Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells (original) (raw)
Abstract
We have recently identified α-galactosylceramide (α-GalCer) as a specific ligand for an invariant Vα14/Vβ8.2 T cell receptor exclusively expressed on the majority of Vα14 NKT cells, a novel subset of lymphocytes. Here, we report that α-GalCer selectively activates Vα14 NKT cells resulting in prevention of tumor metastasis. The effector mechanisms of the ligand-activated Vα14 NKT cells seem to be mediated by natural killer (NK)-like nonspecific cytotoxicity. Indeed, the cytotoxic index obtained by α-GalCer-activated Vα14 NKT cells was reduced by the addition of cold target tumor cells or by treatment with concanamycin A, which inhibits activation and secretion of perforin, but not by mAbs against molecules involved in the NKT cell recognition and conventional cytotoxicity, such as CD1d, Vβ8, NK1.1, Ly49C, Fas, or Fas ligand. These results suggest that the ligand-activated Vα14 NKT cells kill tumor cells directly through a CD1d/Vα14 T cell receptor-independent, NK-like mechanism.
A novel lymphoid lineage, Vα14 natural killer (NK) T cells, distinct from other lymphoid cells including T cells, B cells, and NK cells, is characterized by the early development at day 9.5 of gestation before thymus formation (1) and also by coexpression of the NK receptor and a single, invariant T cell receptor (TCR) encoded by Vα14 and Jα281 gene segments (2–4) in association with a highly skewed set of Vβs, mainly Vβ8.2 (5–13). Moreover, the generation of Vα14 NKT cells is exclusively dependent on the expression of the invariant Vα14 TCR as evidenced by the fact that Vα14 NKT cells do not develop in the invariant Vα14 TCR-deficient mice (14) and that the forced expression of the invariant Vα14 TCR leads exclusively to Vα14 NKT cell generation and blocks conventional T cell development (15, 16).
Although physiological functions of Vα14 NKT cells remain to be elucidated, the extensive analysis has shown that Vα14 NKT cells are able to mediate allograft bone marrow rejection (17), control autoimmune disease development (18, 19), and produce large amounts of both interleukin 4 (IL-4) and interferon γ (16, 20, 21). These findings suggest that Vα14- NKT cells play complicated roles in regulating immune responses more than simply as IL-4 or interferon γ providers for Th2 or Th1 cell development, respectively, so far reported (22, 23). In addition, we have demonstrated recently that Vα14 NKT cells are a primary target of IL-12 and exert a major effector function in IL-12-mediated tumor rejection (14).
A ligand for the invariant Vα14/Vβ8.2 TCR exclusively expressed on Vα14 NKT cells has been identified recently to be α-galactosylceramide (α-GalCer) (16). In addition, α-GalCer is presented by a monomorphic non-major-histocompatibility gene complex (non-MHC) class Ib molecule, CD1d, expressed on dendritic cells (DC), and the ligand/CD1 complex selectively stimulates to proliferate Vα14 NKT cells but not other lymphocytes only if costimulatory signals generated by CD40/CD40 ligand and B7/CD28 interactions are provided (16). The results suggest that activation mechanisms of Vα14 NKT cells are similar to those of conventional T cells. However the effector mechanisms of Vα14 NKT cells after activation with α-GalCer have not yet been determined. Here, we demonstrate that one of the effector functions of α-GalCer-activated Vα14 NKT cells is a target tumor cell lysis mediated by a TCR-independent, NK-like nonspecific mechanism with perforin.
MATERIALS AND METHODS
Mice.
Vα14 NKT-deficient (Jα281−/−) mice were established by specific deletion of the Jα281 gene segment with homologous recombination and aggregation chimera techniques (14). In these mice, only Vα14 NKT cells are not generated, whereas other lymphoid populations, T cells, B cells, and NK cells remain intact. The Vα14 NKT-deficient (Jα281−/−) mice were backcrossed three times with C57BL/6 mice (14). Vα14 NKT (RAG−/−/Vα14tg Vβ8.2tg; tg, transgenic) mice with a C57BL/6 background were established by mating RAG−/−/Vβ8.2tg mice and RAG−/−/Vα14tg mice as described (16). In the Vα14 NKT mice, only transgenic TCRαβ (Vα14tg and Vβ8.2tg) are expressed and result in preferential development of Vα14 NKT cells with no detectable number of T cells or NK cells (16). Pathogen-free C57BL/6 mice were purchased from Japan SLC, Inc. (Shizuoka, Japan). All mice used in this study were maintained in specific pathogen-free conditions.
Evaluation of the Severity of Hepatic Metastasis.
B16 melanoma cells (H-2b, 3 × 106 per mouse) were injected into the spleen of young adult wild-type, Vα14 NKT-deficient, and Vα14 NKT mice. Then, α-GalCer (100 μg/kg) or control vehicle was injected intraperitoneally on days 1, 5, and 9. The treated mice were sacrificed on day 14, and their livers were photographed and subjected to quantitation of GM3 melanoma antigens (24–26).
Quantitation of GM3 Melanoma Antigen in Metastatic Liver.
For measurement of a melanoma antigen (GM3 ganglioside), each liver was homogenized as described (24, 25). The homogenates of normal or metastatic livers (50 mg) or B16 melanoma cells were treated with 1 ml of extraction buffer containing 1% Nonidet P-40. Various concentrations of melanoma cell homogenates were used to make standard curves. GM3 melanoma antigens in the extracts were quantitated by RIA with 125I-labeled M2590 anti-melanoma GM3 mAb as described (24, 25).
51Cr Release Cytotoxicity Assay.
Target cells were labeled with 100 μCi sodium chromate (Amersham) per 5 × 106 cells for 1 hr. α-GalCer-activated spleen cells from Vα14 NKT mice were used as effector cells and seeded into 96-well round-bottomed plates at the indicated effector/target (E/T) ratios on 51Cr-labeled B16 melanoma cells (1 × 104). Radioactivity released from lysed target cells was counted on a γ-counter after 4 hr of incubation at 37°C in 5% CO2. The percentage of specific 51Cr release was calculated by the following formula: % of specific lysis = (sample cpm − spontaneous cpm) × 100/(maximum cpm − spontaneous cpm). Spontaneous cpm was calculated from the supernatant of the target cells alone, and the maximum release was obtained by adding 1 M HCl to target cells. The data are expressed as a mean value of triplicate cultures with standard deviations.
For cold target inhibition experiments, a mixture of αGalCer-activated Vα14 NKT cells and 51Cr-labeled B16 melanoma target cells (at an E/T ratio of 25:1) was incubated with unlabeled tumor cells (B16 or EL-4) at the indicated target/inhibitor ratios. For antibody-blocking experiments, assays were performed at an E/T ratio of 50:1 in the presence of 50 μg/ml of mAbs specific for H-2Kb (AF6–88.5), H-2Db (KH-95), CD1d (1B1), Vβ8 (MR5–2), NK1.1 (PK136), Ly49C (5E6), Fas (Jo2) (PharMingen), and Fas ligand (MFLIII was a kind gifted from H. Yagita, Juntendo University, Tokyo).
Treatment of Vα14 NKT Cells with Concanamycin A (CMA).
Vα14 NKT cells were preincubated with CMA (Wako Pure Chemical, Osaka) or control vehicle (dimethyl sulfoxide) at indicated concentrations as described (14, 27, 28).
The assay was performed at an E/T ratio of 50:1. Rausher virus-induced T cell lymphomas (RMA-S) and their CD1d-transfectants (RMA-S-CD1.1) were generated by L. Brossay, La Jolla Institute of Allergy and Immunology (La Jolla, CA).
RESULTS AND DISCUSSION
Antitumor Activity of Vα14 NKT Cells Activated by αGalCer.
The goal of this study was to clarify the effector mechanisms of the cytotoxic function of ligand-activated Vα14 NKT cells. Recently, we have identified α-GalCer as a ligand for the invariant Vα14 TCR exclusively expressed on Vα14 NKT cells (16). To investigate effector function of α-GalCer-activated Vα14 NKT cells, we used three different types of mice: (i) wild-type mice bearing normal numbers of T cells, B cells, NK cells, and Vα14 NKT cells, (ii) Vα14 NKT-deficient mice that lack only Vα14 NKT cells while other lymphoid populations (T cells, B cells, and NK cells) remain intact (14), and (iii) Vα14 NKT mice in which only Vα14 NKT cells but not T cells, B cells, or NK cells are present (16).
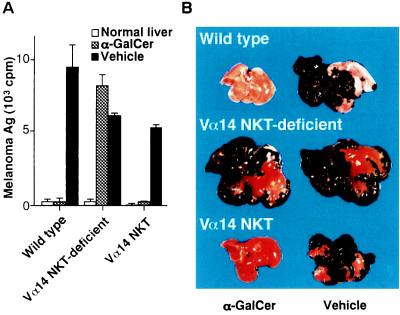
We injected α-GalCer intraperitoneally after intrasplenic inoculation of B16 melanoma cells. The severity of melanoma metastasis was evaluated by measuring amounts of GM3 melanoma antigen in the liver. As shown in Fig. 1A, a negligible level of GM3 melanoma antigen was detected when α-GalCer but not vehicle was administrated in wild-type mice, indicating that α-GalCer treatment prevented liver metastasis of B16 melanoma almost completely. In contrast, the prevention of metastasis was not observed in Vα14 NKT-deficient mice lacking only Vα14 NKT cells. Because T cells and NK cells other than Vα14 NKT cells are generated normally in Vα14 NKT-deficient mice (14), the antitumor effect by α-GalCer treatment appeared to be a consequence of the defect of Vα14 NKT cells. In Vα14 NKT mice bearing only Vα14 NKT cells without other lymphoid cells, as anticipated, the in vivo administration of α-GalCer suppressed the metastasis of B16 melanoma cells as effectively as observed in wild-type mice. Because the number of Vα14 NKT cells in the spleen of Vα14 NKT mice (7–9 × 105) is similar to that in the wild-type mice (6–9 × 105), the efficacy of tumor-killing activity appears to be equivalent. Fig. 1B shows representative photographic views of the metastasized livers. The metastatic melanoma nodules were visible as large black spots, which are not observed in the liver of either wild-type or Vα14 NKT mice if treated with α-GalCer. These results strongly suggest that the Vα14 NKT cells were selectively activated by α-GalCer and revealed an inhibitory effect on tumor metastasis in the liver. Similar in vivo effects of α-GalCer-mediated protection of tumor metastasis were observed in other tumor systems, such as Lewis lung carcinoma, erythroleukemia (FBL-3), Colon26, or RMA-S tested so far (data not shown).
Figure 1.
Inhibition of liver metastasis of B16 melanoma cells by in vivo treatment with α-GalCer. (A) Melanoma antigens in metastasized livers measured by RIA using 125I-labeled M2590 mAb. B16 melanoma cells (3 × 106 per mouse) were inoculated in the spleen of wild-type (Jα281+/+), Vα14 NKT-deficient (Jα281−/−), and Vα14 NKT (RAG−/−/Vα14tg Vβ8.2tg) mice, followed by intraperitoneal injection with either α-GalCer (100 μg/kg) or vehicle on days 1, 5, and 9. The mice were sacrificed and examined on day 14. Each group consisted of three mice. The data are expressed as a mean cpm value of triplicate samples with standard errors. (B) Photographic views of metastasized livers from mice treated with α-GalCer or vehicle.
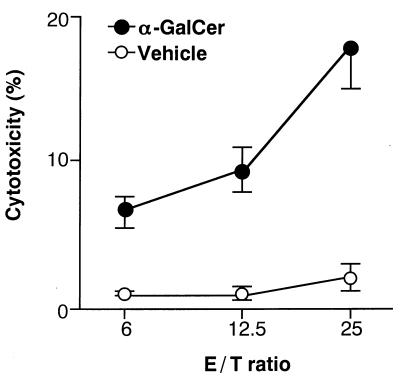
As shown in Fig. 2, cytotoxic activity against B16 melanoma was detected in vitro when Vα14 NKT mice were injected with α-GalCer but not with vehicle, indicating that α-GalCer-activation is required for mediating effector function of Vα14 NKT cells. These findings confirm the in vivo data on the rejection of melanoma after administration of α-GalCer.
Figure 2.
Cytotoxicity of spleen cells from Vα14 NKT mice after in vivo treatment with α-GalCer. Vα14 NKT mice were injected intraperitoneally with either α-GalCer (100 μg/kg) or vehicle, and 24 hr later spleen cells were used as effector cells for cytotoxic assay on B16 melanoma cells at indicated effector/target ratios.
NK-Like Effector Function of α-GalCer-Activated Vα14 NKT Cells.
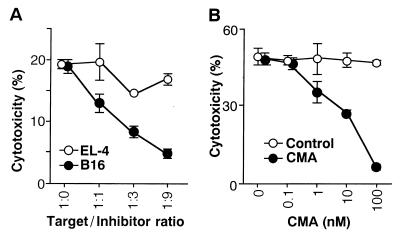
Next, we performed a series of experiments aimed at elucidating the molecular mechanism involved in the effector cytotoxic function of the α-GalCer-activated Vα14 NKT cells. Fig. 3A demonstrates a representative result of a cold target inhibition assay on B16 melanoma cell killing by α-GalCer-activated Vα14 NKT cells. 51Cr release was reduced significantly by the addition of nonlabeled B16 melanoma cells in a dose-dependent manner, but not by EL-4, which is not susceptible to Vα14 NKT cell-mediated target cell lysis, indicating the requirement of direct cell-to-cell contact. In addition, the treatment of Vα14 NKT cells with CMA inhibited antitumor cytotoxic activity of the α-GalCer-induced Vα14 NKT cells in a dose-dependent fashion (Fig. 3B). Because CMA is a specific inhibitor of vacuolar-type H+-ATPase and inhibits the perforin-mediated killing but not Fas-dependent cytotoxic pathway (27, 28), the effector function of Vα14 NKT cells appeared to be mediated by NK-like mechanisms.
Figure 3.
Effector mechanism of α-GalCer-activated Vα14 NKT cells. (A) α-GalCer-activated Vα14 NKT cells were incubated with 51Cr-labeled B16 melanoma cells in the presence of various numbers of unlabeled tumor cells (B16 or EL-4) as cold targets. Cytotoxic assays were performed at an E/T ratio of 25:1. (B) Effect of CMA treatment on α-GalCer-activated Vα14 NKT cells. α-GalCer-activated Vα14 NKT cells were treated with indicated doses of CMA for 2 hr, and their cytotoxic activity was assessed on 51Cr-labeled B16 melanoma at an E/T ratio of 50:1.
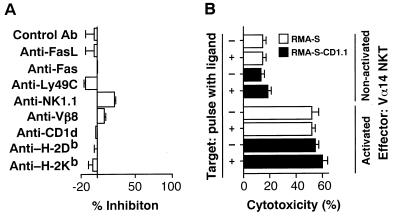
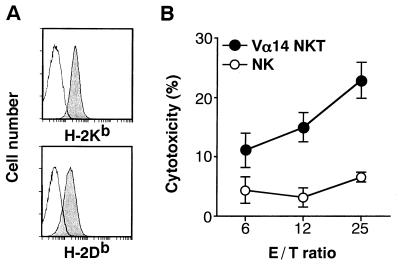
We tried to identify the cell surface molecule essential for direct cell contact involved in the cytotoxic interactions by using various mAbs with a specific blocking activity (Fig. 4A). Interestingly, however, no significant inhibition was observed by the addition of mAbs specific for Fas ligand, Fas, Ly49C, NK1.1, TCRVβ8, CD1d, H-2Db or H-2Kb. The data shown in Fig. 4B in part confirm the results in Fig. 4A on the nonessential requirement of CD1d/invariant Vα14 TCR interactions in the effector phase. The α-GalCer-activated Vα14 NKT cells killed both RMA-S and RMA-S-CD1.1 cells, regardless of their CD1d expression or their antigen pulse with α-GalCer, confirming that the invariant Vα14/Vβ8.2 TCR is not used in the cytotoxic interaction between Vα14 NKT cells and tumor target cells. The system appeared not to involve either Ly49C/NK1.1 molecules essential for conventional NK cell-mediated killing or TCR/MHC class I molecules necessary for CTL-mediated killing mechanisms. In particular, nonactivated Vα14 NKT but not NK cells killed FBL-3 tumor cells expressing MHC class I molecules on the target cell surface (Fig. 5). It is well documented that NK cells killed only MHC class I negative target cells because of their negative signal through interaction of Ly49C molecule on NK cells with MHC class I molecule on target cells (29). Therefore, Vα14 NKT cells killed the target in the different mechanisms from those of NK-mediated cytolysis. These results indeed are in agreement with the effector cytotoxic mechanisms of IL-12-activated Vα14 NKT cells against tumor cells (14), suggesting that the α-GalCer-activated Vα14 NKT cells use a novel effector system mediating antitumor cytotoxicity. Although we could not block cytotoxic activity by the addition of antibodies against interferon γ (data not shown) or Fas ligand (see Fig. 4A), it is still possible that some cytokines are involved in the effector killing mechanisms.
Figure 4.
Molecular requirements in the effector phase of cytotoxic function of α-GalCer-activated Vα14 NKT cells. (A) Effects of various mAbs on Vα14 NKT cell-mediated cytotoxicity. Fifty micrograms per ml of the indicated mAbs were added to the cytotoxic assays. (B) Cytotoxic activity of Vα14 NKT cells on α-GalCer-pulsed class I-deficient RMA-S cells and their CD1d-transfectants. RMA-S and RMA-S-CD1.1 cells were pulsed with α-GalCer and used as target cells. The data are expressed as a mean value of triplicate cultures with standard deviations.
Figure 5.
Cytotoxicity of NK and Vα14 NKT cells against FBL-3 erythroleukemia cells. (A) Fluorescence-activated cell sorter profiles of FBL-3. Cells were stained with anti-H-2Kb (AF6–88.5) or H-2Db (KH95) antibodies. Isotype-matched antibody was used as a negative control. (B) Cytotoxic activity. Freshly isolated spleen cells from Vα14 NKT mice (•) or RAG−/− (NK) mice (○) were assayed for their cytotoxicity by the 4-hr 51Cr-release assay on FBL-3 tumor cells expressing MHC class I molecules on the cell surface.
In the present study, we demonstrated a unique feature of molecular requirements in activation and effector function of Vα14 NKT cells. Similar to the activation of conventional T cells, the specific ligand recognition by TCR is required for the activation of Vα14 NKT cells. The invariant Vα14 TCR recognizes α-GalCer presented by a CD1d molecule and mediates activation signals to Vα14 NKT cells together with costimulatory signals generated by B7/CD28 and CD40/CD40 ligand interactions between dendritic cells and Vα14 NKT cells. Interestingly, however, the activated Vα14 NKT cells kill tumor targets by NK-like mechanisms in a nonspecific TCR-independent fashion. Because α-GalCer so far has not been detected on tumor target cells, it is necessary to provide a ligand stimulation through dendritic cells to activate Vα14 NKT cells. Once they are activated, they express nonspecific cytotoxic functions against a variety of tumors. In light of the successful control of Vα14 NKT cell activation in vivo, α-GalCer is an ideal drug to manipulate tumor eradication.
Acknowledgments
We thank H. Tanabe for the preparation of this manuscript.
ABBREVIATIONS
α-GalCer
α-galactosylceramide
TCR
T cell receptor
CMA
concanamycin A
tg
transgenic
NK
natural killer
MHC
major histocompatibility complex
E/T
effector/target
IL
interleukin
References
- 1.Makino Y, Kanno R, Koseki H, Taniguchi M. Proc Natl Acad Sci USA. 1996;93:6516–6520. doi: 10.1073/pnas.93.13.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai K, Kanno M, Kimoto H, Shigemoto K, Yamamoto S, Taniguchi M. Proc Natl Acad Sci USA. 1986;83:8708–8712. doi: 10.1073/pnas.83.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lantz O, Bendelac A. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Int Immunol. 1995;7:1157–1161. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- 5.Fowlkes B J, Kruisbeek A M, Ton-That H, Weston M A, Coligan J E, Schwartz R H, Pardoll D M. Nature (London) 1987;329:251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- 6.Budd R C, Miescher G C, Howe R C, Lees R K, Bron C, MacDonald H R. J Exp Med. 1987;166:577–582. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballas Z K, Rasmussen W. J Immunol. 1990;145:1039–1045. [PubMed] [Google Scholar]
- 8.Sykes M. J Immunol. 1990;145:3209–3215. [PubMed] [Google Scholar]
- 9.Levitsky H I, Golumbek P T, Pardoll D M. J Immunol. 1991;146:1113–1117. [PubMed] [Google Scholar]
- 10.Takahama Y, Kosugi A, Singer A. J Immunol. 1991;146:1134–1141. [PubMed] [Google Scholar]
- 11.Bix M, Coles M, Raulet D. J Exp Med. 1993;178:901–908. doi: 10.1084/jem.178.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendelac A, Killeen N, Littman D R, Schwartz R H. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 13.Masuda K, Makino Y, Cui J, Ito T, Tokuhisa T, Takahama Y, Koseki H, Tsuchida K, Koike T, Moriya H, et al. J Immunol. 1997;158:2076–2082. [PubMed] [Google Scholar]
- 14.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi M, Koseki H, Tokuhisa T, Masuda K, Sato H, Kondo E, Kawano T, Cui J, Perkes A, Koyasu S, Makino Y. Proc Natl Acad Sci USA. 1996;93:11025–11028. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Dennert G. J Exp Med. 1993;177:155–164. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mieza M A, Itoh T, Cui J Q, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, et al. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- 19.Sumida T, Sakamoto A, Murata H, Makino Y, Takahashi H, Yoshida S, Nishioka K, Iwamoto I, Taniguchi M. J Exp Med. 1995;182:1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arase H, Arase N, Saito T. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimoto T, Paul W E. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul W E. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimoto T, Bendelac A, Hu-Li J, Paul W. Proc Natl Acad Sci USA. 1995;92:11931–11934. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakabayashi S, Okamoto S, Taniguchi M. Gann. 1984;75:427–432. [PubMed] [Google Scholar]
- 25.Gunji Y, Taniguchi M. Jpn J Cancer Res. 1986;77:595–601. [PubMed] [Google Scholar]
- 26.Nores G A, Dohi T, Taniguchi M, Hakomori S. J Immunol. 1987;139:3171–3176. [PubMed] [Google Scholar]
- 27.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 28.Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K, Imawari M. J Immunol. 1997;158:5283–5291. [PubMed] [Google Scholar]
- 29.Yu Y Y, George T, Dorfman J R, Roland J, Kumar V, Bennett M. Immunity. 1996;4:67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]