Lung cancer screening in a community setting: Characteristics, motivations, and attitudes of individuals being screened (original) (raw)
Abstract
We describe the characteristics of individuals being screened in community settings including factors influencing screening decisions and the level of information sought prior to screening. Individuals from two community-based radiology clinics (N = 27) were surveyed after screening. Screening efficacy and salience were the most important factors in screening decisions, whereas healthcare provider recommendations were rated not important. Half of participants reported no or little conversation about screening with their primary care provider, and 61.5 percent had not sought any information on screening. Individuals being screened in a community setting are unlikely to have sufficient information for an informed decision about screening.
Keywords: decision-making, early adoption, information, low-dose computed tomography, lung cancer screening
Introduction
Lung cancer has long been the leading cause of cancer deaths among adults in the United States, with 234,030 new cases and 154,050 estimated for 2018 (American Cancer Society (ACS), 2018). After the National Lung Screening Trial (NLST) findings in 2011 showed a substantial relative reduction in lung cancer mortality with low-dose computed tomography (LDCT) screening (Aberle et al. 2011), the US Preventive Services Task Force (USPSTF) recommended lung cancer screening for specific high-risk populations (Moyer and USPSTF, 2014), and in 2015, Medicare and private insurance began to provide coverage of LDCT screening for lung cancer. Following these policy changes, advocates, researchers, and health care providers anticipated a strong upsurge in screening rates and hoped that lung cancer screening might provide a teachable moment regarding smoking cessation (Lennes et al., 2018). However, anecdotal evidence and two recent studies (Huo et al., 2017; Jemal and Fedewa, 2017) have shown lower than expected rates of screening among individuals for whom screening is recommended based on their smoking history.
It is important to recognize that recommendations for lung cancer screening are more targeted to specific populations than other cancer screening recommendations, as screening has only been shown to be efficacious in reducing mortality in a specific set of individuals. Therefore, a general or population-wide increase in screening rates, such as has been seen with breast cancer screening interventions (Anastasi and Lusher, 2017), is not necessarily desirable. Rather, lung cancer screening programs should aim to raise awareness, provide education, and promote engagement in shared decision-making, so that individuals have the knowledge and support to determine whether screening is right for them. Therefore, provision of information and guidelines for shared decision-making regarding lung cancer screening must be cognizant of the attitudes, motivations, and information needs of individuals who are making decisions about lung cancer screening.
Along these lines, a number of recent studies have explored individuals’ attitudes toward and interest in lung cancer screening. For example, Lillie et al. (2018) found that need for cognitive closure was not associated with lung cancer screening uptake in veterans. However, for the most part, these studies have focused on individuals participating in a lung cancer screening trial (e.g. Patel et al., 2012; Van den Bergh et al., 2009) or are survey studies of individuals who are not and/or have not been screened for lung cancer, but rather have been recruited from the general public or through a primary care setting (e.g. Delmerico et al., 2014; Jonnalagadda et al., 2012; Pallin et al., 2012; Silvestri et al., 2007; Tanner et al., 2013). Therefore, there is a lack of information in the literature about factors affecting screening decisions, attitudes toward screening, and information sought about screening in individuals who are actually undergoing screening lung cancer screening outside of a research setting. Although these data were collected before the NLST results, we nevertheless believe that the choice setting represented in our study is more relevant and comparable to current community-based settings for decision-making about lung cancer screening than other studies undertaken in the context of a trial or hypothetical (survey) choice situation.
The study reported here addresses this gap in knowledge by presenting information collected from individuals receiving lung cancer screening in a community setting. Although these data pre-date the NLST trial results and subsequent policy changes, the decisions made regarding screening reported here closely mirror actual community-based decisions about screening than the more frequently studied situations of screening in a screening trial setting or hypothetical screening questions.
Materials and methods
Participants and procedures
Eligible participants for this study were adults who had previously received at least one LDCT between February 2008 and June 2009 at one of two community-based direct outpatient radiology clinics in Florida and Kentucky. The study sample was recruited through two means: (1) the clinics distributed study flyers as potentially eligible patients arrived for their LDCT and (2) the clinics mailed an invitation letter to the 92 patients who had received an LDCT any time within the past year (Florida study site: 50 patients; Kentucky study site: 42 patients). Interested participants self-referred into the study. Participation was open regardless of gender, age, and race/ethnicity; however, individuals were excluded if they were incapable of completing a survey in English. Eligibility was confirmed and informed consent subsequently obtained either at the study site (Kentucky study site) or via telephone or mail (Florida study site). The survey was completed either during a scheduled study visit (Kentucky study site) or via mail (Florida study site). Participants received a US$60 gift card after completing the survey. The protocol was approved by the Institutional Review Boards of the University of Miami and the University of Kentucky.
Measures
Sample characteristics
Participants reported basic demographics (e.g. age, gender, race/ethnicity, highest educational attainment, marital status, employment status, annual household income), as well as general health, type of insurance, smoking history, and number of previous LDCT scans (including results) on the survey. In addition, individuals were asked whether they believed that they were at risk for developing lung cancer (yes/no).
Factors important in the decision to be screened for lung cancer
To identify possible influences on the decision to be screened in the community setting, participants rated 22 decision-making factors which potentially affected their past decision to be screened for lung cancer and 8 decision-making factors which might affect future LDCT decision-making. The factors were rated on a 10-point Likert-type scale (1 = not at all important to 10 = very important) and addressed barriers to screening, screening clinic and physician characteristics, screening efficacy, fear, social influence, lung cancer screening attributes, and salience (see Table 2 for the complete list of factors and results). The specific factors were selected as likely relevant to lung cancer screening decision-making from a systematic literature review as well as health behavior theory (Ajzen, 1991; Becker, 1974; Carter-Harris et al., 2016). For analyses, factors were categorized into three groups based on the overall mean scores: not important = 1–4.9, somewhat important = 5–7.9, and very important = 8–10. A mean summary importance score was also calculated.
Table 2.
Factors important to lung cancer screening decisions (n = 27).
| Mean (SD)a | Significant associations | |||
|---|---|---|---|---|
| Smoking statusCurrent vs former/never | GenderFemale vs male | Age⩽60 vs >60 | ||
| Previous lung cancer screening decision | ||||
| Factors rated not important | ||||
| A friend thought they should be screened (I) | 2.40 (2.90) | NS | 2.73 vs 1.00* | NS |
| A friend recommended the screening (I) | 2.46 (2.96) | NS | 3.00 vs 1.00* | NS |
| A friend had the same type of screening before (I) | 3.12 (3.54) | NS | 4.07 vs 1.00** | NS |
| A family member had the same type of screening (I) | 3.88 (3.75) | NS | NS | NS |
| A family member recommended the screening (I) | 4.08 (3.98) | NS | NS | NS |
| A family member thought they should be screened (I) | 4.13 (3.87) | NS | NS | NS |
| They had a coupon to reduce the cost of screening (B) | 4.25 (3.97) | NS | 5.21 vs 2.11* | NS |
| A doctor recommended the screening (I) | 4.42 (4.27) | NS | NS | NS |
| They received a discount on the screening (B) | 4.71 (3.84) | NS | 5.93 vs 2.22** | NS |
| Factors rated somewhat important | ||||
| They saw or heard an advertisement for the screening on the TV, newspaper, or radio (I) | 6.04 (3.85) | NS | NS | NS |
| The low false-positive rate (L) | 6.08 (3.58) | NS | 6.87 vs 4.33* | NS |
| The cost of the screening exam (B) | 6.48 (3.30) | NS | NS | NS |
| Their smoking history (S) | 7.62 (3.81) | 9.40 vs 6.50* | NS | NS |
| Whether the lung screening physician is board certified (C) | 7.78 (3.32) | NS | NS | NS |
| That the screening exam was painless (L) | 7.81 (3.06) | NS | 8.63 vs 6.30* | NS |
| Factors rated very important | ||||
| Fear of being diagnosed with lung cancer (F) | 8.16 (3.12) | NS | NS | NS |
| That screening had no major side effects (L) | 8.30 (2.84) | NS | NS | NS |
| The ability of screening to reduce the risk of dying from lung cancer (E) | 9.22 (2.15) | NS | 9.75 vs 8.30* | NS |
| They wanted to be reassured that their lungs were healthy (L) | 9.31 (2.02) | NS | NS | NS |
| Being forewarned of any potential future health problems (L) | 9.37 (1.57) | NS | NS | NS |
| Because their health is very important to them (S) | 9.58 (1.03) | NS | NS | 9.08 vs 10** |
| The ability of screening to detect lung cancer early (E) | 9.93 (0.38) | NS | NS | NS |
| Future lung cancer screening decision | ||||
| Factors rated not important | ||||
| How close the clinic was to work (B) | 3.88 (3.26) | NS | NS | NS |
| Factors rated somewhat important | ||||
| How close the clinic was to home (B) | 6.04 (3.07) | NS | NS | NS |
| How inviting the clinic was (C) | 6.59 (3.07) | NS | 7.31 vs 5.10* | NS |
| The pleasantness of the staff at the center (C) | 7.19 (2.77) | NS | 8.25 vs 5.20** | NS |
| The results were explained by a physician at the center (C) | 7.23 (3.65) | NS | NS | 5.75 vs 8.50* |
| Factors rated very important | ||||
| That they would be seen on time for appointment (C) | 8.22 (2.45) | NS | 9.06 vs 6.70** | NS |
| That it was easy to schedule the appointment (B) | 8.33 (2.35) | NS | NS | NS |
| That they did not have to wait very long to get an appointment (C) | 8.48 (2.34) | NS | NS | NS |
Sources of lung cancer screening information
Main sources of lung cancer screening information were assessed in two ways. First, participants were asked to what degree they had discussed lung cancer screening with any of the following people: family, friends, primary care physician, other physicians, nurses, others (response options: never, a little bit, some, a fair bit, extensively). Second, respondents indicated whether they had sought information on lung cancer screening from any of the following sources: screening center website, other Internet sites, a cancer hotline such as the Cancer Information Service operated by the National Cancer Institute, a community health center, or another source (write-in answer).
Evaluation of lung cancer screening decision
Participants assessed their overall lung cancer screening experience using a 5-point Likert-type scale (1 = very negative to 5 = very positive) and asked whether they would be interested in future lung cancer screening using a 5-point Likert-type scale (1 = definitely no to 5 = definitely yes). Participants also completed three validated scales to evaluate their screening decision: (1) the 5-item Decision Regret Scale (Brehaut et al., 2003), which measures distress or remorse after a healthcare decision (1 = strongly agree to 5 = strongly disagree; higher mean scores indicate lower decision regret), with a sixth lung cancer–specific item (“I am aware of the choices I have to participate in lung cancer screening”); (2) the 6-item Satisfaction with Decision Scale (Holmes-Rovner et al., 1996), which measures participants’ satisfaction with a health care decision (1 = lowest satisfaction to 5 = highest satisfaction; higher mean scores indicate higher decision satisfaction); and (3) the 16-item Decisional Conflict Scale (O’Connor, 1995), which measures personal perceptions of decision uncertainty (0 = strongly agree to 4 = strongly disagree; higher mean scores indicate greater decisional conflict) using five sub-scales that focus on uncertainty, informed, values clarity, support, and effective decisions.
Statistical analyses
We report means and standard deviations for continuous variables and frequencies and percentages for all other data. Group differences in factors important to screening decisions were compared by smoking status, gender, and age. Comparisons of mean differences in decision regret, decision satisfaction, and decisional conflict by smoking status, gender, and age were performed using t tests. Because a significant difference in age was found between the Kentucky and Florida subsets of the sample, we explored conducting analyses stratified by location. However, because of concerns of small sample size and multiple comparisons leading to potentially spurious significant results, we do not include those analyses here. All analyses were performed using STATA v. 13 (StataCorp LP, College Station, TX, USA).
Results
Sample characteristics
There were 27 individuals undergoing LDCT-based lung cancer screening in our two community clinics who participated in our survey. The Florida study site recruited 13 participants through the study invitation letter (26% response rate), and the Kentucky study site recruited 14 participants through the study invitation letter (n = 13; 31% response rate) and the clinic-distributed flyers (n = 1). Participants had an average age of 59.5 years (standard deviation (SD) 9.1). They were primarily female (61.5%), White (92.3%), and living with a spouse (80.8%). Reflecting the lack of insurance or Medicare coverage of screening at the time, our study population was financially stable: 48.2 percent were in the highest income bracket (over US$75,000/year), 63.0 percent had private insurance, and 57.7 percent had at least a college degree. The majority of respondents reported themselves as healthy (92.6%) and 63.0 percent were not current smokers. Just over half (n = 15; 55.6%) of participants had received only one previous LDCT scan, and 74.1 percent believed that they were at risk of developing lung cancer. There were no statistically significant differences between participants drawn from the Kentucky and Miami study sites on gender, race/ethnicity, highest educational attainment, annual household income, general health status, or smoking status (current vs former smoker). However, participants from the Kentucky study site were younger than those from the Florida study site (mean age 56.0 vs 63.7, p = 0.03). Full participant characteristics are presented in Table 1.
Table 1.
Sample characteristics (n = 27).
| Variable | n (%)a |
|---|---|
| Age, mean (SD) (years) | 59.5 (9.06) |
| Gender | |
| Male | 10 (38.5) |
| Female | 16 (61.5) |
| Race/ethnicity | |
| White, non-Hispanic | 24 (92.3) |
| Black | 2 (7.7) |
| Other | 0 (0) |
| Highest level of education | |
| High school graduate or less | 3 (11.5) |
| Some technical or college training | 8 (30.7) |
| College graduate | 6 (23.1) |
| Postgraduate work | 9 (34.6) |
| Marital statusb | |
| Live with spouse | 21 (80.8) |
| Live with children | 2 (7.7) |
| Live alone | 4 (15.4) |
| Other | 3 (11.5) |
| Work outside of the home | |
| Yes, full-time | 13 (48.2) |
| Yes, part-time | 4 (14.8) |
| No | 10 (37.0) |
| Household income | |
| Less than US$20,000 | 2 (7.4) |
| US$20,000–35,000 | 2 (11.1) |
| US$35,000–50,000 | 4 (14.8) |
| US$50,000–75,000 | 2 (7.4) |
| Over US$75,000 | 13 (48.2) |
| Refused | 3 (11.1) |
| General health | |
| Excellent | 6 (22.2) |
| Very good | 8 (29.6) |
| Good | 11 (40.7) |
| Fair | 2 (7.4) |
| Poor | 0 (0) |
| Insuranceb | |
| Private | 17 (63.0) |
| Medicare | 7 (25.9) |
| Medicaid | 1 (3.7) |
| VA | 3 (11.1) |
| No insurance | 5 (18.5) |
| Smoking history | |
| Never smoker | 5 (18.5) |
| Former smoker | 12 (44.4) |
| Current smoker | 10 (37.1) |
| Pack year exposure, mean (SD) | 22.3 (16.6) |
| Previous LDCT | |
| 1 | 15 (57.7) |
| 2 or more | 10 (38.5) |
| Do you believe that you are at risk for lung cancer? | |
| Yes | 20 (74.1%) |
| No | 7 (25.9%) |
| Compared to others your same age, how would you describe your risk for lung cancer? | |
| A lot lower than average | 2 (7.4) |
| Lower than average | 3 (11.1) |
| About average | 6 (22.2) |
| Higher than average | 9 (33.3) |
| A lot higher than average | 7 (25.9) |
We compared the demographic characteristics of our study sample to that of the communities from which they originate (from US Census Bureau 2016 American Community Survey 5-year estimates, Boca Raton, FL (FL) and Lexington, KY (KY), respectively). The study sample is somewhat older (median age 46.3 FL and 34.1 KY), had a higher percentage of women (51.5% FL and 50.9% KY, in Lexington), but had similar racial demographics (88.6% White in FL and 75.8% White in KY).
Factors important in the decision to be screened for lung cancer
Participants’ ratings of the importance of factors affecting past and future lung cancer screening decisions are shown in Table 2. Overall, the most important factor in the decision to be screened was the ability to detect lung cancer early (mean: 9.93, SD 0.38). The majority of factors designated as very important related to lung cancer screening attributes and efficacy: being forewarned of any potential future health problems (mean: 9.37, SD 1.57), reassurance that one’s lungs were healthy (mean: 9.31, SD 2.02), reducing the risk of dying from lung cancer (mean: 9.22, SD 2.15), and screening having no major side effects (mean: 8.30, SD 2.84). Other factors rated very important were considering health to be very important (mean: 9.58, SD 1.03) and the fear of being diagnosed with lung cancer (mean: 8.16, SD 3.12).
The majority of factors relating to social influence were identified as not important in the lung cancer screening decision, including a doctor’s recommendation (mean: 4.42, SD 4.27). Although the participants identified the cost of lung cancer screening as a somewhat important factor (mean: 6.48, SD 3.30), they identified the two factors related to cost-cutting as not important: having a coupon to reduce the cost of screening (mean: 4.25, SD 3.97) and receiving a discount on screening (mean: 4.71, SD 3.84).
With regard to factors potentially affecting future screening, participants identified most barriers or clinic attributes as very important (not waiting long to get an appointment, ease of scheduling, and being seen on time) or somewhat important (that the results were explained by a physician at the center, the pleasantness of the staff at the center, how inviting the clinic was, and how close the clinic was to home). Only one factor, how close the clinic was to work, was identified as not important in future lung cancer screening decision-making (mean: 3.88, SD 3.26).
Variations in factors important to lung cancer screening uptake
As shown in Table 2, there were statistically significant variations in importance ratings by smoking status, gender, and age. In some of these instances, the differences are substantial enough so that stratifying the population responses leads to a different categorization of importance of a factor for the different population strata. These instances are highlighted below in labeling each factor as NI = not important, SI = somewhat important, and VI = very important for those cases where the stratified analyses result in different categorizations for different subpopulations.
Not surprisingly, smokers reported smoking history (SI overall) being of more importance to lung cancer screening decision-making than did non-smokers (9.40 (VI) vs 6.50 (SI)). Using a summary measure, women globally rated all decision-making factors more importantly than did men: 7.10 vs 5.58. Specifically, attributes of the lung cancer screening test and results seemed to be more important to women than to men (i.e. low false-positive rate (SI overall): 6.87 (SI) vs 4.33 (NI); painless screening exam (SI overall): 8.63 (VI) vs 6.30 (SI); ability of screening to reduce the risk of dying from lung cancer: 9.75 vs 8.30), as were clinic/physician attribute associations with future screening decisions (how inviting the clinic was: 7.31 vs 5.10; pleasantness of the staff at the center (SI overall): 8.25 (VI) vs 5.20 (SI); being seen on time for the appointment (VI overall): 9.06 (VI) vs 6.70 (SI)). In addition, women rated the social influence of friends and some financial factors, indicating cost-related barriers, as more important than men. However, despite between-group differences, both men and women identified these factors as generally unimportant. Finally, older adults rated a screening center physician explaining the LDCT results (SI overall) (8.50 (VI) vs 5.75 (SI)) and the importance of their own health (10.00 vs 9.08) as more important than younger adults.
Sources of lung cancer screening information
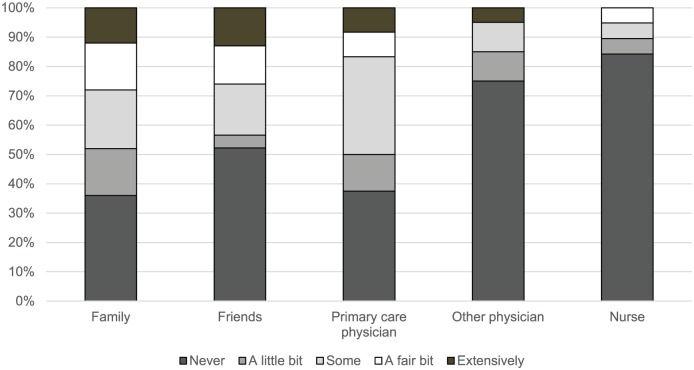
Figure 1 shows the extent of screening discussions that participants engaged in with specific types of people. Overall, most participants did not extensively discuss lung cancer screening with any category of potential discussants before being screened. In describing the extent of discussions about lung cancer screening, 37.5 percent of participants revealed that they had never spoken with a primary care physician before being screened. Similarly, 36.0 percent had never spoken with family members and 52.2 percent never with friends about lung cancer screening before screening. Overall, 18.5 percent of participants selected “never” for all categories in Figure 1, indicating that they had not spoken with anyone before being screened.
Figure 1.
Lung cancer screening discussions (n = 27).
In addition, participants were asked whether they had sought information from a variety of sources such as the Internet, a telephone call in line such as the Cancer Information Service, or other. The majority (61.5%) indicated that they had not sought information from any sources. Of the 11 individuals, 10 who reported seeking information used Internet-based resources, predominantly the webpage of the specific radiology clinic where they were screened.
Evaluation of lung cancer screening decision
The majority of participants indicated that they had a positive or very positive lung cancer screening experience (85.2%) and would “probably” or “definitely” be screened again (88.9%). No participant reported having a very negative screening experience.
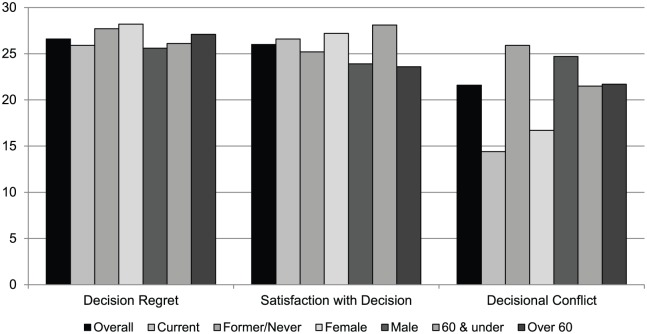
Figure 2 shows how participants evaluated their lung cancer screening decision as measured by several validated survey instruments related to decision-making. Results are given for the overall population and for the population stratified by smoking status, gender, and age category. Participants indicated low decisional regret (mean: 26.58, SD 4.62) and high satisfaction with the decision (mean: 26.04, SD 6.46). For decisional regret, where higher scores indicate less regret, women had significantly lower decisional regret than did men (mean: 28.2 vs 25.6). Younger individuals had higher decision satisfaction than did older individuals (mean: 28.1 vs 23.6). Participants reported low conflict levels based on the Decisional Conflict Scale (mean score: 21.2, SD 18.6), with women and current smokers having lower decisional conflict than men and former smokers (mean: 25.20 vs 32.3, 26.1 vs 31.8, respectively).
Figure 2.
Evaluation of lung cancer screening decision (n = 27).
Discussion
In an effort to understand the factors that were important to individuals who were early adopters of lung cancer screening, we collected novel information from an important and unique sample of individuals who had been screened for lung cancer with LDCT in community settings. Although these individuals were screened prior to the NLST results and coverage of screening, their responses are more relevant than most previous studies reporting this information, as these people had made real—as opposed to hypothetical—decisions about screening and were screened in the community as opposed to being screened within an ongoing screening trial. Overall, these results suggest that participants positively appraised their lung cancer screening, and they elected to pursue screening with an expectation that it would effectively detect lung cancer early, implicitly reduce the risk of lung cancer death, and/or definitively ascertain lung health.
Several findings from this study are especially noteworthy and warrant consideration alongside efforts to implement high-quality lung cancer screening among individuals at high risk of lung cancer across the nation and globally. First, our results identify a largely homogeneous population who received LDCT-based lung cancer screening. Despite being from different locations, the demographics of our sample indicate a White, female, and financially stable group of screeners who, despite their high level of education, did not seek much information prior to screening. In addition, despite their elevated perceived lung cancer risk, these individuals were not uniformly at high risk for lung cancer. In fact, the majority (70%) of our participants would not meet the USPSTF recommended criteria for screening.
Second, we found that there were interesting variations by participant characteristics in both the identification of important lung cancer screening decision-making factors and the evaluation of lung cancer screening. In some cases, stratified analyses resulted in disparate classifications of the importance of a factor for different subpopulations. (Note, however, that the classification definitions were subjective and not statistically determined.) Female participants globally rated decision-making factors more important than their counterparts and specifically rated screening efficacy factors, clinic attribute factors, and cost-related barrier factors more important than did men. Although social influence factors were rated as relatively unimportant to decision-making overall, women valued these factors significantly more than men. In contrast to finding that social influence was not important in lung cancer screening decisions in this population, Braybrook et al. (2011) reported that family members improved men’s awareness of lung cancer screening, especially if a family member already had cancer. Therefore, it is unclear what role family members may play in uptake of lung cancer screening in response to newly crafted screening guidelines. In families with a family history of lung cancer, this might engender support for screening; however, family communication patterns regarding smoking and tobacco cessation efforts might actually complicate efforts to raise awareness or encourage consideration of lung cancer screening.
Women also experienced lower decision regret and lower decisional conflict regarding lung cancer screening participation decisions. Older individuals placed more importance on screening center physicians explaining the lung cancer screening results and the importance of their own health and experienced lower decision satisfaction than younger participants. Not surprisingly, current smokers placed more importance on their smoking history; former and never smokers placed importance on their health in general and experienced higher decisional conflict in the informed and values clarity domains, indicating that non-smokers receiving lung cancer screening were least prepared to make informed choices. This follows previous research findings that current, former, and never smokers have disparate attitudes toward lung cancer screening (Jonnalagadda et al., 2012).
Finally, and perhaps most striking, our results suggest that informed decision-making for lung cancer screening was not occurring among all participants. A healthcare provider recommendation for lung cancer screening was not highly valued, nor was healthcare provider consultation commonly sought. While physicians currently lack detailed knowledge regarding lung cancer screening efficacy, evidence, and guidelines (Klabunde et al., 2012), alternative information sources, such as direct to consumer advertising, commonly focus solely on benefits and do not impart adequate knowledge of screening harms (Illes et al., 2004). Indeed, early adopters emphasized the benefits of lung cancer screening, as they classified these factors as very important but did not call attention to potential harms. This finding follows a general trend of people overestimating benefits of screening and underestimating harms (Schwartz et al., 2004) and is of notable concern in the context of LDCT-based lung cancer screening; there are a number of significant high-probability and low-probability harms associated with screening and follow-up procedures, including overdiagnosis (Patz et al., 2014), high rates of false positives and indeterminate pulmonary nodules, and significant incidental findings (Aberle et al., 2011; Petersen, 2014) that may lead to psychosocial distress (Byrne et al., 2008; Slatore et al., 2014; Van den Bergh et al., 2010; Wiener et al., 2015), increased health care resources (Byrne et al., 2010; Weiner et al., 2014), and the provision of unnecessary biopsies (Wiener et al., 2011), that warrant consideration in the decision-making process and could impact follow-up and program adherence if not properly addressed prior to screening.
Study strengths and limitations
There are several limitations to this largely descriptive study of early lung cancer screening adopters. First, the data are from a period prior to release of the NLST findings and changes in recommendations for lung cancer screening. However, as described in the “Introduction” section, the “choice setting” for this study more closely emulates the current situation for most individuals making decisions about lung cancer screening than more recently collected data which use hypothetical survey responses or individuals being screened in the context of a clinical trial. Second, we report on a small sample size with no ability to comment on survey non-respondents. However, these data may be helpful in designing subsequent research in this emerging area of lung cancer prevention and control. Third, this study describes individuals living in two relatively different communities who paid out-of-pocket for LDCT-based lung cancer screening prior to the updated USPSTF guideline. Results cannot be reliably generalized to other populations but may provide guidance for subsequent development of interventions seeking to support informed and shared decision-making about lung cancer screening participation. Finally, the data collected in this study were self-reported; there may be potential for some degree of responder bias and recall bias, given that some participants had participated in screening up to 12 months prior to participation. Despite these limitations, data collected from lung cancer screening early adopters who sought and received lung cancer screening offer a unique and valuable perspective on lung cancer screening decision-making as implementation expands. To our knowledge, no other studies have reported data from similar populations undergoing lung cancer screening in a community setting and outside of a clinical trial.
Conclusion
This study found that individuals who received LDCT-based lung cancer screening in a community setting generally viewed screening in a highly positive light. However, on the whole, individuals being screened did not seek detailed information about screening nor consulted trusted health care clinicians about this decision. The paucity of discussions between screeners and health care clinicians runs counter to current lung cancer screening guidelines, which strongly emphasize, and in some contexts mandate, integration of patient counseling practices using shared decision-making. These guidelines stress the importance of informed patients making screening choices in collaboration with healthcare providers who communicate the balance of benefits, harms, and the uncertainties of screening (Weiner and Slatore, 2013). To achieve the shared decision-making standards set forth by the Centers for Medicare & Medicaid Services, efforts are needed to develop, rigorously evaluate, and promote the use of evidence-based lung cancer screening decision tools. These tools need to provide patients with unbiased information about the potential sequelae of screening, facilitate informed decision-making, and encourage discussions with healthcare clinicians.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.M.B. was funded through NIH NCI K07 CA 101812 (Pennsylvania), S.E.L. through the VA HSR&D Associated Health Postdoctoral Fellowship (Minnesota), and J.L.S. through an Investigator-Initiated Grant from the Kentucky Lung Cancer Research Program (Kentucky). The funding sources had no involvement in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the article for publication.
References
- Aberle DR, Adams AM, Berg CD, et al. (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine 365: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajzen I. (1991) The theory of planned behavior. Organizational Behavior and Human Decision Processes 50: 179–211. [Google Scholar]
- American Cancer Society (ACS) (2018) Cancer Facts & Figures. Atlanta, GA: ACS. [Google Scholar]
- Anastasi N, Lusher J. (2017) The impact of breast cancer awareness interventions on breast screening uptake among women in the United Kingdom: A systematic review. Journal of Health Psychology. Epub ahead of print 1 March. DOI: 10.1177/1359105317697812. [DOI] [PubMed] [Google Scholar]
- Becker MH. (ed.) (1974) The health belief model and personal health behavior. Health Education Monographs 2: 324–508. [Google Scholar]
- Braybook DE, Witty KR, Robertson S. (2011) Men and lung cancer: A review of the barriers and facilitators to male engagement in symptom reporting and screening. Journal of Men’s Health 8: 93–99. [Google Scholar]
- Brehaut JC, O’Connor AM, Wood TJ, et al. (2003) Validation of a Decision Regret Scale. Medical Decision Making 23: 281–292. [DOI] [PubMed] [Google Scholar]
- Byrne MM, Koru-Sengul T, Zhao W, et al. (2010) Healthcare use after screening for lung cancer. Cancer 116: 4793–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne MM, Weissfeld J, Roberts MS. (2008) Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Medical Decision Making 28: 917–925. [DOI] [PubMed] [Google Scholar]
- Carter-Harris L, Tan AS, Salloum RG, et al. (2016) Patient-provider discussions about lung cancer screening pre- and post-guidelines: Health Information National Trends Survey (HINTS). Patient Education and Counseling 99: 1772–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmerico J, Hyland A, Celestino P, et al. (2014) Patient willingness and barriers to receiving a CT scan for lung cancer screening. Lung Cancer 84: 307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes-Rovner M, Kroll J, Schmitt N, et al. (1996) Patient satisfaction with health care decisions: The satisfaction with decision scale. Medical Decision Making 16: 58–64. [DOI] [PubMed] [Google Scholar]
- Huo J, Shen C, Volk RJ, et al. (2017) Use of CT and chest radiography for lung cancer screening before and after publication of screening guidelines: Intended and unintended uptake. JAMA Internal Medicine 177: 439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes J, Kann D, Karetsky K, et al. (2004) Advertising, patient decision making, and self-referral for computed tomographic and magnetic resonance imaging. Archives of Internal Medicine 164: 2415–2419. [DOI] [PubMed] [Google Scholar]
- Jemal A, Fedewa SA. (2017) Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncology 3(9): 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnalagadda S, Bergamo C, Lin JJ, et al. (2012) Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer 77: 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Marcus PM, Han PK, et al. (2012) Lung cancer screening practices of primary care physicians: Results from a national survey. Annals of Family Medicine 10: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennes IT, Luberto CM, Carr AL, et al. (2018) Project reach: Piloting a risk-tailored smoking cessation intervention for lung screening. Journal of Health Psychology. Epub ahead of print 1 February. DOI: 10.1177/135910538756500. [DOI] [PubMed] [Google Scholar]
- Lillie SE, Fu SS, Fabbrini AE, et al. (2018) Does need for cognitive closure explain individual differences in lung cancer screening? A brief report. Journal of Health Psychology. Epub ahead of print 1 January. DOI: 10.1177/1359105317750253. [DOI] [PubMed] [Google Scholar]
- Moyer VA. and US Preventive Services Task Force (USPSTF) (2014) Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 160: 330–338. [DOI] [PubMed] [Google Scholar]
- O’Connor AM. (1995) Validation of a decisional conflict scale. Medical Decision Making 15: 25–30. [DOI] [PubMed] [Google Scholar]
- Pallin M, Walsh S, O’Driscoll MF, et al. (2012) Overwhelming support among urban Irish COPD patients for lung cancer screening by low-dose CT scan. Lung 190: 621–628. [DOI] [PubMed] [Google Scholar]
- Patel D, Akporobaro A, Chinyanganya N, et al. (2012) Attitudes to participation in a lung cancer screening trial: A qualitative study. Thorax 67: 418–425. [DOI] [PubMed] [Google Scholar]
- Patz EF, Jr, Pinsky P, Gatsonis C, et al. (2014) Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Internal Medicine 174: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen H. (2014) Incidentally discovered pulmonary nodules. JAAPA 27: 25–32. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Woloshin S, Fowler FJ, et al. (2004) Enthusiasm for cancer screening in the United States. JAMA 291: 71–78. [DOI] [PubMed] [Google Scholar]
- Silvestri GA, Nietert PJ, Zoller J, et al. (2007) Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax 62: 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatore CG, Sullivan DR, Pappas M, et al. (2014) Patient-centered outcomes among lung cancer screening recipients with computed tomography: A systematic review. Journal of Thoracic Oncology 9: 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner NT, Egede LE, Shamblin C, et al. (2013) Attitudes and beliefs toward lung cancer screening among US Veterans. Chest 144: 1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. (2010) Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). British Journal of Cancer 102: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh KA, Essink-Bot ML, van Klaveren RJ, et al. (2009) Informed participation in a randomised controlled trial of computed tomography screening for lung cancer. European Respiratory Journal 34: 711–720. [DOI] [PubMed] [Google Scholar]
- Weiner RS, Slatore CG. (2013) Framing discussions about CT scan screening for lung cancer so that patients see the whole picture. Chest 144: 1749–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner RS, Gould MK, Slatore CG, et al. (2014) Resource use and guideline concordance in evaluation of pulmonary nodules for cancer: Too much and too little care. JAMA Internal Medicine 174: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener RS, Gould MK, Woloshin S, et al. (2015) “The thing is not knowing”: Patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect 18: 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener RS, Schwartz LM, Woloshin S, et al. (2011) Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: An analysis of discharge records. Annals of Internal Medicine 155: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]