p53-mediated protective responses to UV irradiation (original) (raw)
Exposure of cells to DNA-damaging agents elicits a complex set of acute cellular responses. Generally, DNA damage-inducible (DDI) responses serve to protect the cell from genotoxic adversity. In Escherichia coli, the SOS regulon frequently has been studied by using UV radiation as a model-inducing agent (1). In this prototypical response, approximately 20 genes are coordinately induced that have roles in DNA repair, mutagenesis, recombination, and growth control (1). This general theme, involving the coupling of cell cycle arrest and DNA repair by coordinate induction of several genes, appears to be conserved between single-cell organisms and mammalian cells; for example, the sulA gene, a component of the SOS response in E. coli, encodes a growth inhibitor analogous to cell cycle inhibitors of mammalian cells (reviewed in ref. 2). A recent example of interspecies conservation of cell cycle checkpoint functions was shown in the case of_Schizosaccharomyces pombe_ Chk1 protein kinase and its human homolog, both of which elicit a G2/M checkpoint response (3). In yeast, 80 or more genes are DDI, representing almost 1% of the genes in this organism (4).
DDI responses in mammalian cells are considerably more complex than those of simpler organisms. The initial expectation, upon the generation of DDI libraries from mammalian cells, was that the isolation of DDI genes from mammalian cells would include a subset of DNA repair genes. However, most mammalian DDI genes appear to function in other cellular functions related to cell and tissue injury such as growth arrest. Evidence for SOS-type responses in mammalian cells stems from certain split-dose experiments, in which exposure of cells to a low dose of a DNA-damaging agent afforded some measure of protection from a second, higher dose of the same agent (reviewed recently in ref.5). By a similar token, experiments that used host-cell reactivation (HCR) of irradiated viruses suggested that mammalian cells might exhibit some measure of inducible repair (6). The molecular basis for these effects, however, could be caused by induction of cell cycle arrest by the first exposure, by simply allowing additional time for repair (7), or it could be caused by the induction of genes whose products are involved in DNA repair, by the first exposure (reviewed in ref. 5). More recently, experiments that used HCR of damaged reporter genes similarly showed that carcinogen-treated cells would repair and thereby reactivate the damaged reporter gene to a greater extent than cells that were not pretreated (8).
The issue of inducible repair in mammalian cells remained, however, controversial, because the observed differences in repair were relatively modest compared with the SOS responses of simpler organisms. Adding to the complexity of the problem, the practical separation of inducible repair from cell cycle effects is not trivial. Some studies that used “liquid holding” experiments had shown that although no differences in repair were observed in G1 vs. G2-arrested cells (ref. 9, and references therein), cells that were prevented from S-phase entry might be relatively protected from cytotoxicity (10). Because repair rates are similar in G1, S, and G2, this protection probably is caused by increased time for repair during the “liquid holding” period. But again, separating inducible repair from cell cycle effects is not a trivial task, and as discussed in the following sections, recent evidence suggests that a number of cell cycle checkpoint proteins have dual roles and also may function in DNA damage processing. Thus, a number of inducible processes can, and probably do, contribute to genotoxic stress resistance.
The paper by Eller et al. in this issue of the_Proceedings_ (11) demonstrates that treatment of cells with thymidine dinucleotide (pTpT) can elicit a protective response to subsequent exposure to UV irradiation. It is likely that pTpT, and perhaps other small nucleic acids, mimics the products of DNA damage or processed DNA-damage intermediates. This compound previously has been shown to evoke a melanogenic (tanning) response in skin, thus recapitulating the melanogenic protective response to UV irradiation, and in the present study, is shown to result in induction of the p53 pathway. This finding raises interesting questions with regard to cellular signaling pathways that are triggered by genotoxic stress. The initial signal triggering the “UV response” is in large part independent of DNA damage, but rather appears to be mediated by a (damage) signal to membrane-associated components of the RAS pathway with activation of one or more mitogen-activated protein kinases (MAPKs) (12). However, others argue that even UV activation of MAPKs may have a DNA-damage signal component (13). In the case of p53, activating signals clearly can involve DNA damage in addition to other nongenotoxic stresses (reviewed in ref. 7). The activation of the p53 pathway by pTpT in the present paper may mean that intermediates of DNA damage processing can provide a stimulus for the activation of cellular stress responses, and thus represents a third type of activation signal.
p53, Guardian of the Genome, Revisited
One important component of mammalian DDI responses is the p53 tumor suppressor gene product. p53 becomes activated by genotoxic and other stresses, and in turn p53 transactivates the transcription of perhaps as many as 100 other genes (14). The best known p53-mediated response is G1 phase cell cycle arrest, which is mediated largely through the cyclin-dependent kinase inhibitor, p21Cip1/Waf1 (15). Cell cycle checkpoints in other phases of the cell cycle also have been reported to be affected by p53 (16). Cell cycle checkpoints are believed to provide additional time for DNA repair (7). The activation of (wild-type) p53 facilitates the repair of at least some classes of DNA damage, such as that caused by UV irradiation (17–23). This point has been demonstrated also by loss of p53 function, such as by mutations occurring in human cancers, or by expression of viral gene products that block p53 (17–27). In these studies, decreased DNA repair was attributed to p53 loss of function. UV irradiation of p53-deficient cells resulted in increased mutagenesis in some studies (21, 22, 24). On the other hand, increased mutagenesis was not observed by using a lacZ reporter in Big Blue mice lacking p53 genes (28). The reason for the discrepancy is not clear, though it may relate to the nature of the transgene target in a whole animal. Along these lines, one group recently reported qualitative differences in repair of a supF reporter in a p53-deficient cellular background, even though quantitative differences between p53+/+ and p53−/− cells were not observed in this particular study (29).
Much emphasis has been placed on chromosomal-scale alterations in p53-deficient cells, such as increased gene amplification and aneuploidy. Recent work implicating p53 in repair of UV lesions extends the implications of the original “guardian of the genome” hypothesis (7, 30). Some mutant p53 genes may confer phenotypes that differ from that of the p53 nullizygous condition. This concept is illustrated by the mutational spectra of UV-damaged supF reporter plasmids replicated in Li-Fraumeni fibroblasts compared with that obtained with mouse embryo fibroblasts lacking p53 genes. Li-Fraumeni cells carry mutant p53 genes and the retrieved_supF_ plasmids exhibit a complex pattern of multiple point mutations, which differs from that of the p53 “knockout” mice, and both of which differ from their respective normal controls (29,31). Given the wide spectrum of p53 mutations occurring in human cancers, it seems reasonable to speculate that different mutant p53 alleles may affect the processing of DNA damage in different ways, especially because mutant p53 proteins vary in other biological respects (32). The protective effect of p53 in response to UV irradiation extends also to important cancer chemotherapy agents such as _cis_-platin and melphalan, agents that, like UV radiation, produce damage that is repaired primarily by the nucleotide excision repair (NER) pathway (33, 34). Downstream effector genes of the p53 pathway such as CIP1/WAF1 and GADD45 also may play a role in the protective response (35–39). Most intriguingly, heterologous expression of human p21Cip1/Waf1 in S. pombe revealed evidence of genetic interactions between the human p21Cip1/Waf1 and endogenous Chk1 (discussed above), PCNA, and RAD3 genes (40). In short, a complex pattern is emerging regarding p53 downstream effector gene products and the coupling of cell cycle checkpoints and DNA repair (reviewed in refs. 41 and 42). Recent studies in yeast provide evidence that several cell cycle checkpoint proteins may have dual roles in the processing of DNA damage. The RAD17 gene product, for example, encodes a putative 3′ to 5′ exonuclease activity (43). In mammalian cells, this coupling of cell cycle control and DNA repair is exemplified by the TFIIH complex, a basal transcription factor that also contains the repair-associated proteins ERCC2 and ERCC3, as well as cyclin H and a cyclin-dependent kinase (44). The proximity of these proteins within the same complex supports the concept that DNA repair could be coupled to cell cycle control. Interestingly, p53 associates with TFIIH in vitro, an interaction that is abrogated in cancer-associated mutant p53 proteins (18, 45). In addition, the p53 protein itself exhibits a 3′ to 5′ exonuclease activity, which perhaps suggests another potential mechanism by which p53 might directly participate in DNA damage processing (46).
Apart from split-dose experiments that used DNA damaging agents, other treatments may modulate cellular protective responses, either at the level of cell cycle perturbation, or repair of the damage. In the paper by Eller et al. (11), pTpT is shown to up-regulate p53 and evoke the protective response associated with tanning. This response is presumably because pTpT mimics some aspect of the DNA damage signal, perhaps because small nucleic acids might resemble intermediates in DNA damage processing, resulting in induction of the pathway in the absence of genotoxic stress. Such examples of “mimicry” may find practical application, such as in chemoprotection from carcinogenesis. Conceptually similar approaches are to be found in the cancer literature, such as in the case of the chemoprotective agent oltipraz, a compound derived from vegetables such as cabbage, broccoli, and cauliflower, which may increase DNA repair activity in normal cells, and thereby protect cells from carcinogenesis (47).
Apoptosis vs. Protective Responses: The “Two Faces” of p53
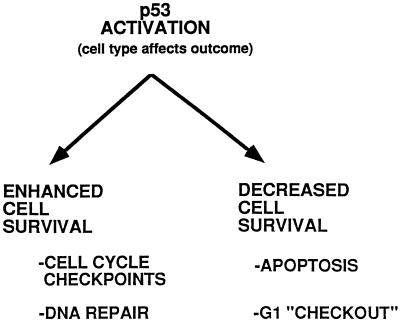
The present discussion ignores for the moment that p53 also can trigger apoptosis in some cell types. Because apoptosis is a terminal phenotype, the induction of apoptosis is an outcome that appears contrary to that of p53-mediated protective responses. We have termed this concept the “two faces of p53” (48) (Fig. 1). If p53 protects by activating cell cycle checkpoints and repair, or evokes an apoptotic response (49), depends on cell type. It would seem that which “face” p53 presents in a given cell type, in response to a given type of DNA damage, would be a critical consideration in carcinogenesis and in cancer chemotherapy, inasmuch as many chemotherapy drugs are themselves DNA-damaging agents. Indeed, it appears that cancers that are most amenable to chemotherapeutic intervention, for example, myeloid and lymphoid malignancies or testicular cancers, are those that are prone to undergo apoptosis after treatment. Unlike many other neoplasms, testicular cancers have a low frequency of p53 mutations (50). Activation of wild-type p53 in these cancers may participate in apoptosis induction by chemotherapy drugs (50).
Figure 1.
The two faces of p53. Activation of p53, such as by DNA damage, elicits either a protective response (Left) or an apoptotic response (Right). A third possible outcome associated with decreased cell survival is “G1 checkout” (55), in which cells undergo an irreversible arrest. These possible outcomes appear to be determined largely by cell type and possibly also by the type of DNA damage incurred. The paper by Eller et al. (11) demonstrates that the protective “face” of p53 can be induced by treatment of cells with thymidine dinucleotide (pTpT), in the absence of DNA damage. Thus, functional inactivation of p53, such as by mutations occurring in cancer cells, can allow cells to escape p53-mediated apoptosis. At the same time, mutant p53 cells exhibit a condition of genomic instability, cell cycle checkpoint loss, and decreased ability to repair subsequent DNA damage.
By contrast, the p53 pathway is associated with protective responses in other cell types. In vitro data suggest that cancers of epithelial origin, such as breast or colon cancers, which often harbor mutant p53 genes, can be sensitized to killing by agents like _cis_-platin or melphalan (33, 34). These agents produce diadducts and interstrand crosslinks that, like UV-induced pyrimidine dimers, are repaired primarily by NER. There is ample evidence that NER is reduced in p53-deficient cells, as discussed above. The magnitude of reduction in NER owing to loss of p53 function correlates with observed decreases in cellular survival in these examples, which is again fairly modest (17, 24, 33, 34, 39). Much of the cytoxicity associated with UV damage is caused by damage within transcriptionally active genes, but it is clear from recent work by Ford and Hanawalt (19, 20) that p53 plays a major role in overall genomic repair, and not transcription-coupled repair. This observation may well account for the modest effects of p53 on UV cytotoxicity. In contrast, DNA crosslinks even in nontranscribed regions that are repaired by global NER (as opposed to transcription-coupled NER) probably produce substantial cytotoxicity, which may be reflected in larger differences between p53 wild type and p53-deficient cells. In experiments that used_cis_-platin, an 8-fold dose-modifying effect was observed for cells with functional p53 compared with cells in which p53 function was blocked by HPV16-E6 (39), whereas only a 2-fold dose-modifying effect was obtained for UV irradiation (17, 24, 39). Differences in cellular survival in the presence or absence of functional p53 was most pronounced when cells were exposed continuously to low doses of_cis_-platin, rather than an acute exposure protocol (39). One possibility is that cells with wild-type p53 function may be able to adapt to continuous exposure, affording them protection from_cis_-platin, whereas mutant p53 cells are unable to elicit this response.
p53 in Carcinogenesis and Chemoprevention
Importantly, the p53 gene is itself a frequent target of mutation in UV carcinogenesis of the skin (reviewed in ref. 51). Cells carrying mutant p53 genes undergo clonal expansion in the carcinogenesis process, as evidenced by immunostaining mutant p53 proteins in premalignant lesions (52), a finding commensurate with other studies (53). Mutant p53 genes isolated from nonmelanoma skin cancers and premalignant lesions exhibit characteristic “fingerprint” mutations associated with UV damage, consisting of C to T and CC to TT transitions (52). It has been suggested that UV can act as an initiator of carcinogenesis by causing mutations, and also a promoter of carcinogenesis by providing selection pressure for cells carrying mutant p53 genes (54). This theory makes sense as selection against p53 function may occur by two mechanisms in UV carcinogenesis, by abrogation of cell cycle checkpoints and decreased capacity for repair (26), and also by allowing those cells harboring mutant p53 genes to escape from p53-mediated apoptosis (48). Thus, both “faces” may act on the same population of cells to promote carcinogenesis. However, if the number of carcinogenesis-initiating mutations can be reduced, such as by augmenting p53’s protective functions in normal cells, then cancer risk may be reduced. Such is the implication of the work by Eller et al. (11) in this issue.
References
- 1.Walker G C. Microbiol Rev. 1984;48:60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fornace A J., Jr Annu Rev Genet. 1992;26:507–526. doi: 10.1146/annurev.ge.26.120192.002451. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 4.Ruby S W, Szostak J W. Mol Cell Biol. 1985;5:75–84. doi: 10.1128/mcb.5.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith M L, Fornace A J., Jr Mutat Res. 1996;340:109–124. doi: 10.1016/s0165-1110(96)90043-3. [DOI] [PubMed] [Google Scholar]
- 6.Ryan D K, Rainbow A J. Mutat Res. 1986;166:99–111. doi: 10.1016/0167-8817(86)90045-3. [DOI] [PubMed] [Google Scholar]
- 7.Hartwell L H, Kastan M B. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 8.Protic M, Roilides E, Levine A S, Dixon K. Somat Cell Mol Genet. 1988;14:351–357. doi: 10.1007/BF01534643. [DOI] [PubMed] [Google Scholar]
- 9.Petersen L N, Orren D K, Bohr V A. Mol Cell Biol. 1995;15:3731–3737. doi: 10.1128/mcb.15.7.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan G L, Little J B. Mutat Res. 1979;63:401–412. doi: 10.1016/0027-5107(79)90072-1. [DOI] [PubMed] [Google Scholar]
- 11.Eller M S, Maeda T, Magnoni C, Atwal D, Gilchrest B A. Proc Natl Acad Sci USA. 1997;94:12627–12632. doi: 10.1073/pnas.94.23.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelberg D, Klein C, Martinetto H, Struhl K, Karin M. Cell. 1994;77:381–390. doi: 10.1016/0092-8674(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 13.Adler V, Fuchs S Y, Kim J, Kraft A, King M P, Pelling J, Ronai Z. Cell Growth Differ. 1995;6:1437–1446. [PubMed] [Google Scholar]
- 14.Tokino T, Thiagalingam S, El-Deiry W S, Waldman T, Kinzler K W, Vogelstein B. Hum Mol Genet. 1994;3:1537–1542. doi: 10.1093/hmg/3.9.1537. [DOI] [PubMed] [Google Scholar]
- 15.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 16.Cross S M, Sanchez C A, Morgan C A, Schimke M K, Ramel S, Idzerda R L, Raskind W H, Reid B J. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- 17.Smith M L, Chen I-T, Zhan Q, O’Connor P M, Fornace A J., Jr Oncogene. 1995;10:1053–1059. [PubMed] [Google Scholar]
- 18.Wang X W, Yeh H, Schaeffer L, Roy R, Moncollin V, Egly J-M, Wang Z, Friedberg E C, Evans M K, Taffe B G, Bohr V A, Weeda G, Hoeijmakers J H J, Forrester K, Harris C C. Nat Genet. 1995;10:188–195. doi: 10.1038/ng0695-188. [DOI] [PubMed] [Google Scholar]
- 19.Ford J M, Hanawalt P C. Proc Natl Acad Sci USA. 1995;92:8876–8880. doi: 10.1073/pnas.92.19.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford J M, Hanawalt P C. J Biol Chem. 1997;272:28073–28080. doi: 10.1074/jbc.272.44.28073. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J, Yeasky T M, Havre P A, Glazer P M. Carcinogenesis. 1995;16:2295–2300. doi: 10.1093/carcin/16.10.2295. [DOI] [PubMed] [Google Scholar]
- 22.Yamagishi N, Miyakoshi J, Yagi T, Takebe H. Mutagenesis. 1997;12:191–194. doi: 10.1093/mutage/12.3.191. [DOI] [PubMed] [Google Scholar]
- 23.McKay B C, Francis M A, Rainbow A J. Carcinogenesis. 1997;18:245–249. doi: 10.1093/carcin/18.2.245. [DOI] [PubMed] [Google Scholar]
- 24.Havre P A, Yuan J, Hedrick L, Cho K R, Glazer P M. Cancer Res. 1995;55:4420–4424. [PubMed] [Google Scholar]
- 25.Mirzayans R, Enns L, Dietrich K, Barley R, Paterson M C. Carcinogenesis. 1996;17:691–698. doi: 10.1093/carcin/17.4.691. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Mitchell D L, Ho V C, Reed J C, Tron V A. Am J Pathol. 1996;148:1113–1123. [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Ho V C, Mitchell D L, Trotter M J, Tron V A. Am J Pathol. 1997;150:1457–1464. [PMC free article] [PubMed] [Google Scholar]
- 28.Sands A T, Suraokar M B, Sanchez A, Marth J E, Donehower L A, Bradley A. Proc Natl Acad Sci USA. 1995;92:8517–8521. doi: 10.1073/pnas.92.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishizaki K, Nishizawa K, Mimaki S, Aizawa S. Mutat Res. 1996;364:43–49. doi: 10.1016/0921-8777(96)00020-1. [DOI] [PubMed] [Google Scholar]
- 30.Lane D P. Nature (London) 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 31.Liu, P. K., Kraus, E., Wu, T. A., Strong, L. C. & Tainsky, M. A. Oncogene 12, 2267–2278. [PMC free article] [PubMed]
- 32.Halevy O, Michalovitz D, Oren M. Science. 1990;250:113–116. doi: 10.1126/science.2218501. [DOI] [PubMed] [Google Scholar]
- 33.Fan S, Smith M L, Rivet D J, Duba D, Zhan Q, Kohn K W, Fornace A J, Jr, O’Connor P M. Cancer Res. 1995;55:1649–1654. [PubMed] [Google Scholar]
- 34.Hawkins D S, Demers G W, Galloway D A. Cancer Res. 1996;56:892–898. [PubMed] [Google Scholar]
- 35.McDonald E R, Wu G S, Waldman T, El-Deiry W S. Cancer Res. 1996;56:2250–2255. [PubMed] [Google Scholar]
- 36.Li R, Hannon G J, Beach D, Stillman B. Curr Biol. 1996;6:189–199. doi: 10.1016/s0960-9822(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 37.Sheikh M S, Chen Y Q, Smith M L, Fornace A J., Jr Oncogene. 1997;14:1875–1882. doi: 10.1038/sj.onc.1201004. [DOI] [PubMed] [Google Scholar]
- 38.Fan S, Chang J K, Smith M L, Duba D, Fornace A J, Jr, O’Connor P M. Oncogene. 1997;14:2127–2136. doi: 10.1038/sj.onc.1201052. [DOI] [PubMed] [Google Scholar]
- 39.Smith M L, Kontny H U, Zhan Q, Sreenath A, O’Connor P M, Fornace A J., Jr Oncogene. 1996;13:2255–2263. [PubMed] [Google Scholar]
- 40.Tournier, S., Leroy, D., Goubin, F., Ducommin, B. & Hyams, J. S. Mol. Biol. Cell 7, 651–662. [DOI] [PMC free article] [PubMed]
- 41.Ko L J, Prives C. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 42.Kelman Z. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- 43.Lydall D, Weinert T. Science. 1995;270:1488–1491. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- 44.Seroz T, Hwang J R, Moncollin V, Egly J-M. Curr Opin Genet Dev. 1995;5:217–221. doi: 10.1016/0959-437x(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 45.Leveillard T, Andera L, Bissonnette N, Schaeffer L, Bracco L, Egly J-M, Waslyk B. EMBO J. 1996;15:1615–1624. [PMC free article] [PubMed] [Google Scholar]
- 46.Mummenbrauuer T, Janus F, Muller B, Wiesmuller L, Deppert W, Grosse F. Cell. 1996;85:1089–1099. doi: 10.1016/s0092-8674(00)81309-4. [DOI] [PubMed] [Google Scholar]
- 47.O’Dwyer P J, Johnson S W, Khater C, Krueger A, Matsumato Y, Hamilton T C, Yao K-S. Cancer Res. 1997;57:1050–1053. [PubMed] [Google Scholar]
- 48.Smith M L, Fornace A J., Jr Am J Pathol. 1996;148:1019–1022. [PMC free article] [PubMed] [Google Scholar]
- 49.Lowe S W, Ruley H E, Jacks T, Housman D E. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 50.Chresta C M, Hickman J A. Nat Med. 1996;2:745–746. doi: 10.1038/nm0796-745. [DOI] [PubMed] [Google Scholar]
- 51.Kraemer K H. Proc Natl Acad Sci USA. 1997;94:11–14. doi: 10.1073/pnas.94.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jonason A S, Kunala S, Price G J, Restifo R J, Spinell H M, Persing J A, Leffell D J, Tarone R E, Brash D E. Proc Natl Acad Sci USA. 1997;93:14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffiths S D, Clarke A R, Healy L E, Ross G, Ford A M, Hooper M L, Wyllie A H, Greaves M. Oncogene. 1997;14:523–531. doi: 10.1038/sj.onc.1200871. [DOI] [PubMed] [Google Scholar]
- 54.Ziegler A, Jonason A S, Leffell D J, Simon J A, Sharma H W, Kimmelman J, Remington L, Jacks T, Brash D E. Nature (London) 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 55.Gadbois D M, Bradbury E M, Lehnert B E. Cancer Res. 1997;57:1151–1156. [PubMed] [Google Scholar]