COVID‐19 from the perspective of dentists: A case report and brief review of more than 170 cases (original) (raw)
Abstract
A novel coronavirus which has appeared from China, has been circulating hastily around the world. We summarized the publications including oral manifestation of coronavirus disease 2019 (COVID‐19) cases based on PubMed and Google Scholar data bases, and also present a case that highlights oral lesions 2 days prior to the first COVID‐19 general symptoms. Two authors independently reviewed the papers, 17 studies of more than 170 confirmed cases between ages of 9 and 90 were included. The most common oral manifestation was dry mouth followed by dysgeusia and pseudomembranous fungal structure. Change in tongue sensation and ulceration, muscle pain during mastication, swelling in oral cavity, and herpetic lesions were other common symptoms. Associated symptoms, latency time, treatment, and prognosis have also been meticulously reviewed. We hope that careful clinical intraoral examination on all COVID‐19 positive patients and equally on any patients who need dental care will pave the way for further studies.
Keywords: coronavirus, COVID‐19, oral examination, oral manifestations, review
1. INTRODUCTION
A novel coronavirus, severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) that triggers a new infectious respiratory disease, coronavirus disease 2019 (COVID‐19) that has appeared from Wuhan, China, has been circulating hastily around the world. It has been declared as pandemic emergency by World Health Organization (WHO), on March 2020. Common clinical features of this virus infection include fever, cough, headache, diarrhea, fatigue, headache, and myalgia. 1 Two new symptoms, anosmia and ageusia, were also found to be widespread, according to the most recent reports. 2
COVID‐19 has an incubation time that ranges between 1 and 14 days. Early detection of symptoms is life‐saving. Some of mucocutaneous manifestations of this viral infection can be helpful for the early diagnosis. Furthermore, recent reports discovered that loss of taste and smell may represent as the first and only manifestations of infection. 3
An increasing number of literatures have been recently published sporadically on oral manifestations of COVID‐19 patients, suggesting that oral lesions could be a possible clinical characteristic of COVID‐19. The epidemiologic significance of the oral manifestations remains uncertain and thus, in this brief review, we have tried to summarize the publications including oral manifestation of COVID‐19 cases.
2. METHODS
An electronic literature search was conducted on Medline (PubMed) and Google Scholar databases on October 2020 with the terms “((novel coronavirus) OR (Coronavirus disease 2019) OR (COVID‐19) OR (SARS‐CoV‐2)) AND ((oral) OR (mucocutaneous) OR (manifestation) OR (mucositis)).” The time for publication was set to 2019 onwards. Two reviewers independently reviewed the articles. Case reports dealing with oral manifestations of COVID‐19 were included. In addition, the patient of the case we report, provided written consent to publish the characteristics and picture of her case and the identity of the patient has been protected.
3. CASE
A 56‐years‐old female with underlying health condition of hypertension and chronic sinusitis, reporting history of inverted papilloma lesion in the left maxillary sinus that was partially resected, presented with vesicles on lower lip mucosa. She was examined and followed up via video consultation and asked to send a picture of the lower lip mucosa. History of the oral manifestation revealed 2 days of dry mouth, acute dysgeusia, mild pain, and burning sensation in lower lip mucosa. She reported appearance of two vesicles in the same region and simultaneous systemic symptoms of COVID‐19 after 2 days (Figure 1). Our subject systemic symptoms included: high fever, fatigue, and lack of appetite. She did not develop any cutaneous lesion. Polymerase chain reacted (PCR) assay resulted positive for COVID‐19, and azithromycin, levofloxacin, montelukast, naproxen, and acetaminophen were immediately prescribed.
FIGURE 1.
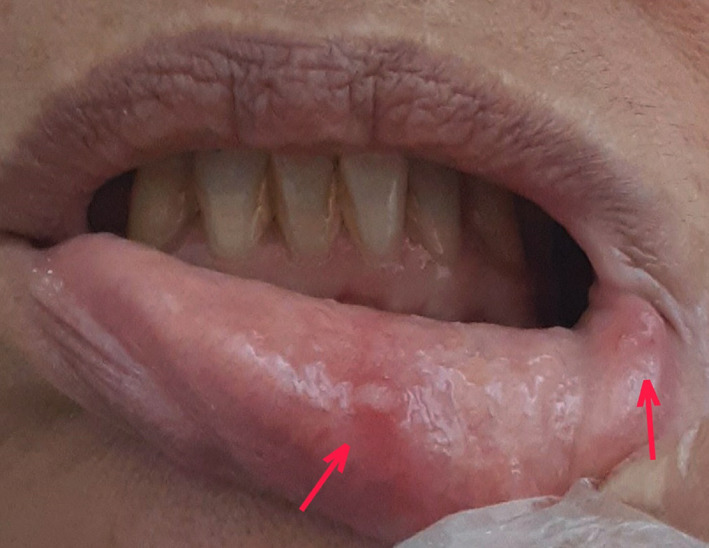
The view of lower lip labial mucosa showing vesicles in the area
The patient reported previous history of herpetic infections. Although, recurrent HSV infections are less common within the mouth and typically involve the keratinized mucosa, such as gingiva and palate, they can occur on any mucosal site in immunocompromised patients. 4 We proposed herpes PCR testing, however, but due to mild symptoms and financial issues, the patient refused to perform. After 7 days, oral lesions were healed and systemic symptoms regressed on day 14.
4. RESULTS
Overall, 17 studies were included in the final analysis. More than 170 COVID‐19 positive Patients developed oral manifestations, ranging from 9 to 90 years of age (Table 1). All the patients, with the exception of 24, 5 , 6 , 7 presented with systemic symptoms such as fever, cough, weakness, shortness of breath, diarrhea, and dysgeusia.
TABLE 1.
Articles on oral manifestations of Coronavirus disease 2019 (COVID‐19)
| Author and year | Nr of Cases | COVID‐19 PCR test | Age and gender | COVID‐19 manifestations | Cutaneous lesions | Treatment for COVID‐19 | Oral manifestation | Location of oral manifestation | Days after COVID‐19 diagnosis | Treatment of oral symptoms | Healing of oral symptoms |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patel et al, 2020 | 1 | Not provided | 35, Female | CGS | None | None | Necrotizing gingivitis | Labial interdental papillae | 3 days | Metronidazole, Chlorhexidine | 5 days |
| Biadsee et al, 2020 | 128 | Positive | Ages NR 55%Female | CGS (especially cough and Impaired sense of smell and taste) | NR | NR | Dry mouth (n = 72) Dysgeusia (n = 67) Change in sensation in the tongue (n = 20) Masticatory muscle pain (n = 15) Swelling of oral cavity (n = 10) Plaque‐like changes in the tongue (n = 9) Oral bleeding (n = 6) | Tongue, Palate, Gums | NR | NR | NR |
| Ansari et al, 2020 | 2 | Positive | 56, Female75, Male | CGS | None | Antibiotics(Azithromycin) Supportive treatment Anti‐viral(Remdesivir[n = 1]) | Painful ulcers with irregular margins (Recurrent herpes simplex) | Tongue, Hard palate | 5 days 7 days | Diphenhydramine, Dexamethasone, Tetracycline and Lidocaine. | 7 days |
| Soares et al, 2020 | 1 | Positive | 42, Male | CGS | Present | Anti‐inflammatory drugs (Dexamethasone, Dipyrone) | Painful ulcer Multiple reddish macules | Tongue, Hard palate, Buccal mucosa, Lips | NR | None | 3 weeks |
| Chaux‐Bodard et al, 2020 | 1 | Positive | 45, Female | CGS (mild stage) | Present | None | Irregular ulcer (it was painful during 24 h, followed by 24 h of erythematous macula, which evolved into asymptomatic ulcer) | Tongue | −8 days | NR | 10 days |
| Rodríguez et al, 2020 | 3 | Positive | 43, 78, Females 53,Male | CGS | None | NR | Dry mouth Aphthous‐like lesions Tongue depapillation Pseudomembranous candidiasis Commissural cheilitis (n = 2) Burning sensation (n = 2) | Tongue, Palate, Lips | since hospitalization, 7 days 42 days | Triamcinolone acetonide, Neomycin, Nystatin | 10 days, not reported, 15 days |
| dos Santos et al, 2020 | 1 | Positive | 67, Male | CGS | None | Hydroxychloroquine Antibiotics (Ceftriaxone, Azithromycin) | Ulcers (Recurrent herpes simplex) white plaque (Saccharomyces cerevisiae) | Tongue | 24 days | Fluconazole, Nystatin, Chlorhexidine, and, Hydrogen peroxide 1% | 14 days |
| Tomo et al, 2020 | 1 | Positive | 37, Female | CGS | None | Anti‐inflammatory drugs (Dexamethasone, Dipyrone) | Erythema and depapillation Dry mouth Dysgeusia | Tongue | 9 days | Chlorhexidine | 14 days |
| Martín Carreras‐Presas et al, 2020 | 3 | Suspicious (n = 2) Positive (n = 1) | 65, Female 56, 58, Males | CGS (One patient has no symptoms except the mucosal manifestation) | Present (n = 1) | Hydroxychloroquine Antibiotics Anti‐inflammatory drugs (Dexamethasone) Antifungal Anti‐viral (Lopinavir, Ritonavir) | Painful ulcers (Recurrent herpes simplex (n = 2)) Blisters (n = 1) Desquamative gingivitis (n = 1) Pain on tongue (n = 1) | Hard palate, Labial mucosa | Single manifestation (n = 1) Since the beginning (n = 1) 2 days (n = 1) | Hyaluronic acid, Chlorhexidine, Prednisolone and Valaciclovir | 10 days, 7 days, 3 days |
| Aghazadeh et al, 2020 | 1 | Positive | 9, Female | CGS | Present | Supportive treatment | Vesicles and erosions | Lips, Tongue, Buccal mucosa | NR | None | 7 days |
| Corchuelo et al, 2020 | 1 | Positive | 40, Female | Lymphadenopa‐thy | Present | Antibiotics(Azithromycin) Anti‐inflammatory drugs (Ibuprofen) Vitamin D2 | Canker sores (Candida albicans) Aphthous‐like lesion Gingival hyperpigmentation | Tongue, Lips, Gingiva | 3 weeks | Nystatin, Chlorhexidine | 20 days, pigmentation not faded |
| Ciccarese et al, 2020 | 1 | Positive | 19, Female | CGS | Present | NR | Erosions and Ulcerations Petechiae | Lips, Palate, Gingiva | 5 days | Palliative | 10 days |
| Cebeci Kahraman et al, 2020 | 1 | Positive | 51, Male | CGS | None | Antibiotics (Clarithromycin) | Erythema, Petechiae, and Pustules | Oropharynx, Palate | 10 days | None | A few days! |
| Sakaidaa et al, 2020 | 1 | Positive | 52, Female | CGS | Present | Antibiotics (Ampicillin/Sulbactam, Clarithromycin) | Erosions | Lips, Buccal mucosa | −10 days | Oral prednisolone | NR |
| Salehi et al. 2020 | 53 | Positive | Mean age = 63 (age ≥ 50:80%) Female 57% | CGS (especially respiratory distress and fever) | NR | Anti‐viral Antibiotics Anti‐inflammatory drugs (Corticosteroids) | Pseudomembranous structures, white plaques | NR | Mean = 8 days | Fluconazole, Nystatin | NR |
| Galvina et al, 2020 | 1 | Positive | 40, Female | CGS (mild stage) | NR | NR | Recurrent herpes simplex Hairy tongue white lesions | Hart palate, Tongue | 7 days | Acyclovir, Antiseptic, Nystatin, Panthenol,Local anesthetic | 3 weeks |
| Riad et al, 2020 | 26 | Positive | Mean age = 37 Female 56% | CGS (n = 4) 22 patients had no symptom except the mucosal manifestation | NR | NR | Ageusia (n = 3) Painful tongue ulcers (all cases) | Tongue (dorsum and side) | Mean = 4 days after PCR test (ranging from 0–5 days) | Oral paracetamol, Chlorhexidine | 7 days (n = 21) 14 days (n = 5) |
| Our case | 1 | Positive | 56, Female | CGS | None | Antibiotics (Azithromycin, Levofloxacin) Anti‐inflammatory and antiasmathic drugs (Montelukast, Naproxen and Acetaminophen) | Vesicles Dry mouth Dysgesia | Lower lip mucosa | −2 days | None | 7 days |
The most common oral manifestation was dry mouth, which was reported in 75 of the cases. 71 patients reported changes in taste sensation and a strong association with burning mouth and taste change was found. 3 , 5 , 6 , 8 , 9 , 10 67 patients developed pseudomembranous structure, white plaque or hairy tongue on the intraoral mucosa, with or without other symptoms like dry mouth and glossalgia, 11 of which on the tongue were identified by questionnaire, the etiology remained unknown, while in 55 other cases specimens were taken from the oral mucosa and fungal infection was confirmed, with Candida albicans as the most prevalent strain. 3 , 5 , 8 , 11 , 12 , 13 48 patients reported a change in their tongue sensation, 28 of whom suffered from painful ulcers in the area, 15 patients suffered from muscle pain during mastication with a mean VAS score of 3.8, and 10 patients indicated swelling in the oral cavity: four in the palate, four in the tongue, and two in the gums. Change in tongue sensation strongly correlated with swollen palate and plaque‐like changes in the tongue. 3 , 7 , 14 , 15
Sporadic reports showed recurrent herpes simplex virus lesions in six cases: four on the hard palate, and two on the tongue. 6 , 12 , 13 , 16 Four patients developed erythema multiforme‐like eruptions, three of whom had palatal macules and petechiae and one of whom showed three blisters in the inner lip mucosa. 6 , 17 Erosions, erythema, ulcers, necrotizing ulcerative periodontitis, and aphthous like lesions were also observed in single cases. 5 , 8 , 9 , 10 , 14 , 15 , 18 , 19 , 20 , 21
The details on the onset of the oral manifestation was recorded for the 95 of the cases, with a mean of 7.21 days after systemic symptoms, varying from −10 to 42 days. In 42 cases, oral lesions resolved spontaneously or with some basic treatment within 3 weeks, except gingival hyperpigmentation in one patient, detailed information was not documented in other cases.
5. DISCUSSION
Several reports and systematic reviews investigated cutaneous lesions due to COVID‐19. Sousa et al 22 recently, highlighted the need for further epidemiological research on oral manifestation of COVID‐19. As the virus has become a pandemic, there has been continuous production of additional data. We therefore conducted a study to briefly review papers on oral and mucosal symptoms of patients suspected or diagnosed with COVID‐19.
The main impact of COVID‐19 on oral mucosa seems to be multi‐factorial, the important role of the immune system is inevitable. Medicine, the nature of the virus and even stress could put the control of the Immune system at risk. A certain state of immune dysregulation may lead to opportunistic infections, drug eruptions, or the risk of exacerbating existing autoimmune conditions via cytokine storm. Moreover, some researchers assume that oral lesions contributed to COVID‐19 are based on the assumption of an Inflammatory response that induces vascular inflammation. 14 Nevertheless, we also noticed that oral manifestations may potentially be other overlapping diseases.
Smell and taste changes are early indicators of the COVID‐19 pandemic and are effective in political decision making. While dry mouth was the most prevalent oral symptom, 3 , 8 , 9 invasion of the salivary ducts has been confirmed by animal model experiments as the first attack of the virus, 23 Provided that it may be the potential reason of acute sialadenitis and related symptoms including discomfort, swelling, pain, or secretory salivary glands dysfunction. Nonetheless, it is possible to cause chronic sialadenitis after recovery. Several studies have shown the possibility of an outbreak of opportunistic infections, 3 , 5 , 8 , 12 especially, fungal co‐infections. Accordingly, some risk factors may play a role, including lymphocytopenia, intensive care unit admission, invasive or non‐invasive ventilation, corticosteroid, and broad‐spectrum antibiotic usage, growing age, or having immunocompromised condition. Exanthematic lesions may be linked to viral etiology, for instance, hand‐foot‐and‐mouth disease, herpetic recurrent stomatitis (affecting keratinized tissue), and erythema multiforme (affecting both keratinized and non‐keratinized tissue) that may be triggered by direct virus invasion.
Various care and treatment protocols and guidelines existed among countries, which caused challenge of data analysis. The most prominent treatment approach for COVID‐19 was the combination of anti‐viral therapy with antibiotics. In addition, analgesics, antipyretics, and antitussives could be applied as Supportive treatment. Corticosteroids and antihistamines were used for anti‐inflammation effects. Some case reports implied medication containing antiseptics mouthwash, corticosteroids, and palliative drugs for mucosal symptoms. Antifungal or antibacterial therapy was also prescribed, where the etiology was relevant. However, as in our case, some researchers refused to consider any therapy for mild or asymptomatic patients due to the nature of spontaneous recovery. Drug eruption can occur within the latency period. 20 Although its pathogenesis is multifactorial, it could be correlated with exacerbated cytokine storm induced inflammation along with thrombocytopenia caused by a direct viral attack to bone marrow, and the use of medicines may further reduce the platelet count.
A limitation of our research is that we were not able to quantitatively analyze oral manifestations and disease patterns due to vast amounts of unprovided and incomparable data.
6. CONCLUSION
In conclusion, our review outlined the clinical characteristics of oral manifestations in patients with COVID‐19 and their potential mechanisms. The oral symptoms often appeared after general symptoms such as fever and asthenia, but can still be the initial or only sign of COVID‐19. Thus, a careful clinical intraoral examination must be performed on COVID‐19 positive patients and equally on any patients who need dental care thoroughly and systematically to ensure that no parts are missing and to obtain further clinical data, which could pave the way for further studies.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Eghbali Zarch R, Hosseinzadeh P. COVID‐19 from the perspective of dentists: A case report and brief review of more than 170 cases. Dermatologic Therapy. 2021;34:e14717 10.1111/dth.14717
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kara C, Çelen K, Dede FÖ, Gökmenoğlu C, Kara NB. Is periodontal disease a risk factor for developing severe COVID‐19 infection? The potential role of Galectin‐3. Exp Biol Med. 2020;245(16):1425‐1427. 10.1177/1535370220953771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID‐19: sex‐related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163:722‐728. 10.1177/0194599820934380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santosh A, Muddana K. Viral infections of oral cavity. J Family Med Prim Care. 2020;9:36 10.4103/jfmpc.jfmpc_807_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID‐19: case report. Int J Infect Dis. 2020;100:154‐157. 10.1016/j.ijid.2020.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martín Carreras‐Presas C, Amaro Sánchez J, López‐Sánchez AF, Jané‐Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS‐CoV‐2 infection. Oral Dis. 2020;1‐3. 10.1111/odi.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riad A, Kassem I, Hockova B, Badrah M, Klugar M. Tongue ulcers associated with SARS‐CoV‐2 infection: a case series. Oral Dis. 2020;1‐3. 10.1111/odi.13635. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez MD, Romera AJ, Villarroel M. Oral manifestations associated to Covid‐19. Oral Dis. 2020;1‐3. 10.1111/odi.13555. [DOI] [PubMed] [Google Scholar]
- 9.Tomo S, Miyahara GI, Simonato LE. Oral mucositis in a SARS‐CoV‐2‐infected patient: secondary or truly associated condition? Oral Dis. 2020;1‐5. 10.1111/odi.13570. [DOI] [PubMed] [Google Scholar]
- 10.Cebeci Kahraman F, ÇaŞkurlu H. Mucosal involvement in a COVID‐19‐positive patient: a case report. Dermatol Ther. 2020;33:e13797 10.1111/dth.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses. 2020;63(8):771‐778. 10.1111/myc.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.dos Santos JA, Normando AGC, da Silva RLC, et al. Oral mucosal lesions in a COVID‐19 patient: new signs or secondary manifestations? Int J Infect Dis. 2020;97:326‐328. 10.1016/j.ijid.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glavina A, Biočina‐Lukenda D, Mravak‐Stipetić M, Markeljević J. Oral symptoms and lesions in SARS‐CoV‐2 positive patient. Oral Dis. 2020;1‐2. 10.1111/odi.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaux‐Bodard A‐G, Deneuve S, Desoutter A. Oral manifestation of Covid‐19 as an inaugural symptom? J Oral Med Oral Surg. 2020;26(2):18 10.1051/mbcb/2020011. [DOI] [Google Scholar]
- 15.Soares CD, de Carvalho RA, de Carvalho KA, de Carvalho MGF, de Almeida OP. Letter to editor: Oral lesions in a patient with Covid‐19. Med Oral Patol Oral Cir Bucal. 2020;25(4):e563‐e564. 10.4317/medoral.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID‐19): a letter‐to‐editor. Oral Dis. 2020;1‐6. 10.1111/odi.13465. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez‐Cauhe J, Ortega‐Quijano D, Carretero‐Barrio I, et al. Erythema multiforme‐like eruption in patients with COVID‐19 infection: clinical and histological findings. Clin Exp Dermatol. 2020;45:892‐895. 10.1111/CED.14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aghazadeh N, Homayouni M, Sartori‐Valinotti JC. Oral vesicles and acral erythema: report of a cutaneous manifestation of COVID‐19. Int J Dermatol. 2020;59(9):1153‐1154. 10.1111/ijd.15047. [DOI] [PubMed] [Google Scholar]
- 19.Ciccarese G, Drago F, Boatti M, Porro A, Muzic SI, Parodi A. Oral erosions and petechiae during SARS‐CoV‐2 infection. J Med Virol. 2020;1‐4. 10.1002/jmv.26221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaida T, Isao T, Matsubara A, Nakamura M, Morita A. Unique skin manifestations of COVID‐19: is drug eruption specific to COVID‐19? J Dermatol Sci. 2020;99:62‐64. 10.1016/j.jdermsci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel J, Woolley J. Necrotizing periodontal disease: Oral manifestation of COVID‐19. Oral Dis. 2020;1‐2. 10.1111/odi.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Sousa F, Paradella TC. Considerations on oral manifestations of COVID‐19. J Med Virol. 2020;1‐2. 10.1002/jmv.26451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Wu H, Ding X, et al. Does infection of 2019 novel coronavirus cause acute and/or chronic sialadenitis? Med Hypotheses. 2020;140:109789 10.1016/j.mehy.2020.109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.