CD81 on B cells promotes interleukin 4 secretion and antibody production during T helper type 2 immune responses (original) (raw)
Abstract
Mice lacking CD81 (TAPA-1), a widely expressed tetraspanin molecule, have impaired antibody responses to protein antigens. This defect is specific to antigens that preferentially stimulate a T helper 2 response (ovalbumin or keyhole limpet hemocyanin in alum) and is only seen with T cell-dependent antigens. Absence of CD81 on B cells is sufficient to cause the defect. Also, antigen-specific interleukin (IL) 4 production is greatly reduced in the spleen and lymph nodes of CD81-null mice compared with heterozygous littermates. Thus, expression of CD81 on B cells is critical for inducing optimal IL-4 and antibody production during T helper 2 responses. These findings suggest that CD81 may interact with a ligand on T cells to signal IL-4 production. By using a soluble form of CD81 as a probe, a putative ligand for CD81 was identified on a subset of B and T cells. Two possible models for the interaction of CD81 on B cells with a potential ligand on either B or T cells are proposed.
The factors controlling the induction of T helper 1 or 2 (Th1 or Th2) immune responses have been the subject of intense recent investigation (1–6). Th1 responses are characterized by cellular immunity and production of IgG2a antibodies. Th2 responses are characterized by humoral immunity, specifically the production of IgG1 and IgE antibodies. For Th1 responses, it is well accepted that interleukin (IL) 12 (7–10) and IL-18 (11–13) are the primary inducers of interferon γ (IFN-γ), which is the major effector cytokine of Th1 development. For Th2 responses, however, it is less clear what factors initially induce IL-4, which is central to the development of a Th2 response. Some work suggests that IL-6 may play such a role (14). In this report, we show that a cell surface molecule, CD81, is crucial to the induction of IL-4 and the development of Th2 responses in vivo.
CD81 (TAPA-1) is a member of the tetraspanin superfamily of cell surface proteins, all of which have four transmembrane domains and two extracellular loops (for review, see ref. 15). These proteins in general, and CD81 in particular, have been linked to the control of cell proliferation, cell adhesion, and cell motility (for review, see ref. 16). CD81 is expressed on virtually all nucleated cells (17) but is highly expressed on germinal-center B cells (ref. 18 and unpublished data). A recent report has shown that antibody stimulation of human CD81 in mixed lymphocyte cultures from allergic individuals caused greatly enhanced IL-4 production (19). This was only true when B cells were used as antigen-presenting cells in these cultures.
Mice lacking CD81 have now been produced in three laboratories (20–22). Such mice undergo normal B and T cell maturation and have grossly normal lymphoid architecture (including germinal centers). However, they have diminished Ig or IgG1 antibody responses to protein antigens [ovalbumin given in alum or keyhole lympet hemocyanin (KLH) in complete Freund’s adjuvant (CFA), respectively] (20, 21). In this report, we examine the generality of the immune response defect of CD81-null mice and propose a model for the role of CD81 in the immune response.
MATERIALS AND METHODS
Mice.
CD81-null mice were generated as described (20). For each experiment shown, homozygous CD81−/− mice and heterozygous CD81+/− littermates were compared. For B cell chimera experiments, CD81−/− embryonic stem cells (ES cells) were generated by culturing CD81+/− ES cells in G418 at 1–2 mg/ml (GIBCO/BRL) to select for clones that had converted their second allele to CD81−. These clones were confirmed by Southern blot analysis (20). One such clone was used to inject into blastocysts from B cell-deficient [heavy chain J region (JH) deletion] mice (23), a gift from GenePharm International (Palo Alto, CA). These blastocysts were reimplanted into pseudopregnant (B6 × CBA)F1 hosts, and agouti offspring were selected. The presence of B cells (derived from the CD81−/− stem cells) was confirmed in these chimeras by flow cytometry analysis, and total serum Ig levels were measured. Similarly, CD81+/+ ES cells that had undergone a similar manipulation in vitro were injected into blastocysts from JH deletion mice, and agouti offspring of these injections were used as controls.
Immunization.
Mice as described above were immunized i.p. with 100 μg of ovalbumin or 25 μg of KLH either precipitated in alum (24) or given with 10 μg of QS-21 or 1 μg of IL-12 (a gift from S. Wolf, Genetics Institute, Cambridge, MA). The mice were bled from their tail veins 4, 8, and 12 weeks after a single immunization, then given a second injection as described above, and bled again 18 days later. To test the response to T independent antigens, mice were injected with either 50 μg of trinitrophenol (TNP)-lipopolysaccharide (Sigma) or 25 μg of TNP-Ficoll (BTI, San Rafael, CA) in saline and then bled 7 and 14 days after immunization.
ELISA Assays for Serum Ig and Antigen-Specific Ig.
Microtiter plates (Maxisorb, Nunc) were coated overnight at 4°C with one of the following in PBS: goat anti-mouse IgG (Caltag Laboratories, South San Francisco, CA; 2 μg/ml), ovalbumin (Sigma; 5 μg/ml), KLH (Pierce; 5 μg/ml), or TNP-BSA (Accurate Chemicals; 1 μg/ml). Plates were washed with 0.1% Triton X-100/0.15 M NaCl and then blocked by incubation for 1 h at room temperature with 2% BSA/PBS. Serum was then titered into the wells in serial dilutions starting from 1:200 and incubated for 1 h. The plates were washed as above and goat anti-mouse Ig (Southern Biotechnology Associates) was added at a 1:5,000 dilution in 2% BSA/PBS for 1 h. For subclass-specific ELISA, goat anti-mouse IgG1 or IgG2a (1:5,000 dilution, also from Southern Biotechnology Associates) was used. After washing again as above, plates were developed with an ABTS substrate (Sigma) and read on a microplate reader (Molecular Devices). Where standards were available (ovalbumin assay), results were plotted as anti-ovalbumin antibody in μg/ml; in other assays, relative concentrations compared with an arbitrary serum standard were plotted. Concentrations were determined by using the softmax program (Molecular Devices).
Determination of Antigen-Specific IL-4 and IFN-γ.
Mice were immunized and given booster injections with ovalbumin or KLH as above, and then spleens were harvested 4 days after the last immunization. Red blood cells were removed by hypotonic lysis in 0.144 M NH4Cl/0.017 M Tris⋅HCl, pH 7.2, for 10 min at room temperature. Recovered cells were washed with PBS and plated at 2.5 × 105 cells per well in flat-bottom microtiter plates (Costar) in RPMI 1640 medium supplemented with 10% fetal calf serum, glutamine (300 μg/ml), 0.1 mM MEM nonessential amino acids, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, and gentamycin at 100 μg/ml (all from GIBCO/BRL). Ovalbumin or KLH was added to the wells to achieve a final concentration of 500 μg/ml or 25 μg/ml, respectively, in a total volume of 200 μl. After 4 days at 37°C, 100 μl of supernatant was harvested and tested for IFN-γ and IL-4 by sandwich ELISA as described (25).
Production of Soluble CD81-Fc and Use in Flow Cytometry.
The two extracellular domains of murine CD81 were separately amplified by PCR and made to be joined with a (Gly)3-Cys-(Gly)3 linker. They were cloned into pCDM87B− (26) in-frame with the human IgG1 Fc region. CD81-Fc protein was produced by stable transfection of this plasmid into CHO cells and purified from the supernatant by using a protein A-agarose column. Lymphocytes were isolated from mouse spleen by density gradient centrifugation and stained for flow cytometry by using fluorescein isothiocyanate-labeled anti-T cell receptor or anti-B220 antibodies (PharMingen), and biotin-labeled CD81-Fc or an irrelevant human IgG1 antibody, followed by streptavidin-phycoerythrin.
RESULTS AND DISCUSSION
CD81-Null Mice Make Diminished Antibody During Th2 but not Th1 Responses.
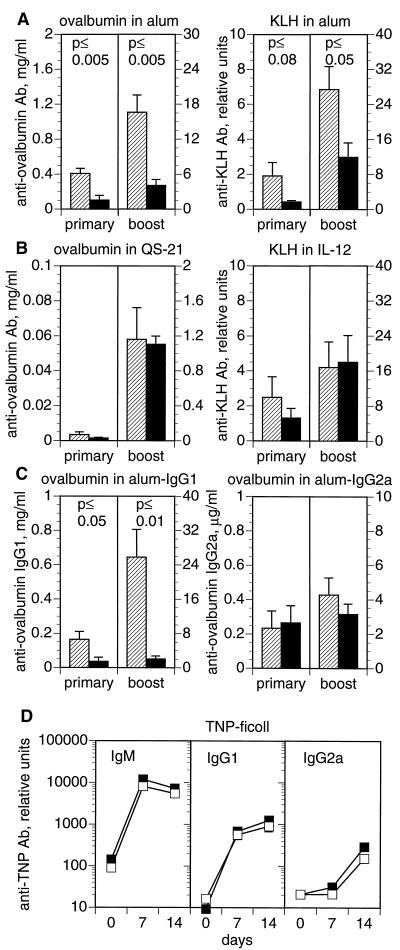
CD81-null mice make diminished Ig and/or IgG1 antibody responses to ovalbumin given in alum (20) or KLH given in CFA (20, 21). To analyze the generality of this defect, we immunized mice with ovalbumin or KLH in three adjuvants, alum (which biases toward a Th2 response), IL-12, or QS-21 (which bias toward a Th1 response). As seen in Fig. 1A, the total antigen-specific Ig level of CD81-null mice was significantly lower than their heterozygous littermates, when immunized with ovalbumin or KLH in alum. However, when ovalbumin or KLH was given with soluble QS-21 or IL-12, no significant difference in antigen-specific Ig was seen (Fig. 1B). The IgG1 and IgG2a subclass responses to these immunizations were also not significantly different between groups (data not shown). Thus, the antibody response of CD81-null mice is dependent upon the adjuvant used. Adjuvants favoring Th2 responses (e.g., alum) lead to reduced antibody production, whereas those favoring Th1 responses (e.g., QS-21 or IL-12) lead to normal antibody production.
Figure 1.
(A–C) Response of CD81-null mice to T dependent antigens varies with adjuvant. CD81-null mice (solid bars) and heterozygous littermates (hatched bars) were immunized i.p. with ovalbumin or KLH either precipitated in alum or given with QS-21 or IL-12 as adjuvants. Antigen-specific serum antibody responses were measured 12 weeks after a single immunization (primary) or 18 days after a second injection (boost). (A) Responses of CD81-null mice were significantly lower than heterozygotes to ovalbumin or KLH in alum, which induces a Th2 response. (B) Responses of CD81-null mice were not significantly different from heterozygotes when immunized with ovalbumin in QS-21 or KLH in IL-12, which stimulate a Th1 response. (C) The IgG1 response to ovalbumin in alum was significantly decreased in CD81-null mice. IgG2a responses to ovalbumin in alum were not significantly different between groups. (D) Response of CD81-null mice to a T independent antigen. CD81-null mice (▪) and heterozygous littermates (□) were immunized i.p. with 25 μg of TNP-Ficoll. The serum antibody response to TNP was measured by ELISA at 7 and 14 days. There were no significant differences between groups. Data are the means ± SEM (n = 3 to 9 animals per group).
The reduced immune response to ovalbumin in alum was further analyzed by quantitating the amount of ovalbumin-specific IgG1 and IgG2a (Fig. 1C). We found that the CD81-null mice made significantly less IgG1, but normal amounts of IgG2a, in response to ovalbumin in alum. Another group (21) has also found that CD81-null mice make less IgG1 in response to KLH given in CFA; the response to CFA is predominantly IgG1 with less IgG2a. Thus, CD81-null mice seem to be impaired in IgG1 antibody production and, therefore, make less overall antibody in response to Th2 stimuli.
Defective Antibody Production Does Not Occur with T Independent Antigens.
To test whether T cells are involved in the defective antibody responses of CD81-null mice, we immunized CD81−/− mice and their heterozygous littermates with a T independent antigen, Ficoll. A T independent type 2 antigen, Ficoll leads to a qualitatively Th2-like response, with much more IgG1 than IgG2a. At 7 and 14 days after immunization, CD81-null mice and their heterozygous littermates made similar amounts of IgM, IgG1, and IgG2a against TNP-Ficoll (Fig. 1D). Analogous results were obtained with lipopolysaccharide, a T independent type 1 antigen (data not shown). Thus, the defect in antibody production in CD81-null mice is dependent upon the involvement of T cells and is not an impairment in the ability of B cells to produce Ig or IgG1.
CD81 on B Cells Is Necessary for Normal Antibody Responses to Th2 Stimuli.
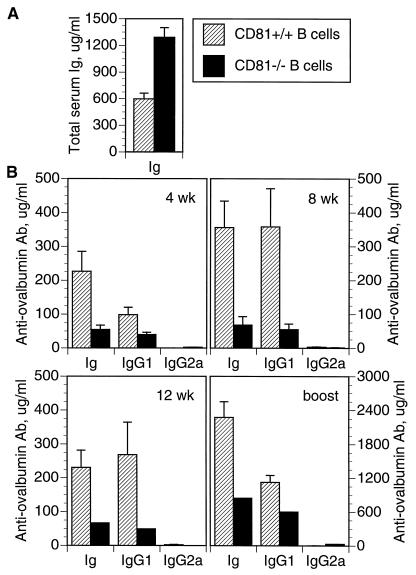
CD81 is expressed on both B and T cells (17, 20) but is highly expressed on germinal center B cells (ref. 18 and unpublished data). To determine the role of B cell CD81 in generating antibody responses to T dependent Th2 antigens, we constructed chimeric mice in which only the B cells lacked CD81. This was done by injecting CD81−/− ES cells into blastocysts from B cell-deficient (JH deletion) mice (23). The only B cells in such mice are derived from CD81−/− ES cells. Before immunization, such chimeras had significantly higher levels of serum Ig compared with control chimeras that were made with CD81+/+ ES cells (Fig. 2A). Despite the capacity to generate higher levels of serum Ig, the chimeras whose B cells lacked CD81 nevertheless made lower antibody responses to ovalbumin in alum (Fig. 2B) at all time points tested. IgG1 responses were also lower than the control chimeras, but IgG2a levels were not significantly different. Thus, a lack of CD81 on B cells is sufficient to cause reduced antibody production to a Th2 stimulus, despite the ability of CD81− B cells to produce higher than normal levels of Ig.
Figure 2.
Chimeric mice were constructed by injection of CD81−/− ES cells (solid bars) or CD81+/+ ES cells (hatched bars) into blastocysts from B cell-deficient mice. (A) Total serum Ig of chimeric mice at 12 weeks of age. (B) Response of chimeric mice to ovalbumin in alum. Chimeric mice were immunized with ovalbumin in alum, and serum anti-ovalbumin antibody was measured at 4, 8, and 12 weeks after a single injection. Mice were then given a booster injection and secondary responses were measured 18 days later. Error bars indicate the SEM (n = 2 to 10 animals per group).
CD81-Null Mice Are Defective in Antigen-Specific IL-4 Production.
The above results implicate CD81 on B cells as important for interaction with T cells to produce optimal antibody responses to Th2 stimuli. Th2 responses are highly dependent on IL-4 production by T cells, which directs the production of IgG1 antibody from B cells. We thus wondered whether CD81-null mice might be impaired in IL-4 production and whether this could be the basis for their decreased Ig and IgG1 responses to Th2 stimuli.
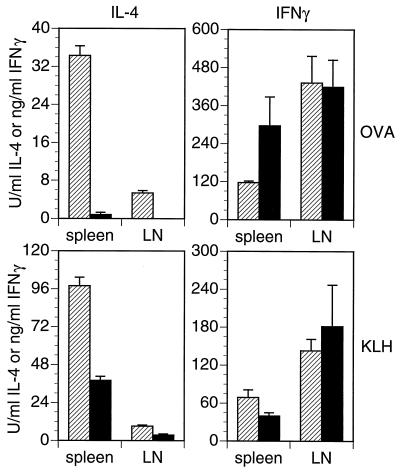
CD81-null and heterozygous mice were immunized with ovalbumin and KLH in alum, and antigen-specific cytokine production from their spleen and lymph node cells was measured. Both spleen and lymph node cells from CD81-null mice made significantly less IL-4 in response to either KLH or ovalbumin than did cells from their heterozygous littermates (Fig. 3). IFN-γ production, however, was not significantly different between groups. Similar results were obtained from these same animals with an ELISPOT assay (data not shown), indicating that the CD81-null mice had fewer IL-4-secreting cells than did heterozygotes.
Figure 3.
Antigen-specific IL-4 and IFN-γ responses in CD81-null mice (solid bars) and heterozygous littermates (hatched bars). Mice were immunized three times with the specified antigen in alum. Spleens and peripheral lymph nodes were removed 4 days after the last injection. Mononuclear cells were restimulated with ovalbumin or KLH for 3 days, then supernatants were harvested, and cytokines measured by ELISA (25). Bars indicate the SD of triplicate cultures.
Thus, our data suggest that CD81 on B cells is required to induce optimal IL-4 secretion by T cells during Th2 responses and also to produce optimal antibody responses to Th2 stimuli. In the human system, Secrist et al. (19) have tested the effect of adding anti-CD81 antibody to cultures of lymphocytes from allergic individuals, which were stimulated with their cognate allergen. In these experiments, stimulation of CD81 with an antibody caused cell adhesion and greatly increased IL-4 production but no change in IFN-γ production from the T cells. Furthermore, this increased IL-4 production was only seen when B cells were used as the antigen-presenting cells and not when monocytes were mixed with T cells in similar cultures. Our present work helps explain this finding by demonstrating the role of CD81 in IL-4 production and IgG1 antibody production in vivo.
Models for CD81 Function and Binding to a Ligand.
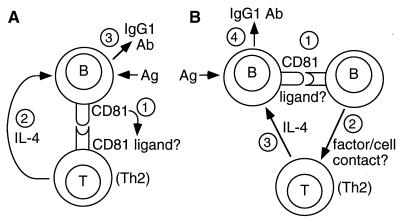
At least two models for this interaction can be put forward (Fig. 4). In the simplest model (Fig. 4A), CD81 on B cells interacts directly with a putative ligand on T cells to induce IL-4 production. Indeed, we have made a soluble CD81-Fc fusion protein and found that it binds to a large proportion (≈70%) of B cells and a smaller proportion (≈10%) of T cells in mouse spleen (data not shown). The T cells that express the putative CD81 ligand could be those that are already committed to Th2 development and IL-4 secretion; alternately, binding of the CD81 ligand on these cells could induce differentiation to the Th2 lineage. In either case, lack of CD81 on B cells would be sufficient to impair IL-4 production from these T cells, leading to reduced IgG1 antibody production and impaired Th2 immune responses.
Figure 4.
Schematic models for the role of CD81 in Th2 responses. In the simplest model (A), CD81 on B cells interacts directly with a putative ligand on T cells, stimulating IL-4 production, which leads to IgG1 antibody production. The interacting T cells expressing CD81 ligand may be those already committed to Th2 development, or they may be recruited into this pathway upon interaction with B cells expressing CD81. In a slightly different model (B), B cells interact first with a subset of other B cells expressing the putative CD81 ligand. These B cells then interact with T cells via cell contact and/or soluble factors to induce IL-4 secretion and IgG1 antibody production.
In the second model, which is not mutually exclusive, CD81 on B cells could interact with its putative ligand on other B cells (Fig. 4B). This is possible because of the observation noted above that soluble CD81-Fc binds to a large subpopulation of mouse B cells. These B cells, on CD81–ligand interaction, could then stimulate T cell IL-4 production by cell contact and/or soluble factor(s). Again, a lack of CD81 on B cells would be sufficient to impair IL-4 production and reduce IgG1 antibody production.
Our findings may help explain the findings of other investigators with regard to the role of B cells in IL-4 production. For example, other investigators have found that mouse or human B cells preferentially induce IL-4 secretion from T cells in various systems (25, 27–30) and that Th2 clones proliferate optimally to antigen presented by B cells (31). Schmitz et al. (32) found that using B cells, but not macrophages, as antigen-presenting cells gave rise to T cells that could differentiate along a Th2 pathway. Also, Taylor-Robinson et al. (33, 34) found that B cells were required to switch responses to Plasmodium chabaudi chabaudi from Th1 to Th2. All of these results could be explained at least in part by the capacity of CD81 on B cells to promote IL-4 secretion from T cells.
Costimulatory proteins such as B7–1 and B7–2 have been shown in some systems to have differential effects on cytokine secretion by T cells (35–38). Our work indicates that nonclassical costimulatory molecules, specifically CD81 on B cells, can control cytokine production by T cells. The involvement of CD81 in directing Th2 responses has important implications for vaccination and the control of Th2 immunity in conditions such as allergic disease and autoimmunity.
Acknowledgments
We thank Irving Weissman for providing support for, and Julie Christianson for performing, blastocyst injection of JH deletion mice; Debra Czerwinski for flow cytometry analysis; Rosemarie DeKruyff for providing reagents for analysis of cytokines; Anne Macaulay for breeding JH deletion mice; and Ronald Levy and Dale Umetsu for support and critical review of the data. This work was supported by Public Health Service Grant CA 34233 from the National Institutes of Health (NIH). H.T.M. was supported by a National Research Service Award from the NIH.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Th1 and Th2 cells, T helper cells 1 and 2, respectively; KLH, keyhole limpet hemocyanin; IL, interleukin; INF-γ, interferon γ; CFA, complete Freund’s adjuvant; ES cell; embryonic stem cell; JH, heavy chain J region; TNP, trinitrophenol.
References
- 1.Nakamura T, Kamogawa Y, Bottomly K, Flavell R A. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- 2.Hosken N A, Shibuya K, Heath A W, Murphy K M, O’Garra A. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabo S J, Jacobson N G, Dighe A S, Gubler U, Murphy K M. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh C S, Macatonia S E, O’Garra A, Murphy K M. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorham J D, Guler M L, Steen R G, Mackey A J, Daly M J, Frederick K, Dietrich W F, Murphy K M. Proc Natl Acad Sci USA. 1996;93:12467–12472. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan D D, Swain S L. Eur J Immunol. 1994;24:2506–2514. doi: 10.1002/eji.1830241037. [DOI] [PubMed] [Google Scholar]
- 7.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O’Garra A. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 8.Schmitt E, Hoehn P, Germann T, Rüde E. Eur J Immunol. 1994;24:343–347. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rüde E, Germann T. Eur J Immunol. 1994;24:793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 10.Germann T, Gately M K, Schoenhaut D S, Lohoff M, Mattner F, Fischer S, Jin S C, Schmitt E, Rüde E. Eur J Immunol. 1993;23:1762–1770. doi: 10.1002/eji.1830230805. [DOI] [PubMed] [Google Scholar]
- 11.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 12.Micallef M J, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 13.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Nature (London) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 14.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maecker H T, Todd S C, Levy S. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 16.Levy S, Todd S C, Maecker H T. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- 17.Oren R, Takahashi S, Doss C, Levy R, Levy S. Mol Cell Biol. 1990;10:4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engel, P. & Tedder, T. F. (1994) Leuk. Lymphoma 13 Suppl. 1, 61–64. [DOI] [PubMed]
- 19.Secrist H, Levy S, DeKruyff R H, Umetsu D T. Eur J Immunol. 1996;26:1435–1442. doi: 10.1002/eji.1830260706. [DOI] [PubMed] [Google Scholar]
- 20.Maecker H T, Levy S. J Exp Med. 1997;185:1505–1510. doi: 10.1084/jem.185.8.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki T, Muller U, Campbell K S. EMBO J. 1997;16:4217–4225. doi: 10.1093/emboj/16.14.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsitsikov E N, Gutierrez-Ramos J C, Geha R S. Proc Natl Acad Sci USA. 1997;94:10844–10849. doi: 10.1073/pnas.94.20.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Trounstine M, Alt F W, Young F, Kurahara C, Loring J F, Huszar D. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 24.Mishell B B, Shiigi S M. Selected Methods in Cellular Immunology. New York: Freeman; 1980. [Google Scholar]
- 25.Macaulay A E, DeKruyff R H, Goodnow C C, Umetsu D T. J Immunol. 1997;158:4171–4179. [PubMed] [Google Scholar]
- 26.Hollenbaugh D, Grosmaire L S, Kullas C D, Chalupny N J, Braesch-Andersen S, Noelle R J, Stamenkovic I, Ledbetter J A, Aruffo A. EMBO J. 1992;11:4313–4321. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelman F D, Snapper C M, Mountz J D, Katona I M. J Immunol. 1987;138:2826–2830. [PubMed] [Google Scholar]
- 28.Morris S C, Lees A, Finkelman F D. J Immunol. 1994;152:3777–3785. [PubMed] [Google Scholar]
- 29.Secrist H, DeKruyff R H, Umetsu D T. J Exp Med. 1995;181:1081–1089. doi: 10.1084/jem.181.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeKruyff R H, Fang Y, Umetsu D T. J Immunol. 1992;149:3468–3476. [PubMed] [Google Scholar]
- 31.Gajewski T F, Pinnas M, Wong T, Fitch F W. J Immunol. 1991;146:1750–1758. [PubMed] [Google Scholar]
- 32.Schmitz J, Assenmacher M, Radbruch A. Eur J Immunol. 1993;23:191–199. doi: 10.1002/eji.1830230130. [DOI] [PubMed] [Google Scholar]
- 33.Taylor-Robinson A W, Phillips R S. Infect Immun. 1994;62:2490–2498. doi: 10.1128/iai.62.6.2490-2498.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor-Robinson A W, Phillips R S. Infect Immun. 1996;64:366–370. doi: 10.1128/iai.64.1.366-370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Natesan M, Razi-Wolf Z, Reiser H. J Immunol. 1996;156:2783–2791. [PubMed] [Google Scholar]
- 36.Freeman G J, Boussiotis V A, Anumanthan A, Bernstein G M, Ke X Y, Rennert P D, Gray G S, Gribben J G, Nadler L M. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 37.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 38.Ranger A M, Das M P, Kuchroo V K, Glimcher L H. Int Immunol. 1996;8:1549–1560. doi: 10.1093/intimm/8.10.1549. [DOI] [PubMed] [Google Scholar]