The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials (original) (raw)
Supplemental digital content is available in the text.
Key words/Abbreviations: affective disorders, mental illness, mood, nutrients, nutrition, CI = confidence interval, MDD = major depressive disorder, RCT = randomized control trial
ABSTRACT
Objective
Poor diet can be detrimental to mental health. However, the overall evidence for the effects of dietary interventions on mood and mental well-being has yet to be assessed. We conducted a systematic review and meta-analysis examining effects of dietary interventions on symptoms of depression and anxiety.
Methods
Major electronic databases were searched through March 2018 for all randomized controlled trials of dietary interventions reporting changes in symptoms of depression and/or anxiety in clinical and nonclinical populations. Random-effects meta-analyses were conducted to determine effect sizes (Hedges' g with 95% confidence intervals [CI]) for dietary interventions compared with control conditions. Potential sources of heterogeneity were explored using subgroups and meta-regression analyses.
Results
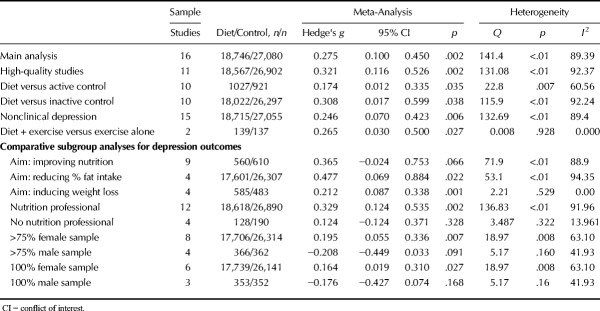
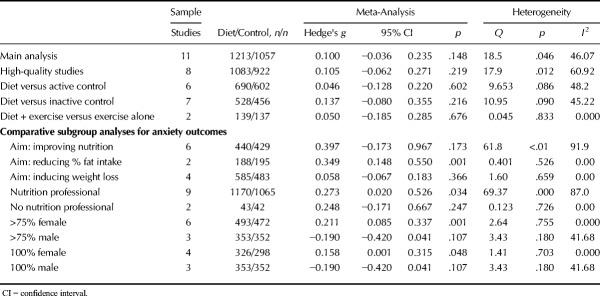
Sixteen eligible randomized controlled trials (published in English) with outcome data for 45,826 participants were included; the majority of which examined samples with nonclinical depression (n = 15 studies). Nonetheless, dietary interventions significantly reduced depressive symptoms (g = 0.275, 95% CI = 0.10 to 0.45, p = .002). Similar effects were observed among high-quality trials (g = 0.321, 95% CI = 0.12 to 0.53, p = .002) and when compared with both inactive (g = 0.308, 95% CI = 0.02 to 0.60, p = .038) and active controls (g = 0.174, 95% CI = 0.01 to 0.34, p = .035). No effect of dietary interventions was observed for anxiety (k = 11, n = 2270, g = 0.100, 95% CI = −0.04 to 0.24, p = .148). Studies with female samples observed significantly greater benefits from dietary interventions, for symptoms of both depression and anxiety.
Conclusions
Dietary interventions hold promise as a novel intervention for reducing symptoms of depression across the population. Future research is required to determine the specific components of dietary interventions that improve mental health, explore underlying mechanisms, and establish effective schemes for delivering these interventions in clinical and public health settings.
Registration
PROSPERO Online Protocol: CRD42018091256.
INTRODUCTION
Depressive disorders affect more than 300 million people around the world and are associated with unemployment, poor physical health, impaired social functioning, and, in its most severe forms, suicide (1). Thus, depressive disorders incur considerable burden not only for individuals but also for society because of the high economic cost from lost productivity and demand on healthcare services (2). The same can be said for anxiety disorders, which, along with depression, are also classified as “common mental disorders” because of their prevalence across the globe, with approximately one in five people experiencing one of these conditions for any given year (3). Standard treatments for common mental disorders comprise psychopharmacological and psychotherapeutic interventions. Although these have established efficacy in depression, a substantial proportion of people do not achieve remission using such strategies (4).
Furthermore, subclinical symptoms of depression and anxiety are also highly prevalent across the general population, among those without clinically diagnosed common mental disorders. These symptoms, although falling short of diagnostic thresholds, still impede upon quality of life and socio-occupational functioning, incurring even further personal and economic burden on a population scale (5). Therefore, new primary and/or adjunctive methods to address symptoms of depression and anxiety across the population are urgently needed.
Emerging evidence suggests that diet may influence the onset of mood disorders and specifically depression. For instance, many studies described in recent systematic reviews have demonstrated associations between measures of diet quality and the probability of and risk for depression (6,7). Moreover, proinflammatory dietary patterns are also associated with a significantly higher incidence of depressive symptoms, even among those without diagnosed mental disorders (8–10). A previous systematic review examined the benefits of various dietary interventions for depressive symptoms and anxiety, but using only narrative synthesis (11). Results generally suggested positive effects of dietary interventions on subclinical depression and anxiety, measured as secondary outcomes (11). However, the previous review did not apply meta-analytic techniques to quantify the findings and the results did not include recent interventions in clinical populations, Thus, it remains unclear if dietary interventions can improve symptoms of depressive and anxiety (in either clinical or nonpsychiatric samples) and the magnitude of any effects. Moreover, the potential influence of moderators such as sex, professional delivery, or the quality of studies on treatment outcomes is uncertain. Therefore, we aimed to determine the efficacy of dietary interventions for symptoms of depression and anxiety by conducting a meta-analysis of all randomized controlled trials (RCTs) examining this therapeutic strategy to date. We also used subgroup analyses to examine effects of dietary interventions on depression/anxiety in both clinical and nonclinical populations and to explore which aspects of these are associated with any potential greater efficacy. The findings of this meta-analysis will provide the first overall estimate of the efficacy of dietary interventions for reducing symptoms of depression and anxiety, along with informing self-management strategies for people with these conditions, and suggest directions for future research.
METHODS
This meta-analysis followed the PRISMA statement for transparent, comprehensive reporting of methodology and results (12). To eliminate researcher bias, the search strategy, inclusion criteria, and data extraction, overall and prespecified subgroup analyses used in this meta-analysis were prospectively registered with PROSPERO (CRD42018091256).
Search Strategy
The primary search was performed using OVID Medline on December 03, 2018, in line with the preregistered protocol, using the key word terms “Diet” with “Mediterranean” or “Therapy” or “Educat*” or “Counsel*” or “Intervention*” or “Treatment*” AND “Randomized Controlled Trial” or “Random Allocation” or “Clinical Trial” or “Control Groups” AND “Depression” or “Anxiety” or “Depressive Disorder.” We performed additional searches of Cochrane Central Register of Controlled Trials, Health Technology Assessment Database, Allied and Complementary Medicine (AMED), Embase, Health Management Information Consortium (HMIC), and PsycINFO, using the same keywords, along with a further general search of “Google Scholar” to capture any articles not captured by the main search. The full search details are presented in Supplemental Digital Content 1, http://links.lww.com/PSYMED/A537.
Eligibility Criteria
Only English articles published in peer-reviewed journals were included. We aimed to determine effects of dietary interventions on symptoms of depression and anxiety in all clinical and nonclinical populations, including depression (e.g., major depressive disorder [MDD]) or anxiety, comorbid depression, and anxiety, and in samples with depressive/anxiety symptoms that did not reach clinical thresholds. No restrictions were placed on diagnosis or any other clinical or demographic characteristics of eligible samples.
Eligible studies were RCTs comparing the effect of dietary interventions to nondietary control conditions. All “whole-of-diet” dietary interventions were eligible, delivered via any format, including individualized dietary counseling, group dietary classes, and standardized dietary prescription. In addition, all “types” of diet were eligible, including those primarily aiming to decrease the intake of unhealthy foods, improve nutrient intake, and/or those designed to restrict calorie intake to order induce weight loss. Because we aimed to establish the effects of whole-of-diet interventions for depression and anxiety, rather than examining only individual foods/nutrients, interventions focusing only on a single food component (e.g., eating more fish) were not included. Multicomponent life-style interventions were only eligible where comparator conditions had adequately controlled for active nondietary aspects of the intervention. For instance, multicomponent interventions such as “exercise with diet” would only be eligible if compared with an “exercise alone” control condition, so that the effects of the dietary component could be accurately determined. Crossover trials were only included where between-group differences from the first leg of the crossover trial were reported (so that parallel groups comparisons could be performed from the data).
Studies using both “inactive control groups” and “active control groups” were eligible for inclusion. Inactive control groups were classified as those in which participants maintained their habitual diets and received no additional active intervention during the trial period (or put onto a “waitlist” until pre- and postmeasures had been collected from both groups). Conversely, “active control groups” were categorized as any that compared diet with other active interventions or used comparator conditions designed to control for general “intervention effects” using either (a) benign interventions not aiming to treat depression/anxiety, (b) psychosocial interventions, e.g., social support, counseling, or exercise, or (c) other forms of activities, such as “time and attention”–matched patient contact.
All studies matching the previous criteria and reporting changes in at least one quantitative measure of depression or anxiety with sufficient detail for meta-analysis were included. Two independent investigators judged article eligibility (JF and RC) with any disagreements resolved through discussion. Where study design matched eligibly criteria, but data were insufficiently reported, study authors were contacted twice for 2 months to request the necessary data.
Data Extraction
A systematic extraction form was used to extract the following data from each eligible study:
- (i) Sample information: sample size (n), sex (% females), mean age of participants (years), population sampled health status (diagnostic information or relevant inclusion criteria),
- (ii) Intervention: primary aim of dietary change (e.g., weight loss or increasing nutritional intake), dietary program summary, individual delivering the intervention (e.g., dietitian or researcher), any additional intervention components (e.g., in-person or remotely delivered nondietary additions), control condition, intervention length (weeks).
- (iii) Effects on depressive or anxiety symptoms: changes in total depressive/anxiety symptoms before and after dietary and control conditions, using any clinically validated rating scale. For studies that used more than 1 measure of depression, a mean total change was calculated by pooling outcomes from each measure.
Study quality was determined through applying the quality criteria from the Academy of Nutrition and Dietetics (formerly the American Dietetic Association [ADA]) in the ADA quality assessment tool (13). This applies set criteria for examining allocation bias, selection bias, blinding, data collection, trial retention (along with methods of handling dropouts), and interventional adherence. Each study was categorized as positive, negative, or neutral using the standardized “quality consideration questions” described in the ADA Evidence Analysis Manual (13). All studies were included in the meta-analysis, regardless of ADA rating.
Statistical Analyses
Meta-analyses were conducted using Comprehensive Meta-Analysis 2.0 (14), using a random-effects model (15) to account for the expected heterogeneity between studies. The total difference in changes in symptoms of depression and anxiety from dietary interventions versus control conditions were pooled to compute the overall effect size of dietary interventions (as Hedges g), with 95% confidence intervals (CI). For RCTs reporting comparisons of dietary interventions with more than one control group, we pooled comparisons with each control group to generate an overall estimated effect of dietary interventions, to make use of all available data. For the one study reporting sex groups separately (16), a combined estimate across both sexes was calculated as Hedges g effect size and used for primary analyses. After computing main effects, a sensitivity analysis was applied to investigate effects of dietary interventions in RCTs that had a “positive” ADA rating.
The degree of statistical heterogeneity in the meta-analyses was quantified using Cochran's Q and _I_2 values. Risk of publication bias was examined by applying Eggers' regression to all previously mentioned analyses. Furthermore, a Duval and Tweedie's “trim-and-fill” analysis was applied to the random-effects models, to recalculate the pooled effect size after statistically accounting for any studies that may introduce publication bias (e.g., small studies with large effect sizes). In addition, a funnel plot of study effect sizes was generated from primary analyses, for a visual inspection of publication bias.
Prespecified subgroup analyses were conducted to examine how effects of dietary interventions differed when (a) comparing diet with either waitlist/inactive control conditions or active control conditions, (b) in “clinical” (i.e., patients with diagnosed depressive/anxiety disorder) and “nonclinical” (i.e., people without diagnoses of depression or anxiety), or (c) comparing interventions that had combined “diet with exercise” to control groups using exercise alone. In addition, we conducted a range of post hoc analyses, to examine putative factors that may influence the effects of dietary interventions. Specifically, we examined how changes in depressive symptoms were influenced the following factors: studies' sex distribution, mean sample age, type of diet used, how the intervention was delivered, intervention length (weeks), and study quality (measured with ADA scale).
RESULTS
Included Studies and Participant Details
The full search and screening process are shown in Supplemental Digital Content 1, http://links.lww.com/PSYMED/A537. After the removal of duplicate articles from the systematic search of electronic databases, 26 articles were identified as potentially eligible after the title and abstract screening stage. Screening of the full-text versions resulted in 10 of these being excluded, and 16 identified as eligible for inclusion. The additional search of Google Scholar identified further two possible trials, although these were deemed ineligible after full-text screening. Details on the ineligible articles and reasons for exclusion are displayed in Supplement 1, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A537.
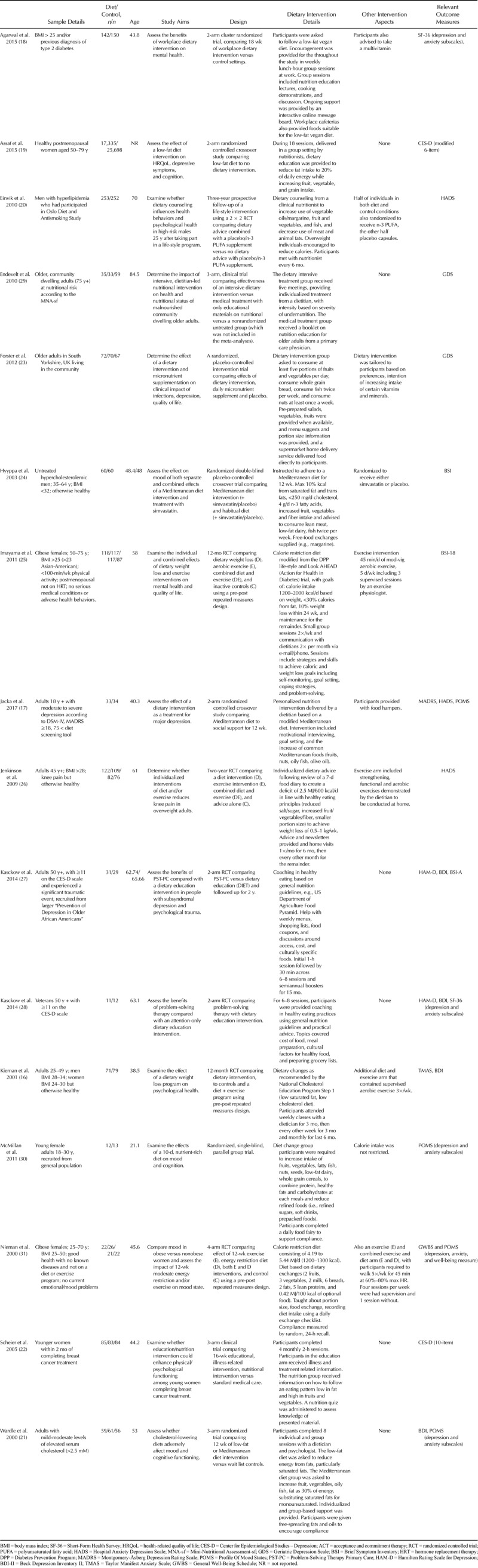
Therefore, a total of 16 RCTs were included in the analyses; reporting outcome data from 45,826 individuals (median average age = 55 years, range = 21–85 years). The results from the ADA Quality Assessments for each study are displayed in Supplemental Digital Content 2, http://links.lww.com/PSYMED/A538. This showed that only one study scored 12/12 for study quality (17), 10 others met the criteria for positive on ADA scale by scoring 9 or higher (categorized as “high quality”) (18–27), and five studies scored lower than 9 (categorized as low/neutral quality) (16,28–31). One reported outcome data in a format not suited for meta-analysis, but the corresponding authors provided the required data for inclusion (23).
Depressive symptoms were measured by all 16 studies, whereas anxiety outcomes were measured by only 11 of the 16 eligible trials. Changes in symptoms were assessed using the total scores from the following measures: “Centre for Epidemiological Studies Depression” (19,22,32); the “Beck Depression Inventory” (16,21,27,28,33); the “Hamilton Rating Scale for Depression” (28,34); the “Montgomery-Åsberg Depression Rating Scale” (17,35); the Geriatric Depression Scale (23,29,36), the Taylor Manifest Anxiety Scale (16,37), and the subscale scores for depression/anxiety from the following measures: the “Hospital Anxiety Depression Scale” (17,20,26,38); the Short-Form Health Survey (18,27,39); the Brief Symptom Inventory (24,25,28,40); the Profile Of Mood States (41) (17Wardle, 2000 #10083, 30, 31), and the General Well-Being Schedule (31,42). However, only one study examined the effects of a dietary intervention in a sample with primary diagnosis of clinical depression (17), with all the remaining studies examining effects on comorbid, subclinical, or secondary symptoms of depression/anxiety (see Table 1 for details). Across the different types of diets used by the studies, nine interventions were primarily aimed at improving nutrient intake (n = 9), four aimed to decrease fat intake (n = 4), and four were designed to reduce bodyweight (n = 4). The specifics of dietary interventions differed substantially across studies, and summaries for each are displayed in Table 1. Interventions ranged from 10 days to 3 years in length.
TABLE 1.
Details of Included Studies
Overall Effects of Dietary Interventions on Depression
Figure 1 displays the pooled effect size from dietary interventions on depressive symptoms, along with individual effects from each study. Table 2 displays the full results of all meta-analyses. A random-effects meta-analysis of 16 RCTs, reporting outcome data from 45,826 individuals, revealed that dietary interventions significantly reduced depressive symptoms in comparison to control conditions, with a small pooled effect (g = 0.275, 95% CI = 0.10 to 0.45, p = .002). There was significant heterogeneity across the study data (Q = 141.4, p < .01, _I_2 = 89.4%) and some indication of publication bias (Egger's regression intercept = 1.67, p = .025; see funnel plot in Supplemental Digital Content 3, http://links.lww.com/PSYMED/A539). Nonetheless, the random-effects trim-and-fill analysis found the estimated effect size to be larger and still statistically significant when accounting for publication bias (recalculated at g = 0.408, 95% CI = 0.2 to 0.60, p < .01). Furthermore, significant effects from dietary interventions on depression were also observed in the sensitivity analysis including only the RCTs with high-quality ratings from the ADA Quality Assessment (n = 11, n = 45,469, g = 0.321, 95% CI = 0.12 to 0.53, p = .002, Q = 131.1, _I_2 = 92.4%).
FIGURE 1.
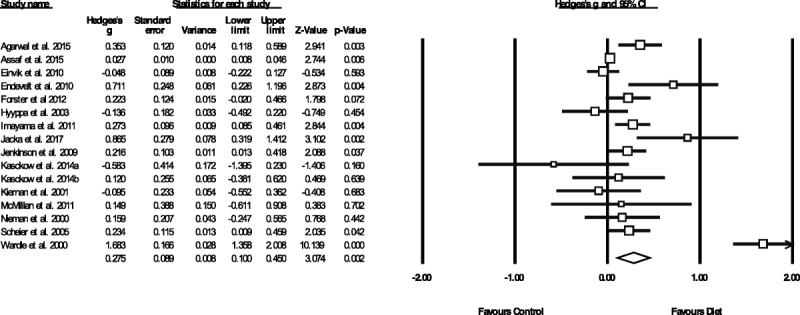
Meta-analysis of the effects of dietary interventions on depressive symptoms. Box size represents study weighting. Diamond represents overall effect size and 95% CIs.
TABLE 2.
Effects of Dietary Interventions on Symptoms of Depression
Prespecified Subgroup Analyses for Depression
Table 2 displays full results of all meta-analyses on depression outcomes in primary and subgroup analyses. The pooled effect size (g) on depressive symptoms across 10 dietary interventions that compared with habitual diet alone (or “inactive” control conditions) was 0.308 (n = 44,319, 95% CI = 0.02 to 0.6, p = .038), indicating a small to moderate significant effect. Effects were slightly smaller but still statistically significant, when compared with “active” control conditions (n = 10, n = 1,948, g = 0.174, 95% CI = 0.01 to 0.34, p < .001). Both waitlist- and active-controlled subgroups had high heterogeneity among included studies, with no evidence of publication bias significantly altering the findings (Table 2).
For prespecified subgroup analyses on clinical versus nonclinical populations, only one study used a clinically depressed sample (n = 67), showing significantly greater reduction in depressive symptoms from a 12-week modified Mediterranean diet intervention in comparison with “social support” (17). Dietary interventions reduced depressive symptoms significantly more than control conditions among the remaining 15 trials in nonclinically depressed individuals (n = 45,770, g = 0.246, 95% CI = 0.07 to 0.423, p = .006). In addition, preplanned subgroup analyses comparing “diet plus exercise” combination interventions to exercise alone found a small positive effect on depressive symptoms from the interventions that had the dietary component (g = 0.265, 95% CI = 0.03 to 0.50, p = .027) although this was based only on two studies (n = 276).
Post hoc Analyses of Factors Influencing Dietary Intervention Effects on Depression
Post hoc subgroup analyses were applied to explore, where possible, how interventional and participant characteristics may affect study findings. Full results are shown in Table 2. Regarding the design of dietary interventions, significant reductions in depression were observed from those primarily aiming to induce bodyweight loss (n = 4, n = 1068, g = 0.212, 95% CI = 0.09 to 0.34, p = .001) and those aiming to reduce fat intake (n = 4, n = 43,638, g = 0.477, 95% CI = 0.07 to 0.89, p = .022). Similar sized effects were observed from interventions primarily aiming to improve nutritional intake (n = 9, n = 1170, g = 0.365, 95% CI = −0.02 to 0.75), although this subgroup fell short of statistical significance (p = .066). Studies specifying the involvement of a nutritional professional (e.g., dietitians or nutritionists) in the delivery of dietary interventions observed a significant effect on depressive symptoms (n = 12, n = 45,508, g = 0.329, 95% CI = 0.12 to 0.54, p = .002), whereas those that were delivered without dietitian/nutritionist professional involvement had no greater effects than control conditions (n = 4, n = 318, g = 0.124, 95% CI = −0.12 to 0.37, p = .328).
Finally, as shown in Figure 2, studies with mostly female samples (i.e., >75% female; eight studies) observed significant positive effects on depressive symptoms from dietary interventions (g = 0.195, 95% CI = 0.06 to 0.37, p = .007), whereas those with mostly male samples (>75% male, four studies) observed a slight worsening of depressive symptoms from dietary interventions, which approached statistical significance (g = −0.208, 95% CI = −0.45 to 0.03 p = .091). This finding persisted when examining only the studies with 100% female samples (six studies, g = 0.164, 95% CI = 0.02 to 0.31, p = .027) or 100% male samples (three studies, g = −0.176, 95% CI = −0.43 to 0.07, p = .17), with significantly greater effects from dietary interventions on depression observed in female sample studies (p = .021 between subgroups). Exploratory meta-regression analyses examining intervention length (weeks), study quality (ADA scale) and sample age (mean average, years) found no relationships between these variables and observed effects of diet on depression (full results presented in Supplemental Digital Content 4, http://links.lww.com/PSYMED/A540).
FIGURE 2.
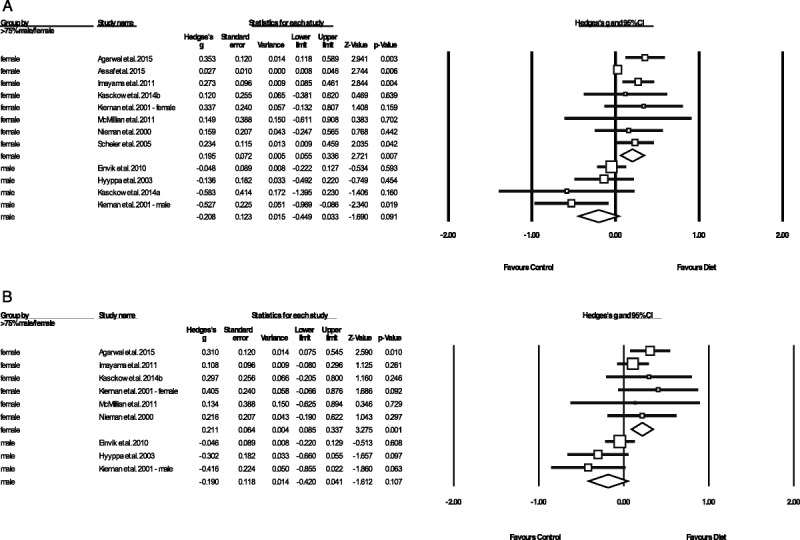
Meta-analysis showing differential effects of dietary interventions in male versus female samples, on (A) a symptoms of depression and (B) symptoms of anxiety. Box size represents study weighting. Diamond represents overall effect size and 95% CIs.
The Effects of Dietary Interventions on Anxiety
As shown in Figure 3, random-effects meta-analysis of 11 RCTs reporting outcome data from 2270 individuals found no overall effect of dietary interventions on anxiety compared with control conditions (g = 0.100, 95% CI = −0.036 to 0.235, p = .148, Q = 18.5, _I_2 = 46.1). A sensitivity analysis including only studies with high-quality ADA ratings also found no effect of dietary interventions on anxiety (n = 8, n = 2005, g = 0.105, 95% CI = −0.06 to 0.27, p = .219, Q = 17.9, _I_2 = 60.92). Furthermore, there were no effects from dietary interventions on anxiety when compared with either active control conditions (n = 6, n = 1,292, g = 0.046, 95% CI = −0.13 to 0.22, p = .602) or habitual diet/inactive controls (n = 7, n = 984, g = 0.137, 95% CI = −0.08 to 0.36, p = .216), and no additional effect of diet on anxiety were observed from studies comparing diet and exercise combinations to exercise alone (n = 2, n = 175, g = 0.05, 95% CI = −0.19 to 0.29, p = .676). Full meta-analytic results are displayed in Table 3. Moderate heterogeneity was present across all of the analyses (_I_2 = 45.22%–48.2%), and there was some indication of publication bias (Eggers regression intercept = 1.19, p = .093) although recalculating the results with trim-and-fill analyses did not change the findings (i.e., no significant benefits from dietary interventions for anxiety outcomes, all p > .05). No studies examined effects of dietary interventions in clinical anxiety disorder samples.
FIGURE 3.
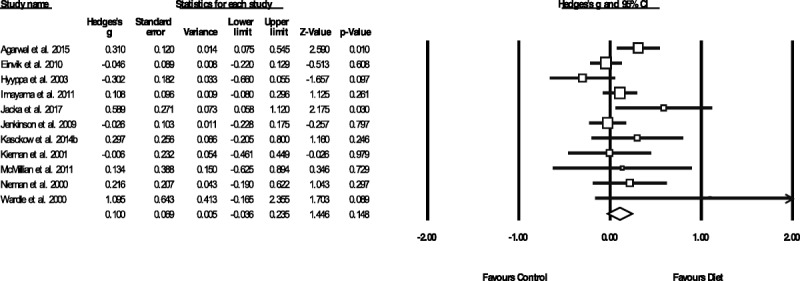
Meta-analysis of the effects of dietary interventions on symptoms of anxiety. Box size represents study weighting. Diamond represents overall effect size and 95% CIs.
TABLE 3.
Effects of Dietary Interventions on Symptoms of Anxiety
Post hoc Analyses of Factors Influencing Dietary Intervention Effects on Anxiety
No significant effects on anxiety were observed from the subgroups of dietary interventions that primarily aimed to improve nutrition (n = 6, n = 869, g = 0.397, 95% CI = −0.17 to 0.97 p = .174) or those aiming to reduce bodyweight (n = 4, n = 1068, g = 0.058, 95% CI = −0.07 to 0.18, p = .366). A significant reduction in anxiety was observed from those aiming to reducing fat intake (g = 0.349, 95% CI = 0.15 to 0.55, p = .001), but the result must be interpreted with caution given the small number of studies in this subgroup (n = 2, n = 383). Studies specifying the involvement of a nutritional professional in dietary interventions did observe a significant, small positive effect on symptoms of anxiety (n = 9, n = 2235, g = 0.273, 95% CI = 0.0.02 to 0.53, p = .034), whereas those who did not report dietitian/nutritionist involvement had no effects (n = 2, n = 85, g = 0.242, 95% CI = −0.17 to 0.67, p = .247).
As with the depression outcomes, subgroups of studies using mostly (>75%) female samples observed significant positive effects on anxiety from dietary interventions (n = 6, n = 965, g = 0.211, 95% CI = 0.09 to 0.34, p = .001), whereas those in mostly male samples observed nonsignificant negative effects (g = −0.19, 95% CI = −0.42 to 0.04, p = .107). Inspection of both individual and pooled study effects revealed that dietary interventions in mostly/entirely female samples consistently had a positive direction of effect on both symptoms of depression (Figure 2A) and anxiety (Figure 2B). Conversely, effects of dietary interventions in the mostly (or entirely) male samples were consistently negative for both depression and anxiety (Figure 2A) and anxiety (Figure 2B).
DISCUSSION
To our knowledge, this is the first meta-analysis to examine the efficacy of dietary interventions for depression and anxiety. Our systematic search identified 16 independent studies, reporting outcomes of dietary intervention RCTs across 45,826 participants. The main analysis found that dietary interventions had a small positive effect on depressive symptoms (g = 0.275, 95% CI = 0.10 to 0.45), which remained significant even after adjusting for study quality and publication bias. However, only one of the 16 trials used a sample with primary diagnosis of clinical depression (17), with all the remaining 15 studies investigating effects of dietary interventions on symptoms of depression in nonclinical depression samples. A further limitation to this is the publication bias found in the primary analysis. However, the effects of dietary interventions were still statistically significant after correcting for this. In addition, our subgroup analyses found that positive effects of dietary interventions for depressive symptoms were observed in both studies using inactive control conditions (g = 0.308, p = .038) and active control conditions (g = 0.174, p = .035), indicating the beneficial effects of dietary interventions on mood extend beyond just general intervention effects.
A final limitation is the significant heterogeneity in the meta-analyses, likely stemming from the broad inclusion criteria. Because substantial heterogeneity was also present in the subgroup analyses, this indicates that significant between-study differences in dietary effect sizes also existed when grouping by specific intervention types. Thus, it was difficult to establish the most effective components of dietary interventions for depression, because we found no significant differences between dietary interventions primarily aimed at (a) reducing bodyweight, (b) improving nutrition, or (c) decreasing dietary fat intake. However, this is perhaps unsurprising, because although the primary aims of the interventions did vary, the actual content of the all dietary intervention generally hold some common features, such as aiming to reduce the intake of “junk” foods (e.g., high-fat, high-sugar discretionary foods and takeaways), while replacing these with high-fiber, nutrient-dense alternatives, such as vegetables.
Implications and Recommendations for Future Research
The mechanisms through which these dietary changes can benefit mental health have yet to be fully established. However, diet may act via several pathways that are implicated in mental health. These include pathways related to oxidative stress, inflammation, and mitochondrial dysfunction, which are disrupted in people with mental disorders (43). Gut microbiota dysbiosis has also been implicated because of emerging research demonstrating involvement of the microbiome in the modulation of stress response, immune function, neurotransmission, and neurogenesis (44). A healthy diet typically contains a wide variety of bioactive compounds that can beneficially interact with these pathways. For example, vegetables and fruits contain, in addition to beneficial vitamins, minerals and fiber, a high concentration of various polyphenols that seem to be associated with reduced rates of depression in limited observational studies, potentially because of their anti-inflammatory, neuroprotective, and prebiotic properties (45,46). Furthermore, vitamins (e.g., B vitamins), fatty acids (e.g., omega 3 fatty acids), minerals (e.g., zinc, magnesium), and fiber (e.g., resistant starch) as well as other bioactive components (e.g., probiotics), which are typically abundant in healthy dietary patterns, may also be protective from mental illness (44). Along with increasing the intake of beneficial nutrients, dietary interventions may also impact on mental well-being by reducing the consumption of unhealthy food associated with increased risk for depression, such as processed meats, refined carbohydrates, and other inflammatory foods (8,9). Unhealthy diets are also high in other compounds that may negatively affect these pathways. For example, elements commonly found in processed foods such as saturated fatty acids, artificial sweeteners, and emulsifiers may alter the gut microbiome, which may activate inflammatory pathways (47).
Our results showed that dietary interventions that primarily targeted weight loss also significantly reduced symptoms of depression. The psychological benefits of weight loss diets observed in our meta-analysis could be linked with reductions in obesity, because there is robust evidence from epidemiological data that overweight status is consistently associated with an elevated risk of depression (48,49). Indeed, all four of the weight loss interventions included in our meta-analysis were conducted in overweight/obese samples. Although only three of these trials examined the correlations between mental health and weight loss, these consistently found that individuals who lost most weight during the trial also had the greatest improvements in measures of psychological well-being (16,25,31). Previous trials of multicomponent weight loss interventions (which were ineligible for our meta-analysis) have also shown that reductions in depressive symptoms following health behavior programs are significantly correlated with reductions in bodyweight (50). The leading hypothesis for why obesity is associated with depression is through inflammation, because this is a core feature of depressive illness (51) and excessive adipose tissue increases the production of proinflammatory cytokines (52). Indeed, recent preclinical research has shed further light on pathways through which obesogenic diets impacts on mental health, demonstrating that dietary-induced obesity reduces insulin signaling in the brain and increases neuroinflammation, resulting in depressive-like behaviors in rodent models (53). This is supported by recent research in human adolescent samples, which has demonstrated that the protective effects of healthy diet on depression risk is conferred through reduced body mass index and associated inflammation (10). However, it is important to note that the significant effects of weight loss diets on symptoms of depression in this meta-analysis were all observed in nonclinical samples (i.e., individuals with mostly subthreshold depression). In those with clinical depression, the recent SMILES trial showed large positive effects of a dietary intervention in MDD without altering the weight of participants (17). Instead, the trial found that changes in diet quality for the 12-week period correlated closely with changes in depressive symptoms. This is in accordance with the weight of evidence in the extensive observational literature showing that the association between diet quality and major depression exists even independently of body weight (7) and the emerging evidence from preclinical studies indicating poor diet can also influence brain health and function in absence of obesity (54).
None of our prespecified analyses found notable effects from dietary interventions on symptoms of anxiety. This could be due to a “floor effect,” whereby the low levels of anxiety in the nonclinical samples examined to date make it difficult to observe any notable effects of dietary interventions. Indeed, in the single trial to use a sample of individuals with diagnosed affective disorders (although of major depression), the participants also had borderline clinical levels of anxiety at baseline, and these symptoms were significantly reduced by the dietary intervention (17). Future RCTs are required to confirm or refute the effects of dietary interventions on those with clinically diagnosed anxiety disorders.
Clinical Implications
A key issue in clinical applicability of our findings is the lack of studies in clinically depressed samples meaning that most evidence of dietary interventions reducing depressive symptoms only applies to nonclinical depression to date. Although the SMILES trial was the first to examine the efficacy of dietary interventions in a clinically depressed sample, another more recent RCT (the HELFIMED trial) has also indicated the efficacy of a Mediterranean diet for treating depression (55). However, this study was ineligible for our meta-analysis because of the intervention also including fish oil supplements (an active treatment for depression) (56), thus making it impossible to determine whether reductions in depression were due to dietary changes or fish oil treatment. Furthermore, a recent economic evaluation of the SMILES trial provides support for the cost-effectiveness of such an approach to treating depression, with participants in the dietary support condition generating substantially reduced societal and health sector costs compared with the social support condition (57). However, it is important to consider that, to date, no trials have yet compared the efficacy of dietary interventions to antidepressant medications. Thus, dietary intervention can only be considered an adjunctive strategy for managing depressive symptoms at this point.
Nonetheless, the significant benefits observed for subclinical/secondary depression are also of considerable value. The benign nature of dietary interventions, along with the established benefits of diet for physical health, suggests that dietary improvement could be an ideal option for low-intensity treatment or for individuals to adopt themselves as a self-management approach for reducing subclinical depressive symptoms. Furthermore, diet seems to improve depression even when used alongside other more established self-management strategies, such as physical activity (50), because pooled data from studies examining “diet plus exercise” combinations showed significant additional benefits compared with exercise alone. However, this result should be interpreted with caution because of the low number of studies included in the subgroup analysis (n = 2, n = 276). Our subgroup analyses also indicated that interventions delivered by registered dietitians and professional nutritionists have significant benefits for both depression and anxiety, whereas those delivered by other individuals (e.g., research staff) did not. Although preliminary, the finding from this subgroup analysis is in line with a previous research showing that interventions that use dietitians have significantly better effects on weight management in severe mental illness compared with those who use other types of health professionals (58,59).
Our meta-analysis also found that studies using primarily female samples observed significant mental health benefits from dietary interventions (for depression and anxiety), whereas those with male samples did not, even indicating a trend toward a negative effect (Figure 2). Again, because these subgroup analyses consisted of only few studies for each sex (n = 8 studies in females, n = 4 studies in males), definitive conclusions cannot be drawn from these data. However, these findings could be potentially be explained by three sex-specific factors. First, because females have a higher presence of mood disorders across the population, this may create greater scope for a significant benefit from dietary interventions (60). Second, differences in dietary effects on mood could be linked to sex differences in metabolism and body composition, whereby women may be more responsive to diets that alter glucose or fat metabolism (61). Third, sociocultural sex differences in expectations surrounding diet and health beliefs may influence outcomes of dietary interventions. For example, men rate certain health behaviors, including diet, as less important than women, have lower nutrition knowledge, and women seek nutrition counseling more frequently than men (62,63). Thus, women may be more likely than males to adopt health behaviors as recommended. Future research should examine the extent to which sex differences in adherence to dietary interventions explain the differential effects between sexes.
Beyond sex differences, future research should also aim to determine the influence of several other confounding factors, which have so far been overlooked. One key factor for future research to examine is the interaction between dietary interventions with psychotropic medications. Because depressive symptoms were used as secondary outcomes in most studies here and conducted in nonclinical samples, few studies have examined this to date. However, preliminary insights on this issue can be gained by comparing trials, which excluded individuals taking antidepressants, with those studies that included high proportions of antidepressant users. For instance, the single trial of an MDD sample (in which >75% of the intervention group were taking antidepressants) observed large, significant benefits of dietary intervention compared with the counseling control group (17), whereas the two trials that specifically excluded individuals taking antidepressants from their analyses observed no significant differences between dietary interventions and problem-solving therapy for symptoms of depression (27,28). Other important confounding factors to be examined in future research include medical comorbidities (particularly cardiometabolic complications) and substance abuse, both of which could modify the impact of dietary interventions on mental well-being.
SUMMARY AND CONCLUSIONS
The consistently significant and positive effects of dietary interventions on depressive symptoms observed across all random-effects meta-analyses, even in high-quality studies, strongly suggests that diet can play a role in the treatment and also self-management of depressive symptoms across the population. Because pooled effect sizes were mostly classified as “small,” further research is warranted to distil both the key components and mechanistic actions of diet for mental health to develop more refined, targeted, and thus perhaps more effective interventions. In addition, given the potentially cumulative effects of diet and exercise together, future research should explore the modification of diet in concert with multiple other life-style modifications to provide a more integrated approach (64). Finally, further research should also be directed toward determining cost-effective and sustainable methods for providing dietary interventions within mental healthcare services, along with developing and evaluating public health schemes for dietary improvement across the population.
Supplementary Material
SUPPLEMENTARY MATERIAL
Acknowledgments
The authors thank Dr. Elizabeth Williams (Human Nutrition Unit, University of Sheffield) for kindly providing additional data required for the analyses.
Source of Funding and Conflicts of Interest: J.F. is supported by a Blackmores Institute Fellowship. W.M. is funded by a Deakin University Dean's Postdoctoral Research Fellowship. F.N.J. is supported by an NHMRC Career Development Fellowship (2) (APP1108125). J.S. is funded by an NHMRC Research Fellowship (APP1125000). S.T. is funded by the South Eastern Sydney Local Health District in a clinical position. B.S. is supported by the Health Education England and the National Institute for Health Research HEE/NIHR ICA Programme Clinical Lectureship (ICA-CL-2017-03-001). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health and Social Care. F.J. has received grant/research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, Eli Lilly, Meat and Livestock Australia, Woolworths Limited, The Fernwood Foundation, The Wilson Foundation, GMHBA, and The University of Melbourne and has received speakers honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Health Ed, Network Nutrition, Angelini Farmaceutica, Eli Lilly, and Metagenics. J.S. has received either presentation honoraria, travel support, clinical trial grants, book royalties, or independent consultancy payments from: Integria Healthcare & MediHerb, Pfizer, Scius Health, Key Pharmaceuticals, Taki Mai, Bioceuticals & Blackmores, Soho-Flordis, Healthworld, HealthEd, HealthMasters, Kantar Consulting, Research Reviews, Elsevier, Chaminade University, International Society for Affective Disorders, Complementary Medicines Australia, SPRIM, Terry White Chemists, ANS, Society for Medicinal Plant and Natural Product Research, Sanofi-Aventis, Omega-3 Centre, the National Health and Medical Research Council, CR Roper Fellowship.
Footnotes
FJ and JS are co-final authors.
All authors contributed substantially toward the writing of the article and interpretation of the findings. JF conducted statistical analyses. WM, SD, RC, and ST conducted searches and data extraction. MS, BS, FS, FJ, and JS contributed to the intellectual conception of the article and the interpretation of results.
Supplemental Content
REFERENCES
- 1.Hawton K, Casañas I Comabella C, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord 2013;147:17–28. [DOI] [PubMed] [Google Scholar]
- 2.McCrone PR, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S. Paying the price: the cost of mental health care in England to 2026. London, UK: King's Fund; 2008. [Google Scholar]
- 3.Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, Silove D. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980–2013. Int J Epidemiol 2014;43:476–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casacalenda N, Perry JC, Looper K. Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry 2002;159:1354–60. [DOI] [PubMed] [Google Scholar]
- 5.Johnson J, Weissman MM, Klerman GL. Service utilization and social morbidity associated with depressive symptoms in the community. JAMA 1992;267:1478–83. [PubMed] [Google Scholar]
- 6.Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG, Li B. Dietary patterns and depression risk: a meta-analysis. Psychiatry Res 2017;253:373–82. [DOI] [PubMed] [Google Scholar]
- 7.Lassale C, Batty GD, Baghdadli A, Jacka F, Sánchez-Villegas A, Kivimäki M, Akbaraly T. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips CM, Shivappa N, Hébert JR, Perry IJ. Dietary inflammatory index and mental health: a cross-sectional analysis of the relationship with depressive symptoms, anxiety and well-being in adults. Clin Nutr 2018;37:1485–91. [DOI] [PubMed] [Google Scholar]
- 9.Shivappa N, Hébert JR, Veronese N, Caruso MG, Notarnicola M, Maggi S, Stubbs B, Firth J, Fornaro M, Solmi M. The relationship between the dietary inflammatory index (DII®) and incident depressive symptoms: a longitudinal cohort study. J Affect Disord 2018;235:39–44. [DOI] [PubMed] [Google Scholar]
- 10.Oddy WH, Allen KL, Trapp GSA, Ambrosini GL, Black LJ, Huang RC, Rzehak P, Runions KC, Pan F, Beilin LJ. Dietary patterns, body mass index and inflammation: pathways to depression and mental health problems in adolescents. Brain Behav Immun 2018;69:428–39. [DOI] [PubMed] [Google Scholar]
- 11.Opie RS, O'Neil A, Itsiopoulos C, Jacka FN. The impact of whole-of-diet interventions on depression and anxiety: a systematic review of randomised controlled trials. Public Health Nutr 2015;18:2074–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Association AD. Evidence Analysis Manual: Steps in the ADA Evidence Analysis Process. Chicago, IL: American Dietetic Association; 2008. [Google Scholar]
- 14.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2. Englewood, NJ: Biostat; 2005:104. [Google Scholar]
- 15.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105–14. [DOI] [PubMed] [Google Scholar]
- 16.Kiernan M, King AC, Stefanick ML, Killen JD. Men gain additional psychological benefits by adding exercise to a weight-loss program. Obesity 2001;9:770–7. [DOI] [PubMed] [Google Scholar]
- 17.Jacka FN, O'Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, Castle D, Dash S, Mihalopoulos C, Chatterton ML, Brazionis L, Dean OM, Hodge AM, Berk M. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med 2017;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal U, Mishra S, Xu J, Levin S, Gonzales J, Barnard ND. A multicenter randomized controlled trial of a nutrition intervention program in a multiethnic adult population in the corporate setting reduces depression and anxiety and improves quality of life: the GEICO study. Am J Health Promot 2015;29:245–54. [DOI] [PubMed] [Google Scholar]
- 19.Assaf AR, Beresford SA, Risica PM, Aragaki A, Brunner RL, Bowen DJ, Naughton M, Rosal MC, Snetselaar L, Wenger N. Low-fat dietary pattern intervention and health-related quality of life: The Women's Health Initiative Randomized Controlled Dietary Modification Trial. J Acad Nutr Diet 2016;116:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Einvik G, Ekeberg O, Lavik JG, Ellingsen I, Klemsdal TO, Hjerkinn EM. The influence of long-term awareness of hyperlipidemia and of 3 years of dietary counseling on depression, anxiety, and quality of life. J Psychosom Res 2010;68:567–72. [DOI] [PubMed] [Google Scholar]
- 21.Wardle J, Rogers P, Judd P, Taylor MA, Rapoport L, Green M, Nicholson Perry K. Randomized trial of the effects of cholesterol-lowering dietary treatment on psychological function. Am J Med 2000;108:547–53. [DOI] [PubMed] [Google Scholar]
- 22.Scheier MF, Helgeson VS, Schulz R, Colvin S, Berga S, Bridges MW, Knapp J, Gerszten K, Pappert WS. Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early-stage breast cancer. J Clin Oncol 2005;23:4298. [DOI] [PubMed] [Google Scholar]
- 23.Forster SE, Powers HJ, Foulds GA, Flower DJ, Hopkinson K, Parker SG, Young TA, Saxton J, Pockley AG, Williams EA. Improvement in nutritional status reduces the clinical impact of infections in older adults. J Am Geriatr Soc 2012;60:1645–54. [DOI] [PubMed] [Google Scholar]
- 24.Hyyppä MT, Kronholm E, Virtanen A, Leino A, Jula A. Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology 2003;28:181–94. [DOI] [PubMed] [Google Scholar]
- 25.Imayama I, Alfano CM, Kong A, Foster-Schubert KE, Bain CE, Xiao L, Duggan C, Wang C-Y, Campbell KL, Blackburn GL, McTiernan A. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int J Behav Nutr Phys Act 2011;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson CM, Doherty M, Avery AJ, Read A, Taylor MA, Sach TH, Silcocks P, Muir KR. Effects of dietary intervention and quadriceps strengthening exercises on pain and function in overweight people with knee pain: randomised controlled trial. BMJ 2009;339:b3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasckow J, Klaus J, Morse J, Oslin D, Luther J, Fox L, Reynolds C, Haas GL. Using problem solving therapy to treat veterans with subsyndromal depression: a pilot study. Int J Geriatr Psychiatry 2014;29:1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasckow J, Morse J, Begley A, Anderson S, Bensasi S, Thomas S, Quinn SC, Reynolds CF., 3rd Treatment of post traumatic stress disorder symptoms in emotionally distressed individuals. Psychiatry Res 2014;220:370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endevelt R, Lemberger J, Bregman J, Kowen G, Berger-Fecht I, Lander H, Karpati T, Shahar D. Intensive dietary intervention by a dietitian as a case manager among community dwelling older adults: the EDIT study. J Nutr Health Aging 2011;15:624–30. [DOI] [PubMed] [Google Scholar]
- 30.McMillan L, Owen L, Kras M, Scholey A. Behavioural effects of a 10-day Mediterranean diet. Results from a pilot study evaluating mood and cognitive performance. Appetite 2011;56:143–7. [DOI] [PubMed] [Google Scholar]
- 31.Nieman DC, Custer WF, Butterworth DE, Utter AC, Henson DA. Psychological response to exercise training and/or energy restriction in obese women. J Psychosom Res 2000;48:23–9. [DOI] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur 1977;1:385–401. [Google Scholar]
- 33.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio 1996;78:490–8. [Google Scholar]
- 34.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- 36.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. J Aging Mental Health 1986;5:165–73. [Google Scholar]
- 37.Taylor JA. Manifest Anxiety Scale. American Psychological Association; 1953. [Google Scholar]
- 38.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 1992;473–83. [PubMed] [Google Scholar]
- 40.Derogatis LR, Spencer P. Brief symptom inventory: BSI. Upper Saddle River, NJ: Pearson; 1993. [Google Scholar]
- 41.McNair DM, Droppleman LF, Lorr M. Edits manual for the profile of mood states: POMS San Diego, CA: Edits/Educational and Industrial Testing Service; 1992.
- 42.Fazio AF. A concurrent validational study of the NCHS General Well-Being Schedule. Vital Health Stat 2 1977;1–53. [PubMed] [Google Scholar]
- 43.Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, Berk M. A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev 2017;74:1–20. [DOI] [PubMed] [Google Scholar]
- 44.Marx W, Moseley G, Berk M, Jacka F. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc 2017;76:427–36. [DOI] [PubMed] [Google Scholar]
- 45.González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, Sánchez de Medina F. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr 2011;51:331–62. [DOI] [PubMed] [Google Scholar]
- 46.Chang S-C, Cassidy A, Willett WC, Rimm EB, O'Reilly EJ, Okereke OI. Dietary flavonoid intake and risk of incident depression in midlife and older women. Am J Clin Nutr 2016;104:704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacka FN. Nutritional psychiatry: where to next? EBioMedicine 2017;17:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010;67:220–9. [DOI] [PubMed] [Google Scholar]
- 49.Solmi M, Köhler CA, Stubbs B, Koyanagi A, Bortolato B, Monaco F, Vancampfort D, Machado MO, Maes M, Tzoulaki I, Firth J, Ioannidis JP, Carvalho AF. Environmental risk factors and non-pharmacological interventions for obesity: an umbrella review of meta-analyses of cohort studies and controlled trials. _In Press_2018. [DOI] [PubMed]
- 50.Swencionis C, Wylie-Rosett J, Lent MR, Ginsberg M, Cimino C, Wassertheil-Smoller S, Caban A, Segal-Isaacson C-J. Weight change, psychological well-being, and vitality in adults participating in a cognitive–behavioral weight loss program. Health Psychol 2013;32:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Köhler C, Freitas T, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctôt KL, Carvalho AF. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 2017;135:373–87. [DOI] [PubMed] [Google Scholar]
- 52.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772. [DOI] [PubMed] [Google Scholar]
- 53.Soto M, Herzog C, Pacheco JA, Fujisaka S, Bullock K, Clish CB, Kahn CR. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psychiatry 2018;23:2287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, 4th, Taylor CM, Welsh DA, Berthoud H-R. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 2015;77:607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, Itsiopoulos C, Niyonsenga T, Blunden S, Meyer B. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutr Neurosci 2017;1–14. [DOI] [PubMed] [Google Scholar]
- 56.Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173:575–87. [DOI] [PubMed] [Google Scholar]
- 57.Chatterton ML, Mihalopoulos C, O'Neil A, Itsiopoulos C, Opie R, Castle D, Dash S, Brazionis L, Berk M, Jacka F. Economic evaluation of a dietary intervention for adults with major depression (the “SMILES” trial). BMC Public Health 2018;18:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teasdale SB, Ward PB, Rosenbaum S, Samaras K, Stubbs B. Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness. Br J Psychiatry 2017;210:110–8. [DOI] [PubMed] [Google Scholar]
- 59.Teasdale SB, Latimer G, Byron A, Schuldt V, Pizzinga J, Plain J, Buttenshaw K, Forsyth A, Parker E, Soh N. Expanding collaborative care: integrating the role of dietitians and nutrition interventions in services for people with mental illness. Australas Psychiatry 2018;26:47–9. [DOI] [PubMed] [Google Scholar]
- 60.Parker G, Brotchie H. Gender differences in depression. Int Rev Psychiatry 2010;22:429–36. [DOI] [PubMed] [Google Scholar]
- 61.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr 2008;99:931–40. [DOI] [PubMed] [Google Scholar]
- 62.Kiefer I, Rathmanner T, Kunze M. Eating and dieting differences in men and women. J Men Health Gender 2005;2:194–201. [Google Scholar]
- 63.Wardle J, Haase AM, Steptoe A, Nillapun M, Jonwutiwes K, Bellisie F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med 2004;27:107–16. [DOI] [PubMed] [Google Scholar]
- 64.Sarris J, O'Neil A, Coulson CE, Schweitzer I, Berk M. Lifestyle medicine for depression. BMC Psychiatry 2014;14:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY MATERIAL