-type (WT) mice with fluconazole in the drinking water for three weeks, followed by intranasal immunization with HDM 24 hours after its withdrawal (Post-Fluc group, Figure 1A). Since continuous fluconazole treatment has detrimental effects on AAD (Wheeler et al., 2016), a separate group of mice received the drug for the duration of the HDM immunization protocol (Fluc group, Figure 1A). As expected, continuous fluconazole treatment was sufficient to exacerbate AAD. Interestingly, mice immunized with HDM after fluconazole cessation experienced similar degree of disease severity as evidenced by inflammatory immune cell infiltration in bronchoalveolar lavage fluid (BAL) (Figure 1C and D, Figure S1B). While lung infiltration of “first line” responders such as neutrophils, macrophages and eosinophils decreased in the post-fluconazole group when compared to the group receiving continuous fluconazole treatment, the levels of IL-4 producing by T helper 2 (Th2) cells in mediastinal lymph node (medLN), serum IgE and anti-HDM IgG1 were comparable between fluconazole and post-fluconazole groups (Figure 1D-G). This result indicates that changes in the adaptive immune response were maintained for several weeks after fluconazole removal. IL-17+ and IFN-γ+ CD4+ T cells on the other hand remained similar between all groups (Figure1E and Figure S1C). Antifungal therapeutics can have a static effect on fungal populations as well as potential off-target effects on bacteria. We thus asked whether the initial effect observed during continuous exposure to fluconazole would have persistent consequences on fungal populations. Continuous administration of fluconazole for three weeks lead to increased alpha diversity of fungal populations that returned to the baseline level upon its removal. Yet, the reduction in the total amount of fungal 18S rDNA persisted three weeks after fluconazole cessation (Figure 1H-I). Non-metric multidimensional scaling (NMDS) analysis of fungal operational taxonomic units (OTUs) further revealed a dramatic shift of the community composition that persisted weeks after the removal of the drug (Figure 1J). These data demonstrate that fluconazole treatment resulted in alterations of gut mycobiota, skewing the intestinal fungal community from a homeostatic state towards the expansion of fluconazole-resistant species even after discontinuation of the drug (Figure 1J and Figure S2A). Altogether, our data suggests that fluconazole-induced fungal dysbiosis exerts persistent effects on gut fungal communities and on lung airway inflammation.
Figure 1. Fluconazole-induced gut fungal dysbiosis has persistent immune effects on lung allergic airway disease.
(A) Layout of the experiment setup, all mice from normal drinking water (Ctrl), fluconazole (Fluc) and post-fluconazole treated groups (Post-Fluc) were intranasally immunized with HDM. (B) Blood samples were collected for serum fluconazole assessment by liquid chromatography-mass spectrometry (LC-MS/MS). Ctrl (n=8), Fluc (n=8). (C-E) All mice (n=5 per group) were intranasally immunized with HDM. Representative eosinophil staining (pre-gated on CD45+CD11c− cells; left) and frequencies of Siglec-F+SSChi eosinophils in BAL (right). (D) Left to right: total cells, eosinophil, macrophage and neutrophil numbers in BAL. (E) Frequencies of CD4+ IL-4+ Th2 cells (left) and IL-17+ Th17 cells (right) in the medLN. (F-G) ELISA detection of total serum IgE and anti-HDM IgG1. OD value relative to control group. Ctrl (n=10), Fluc (n=5), Post-Fluc (n=10). (H-I) Feces were collected from mice in A) before (Ctrl, n=10), during (Fluc, n=10), and 3 weeks after removal (Post-Fluc, n=10) of fluconazole. (H) Quantitative real-time PCR (q-PCR) for fungal 18S rDNA in feces. (n=5 per group). (I) Alpha diversity (Observed OTU index) and (J) NMDS plot (Bray-Curtis) derived from ITS amplicon sequencing data. Dots represent individual mice, data are represented as mean ± SEM. Data are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA.
Antifungal treatment does not exacerbate AAD in absence of gut fungi.
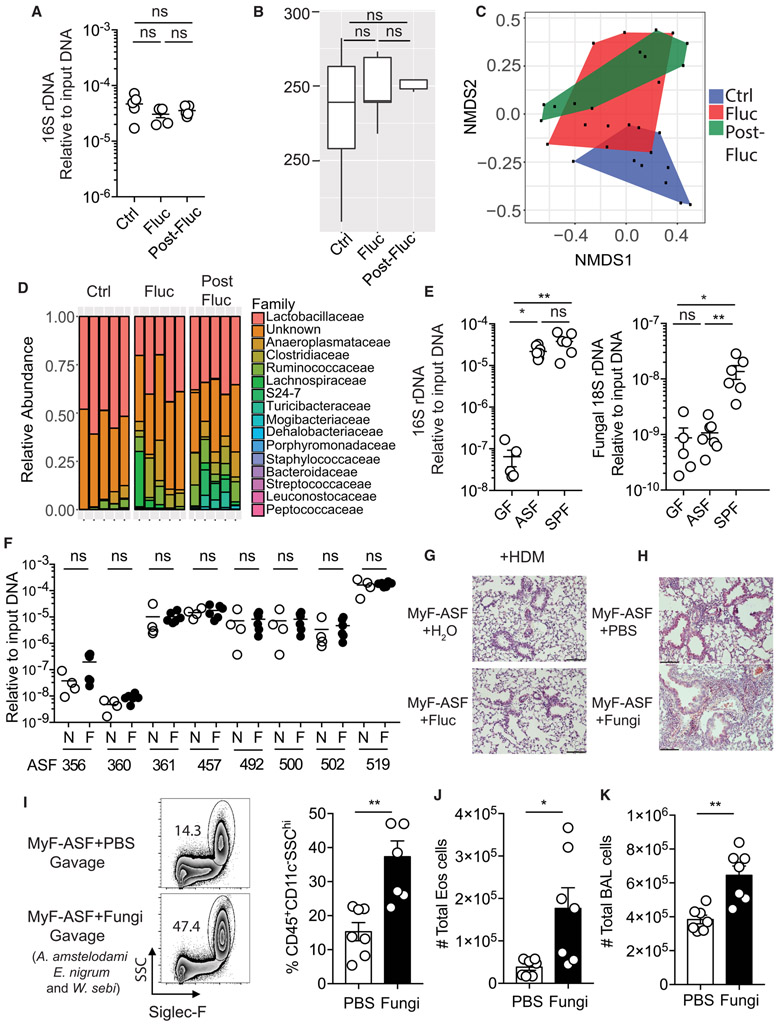
Azoles such as fluconazole, which inhibit the fungal cytochrome system, have not yet been reported to directly target gut commensal bacteria. Consistently, we found that the amount of bacteria in the feces and the overall alpha diversity of the bacterial community remained unchanged after fluconazole treatment (Figure 2A-B). However, fluconazole induced a change in overall bacterial community structure. In contrast to the continuous shift of the fungal community, the bacterial community structure did not shift further after the withdrawal of fluconazole (Figure 2C). At the taxonomic level, we observed small, but consistent relative increase in two Clostridiales families, Clostridiaceae and Lachnospiraceae, and a decrease of Lactobacillaceae (Figure 2D) both upon and after fluconazole treatment (Figure 2A-D). We reasoned that there might be several explanations for this phenomenon. First, fluconazole might have off-targets effect on host immunity that indirectly influence the bacterial community. Second, fluconazole might have a direct effect on bacteria as recently shown for other non-antibiotic drugs that inhibit the growth of gut bacteria (Maier et al., 2018). Third, as bacterial and fungal populations share similar gastrointestinal niches, they might be interdependent and the increase or reduction of particular fungi might influence the abundance of particular bacteria. To explore fungal dependence in a controlled environment, we used gnotobiotic mice colonized with altered Schaedler flora (ASF) for several generations. These mice were born with a minimal bacterial community that includes a defined set of functionally diverse anaerobic and aerobic bacteria (Robertson et al., 2005), sufficient to establish normal physiological functions in germ-free mice (Schaedler et al., 1965). Our analysis of feces by quantitative PCR (Figure 2E), showed that ASF mice lack intestinal fungi, and can be utilized as a “mycobiota-free” model (MyF-ASF). Therefore, we treated MyF-ASF mice with fluconazole for three weeks and assessed the abundance of the eight-bacterial species, including two strains of Lactobacillaceae family, and two strains of Clostridiaceae family. Notably, fluconazole treatment failed to induce change within the ASF community over three weeks of treatment (Figure 2F), indicating that fluconazole does not directly induce bacterial dysbiosis under mycobiota-free condition. Consistently, fluconazole treatment of MyF-ASF mice did not influence HDM-induced allergic airway inflammation as measured by lung histology, eosinophil infiltration in BAL, IL-4+ Th2 cells frequencies in medLN, serum IgE and anti-HDM IgG1 antibody titers (Figure 2G and Figure S2B-G). Collectively, the data suggest that the effects of fluconazole on AAD are conferred through direct targeting of gut fungal communities.
Figure 2. Antifungal treatment does not exacerbate allergic airway disease in mycobiome-free (MyF) mice while oral supplementation with fungi is detrimental.
(A) Q-PCR for bacterial 16S rDNA in feces from mice in Figure 1H. (B) Alpha diversity (Observed OTU index) by 16S rDNA sequencing. (C) NMDS plot (Bray-Curtis) for bacterial OTUs in feces. (D) Relative bacterial abundance at the family level (n=5 per group). (E) Q-PCR for 16S rDNA (left) and 18S rDNA (right) in feces from germ-free (GF) mice (n=5), MyF-ASF mice (n=8) and WT SPF mice (n=6). (F) Species specific q-PCR for bacterial species of altered Schaedler flora in feces from MyF-ASF mice administrated normal water (n=4) or fluconazole for 3 weeks (n=5). (G) MyF-ASF mice were administrated water (Ctrl, n=4) or fluconazole water (Fluc, n=4) 7 days prior to HDM immunizations. H&E stained lung sections (20X), scale bar= 100 μM. (H-K) Mice were fed either with PBS (n=7) or a mixture of three fungal species (A. amstelodami, E. nigrum and W. sebi; n=7) for a week prior to exposure to HDM. (H) H&E stained lung histology (20X), scale bar= 100 μM. (I) Representative eosinophil staining (left), frequencies (right), (J) number of eosinophils and (K) total number of cells in BAL. Dots represent individual mice, data are represented as mean ± SEM. Data are representative of at least two or three independent experiments. *p < 0.05, **p < 0.01, Mann-Whitney test.
Oral supplementation of mycobiota-free ASF mice with a dysbiotic fungal community is sufficient to exacerbate AAD.
ASF mice are a reliable model to study how the addition of a specific bacterial strain to a stable community influences immunological and metabolic pathways or affects disease outcomes (Gomez de Aguero et al., 2016; Shen et al., 2015)(Gomes-Neto et al., 2017). Having observed that fluconazole-induced fungal dysbiosis exerts persistent impacts on systemic immunity and AAD in mycobiota-sufficient specific pathogen-free (SPF) mice, without directly targeting either commensal bacteria or the host, we asked whether a supplementation with intestinal fungi can recapitulate the disease phenotype in MyF-ASF mice. We thus used a dysbiotic fungal community (Aspergillus amstelodami, Epicoccum nigrum, and Wallemia sebi) that we previously found to recapitulate the exacerbating effects of fluconazole on AAD in SPF mice (Wheeler et al., 2016). Such ex-MyF-ASF mice were subsequently immunized with HDM. Interestingly, gut colonization with these three fungi exacerbated HDM-induced AAD, evidenced by elevated eosinophil infiltration, total inflammatory cell infiltration and worsened lung histology (Figure 2H-K). We also observed elevated percentages of both RORγt+ and GATA3+ CD4+ T cells in the lung (Figure S3A), suggesting the induction of a mixed Th2/Th17 driven-allergic airway inflammation in MyF-ASF mice upon gut colonization with a dysbiotic fungal community. Oral administration of a single member of this community (A. amstelodami) also aggravated HDM-triggered BAL cells infiltration and eosinophilia in MyF-ASF mice, but to a lesser extent when compared to the dysbiotic fungal community (Figure S3B-D). In summary, we revealed that gut colonization of MyF-ASF mice with dysbiotic fungi was sufficient to exacerbate airway inflammation.
The systemic effects of gut fungal dysbiosis on AAD are mediated by CX3CR1+ MNPs.
Having shown that the effects of gut fungal dysbiosis on AAD are likely driven by the direct interaction of fungi or fungal derived products with the host, we reasoned that two possible mechanisms might take place. First, fungi-produced metabolites might translocate from the gut to the lung during dysbiosis-induced fungal overgrowth. Fungi-released prostaglandins have been shown as a likely candidate in this case (Kim et al., 2014; Noverr et al., 2002). While many fungi including Aspergillus and Penicillium species can produce prostaglandins (Noverr et al., 2002), intestinal colonization with A. amstelodami exacerbates AAD, while colonization with P. bervicompactum does not (Wheeler et al., 2016), rendering the possibility of fungi-produced prostaglandins as mediators of the systemic effects we observe less likely. Second, the direct recognition of fungal dysbiosis derived signals by gut immune cells might remotely initiate the detrimental effects on the lung allergy development. However, the potential mechanisms through which immune cells recognize fungal dysbiosis-derived signals to regulate distal allergic airway immune responses remain poorly characterized. We have recently shown that CX3CR1+ MNPs that express several antifungal receptors can efficiently recognize and uptake gut commensal fungi (Cook and MacDonald, 2016; Leonardi et al., 2018). Therefore, we next hypothesized that the direct sensing of fungi by gut CX3CR1+ MNPs might be responsible for the distal effects of fungal dysbiosis on systemic immunity and lung airway inflammation. Since CX3CR1+ MNPs are an abundant cell type in both the gut and the lung, we developed a strategy allowing us to efficiently deplete CX3CR1+ MNPs in the gut without affecting lung CX3CR1+ MNPs or other cell types previously shown to play a role in allergic airway inflammation (Cook and MacDonald, 2016; Gibbings et al., 2017; Mionnet et al., 2010; Tweedle and Deepe, 2018) (Figure S4A-C, and Figure 3A). Specifically, we used diphtheria toxin (DT) injected intraperitoneally (i.p.) into CD11c-Cre+/− x Cx3cr1 DTR mice (ΔCX3CR1) to delete CX3CR1+ MNPs in intestinal lamina propria (Figure 3A). ΔCX3CR1 mice and their littermate controls were treated with fluconazole prior to HDM exposure to induce airway disease (Figure S4D). We found that depletion of gut CX3CR1+ MNPs in this model did not influence the severity of HDM-induced airway disease as measured by eosinophil infiltration and medLN cell numbers in ΔCX3CR1 mice and littermate controls when treated with normal drinking water (Figure 3B-D, Figure S4E). In contrast, fluconazole treatment led to a dramatic reduction in eosinophil infiltration in the lung of ΔCX3CR1 mice, when compared to littermate controls. Consistently, mice with deletion of intestinal lamina propria CX3CR1+MNPs also had reduced medLN cell numbers and decreased type 2 immune responses after fluconazole treatment (Figure 3E-F). Together these results support the hypothesis that fungal sensing through CX3CR1+ MNPs is essential to mediate the effects of fluconazole-induced gut fungal dysbiosis on peripheral immune responses.
Figure 3. The systemic effects of gut fungal dysbiosis on lung allergy are mediated by CX3CR1+ MNPs.
(A) _Cd11c-Cre_−/− Cx3cr1 DTR littermates (Litt) (n=14) and Cd11c-Cre+/− Cx3cr1 DTR (ΔCX3CR1) (n=12) mice were treated with DT as described in Figure S4A. CD11b+CX3CR1+ MNPs were gated within live CD45+MHC+II+CD11c+ cells (left) and CX3CR1+MNP frequencies of in the colonic lamina propria (cLP) and lung are shown (right). (B-F) Litt and ΔCX3CR1 mice were administrated normal water (open bars, Litt (n=6), ΔCX3CR1 (n=6)) or fluconazole water (filled black bars, Litt (n=7), ΔCX3CR1 (n=6)) and immunized with HDM (Figure S4D). (B-C) Representative eosinophil staining (B, left), frequencies (B, right) and (C) total number of cells in BAL. (D) medLN total cell numbers. (E) Representative staining (left) and frequencies (right) of CD4+ GATA3+Th2 cells in the lung. (F) Frequencies of CD4+IL-4+ Th2 cells in medLN. Dots represent individual mice, data are represented as mean ± SEM. Data are representative of two independent experiments. *p < 0.05, **p < 0.01, Mann-Whitney test.
Syk signaling in intestinal CX3CR1+ MNPs contributes to allergic airway inflammation during gut fungal dysbiosis.
Gut-resident CX3CR1+ MNPs play a critical role in regulation of intestinal immune response to bacteria and fungi under homeostatic and dysbiotic conditions (Diehl et al., 2013; Kim et al., 2018; Leonardi et al., 2018; Panea et al., 2015). Although fluconazole treatment contributed to increases of CD4+ T cell frequencies in the lung of HDM-immunized mice, we did not observe changes in CX3CR1+ MNPs frequencies in the lung of those mice (Figure 4A). We therefore reasoned that CX3CR1+ MNPs local responses to fungi in the gut might be responsible for the observed phenotypes during fungal dysbiosis. CX3CR1+MNPs express Syk that is crucial for the initiation of the signaling cascades downstream of anti-fungal C-type lectin receptors (CLRs) activation (Leonardi et al., 2018). We have previously shown that selective depletion of Syk in CX3CR1+ MNPs can be achieved by activating tamoxifen-inducible Cre recombinase in Syk fl/fl x Cx3cr1-Cre/ERT mice with tamoxifen (Leonardi et al., 2018). Here, we developed a protocol consisting of intraperitoneal injections of 4-hydroxytamoxifen (4-OHT, Figure S4F), the active metabolite of tamoxifen, that efficiently depleted Syk within intestinal lamina propria CX3CR1+ MNPs of Syk fl/fl Cx3cr1-Cre/ERT (ΔSyk) mice while Syk expression in lung CX3CR1+ MNPs remained unaffected between littermate controls and ΔSyk mice (Figure 4C, D). This system allowed us to test whether specific depletion of Syk in intestinal CX3CR1+ MNPs influences AAD aggravated by gut fungal dysbiosis. Surprisingly, Syk inhibition in intestinal CX3CR1+ MNPs of fluconazole-treated mice ameliorated disease, characterized by decreased inflammatory cellular infiltration into the lung compared to their littermate controls (Figure 4E- G). This correlated with a decrease of GATA3+ Th2 cells in the intestinal lamina propria (Figure 4H-I) and reduced Th2 cells in lung upon Syk depletion in intestinal CX3CR1+ MNPs (Figure 4F). These findings indicate that Syk mediated activation of intestinal CX3CR1+ MNPs during fungal dysbiosis might be involved in priming of fungal specific-Th2 responses in the gut-a process that warrants further investigation. Together these results suggest that a selective inhibition of Syk signaling within intestinal CX3CR1+ MNPs mediates the effects of fluconazole-induced gut fungal dysbiosis on AAD.
Figure 4. Syk signaling in intestinal CX3CR1+ MNPs contributes to allergic airway inflammation during gut fungal dysbiosis.
(A-B) Mice were treated following experimental setup outlined in Figure 1A, Ctrl (n=6), Fluc (n=8). Frequencies of CD4+ T cells (A) and CX3CR1+MNPs (B) in the lung are shown. (C-D) Littermates (Litt, n=8) and Syk fl/fl Cx3cr1-Cre-ERT mice (ΔSyk, n=7) were treated (i.p) with 4-OHT as described in Figure S4F. (C) Representative Syk staining of CX3CR1+ MNPs and (D) mean fluorescence intensity (MFI) in intestinal cLP and lung. (E-I) Litt and ΔSyk mice were administrated normal water (open bars, Litt (n=5), ΔSyk (n=8)) or fluconazole water (filled black bars, Litt (n=8), ΔCX3CR (n=7)) and immunized with HDM. (E) Representative plots and frequencies of eosinophils in BAL, (F) Representative plots (left) and frequencies (right) of CD4+ GATA3+ Th2 cells in the lung. (G) H&E stained lung sections. (20X), scale bar= 100 μM. (H-I) Representative plots (H, left), frequencies (H, right) and total number of CD4+ GATA3+ Th2 cells (I) in the cLP. Dots represent individual mice, data are represented as mean ± SEM. Data are representative of two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 Mann-Whitney test.
Discussion
In this study, we utilize antifungal treatment to induce intestinal microbiota instability and show that the resulting fungal dysbiosis exert effects on the microbiota composition and experimental lung allergy weeks after drug withdrawal. We use mice colonized with ASF mouse model to show that antifungal treatment does not affect bacteria or exacerbate lung allergy in mycobiota-free ASF mouse. Intestinal colonization of MyF-ASF mice with a community of dysbiotic fungi, such as A. amstelodami, E. nigrum and W. sebi, aggravated the sympthoms of AAD, suggesting that gut fungi can activate gut–lung axis mechanisms to affect peripheral immunity and disease. We further explored the innate immune mechanisms of fungal dysbiosis sensing in the gut. We show that depletion of gut CX3CR1+ MNPs or specific Syk deletion in these cells abrogates the exacerbating effects of gut fungal dysbiosis on AAD. Our findings provide evidence for the influence of drug-induced dysbiosis and innate fungal sensing through intestinal CX3CR1+ MNPs on gut-lung directed immune activation.
Since fungi and bacteria co-exist in similar intestinal niches in the gut and several studies have demonstrated that these communities are interdependent on each other (Iliev and Leonardi, 2017; Noverr et al., 2004), we aimed to determine whether fungal-bacterial crosstalk was involved in the effects of fluconazole-induced dysbiosis on AAD. High-throughput internal transcribed spacer (ITS) and 16S sequencing showed that mycobiota targeting in SPF mice with common antifungal drug affected both gut fungal and bacterial communities. This raised the question whether the observed microbiota changes and disease phenotypes were the result of primary alterations in the fungal community or were due to secondary effects on bacterial populations. Our findings favor a model where the effect of fluconazole-induced dysbiosis on AAD are induced by gut fungi and bacterial population changes occur as a secondary phenomenon. Indeed, gut microbiota targeting with fluconazole lead to the relative increase of two clostridia families, Clostridiaceae and Lachnospiraceae. This data was surprising as members of these two families are known major short chain fatty acids (SCFA) producers (Vital et al., 2014) and systemic translocation of SCFA has been shown to play a key role in gut-lung axis mediated suppression of allergic inflammation (Cait et al., 2017; Trompette et al., 2014). Future studies on fungal interaction with members of these families in a controlled setting would shed a light on whether the observed correlation represents a real interaction or a secondary phenomenon.
We have recently demonstrated that a population of CX3CR1+ mononuclear phagocytes is crucial for the induction of adaptive immune responses to intestinal fungi (Leonardi et al., 2018). Thus, we wondered if gut fungal sensing through these phagocytes would modulate immune gut–lung axis-directed mechanisms to affect systemic immunity targeting distal body sites. CX3CR1+ MNPs originate from Ly6Chi monocytes, however their phenotypic differentiations appear to be tissue specific (Mowat and Agace, 2014). This population of phagocytes is also patrolling other body sites including the lung (Gibbings et al., 2017; Plantinga et al., 2013), rendering tissue specific targeting of these cells challenging. Here we present mouse models that allow targeting of CX3CR1+ MNPs in the gut without affecting their numbers in the lung. Using these models, we determined a role of gut CX3CR1+ MNPs and Syk-dependent signaling in these cells in mediating the exacerbating effects of fluconazole-induced intestinal fungal dysbiosis on HDM-induced AAD. We anticipate that there are additional factors and immune cell networks downstream of CX3CR1+ MNPs that may contribute to the effect of gut fungal dysbiosis on AAD. A recent study has indeed demonstrated that lung autoimmune inflammation can be triggered by segmented filamentous bacteria gut-lung axis Th17 cell induction (Bradley et al., 2017). While our study exploring the innate immune events affecting lung allergy during gut fungal dysbiosis, it will be important for future studies to determine how adaptive antifungal responses generated upon CX3CR1+ MNP activation affect systemic and distal site immunity. Gut–lung axis migration of T cells, innate lymphoid cells or inflammation-related factors have been shown to occur (Bradley et al., 2017; Huang et al., 2018; Kim et al., 2014) and could be possible avenues to pursue in future studies investigating the role of fungal dysbiosis on peripheral immunity and inflammatory diseases at gut distal sites.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for reagents may be directed to and will be fulfilled by Lead Contact, Iliyan D Iliev (iliev@med.cornell.edu).
Experimental Model and Subject Details
Laboratory Animals
Animal experiments were performed with 8–12-week-old mice and used approximately equal ratios of male and female mice. All animals were housed under specific pathogen-free conditions (Helicobacter pylori free) and raised on a 12-hour light/dark cycle with access to water and food (5053 Rodent Diet 20, PicoLab) ad libitum. All animal experiments were approved and are in accordance with the Institutional Animal Care and Use Committee guidelines at Weill Cornell Medicine.
Wild-type C57BL/6, Cx3cr1tm3(DTR)Litt/J (Cx3cr1 DTR) and Cd11c-Cre, Syk fl/fl, Cx3cr1-Cre/ERT2 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Altered Schaedler flora (ASF) mice were generated from germ-free C57BL/6 mice with inoculation of ASF and breeding such colonized mice for at least 5 generations to obtain fully immunocompetent progeny. ASF mice were maintained within sterile vinyl isolators at Weill Cornell Medical College Gnotobiotic Mouse Facility. Hemizygous Cd11c-Cre+/− mice were crossed with Cx3cr1 DTR mice to generate littermate controls (Cd11c-Cre−/−Cx3cr1 DTR) and Cd11c-Cre+/− Cx3cr1 DTR mice. Syk fl/fl mice were crossed with Cx3cr1-Cre/ERT2 to generate Syk fl/fl Cx3cr1-Cre/ERT2 mice, Syk fl/fl mice were used as littermate controls.
Fungal Strains and Culture Conditions
A. amsteodami (ATCC 46362), E. nigrum (ATCC 42773), W. sebi (ATCC 42694) were cultured on Sabouraud dextrose agar for 5-7 days and E. nigrum further cultured in liquid Sabouraud dextrose broth (at 28°C) prior to oral gavage into mice.
Method Details
Anti-fungal Treatment and Fungal Inoculation
Fluconazole water (0.5 mg/mL) was provided to mice ad libitum for 3 weeks before feces were collected for analysis prior to the initiation of HDM-induced allerigic airway diseases. Fungi were mixed at equal ratios, Mice were oral gavaged with 200 μL of the fungi cocktail (0.5g/kg in PBS) every other day.
Fungal and Bacterial DNA Isolation
Fecal pellets were collected and immediately frozen on dry ice. DNA for fungal and bacterial sequencing and validation quantitative Real-Time PCR was isolated from 2-3 fecal pellets following lyticase treatment, bead beating, and processing using Quick-DNA Fungal/Bacterial Kit (Zymo Research) as described (Leonardi et al., 2018).
Microbiome Library Generation and Sequencing
Mouse fungal and bacterial microbiomes were sequenced using the Illumina MiSeq platforms. Fungal ITS1-2 regions and bacterial 16S regions were amplified by PCR using primers (Table S1. Oligonucleotides used in the study, Related to Figure 1 and 2) modified to include sample barcodes and sequencing adaptors.
ITS amplicons were generated with 35 cycles, whereas 16S amplicons were generated with 25 cycles using Invitrogen AccuPrime PCR reagents (Carlsbad). Amplicons were then used in the second PCR reaction, using Illumina Nextera XT v2 (Illumina) barcoded primers to uniquely index each sample and 2×300 paired end sequencing was performed on the Illumina MiSeq (Illumina). DNA was amplified using the following PCR protocol: Initial denaturation at 94°C for 10 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and elongation at 72°C for 2 min, followed by an elongation step at 72°C for 30 min. All libraries were subjected to quality control using qPCR, DNA 1000 Bioanalyzer (Agilent), and Qubit (Life Technologies) to validate and quantitate library construction prior to preparing a Paired End flow cell.
Quantitative Real-Time PCR
Isolated total bacterial DNA, fungal DNA, specific bacteria species DNA were detected by quantitative PCR using SYBR Green Supermix (Applied Biosystems). Samples were analyzed for gene expression using the primers (Table S1, Oligonucleotides used in the study, Related to Figure 2).
Administration of Diphtheria Toxin and 4-OHT
In order to selectively delete intestinal lamina propria CX3CR1+ MNPs, diphtheria toxin (200 ng/mouse, Sigma-Aldrich) were intraperitoneally (i.p.) injected into littermate controls and Cd11c-Cre+/− Cx3cr1 DTR mice for two consecutive days before fluconazole treatment. For induction of CreERT recombinase in Syk fl/fl Cx3cr1-Cre/ERT2 mice, 4-hydroxytamoxifen (4-OHT, Sigma-Aldrich) was dissolved in sunflower oil (Sigma-Aldrich) to a final concentration of 2 mg/ml, and 100 μl 4-OHT solution were injected i.p. into each mouse (200 μg/mouse) for two consecutive days before fluconazole treatment. The maintenance DT and 4-OHT injections every three days was continued for the duration of the experiments.
House Dust Mite-induced Allergic Airway Disease
HDM extract (Greer Laboratories) was dissolved into PBS at a concentration of 2 mg/mL. Mice were initially started with indicated treatment prior to HDM intranasal immunization. Each mouse was exposed to 50 μL HDM solution (100 μg) three times with 7-day intervals. BAL were washed and stained for infiltrating inflammatory cells. The right lobe of the lung was taken for digestion with Liberase (Sigma-Aldrich) and DNAse I (Sigma-Aldrich). The left lobe of the lung was fixed with 10% formalin for H&E histology. Mediastinal lymph nodes were collected and smashed through a 70μm mesh for further cytokine staining. Briefly, the right lobe of the lung was cut into small pieces using scissors and transferred in 2 mL digestion media per lung with Liberase (Sigma-Aldrich, 0.5 mg/mL) and DNAse (Sigma-Aldrich, 0.2mg/mL) for 45 min-digestion at 37°C with manual vortexing every 15 minutes. Then lung pieces were smashed and filtered through 70 μm cell strainer, washed with Dulbecco's Modified Eagle's medium (supplemented with 2% FBS, 1% L-glutamine, 1% Penicillin/Strep (Thermo Fisher Scientific). Red blood cells were lysed by RBC Lysis Buffer (Biolegend) for 1 min. Cells were washed and stained with antibodies as indicated.
Isolation of Lamina Propria Lymphocytes
Colonic lamina propria cells (cLP) were isolated as previously described (Leonardi et al., 2018). Briefly, colons were excised, opened longitudinally, washed of fecal contents and then cut into 1 cm pieces. Intestinal pieces were transferred into Hank's Balanced Salt Solution (HBSS) medium (Thermo Fisher Scientific), supplemented with 2 mM EDTA, and were shaken for 8 min at 37°C. The remaining tissue was washed, minced and subsequently incubated in digestion medium consisting of RPMI 1640 (Thermo Fisher Scientific), 5% FBS, 0.5 mg/ml collagenase type VIII (Sigma-Aldrich), 5 U/ml DNase (Sigma-Aldrich), 100 IU/ml penicillin and 100 μg/ml streptomycin (Thermo Fisher Scientific), for 25 min at 37°C by gentle shaking. The cell suspensions were filtered through a 100μm mesh and centrifuged at 1700 rpm. The obtained cells were filtered through a 70 μm filter, washed twice with PBS and used as LP cells.
Flow Cytometry and Antibodies
Fluorophore-conjugated antibodies directed against the following molecules were used to stain cells: all antibodies as listed in the Key Resources Table. Bronchioalveolar lavage (BAL), lung cells, and cLP lymphocytes were harvested and stained with a panel of fluorescently conjugated antibodies. For cell surface staining, cells were incubated with antibodies at 4°C for 20 min. The FoxP3 Fix/Perm kit (Thermo Fisher Scientific) was used for intracellular transcription factors staining in accordance with the manufacturer’s instructions. For cytokine staining, cells were re-stimulated with 20 nM phorbol 12-myristate 13- acetate (Sigma-Aldrich), 1.3 uM ionomycin (Sigma-Aldrich) and Brefeldin A Solution (Thermo Fisher Scientific) in RPMI-1640 medium (Corning) supplemented with 10% FBS and penicillin/streptomycin for 4 hours. Intracellular staining for indicated cytokines was carried out with a Cytofix/Cytoperm kit (BD Biosciences) and fluorescent antibodies against mouse IL-4, IL-17 and IFN-γ according to manufacturer’s instructions. Flow cytometry was performed using a BD LSRFortessa cell analyzer (BD Biosciences) and data were analyzed with FlowJo software (TreeStar Inc).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD16/CD32 Monoclonal Antibody (93), eBioscience™ | Thermo Fisher Scientific (eBioscience™) | Cat # 14-0161-81; RRID: AB_467132 |
| Anti-mouse I-A/I-E Antibody (M5/114.15.2), Brilliant Violet 605™ | BioLegend | Cat # 107639; RRID: AB_2565894 |
| CD11b Monoclonal Antibody (M1/70), PE-Cyanine7 | Thermo Fisher Scientific (eBioscience™) | Cat # 25-0112-82; RRID: AB_469588 |
| Ly-6C Monoclonal Antibody (HK1.4), eFluor 450 | Thermo Fisher Scientific (eBioscience™) | Cat # 48-5932-82; RRID: AB_10805519 |
| Rat Anti-Mouse Siglec-F (E50-2440), PE | BD Biosciences | Cat # 552126; RRID: N/A |
| Anti-mouse F4/80 Antibody (BM8), Alexa Fluor 488 | BioLegend | Cat # 123120; RRID: AB_893479 |
| Anti-Syk Antibody (5F5), PE | BioLegend | Cat # 646004; RRID: AB_2565306 |
| Ly-6G Monoclonal Antibody (1A8-Ly6g), APC | Thermo Fisher Scientific (eBioscience™) | Cat # 17-9668-80; RRID: AB_2573306 |
| Anti-mouse CX3CR1 Antibody (SA011F11), BV421 | BioLegend | Cat # 149023; RRID: AB_2565706 |
| Anti-mouse CD11c Antibody (N418), Alexa Fluor 700 | BioLegend | Cat # 117319; RRID: AB_528735 |
| CD45 Monoclonal Antibody (30-F11), APC-eFluor 780 | Thermo Fisher Scientific (eBioscience™) | Cat # 47-0451-82; RRID: AB_1548781 |
| Brilliant Violet 605™ anti-mouse CD4 Antibody (RM4-5) | BioLegend | Cat # 100548; RRID: AB_2563054 |
| Gata-3 Monoclonal Antibody (TWAJ), PerCP-eFluor 710 | Thermo Fisher Scientific (eBioscience™) | Cat # 46-9966-42; RRID: AB_10804487 |
| FOXP3 Monoclonal Antibody (FJK-16s), APC | Thermo Fisher Scientific (eBioscience™) | Cat # 17-5773-82; RRID: AB_469457 |
| ROR gamma (t) Monoclonal Antibody (B2D), PE | Thermo Fisher Scientific (eBioscience™) | Cat # 12-6981-82; RRID: AB_10807092 |
| IL-4 Monoclonal Antibody (11B11), PE | Thermo Fisher Scientific (eBioscience™) | Cat # 12-7041-82; RRID: AB_466156 |
| IFN gamma Monoclonal Antibody (XMG1.2), FITC | Thermo Fisher Scientific (eBioscience™) | Cat # 11-7311-82; RRID: AB_465412 |
| Anti -mouse IL-17A Antibody (TC11-18H10.1) Pacific Blue™ | BioLegend | Cat # 506918; RRID: AB_893544 |
| eBioscience™ Fixable Viability Dye eFluor™ 506 | Thermo Fisher Scientific (eBioscience™) | Cat # 65-0866-14 |
| Bacterial and fungal Strains | ||
| Altered Schaedler Flora (ASF) | N/A | (Gomes-Neto et al., 2017) |
| Aspergillus amstelodami | ATCC | ATCC 46362 |
| Epicoccum nigrum | ATCC | ATCC 42773 |
| Wallemia sebi | ATCC | ATCC 42694 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Diptheria Toxin | Sigma-Aldrich | Cat# D0564 |
| Collagenase type VIII | Sigma-Aldrich | Cat# C2139 |
| Liberase | Sigma-Aldrich | Cat# 5401119001 |
| DNAse I | Sigma-Aldrich | Cat# D5025 |
| Brefeldin A | Thermo Fisher Scientific (eBioscience™) | Cat# 00-4506-51 |
| Ionomycin | Sigma-Aldrich | Cat# I0634 |
| Phorbol 12-myristate 13-acetate (PMA) | Sigma-Aldrich | Cat# P8139 |
| (Z)-4-Hydroxytamoxifen | Tocris Bioscience | Cat# 3412 |
| Fetal Bovine Serum | Corning | Cat# MT35016CV |
| Fluconazole | Sigma-Aldrich | Cat# PHR1160 |
| Sabouraud Dextrose Broth | VWR | Cat# 89406-400 |
| Sabouraud 4% Dextrose Agar | VWR | Cat# EM1.05438.0500 |
| Diptheria Toxin | Sigma-Aldrich | Cat# D0564 |
| Mite, House Dust (freeze-dried extract) Dermatophagoides pteronyssinus | GREER LABORATORIES INC | Cat# B82 |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | Cat# 15140122 |
| Fluconazole-d4 | Cayman Chemical | Cat# 18252 |
| Formic acid | Sigma-Aldrich | Cat# F0507 |
| Critical Commercial Assays | ||
| FoxP3 /Transcription Factor Staining Buffer set | Thermo Fisher Scientific (eBioscience™) | Cat# 00-5523-00 |
| BD Fixation/Permeabilization Solution Kit | BD Biosciences | Cat# 554714 |
| SYBR Green Supermix | BioRad | Cat#1725121 |
| Mouse IgE ELISA Set | BD Biosciences | Cat# 555248 |
| Deposited Data | ||
| 16S Sequencing | NCBI | https://www.ncbi.nlm.nih.gov/sra/docs/; GenBank:PRJNA492500 |
| ITS Sequencing | NCBI | https://www.ncbi.nlm.nih.gov/sra/docs/GenBank:PRJNA492905 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6 | The Jackson Laboratory | JAX:000664 |
| Mouse: CX3CR1-CreERT2 | The Jackson Laboratory | JAX# 021160 |
| Mouse Cx3cr1tm3(DTR)Litt/J (Cx3cr1DTR) | The Jackson Laboratory | JAX# 025629 |
| Mouse: CD11c-Cre | The Jackson Laboratory | JAX# 008068 |
| Mouse: Sykfl/fl (B6.129P2-Syktm1.2Tara/J) | The Jackson Laboratory | JAX# 017309 |
| Oligonucleotides | ||
| Please refer to Table S1 for Oligonucleotides used in this study. | Integrated DNA Technologies, Inc. (IDT) | N/A |
| Software and Algorithms | ||
| GraphPad Prim 5.0 | GraphPad Software | N/A |
| FlowJo LLC version 10.1. | Becton Dickinson | N/A |
| R version 3.3.1 | www.r-project.org | N/A |
| MassHunter Software | Agilent Technologies | N/A |
Collection of Mouse Serum
Blood samples were collected from mice at the indicated time points, and allowed to coagulate at room temperature for 2 hours. The coagulated samples were centrifuged (21,130 x G, 5 min) and the supernatant serum was collected and frozen at −80 °C.
ELISA Measurement of Cytokine and Antibody
Total serum IgE was measured by ELISA kit (BD Biosciences) following the manufacturer’s instructions. For HDM IgG1, 96-well plates (Corning) were coated overnight at 4 °C with 25 mg/mL HDM in PBS (100 μL) to capture serum HDM-specific antibodies followed by the detection with anti-mouse IgG1 kit (BioLegend). OD (450-570 nm) measurements were recorded.
LC-MS/MS Sample Preparation and Method
Mouse serum (10 uL) was mixed with mobile phase B (0.1 % formic acid (Sigma-Aldrich) in acetonitrile (Sigma-Aldrich), 100 μL) and internal standard solution (fluconazole-d4 (1.63 μM in acetonitrile) 100 μL, Cayman Chemical). An aliquot of the supernatant was diluted in 1:1 methanol-mobile phase A (0.1% formic acid in water). The standard curve for fluconazole was created using serum from untreated mice. A 1290 Infinity II HPLC system (Agilent Technologies) coupled with a Triple Quad LC/MS (Agilent 6495) was used for analyte separation and identification by tandem mass spectrometry. Aliquots (2 μL) of the samples were injected into the HPLC and run with an isocratic method (40% mobile phase A, 60% mobile phase B) at 4 mL/min on a Zorbax Eclipse Plus C18 (2.1 × 50 mm, 1.8 μm particle size, Agilent Technologies) stationary phase. Fluconazole and fluconazole-d4 eluted at 0.475 min. Integrations were calculated using MassHunter Software (Agilent Technologies) for the Q1 to Q3 transition (CE = 14 V). Qualifier fragmentations (220 or 223 m/z) were used to identify the analyte. The analyte/internal standard area ratio was used to create the standard curve used for quantification of serum fluconazole levels.
Quantification and Statistical Analysis
OTU Construction and Taxonomic Assignment
Raw FASTQ ITS1 sequencing data were filtered to enrich for high-quality reads, removing the adapter sequence by cutadapt v1.4.1 or any reads that do not contain the proximal primer sequence (Wheeler et al., 2016). Sequence reads were then quality-trimmed by truncating reads not having an average quality score of 20 (Q20) over a 3-base pair sliding window and removing reads shorter than 100 bp. These high-quality reads were then aligned to Targeted Host Fungi (THF) ITS1 database, using BLAST v2.2.22 and the pick_otus.py pipeline in the QIIME v1.6 wrapper with an identity percentage ≥97% for operational taxonomic unit (OTU) picking. The alignment results were then tabulated across all reads, using the accession identifier of the ITS reference sequences as surrogate OTUs and using a Perl script. For Illumina bacterial analysis, because of the abundance of Illumina reads and higher overall sequence quality of the reads, we used the QIIME package with minimal customization. The R packages Phyloseq (1.16.2) and Vegan (2.4-3) were used for determining community properties such as alpha diversity, Bray-Curtis index, NMDS scaling of Bray-Curtis dissimilarities, and relative abundance at various taxonomic levels. R version 3.3.1 was used.
Statistical analysis
N indicated in the figure legends means the number of mice in the experiments. Data are representative of at least two independent experiments as indicated, and error bars mean standard error of the mean (SEM). P values were calculated using unpaired nonparametric Mann-Whitney test or one-way analysis of variance (ANOVA) with a multiple comparisons correction by GraphPad Prism (GraphPad Software) at indicated in the figure legends. The mouse gender and age were randomized. The investigators were not blinded during the experiments and assessment except for the lung histological evaluation. No data or subject were excluded in the study. No statistical analysis was used to determine the appropriate sample size.
Data and Software Availability
The accession number for 16S sequencing data (GenBank: PRJNA492500, https://www.ncbi.nlm.nih.gov/sra/docs/) and ITS sequencing data (GenBank: PRJNA492905, https://www.ncbi.nlm.nih.gov/sra/docs/).
Supplementary Material
1
Table S1. Oligonucleotides Used in the Study, Related to Figure 1 and 2.
2
Highlights.
- Fungal dysbiosis persistently aggravates allergic airway disease (AAD) in mice
- Gut colonization by commensal fungi is both required and sufficient to aggravate AAD
- Intestinal CX3CR1+ mononuclear phagocytes (MNP) are essential for the effects.
- Inhibition of Syk mediated-fungal sensing in intestinal CX3CR1+ MNPs ameliorates AAD
Acknowledgements
This work was funded by the US National Institutes of Health (grants DK113136 and AI137157 to I.D.I), Kenneth Rainin Foundation, Irma T. Hirschl Career Scientist award to I.D.I, and support from the Jill Roberts Institute for Research in IBD. We thank Dr. J. David Warren for assistance with the LC-MS/MS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interest.
References
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, et al. (2015). Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7, 307ra152. [DOI] [PubMed] [Google Scholar]
- Blander JM, Longman RS, Iliev ID, Sonnenberg GF, and Artis D (2017). Regulation of inflammation by microbiota interactions with the host. Nature immunology 18, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CP, Teng F, Felix KM, Sano T, Naskar D, Block KE, Huang H, Knox KS, Littman DR, and Wu HJ (2017). Segmented Filamentous Bacteria Provoke Lung Autoimmunity by Inducing Gut-Lung Axis Th17 Cells Expressing Dual TCRs. Cell host & microbe 22, 697–704 e694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, and White TC (2012). Hidden killers: human fungal infections. Sci Transl Med 4, 165rv113. [DOI] [PubMed] [Google Scholar]
- Cait A, Hughes MR, Antignano F, Cait J, Dimitriu PA, Maas KR, Reynolds LA, Hacker L, Mohr J, Finlay BB, et al. (2017). Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. [DOI] [PubMed] [Google Scholar]
- Cook PC, and MacDonald AS (2016). Dendritic cells in lung immunopathology. Seminars in immunopathology 38, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, Schwab SR, and Littman DR (2013). Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 494, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, and Koh AY (2015). Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nature medicine 21, 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T, Leach SM, Bratton DL, Henson PM, Janssen WJ, et al. (2017). Three Unique Interstitial Macrophages in the Murine Lung at Steady State. American journal of respiratory cell and molecular biology 57, 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Neto JC, Mantz S, Held K, Sinha R, Segura Munoz RR, Schmaltz R, Benson AK, Walter J, and Ramer-Tait AE (2017). A real-time PCR assay for accurate quantification of the individual members of the Altered Schaedler Flora microbiota in gnotobiotic mice. Journal of microbiological methods 135, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. (2016). The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302. [DOI] [PubMed] [Google Scholar]
- Hamad B (2010). The antibiotics market. Nature reviews Drug discovery 9, 675–676. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mao K, Chen X, Sun MA, Kawabe T, Li W, Usher N, Zhu J, Urban JF Jr., Paul WE, et al. (2018). S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359, 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle GB (2010). The microbiota and allergies/asthma. PLoS Pathog 6, e1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, and Leonardi I (2017). Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol 17, 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Galan C, Hill AA, Wu WJ, Fehlner-Peach H, Song HW, Schady D, Bettini ML, Simpson KW, Longman RS, et al. (2018). Critical Role for the Microbiota in CX3CR1(+) Intestinal Mononuclear Phagocyte Regulation of Intestinal T Cell Responses. Immunity 49, 151–163 e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Udayanga KG, Totsuka N, Weinberg JB, Nunez G, and Shibuya A (2014). Gut Dysbiosis Promotes M2 Macrophage Polarization and Allergic Airway Inflammation via Fungi-Induced PGE2. Cell host & microbe 15, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi I, Li X, Semon A, Li D, Doron I, Putzel G, Bar A, Prieto D, Rescigno M, McGovern DPB, et al. (2018). CX3CR1(+) mononuclear phagocytes control immunity to intestinal fungi. Science 359, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori G, Lamas B, Richard ML, Brandi G, da Costa G, Hoffmann TW, Di Simone MP, Calabrese C, Poggioli G, Langella P, et al. (2016). Fungal Dysbiosis in Mucosa-associated Microbiota of Crohn's Disease Patients. Journal of Crohn's & colitis 10, 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, et al. (2018). Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mionnet C, Buatois V, Kanda A, Milcent V, Fleury S, Lair D, Langelot M, Lacoeuille Y, Hessel E, Coffman R, et al. (2010). CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nature medicine 16, 1305–1312. [DOI] [PubMed] [Google Scholar]
- Mowat AM, and Agace WW (2014). Regional specialization within the intestinal immune system. Nat Rev Immunol 14, 667–685. [DOI] [PubMed] [Google Scholar]
- Noverr MC, Noggle RM, Toews GB, and Huffnagle GB (2004). Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun 72, 4996–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Toews GB, and Huffnagle GB (2002). Production of prostaglandins and leukotrienes by pathogenic fungi. Infect Immun 70, 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panea C, Farkas AM, Goto Y, Abdollahi-Roodsaz S, Lee C, Koscso B, Gowda K, Hohl TM, Bogunovic M, and Ivanov II (2015). Intestinal Monocyte-Derived Macrophages Control Commensal-Specific Th17 Responses. Cell reports 12, 1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, et al. (2013). Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38, 322–335. [DOI] [PubMed] [Google Scholar]
- Robertson BR, O'Rourke JL, Neilan BA, Vandamme P, On SL, Fox JG, and Lee A (2005). Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. International journal of systematic and evolutionary microbiology 55, 1199–1204. [DOI] [PubMed] [Google Scholar]
- Schaedler RW, Dubs R, and Costello R (1965). Association of Germfree Mice with Bacteria Isolated from Normal Mice. J Exp Med 122, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TC, Albenberg L, Bittinger K, Chehoud C, Chen YY, Judge CA, Chau L, Ni J, Sheng M, Lin A, et al. (2015). Engineering the gut microbiota to treat hyperammonemia. The Journal of clinical investigation 125, 2841–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature medicine 20, 159–166. [DOI] [PubMed] [Google Scholar]
- Tweedle JL, and Deepe GS Jr. (2018). Tumor Necrosis Factor Alpha Antagonism Reveals a Gut/Lung Axis That Amplifies Regulatory T Cells in a Pulmonary Fungal Infection. Infect Immun 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital M, Howe AC, and Tiedje JM (2014). Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 5, e00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, et al. (2016). Immunological Consequences of Intestinal Fungal Dysbiosis. Cell host & microbe 19, 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wypych TP, and Marsland BJ (2018). Antibiotics as Instigators of Microbial Dysbiosis: Implications for Asthma and Allergy. Trends in immunology. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
Table S1. Oligonucleotides Used in the Study, Related to Figure 1 and 2.
2
Data Availability Statement
The accession number for 16S sequencing data (GenBank: PRJNA492500, https://www.ncbi.nlm.nih.gov/sra/docs/) and ITS sequencing data (GenBank: PRJNA492905, https://www.ncbi.nlm.nih.gov/sra/docs/).