Secretory Phospholipase A2 Is the Principal Bactericide for Staphylococci and Other Gram-Positive Bacteria in Human Tears (original) (raw)
Abstract
We examined human tears for molecules that killed gram-positive bacteria. The principal mediator of bactericidal activity against staphylococci proved to be a calcium-dependent enzyme, secretory phospholipase A2. Whereas the concentration of secretory phospholipase A2 in the normal tear film exceeded 30 μg/ml, only 1.1 ng (<0.1 nM) of the enzyme per ml sufficed to kill Listeria monocytogenes and 15 to 80 ng/ml killed Staphylococcus aureus. Despite its efficacy against gram-positive bacteria, secretory phospholipase A2 lacked bactericidal activity against gram-negative organisms (Escherichia coli, Salmonella typhimurium, and Pseudomonas aeruginosa) when tested in the ionic environment of tears. Given the presence of secretory phospholipase A2 in tears, intestinal secretions, and leukocytes, this enzyme may play a substantial role in innate mucosal and systemic bactericidal defenses against gram-positive bacteria.
Because the normal cornea lacks blood vessels, many of its host defense needs are supplied by the tear film. Alexander Fleming reported the presence of lysozyme in human tears over 75 years ago and described its ability to lyse and kill Micrococcus leisodeikticus (7, 8). Later investigators reported that human tears contained a nonlysozyme antistaphylococcal factor that also killed M. leisodeikticus (10, 43) Ford, et al. have advanced (9) and others have challenged (17, 38) the possibility that the additional bactericidal factor is “beta-lysin”—an ill-defined, heat-stable antimicrobial molecule reportedly present in platelets and plasma (4). In the absence of more precise knowledge, contemporary ophthalmology texts (23) attribute the antimicrobial properties of tears to their high concentrations of lysozyme, lactoferrin, and immunoglobulin A (IgA) (20). The experiments described below demonstrate that this conventional belief requires modification and that secretory phospholipase A2 (sPLA2) is an important host defense molecule in the external eye.
MATERIALS AND METHODS
Tear collection.
Tears were collected from six healthy donors, under a protocol approved by the UCLA Institutional Review Board. After approximately 5 μl of basal, unstimulated tears had been collected over 2 min into a calibrated 5-μl pipette (Accupette; Baxter, McGaw Park, Ill.), the donors were briefly exposed to the vapors of freshly minced onions, and between 100 to 250 μl of stimulated tears was collected over the next 5 to 10 min. These tear specimens were stored at −20°C until tested.
Bacteria.
Six strains of Staphylococcus aureus were used. S. aureus GM-1 and 67395 were ocular isolates obtained from the UCLA Clinical Laboratory. The GM-1 strain was gentamicin resistant. Micrococcus luteus ATCC 4698 (previously called Micrococcus leisodeikticus) and a methicillin-resistant S. aureus (MRSA) strain, ATCC 33591, were purchased from the American Type Culture Collection (Rockville, Md.). Six strains (three MRSA, one S. epidermidis, one group B streptococcus, and one vancomycin-resistant Enterococcus faecium strain [VREF 94.132]) were clinical isolates obtained from the UCLA Clinical Microbiology Laboratory. Bacillus subtilis was a laboratory reference strain, and Listeria monocytogenes EGD was a gift from Pieter Hiemstra.
Artificial tear solution.
Many of our studies were performed with an artificial tear solution (ATS), a balanced salt solution whose composition simulated that of normal human tears (1). The composition of ATS was 124 mM Na+, 133 mM Cl−, 24 mM HCO3−, 30 mM K+, 0.7 mM Mg2+, 0.7 mM Ca2+, 0.35 mM glucose, 4.5 mM urea, 3.5 mM lactate, and 0.2 mM pyruvate.
Colony counting assays.
Mid-logarithmic-phase bacteria were washed with ATS containing 3 mg of Trypticase soy broth powder ml−1 and adjusted to contain approximately 107 CFU ml−1. Samples of pooled tears, purified tear sPLA2, lysozyme, or lactoferrin were serially diluted in ATS that had been supplemented with 0.1% bovine serum albumin (ATS-BSA). Bacteria (10 μl) were mixed with 90 μl of the pooled tear or purified protein samples, so that the final volume (100 μl) of ATS contained 0.3 mg of Trypticase soy broth powder ml−1. After 15 min, 1 h, or 3 h, aliquots were transferred to Trypticase soy agar plates with a Spiral Plater (SpiralTech, Rockville, Md.), and surviving colonies were counted after overnight incubation at 37°C.
Radial diffusion assays.
An extensive description of the assay recently appeared elsewhere (40). The principal modification introduced for this study involved incorporating ATS and 0.1 mg of albumin ml−1 (Sigma A-7030) into the underlay gels. The ATS simulated a lachrymal environment, whereas the albumin minimized nonspecific adsorption of sPLA2 to agarose, especially when ultralow (nanogram) quantities were tested. The underlay contained 4 × 106 bacterial CFU dispersed in 10 ml of a gel that contained full-strength ATS, 1% agarose, 0.3 mg of Trypticase soy broth powder ml−1, and 0.01% BSA. To prepare bacteria in the logarithmic growth phase, overnight cultures in Trypticase soy broth were subcultured in fresh broth for 2.5 h. These organisms were washed twice with ATS and adjusted to the desired concentrations, based on optical density at 620 nm measurements. Serial “half-log” (i.e., 3.16-fold) dilutions of purified PLA2 were prepared in ATS-BSA.
A regularly spaced array of 3.2-mm-diameter sample wells, each with a capacity of 9.9 μl, was punched into the underlay gels, and 5-μl aliquots of the various samples were introduced. After 3 h at 37°C, a 10-ml overlay gel consisting of double-strength (60 g/liter) Trypticase soy broth powder (BBL Microbiology Systems, Cockeysville, Md.) and 1% agarose was poured to enable the surviving bacteria to form visible microcolonies. After overnight incubation at 37°C, the diameters of the clear zones were measured to the nearest 0.1 mm and, after subtraction of the diameter of the well, the difference was expressed in units (1 mm = 10 U). We estimated the minimal bactericidal concentrations by performing linear regression analyses (units vs log10 concentration) and determining the _x_-intercepts. The absence of microcolonies in the clear zones surrounding the wells was confirmed by direct microscopy at a ×40 magnification.
PLA2 and lysozyme purification.
Tears collected from different donors were pooled and subjected to reverse-phase high-performance liquid chromatography on a Vydac C18 column (10 by 250 mm) (The Separations Group, Hesperia, Calif.) with a linear gradient of 0 to 60% acetonitrile that contained 0.1% trifluoroacetic acid. Each fraction was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by the sPLA2 enzyme assay described below. Fractions with phospholipase activity were combined and rechromatographed on a Vydac C18 column (4.6 by 250 mm) with a linear gradient of acetonitrile that contained 0.13% heptafluorobutyric acid (HFBA) as the ion-pairing agent. To prepare highly purified human tear lysozyme, we subjected pooled tears to preparative acid urea-PAGE (13) and performed the two-stage RP-HPLC procedure described above for PLA2.
Enzymatic assay of PLA2.
PLA2 activity was measured essentially as described by Elsbach and Weiss (5). Briefly, E. coli ML-35 was labeled with [14C]oleic acid (50 mCi mmol−1 [NEN/Dupont]), autoclaved, and used as the substrate. Each sample was mixed with 50,000 cpm of 14C-labeled E. coli, equivalent to 2.5 × 108 bacteria (adjusted by addition of nonradioactive autoclaved E. coli) in a 250-μl volume that contained 250 mM Tris, 10 mM CaCl2, and 1 mg of BSA per ml (pH 9.5). After a 1-h incubation in a 37°C shaking water bath, 100 μl of 2 N HCl was added to stop the reaction, and the product (free fatty acids and lysophospholipids) was trapped by adding 100 μl of 20 mg of fatty acid-free BSA ml−1. The mixture was kept at 4°C for 30 min and centrifuged at 10,000 × g for 5 min. The pellet was washed twice with 0.1% acetic acid. The supernatant and washings were combined, and their PLA2 activity was measured by liquid scintillation counting and converted to arbitrary units (AU [1 AU = 1% release of 14C]).
Immunological assay of PLA2.
Serially diluted tears and purified tear PLA2 standards were prepared in 5 μl of ATS containing 0.1% BSA. The standards contained 15, 12.5, 10, 7.5, and 5 ng of sPLA2 per 5 μl. One hundred fifty microliters of Tris-buffered saline (20 mM Tris [pH 7.5], 500 mM NaCl) was added to each well of a Bio-Dot SF microfiltration apparatus (Bio-Rad, Hercules, Calif.). Five microliters of the purified tear PLA2 standards or of a dilution series of tears was added, followed by another 100 μl of Tris-buffered saline. The samples were blotted onto a Hybond-ECL (enhanced chemiluminescence) nitrocellulose membrane (Amersham, Arlington Heights, Ill.) at unit gravity. ECL assay reagents were purchased from Amersham, and ECL Western blotting was performed by strictly following the manufacturer’s protocols. A murine monoclonal IgG1κ antibody to human sPLA2 was purchased from Upstate Biotechnology (Lake Placid, N.Y.) and used as the primary antibody at 0.5 μg/ml. A 1:2,000 dilution of sheep anti-mouse IgG that had been conjugated to horseradish peroxidase (Amersham) was used as the secondary antibody. Light emitted from the luminol substrate after its oxidation by horseradish peroxidase was detected with autoradiography film that was sensitive to blue light (Hyperfilm ECL; Amersham). Densitometry was performed on a Personal Densitometer SI instrument (Molecular Dynamics, Sunnyvale, Calif.) with the manufacturer’s ImageQuant software.
Lysozyme.
Lysozyme activity was measured by a radial diffusion (lysoplate) assay (30). The assay plates contained 1% agarose and 0.5 mg of lyophilized M. lysodeikticus per ml in 15 ml of 66 mM sodium phosphate buffer (pH 7.0). Serially diluted samples were dissolved in 5 μl of 0.01% acetic acid, placed into 3.2-mm-diameter wells, and incubated overnight at room temperature. Clear zones were measured, and activity was expressed relative to that of highly purified human tear lysozyme standards.
Protein microsequencing.
N-terminal sequencing was performed with a Porton model 2090E sequencer (Beckman Instruments, Fullerton, Calif.) after the protein had been reduced, carboxymethylated, and transferred to a polyvinylidene difluoride membrane. Quantitative amino acid analysis was performed by the PicoTag method. The molecular mass of the purified secretory PLA2 was determined by electrospray ionization-mass spectrometry with a Perkin-Elmer Sciex (Thornhill, Canada) triple-quadrupole electrospray mass spectrometer. The instrument was tuned to resolve the isotopes of the polypropylene glycol-NH4+ (singly charged ion at m/z 906 with 40% valley) and calibrated by flow injection of a mixture of polypropylene glycol 425, 1000, and 2000 in water-methanol (1/1 [vol/vol]) containing 2 mM ammonium formate and 0.1% acetonitrile. Samples were dissolved in water-acetonitrile-formic acid (50/50/0.1) and injected into a 10-μl/min stream of this solvent. Spectra were averaged, and the multiply charged ion series were deconvoluted with the MacSpec and MacBiospec software supplied with the instrument.
Other assays.
Protein was measured by the micro-bicinchoninic acid assay, with BSA standards (Pierce, Rockford, Ill.).
RESULTS
General composition of tears.
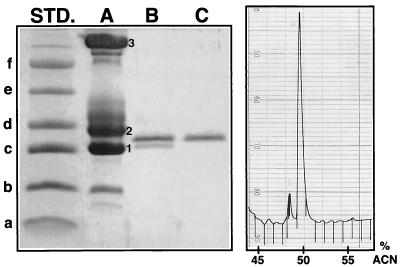
As shown in Table 1, basal tears contained 13.4 ± 1.28 mg of protein ml−1 (mean ± standard error) and contained from 8.3 to 17.1 mg of protein ml−1. Onion vapor-stimulated tears contained 8.97 ± 0.93 mg of protein ml−1 and ranged from 5.0 to 11.3 mg of protein ml−1. SDS-PAGE analysis confirmed that lysozyme, lactoferrin, and lipocalin were especially abundant (Fig. 1).
TABLE 1.
Composition of normal tears in this studya
| Patient no. | Sex | Age (yr) | Ethnicity | Concn of protein (mg/ml) | Concn of PLA2 (μg/ml) | Lysozyme activity (mg/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ECL | 14C-E. coli | ||||||||||
| Basal | Stimulated | Basal | Stimulated | Basal | Stimulated | Basal | Stimulated | ||||
| 1 | Female | 34 | Asian | 14.6 | 10.8 | 41.0 | 33.8 | 48.5 | 24.6 | 1.56 | 1.10 |
| 2 | Male | 28 | Asian | 17.1 | 9.2 | 47.2 | 33.4 | 44.0 | 19.0 | 2.12 | 1.54 |
| 3 | Male | 36 | Caucasian | 15.8 | 7.9 | 36.5 | 26.9 | 37.2 | 16.2 | 1.74 | 1.49 |
| 4 | Female | 34 | Finnish | 8.3 | 5.0 | 26.4 | 24.5 | 15.6 | 8.8 | 1.10 | 0.78 |
| 5 | Female | 25 | Finnish | 12.1 | 9.6 | 31.1 | 19.9 | 27.3 | 7.5 | 1.32 | 0.93 |
| 6 | Female | 33 | Caucasian | 12.5 | 11.3 | 38.0 | 25.9 | 20.2 | 13.5 | 1.88 | 1.36 |
| Mean ± SE | 13.4 ± 1.28 | 8.97 ± 0.93 | 36.7 ± 2.99 | 27.4 ± 2.19 | 32.1 ± 5.40 | 14.9 ± 2.62 | 1.62 ± 0.15 | 1.20 ± 0.13 |
FIG. 1.
Purification of secretory PLA2 from tears. The left panel shows an SDS-PAGE gel (16.5% polyacrylamide) run under reducing conditions and stained with Coomassie blue. Its left lane contained molecular mass standards (STD.) with masses of 3.0 (a), 6.2 (b), 14.3 (c), 18.4 (d), 29.0 (e), and 43.0 (f) kDa. Lane A (5 μl of pooled tears) displays three major bands, which have been numbered. They correspond to the following proteins: 1, lysozyme; 2, tear-specific prealbumin (also called lipocalin); and 3, lactoferrin. Lane B shows the PLA2-containing fractions after the first stage of RP-HPLC purification. Lane C shows highly purified PLA2 after the second stage of RP-HPLC purification, with an acetonitrile (ACN) gradient in 0.13% HFBA. The panel on the right shows the sPLA2 peak, monitored at 230 nm, and is from the second HPLC purification step.
Antibacterial activity of tears.
We performed colony count experiments to test the antimicrobial activity of whole tears against various gram-positive bacteria, including six strains of S. aureus (two clinical ocular isolates and four MRSA strains), L. monocytogenes, B. subtilis, group B Streptococcus, and a vancomycin-resistant E. faecium strain, 94.132. In these studies, 90 μl of pooled tears and 10 μl (105 CFU) of bacteria were mixed together and incubated for 3 h at 37°C in a shaking water bath. As shown in Table 2, over 99% of the bacteria had been eradicated after 1 h, and essentially complete (99.99%) eradication of the organisms was accomplished by 3 h.
TABLE 2.
Activity of tears against gram-positive bacteria
| Bacteria | Result with incubation timea | |||
|---|---|---|---|---|
| 1 h | 3 h | |||
| % Survival | Log10 reduction | % Survival | Log10 reduction | |
| E. faecium 94.132 | <0.01 | >4.19 | <0.01 | >4.43 |
| B. subtilis | <0.01 | >4.33 | <0.01 | >4.98 |
| L. monocytogenes EGD | <0.01 | >4.17 | <0.01 | >4.40 |
| Group B Streptococcus | <0.01 | >4.00 | <0.01 | >4.43 |
| S. aureus 67395 | <0.01 | >4.04 | <0.01 | >4.40 |
| S. aureus GM-1 | <0.03 | 3.60 | <0.01 | >3.83 |
| MRSA ATCC 33591 | 0.02 | 3.64 | <0.01 | >4.00 |
| MRSA 28841 | 0.17 | 2.77 | <0.01 | >4.04 |
| MRSA 30371 | 0.08 | 3.09 | <0.01 | >4.36 |
| MRSA 54424-1 | 0.76 | 2.12 | <0.01 | >4.43 |
Fractionation of tears.
To ascertain which components of human tears were responsible for their activity against gram-positive bacteria, we fractionated normal tears by RP-HPLC. Fractions were collected each minute, lyophilized by vacuum centrifugation, redissolved in acidified water (0.01% acetic acid), and tested against L. monocytogenes EGD in radial diffusion assays. The underlay gels contained 10 mM sodium phosphate buffer, 1% agarose, and 0.3 mg of Trypticase soy broth powder per ml ± 100 mM NaCl, without supplemental calcium. As shown in Fig. 2, two distinct antibacterial peaks were present, one centered around fraction 42 and the other centered around fraction 52. The antimicrobial molecules in fraction 42 were almost equally active under low- and high-salt conditions (10 mM phosphate buffer ± 100 mM NaCl). In contrast, those in fraction 52 were considerably more active under the low-salt conditions (10 mM phosphate buffer). Enzymatic assays revealed that fraction 42 contained the highest PLA2 activity and that fraction 52 corresponded to the lysozyme peak.
FIG. 2.
Activity of HPLC fractions. Antimicrobial activity was tested against L. monocytogenes with radial diffusion assays by using low-salt (□) or high-salt (○) underlay gels that contained 10 mM sodium phosphate ± 100 mM NaCl. Enzymatic PLA2 activity (▴) was measured with 14C-labeled E. coli. Lysozyme activity (▾) was tested with a lysoplate assay.
Purification of PLA2.
Tears were collected from the six donors, pooled, and subjected to RP-HPLC. When the partially purified fractions with PLA2 activity were examined by SDS-PAGE, they contained two principal components, both with apparent masses of approximately 14 kDa (Fig. 1, lane B). These proteins were resolved and purified by rechromatography of the fractions on the same C18 column with 0.13% HFBA as the ion-pairing reagent. The partial N-terminal sequence (residues 1 to 22) of the more abundant protein was identical to that of human type II sPLA2: NLVNF HRMIK LTTGK EAALS YG. The molecular mass of the purified molecule was 13,905.2 Da by electrospray ionization-mass spectrometry which was in close agreement with the expected mass of 13,903.7, calculated from the molecule’s primary sequence (36). In addition, the purified molecule reacted with a monoclonal antibody to human sPLA2 in Western blots (data not shown). This constellation of findings (N-terminal sequence, mass, and immunological reactivity) securely established the molecule’s identity as sPLA2. The N-terminal sequence of the other peptide present in Fig. 1, lane B, corresponded precisely to that of serum leukoprotease inhibitor (SLPI), and this molecule also reacted strongly to a polyclonal antibody against human recombinant SLPI (data not shown). Figure 1, lane C, shows highly purified sPLA2, after its resolution from SLPI by the second RP-HPLC. The right panel of Fig. 1 shows the RP-HPLC chromatogram of purified human tear sPLA2.
Quantitation of tear sPLA2.
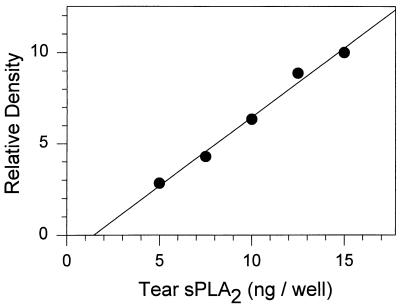
The sPLA2 concentration in tears of six healthy individuals was measured by two methods: one enzymatic and the other immunochemical. The enzymatic assay used [14C]oleate-labeled autoclaved E. coli as a substrate. The immunoassay was an ECL procedure using a murine monoclonal antibody to human sPLA2. Highly purified human tear PLA2 was used as the standard in both assays. In Western blotting experiments with whole tears, the monoclonal antibody stained only sPLA2 and detected <1 ng of sPLA2 when we used an alkaline phosphatase-conjugated secondary antibody (data not shown). The ECL assay was even more sensitive, and its output on film was better suited to densitometry. Figure 3 shows a representative standard curve from the ECL assay of sPLA2.
FIG. 3.
ECL assay. A standard curve is shown for sPLA2 purified from human tears.
As shown in Table 1, basal tears contained 36.7 ± 2.99 μg of PLA2 ml−1 determined by ECL immunoassay and 32.1 ± 5.4 μg of sPLA2 ml−1 determined by the enzymatic assay. Stimulated tears contained 27.4 ± 2.19 μg of PLA2 ml−1 by the ECL immunoassay system but only 14.9 ± 2.62 μg of sPLA2 ml−1 by enzymatic assay. In two separate purifications, we recovered a total of 218 μg of highly purified PLA2 from 21 ml of pooled stimulated tears (10.4 μg ml−1), as determined by quantitative amino acid analysis, indicating that our sPLA2 purification procedure was reasonably efficient. We also measured the lysozyme content of human tears by an enzymatic (lysoplate) assay. As shown in Table 1, basal tears contained 1.62 ± 0.15 mg of lysozyme ml−1, and stimulated tears contained 1.2 ± 0.13 mg of lysozyme ml−1. Thus, lysozyme was approximately 50-fold more abundant than sPLA2 in tears.
Antimicrobial activity of tear PLA2.
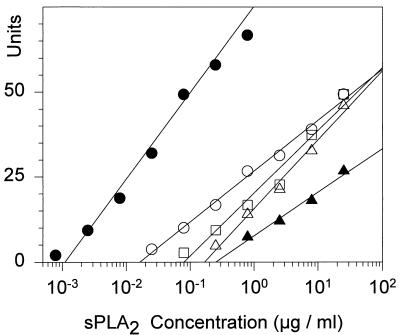
Purified PLA2 was highly effective against all of the gram-positive bacteria we tested (Fig. 4 and 5). The minimal effective concentration of sPLA2 against the various organisms, indicated by their respective _x_-intercepts, varied over a large range in the experiments shown in Fig. 4. Whereas L. monocytogenes EGD was killed by 1.1 ng of sPLA2 ml−1 (≈0.08 nM), approximately 250 ng of sPLA2 ml−1 (18 nM) was needed to kill the least-sensitive organism in this group, E. faecium 94.132. Two S. aureus strains showed intermediate sensitivity, requiring 15 and 80 ng of sPLA2 ml−1 (1.1 and 5.8 nM, respectively).
FIG. 4.
Antibacterial activity of purified sPLA2. Two-stage radial diffusion assays were performed with highly purified tear sPLA2 and five gram-positive bacteria: L. monocytogenes (•), S. aureus 67395 (○), S. aureus GM-1 (□), group B streptococcus (▵), and E. faecium 94.132 (▴). Each symbol represents a mean value derived from three separate experiments with each organism. The regression lines were fit by the method of least mean squares. The _x_-intercepts indicate the minimal effective concentration.
FIG. 5.
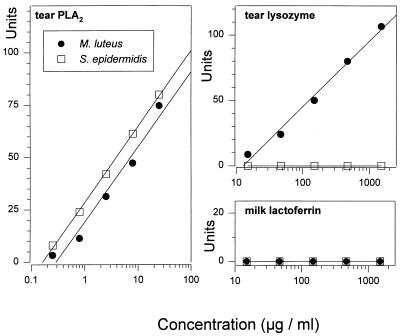
Susceptibility of S. epidermidis and M. luteus. Radial diffusion assays were performed with purified human tear sPLA2, human tear lysozyme, and human milk lactoferrin. Note that the MIC (_x_-intercept) of sPLA2 for M. luteus (•) was approximately 0.3 μg/ml, whereas that of lysozyme was 13 μg/ml. The MIC of sPLA2 for S. epidermidis (□) was approximately 0.15 μg/ml, whereas lysozyme was not effective (MIC of >1.5 mg/ml).
Figure 5 compares the effects of sPLA2, lysozyme, and lactoferrin on two additional gram-positive bacteria, S. epidermidis and M. luteus. The latter is the same strain (originally called M. leisodeikticus) that Fleming used in his pioneering studies with lysozyme. The M. luteus strain was killed by ≈13 μg of lysozyme ml−1 and by ≈0.3 μg of sPLA2 ml−1. Both of these concentrations are approximately 100-fold lower than the concentrations of these enzymes in normal tears (Table 1). sPLA2, but not lysozyme, showed bactericidal activity against S. epidermidis. Lactoferrin was inactive against both of these organisms.
In contrast to its efficacy against gram-positive bacteria, even 25 μg of sPLA2 ml−1 failed to kill gram-negative bacteria, including E. coli, S. typhimurium, and P. aeruginosa in radial diffusion assays performed with underlay gels that contained ATS. However, when these assays were performed with low-salt underlays (10 mM sodium phosphate buffer), sPLA2 showed bactericidal activity against these gram-negative bacteria (data not shown), consistent with our previous report on intestinal sPLA2 (14).
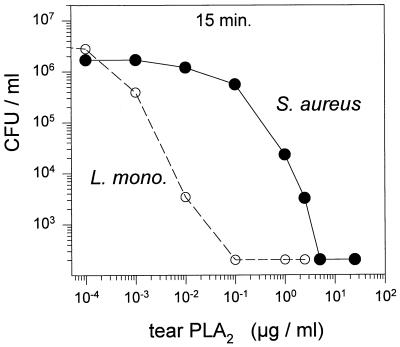
Rapidity of the effect.
Figure 6 shows that sPLA2 acted rapidly after addition to 1.5 × 106 to 3 × 106 bacteria ml−1 in artificial tear fluid. After 15 min of incubation, as little as 100 ng of sPLA2 ml−1 totally eradicated L. monocytogenes EGD and 5 μg ml−1 eradicated S. aureus 67395.
FIG. 6.
Colony count assay. Approximately 106 CFU per ml of L. monocytogenes (L. mono.) EGD or S. aureus 67395 was incubated for 15 min at 37°C with the indicated concentrations of tear sPLA2.
Effects of divalent cations.
To determine the degree to which the bactericidal activity of sPLA2 was calcium dependent, we performed the experiments shown in Fig. 7. The basic unsupplemented underlay gels contained 1% agarose, full-strength calcium- and magnesium-free ATS, and 0.3 mg of Trypticase soy broth powder per ml. The addition of 0.7 mM CaCl2 to these underlay gels enhanced the bactericidal potency of PLA2 over 1,000-fold, whereas addition of 2 mM EGTA, a selective Ca2+ chelator, abolished its antimicrobial activity even when the gels also contained 0.7 mM calcium. The slight activity of sPLA2 in underlay gels without specifically added calcium (Fig. 7, solid circles) probably reflects the effects of the calcium contained in Trypticase soy broth powder, since it was also abolished by addition of EGTA. In retrospect, we were lucky to have selected L. monocytogenes, which is exquisitely sensitive to sPLA2, for the preliminary studies shown in Fig. 2, since the very low concentrations of calcium that were present in the underlay were markedly suboptimal for sPLA2.
FIG. 7.
Calcium dependence of antibacterial activity. Tear sPLA2 was tested against two gram-positive bacteria, L. monocytogenes and S. aureus. The underlay gels contained ATS without Ca2+ or Mg2+ and were supplemented with divalent cations (0.7 mM) or EGTA (2 mM), as indicated by the inset. Each symbol depicts a mean value derived from four separate experiments.
Antimicrobial activity of other tear components.
The calcium dependence of sPLA2-mediated antimicrobial activity against gram-positive bacteria and especially its abolition by EGTA provided a facile method for determining if additional antimicrobial molecules contributed to the antimicrobial properties of tears against gram-positive bacteria. As shown in Table 3, the addition of 2 mM EGTA to normal tears completely blocked its activity against all six S. aureus strains, including the four MRSA strains. In contrast, the activity of tears against L. monocytogenes and group B Streptococcus was only partially blocked, and EGTA-containing tears retained their activity against E. faecium and B. subtilis.
TABLE 3.
Effect of 2 mM EGTA on the activity of tears against gram-positive bacteria
| Bacteria | Result with incubation timea | |||
|---|---|---|---|---|
| 1 h | 3 h | |||
| % Survival | Log10 reduction | % Survival | Log10 reduction | |
| E. faecium 94.132 | <0.01 | >4.11 | <0.01 | >4.18 |
| B. subtilis | <0.01 | >4.34 | <0.01 | >4.94 |
| L. monocytogenes EGD | 30.7 | 0.51 | 2.20 | 1.56 |
| Group B Streptococcus | 0.19 | 2.72 | 0.03 | 3.59 |
| S. aureus 67395 | 94.4 | 0.03 | 125 | −0.10 |
| S. aureus GM-1 | 98.7 | 0.01 | 76.1 | 0.11 |
| MRSA ATCC 33591 | 93.8 | 0.02 | 108.0 | −0.04 |
| MRSA 28841 | 100.0 | 0.00 | 100.0 | 0.00 |
| MRSA 30371 | 109.0 | −0.04 | 106.0 | −0.03 |
| MRSA 54424-1 | 118.0 | −0.07 | 182.0 | −0.26 |
Since both lactoferrin and lysozyme are both abundant in human tears and have antimicrobial potential, we tested them against the four bacterial species killed in EGTA-supplemented tears (Table 4). Although a high (1.5 mg/ml), but physiological concentration of highly purified human tear lysozyme eradicated more than 99.99% of E. faecium and B. subtilis in 1 h, it showed no activity against group B Streptococcus and only bacteriostatic activity against L. monocytogenes (data not shown). Human milk lactoferrin (1.5 mg/ml) displayed modest bactericidal activity only against B. subtilis, and it was inactive against the other three bacteria. Thus, whereas lysozyme might account for the activity of EGTA-treated tears against E. faecium and B. subtilis, the residual activity of EGTA-treated tears against L. monocytogenes and group B Streptococcus may reflect the actions of other, as yet unidentified, tear components.
TABLE 4.
Effect of 2 mM EGTA on the activity of tears, lysozyme, and lactoferrin against gram-positive bacteriaa
| Bacteria | Log10 reduction with: | |||||
|---|---|---|---|---|---|---|
| Whole tears | Lysozyme | Lactoferrin | ||||
| 1 h | 3 h | 1 h | 3 h | 1 h | 3 h | |
| E. faecium 94.132 | >4.11 | >4.18 | >4.11 | >4.80 | 0 | 0 |
| B. subtilis | >4.34 | >4.94 | >4.34 | >4.94 | 0.67 | 1.76 |
| L. monocytogenes EGD | 0.51 | 1.56 | 0.10 | 0.29 | 0 | 0 |
| Group B Streptococcus | 2.72 | 3.59 | 0 | 0 | 0 | 0 |
DISCUSSION
Human sPLA2 is a 13.9-kDa molecule composed of 124 amino acid residues. It has been found in many tissues and secretions, including rheumatoid synovial fluids (37), platelets (21), seminal plasma (29, 41), intestinal Paneth cells (27), and neutrophils (32). The levels of sPLA2 in seminal plasma were reported to range between 15 and around 30 μg/ml (29)—very similar to the levels we found in the tear film (Table 1). It is noteworthy that sPLA2 hydrolyzes phosphatidylglycerol several hundred times more rapidly than phosphatidylcholine, since the former is a principal phospholipid of microbial membranes, whereas the latter is typically abundant in mammalian cell membranes (24, 25, 44).
All PLA2 enzymes (EC 3.1.1.4) hydrolyze the _sn_-2 fatty acyl moiety from phospholipids, releasing equimolar amounts of free fatty acids and lysophospholipids. Over 60 secretory PLA2 enzymes were reviewed by Scott and Sigler (34, 35), who described sPLA2 enzymes as robust, small molecules that were highly resistant to denaturation and preferred substrates that were organized into micelles, monolayers, or membranes. All sPLA2 enzymes bind calcium ions with a Kd of >10−4 M, and this cation is essential for catalysis. The various PLA2 enzymes show strongly conserved three-dimensional structures that are stabilized by multiple intramolecular cystine disulfide bonds. Although mammalian sPLA2s are often described according to their tissues of origin, (e.g., pancreatic, splenic, or intestinal PLA2), only a single sPLA2 gene exists in humans (37). Type I and type II PLA2 enzymes are distinguished by the location of one of their seven disulfide bridges and by a seven-residue C-terminal extension found only in the type II PLA2s. Type I PLA2 is exemplified by mammalian pancreatic enzymes and homologs found in Old World elapid snake venoms, whereas the type II enzymes include intestinal and splenic PLA2s and venom constituents of New World viperid and crotalid snakes (16, 18). Additional PLA2 enzymes that are structurally and functionally distinct from the type I and II enzymes are also found in mammalian cells and may be especially important in mediating cellular injury (3, 33).
Our experiments demonstrated that remarkably large concentrations of type II sPLA2 are present in normal human tears. Only one previous report described the presence of sPLA2 in normal human tears (26). Using a time-resolved fluoroimmunoassay procedure, these investigators reported that tears contained 1.45 μg of sPLA2 ml−1, a value 10- to 20-fold lower than the sPLA2 concentrations shown in Table 1. Since we used both an enzymatic assay and an immunoassay procedure to determine these sPLA2 concentrations, we are confident that the concentrations shown in Table 1 are accurate. This belief is reinforced by our ability to recover 10.4 μg of highly purified sPLA2 ml−1 from tears by the two-stage HPLC procedure described above. The earlier study of sPLA2 in tears was performed in a country (Finland) whose population is unusually homogeneous (15). Consequently, it is noteworthy that the two ethnic Finns in our donor group had sPLA2 concentrations in their tears similar to those found in the Asian or Caucasian donors (Table 1).
Whereas we obtained nearly identical values (36.7 versus 32.1 μg/ml) for the concentration of sPLA2 in basal tears with the enzymatic and immunological assays, the assays gave divergent results (27.4 versus 14.9 μg/ml) when applied to onion vapor-stimulated tears. Our preliminary evidence suggests that the lower sPLA2 activity in stimulated tears reflects the presence of an sPLA2 inhibitor, as yet unidentified (data not shown). Endogenous sPLA2 inhibitors also exist in bovine seminal plasma (22).
Our data provide compelling evidence that sPLA2 is principally responsible for the ability of tears to kill a broad spectrum of gram-positive bacteria, notwithstanding the presence of lysozyme and lactoferrin in much higher concentrations. This inference is supported by several lines of evidence. First, concentrations of purified human sPLA2 much lower than those present in tears showed potent bactericidal activity against each of the gram-positive bacteria in our panel. This bactericidal activity was calcium dependent and was also inhibited by EGTA, suggesting that the enzymatic effects of sPLA2 were critical for its bactericidal properties. Second, 2 mM EGTA abolished the bactericidal effect of normal tears against normal and MRSA strains and greatly reduced their activity against group B streptococci and L. monocytogenes (Tables 2 and 3). Lysozyme and lactoferrin displayed little or no activity against these organisms, even when tested at 1.5 mg/ml. As might be expected, EGTA did not inhibit the activity of tears against highly lysozyme-susceptible bacteria, such as B. subtilis, and a vancomycin-resistant strain of E. faecium.
Not withstanding its high concentration, our data indicate that lysozyme acts in a secondary (backup) manner with respect to the bactericidal properties of human tears against gram-positive bacteria. The reported absence of lysozyme from the tears of cattle is consistent with its secondary role in this respect (31). Inbred mouse strains, including the widely used C57BL/6 strain, that are naturally deficient in sPLA2 because of a frameshift mutation in exon 3 of the gene (19) may afford useful models for defining the role of sPLA2 in mucosal and secretory host defenses.
Both murine intestinal (14) and rabbit leukocyte (45) sPLA2 possess bactericidal properties. The potent activity of rabbit leukocyte sPLA2 against S. aureus largely accounted for the staphylocidal activity of a sterile inflammatory peritoneal exudate fluid which contained 10 nM (0.14 μg/ml) of sPLA2 (45). Whereas normal human serum contains low levels of sPLA2 that circulate mostly in high-molecular-weight complexes (28), the concentration of sPLA2 in human serum rises sharply during sepsis (6, 12). sPLA2 causes bacterial phospholipid degradation during phagocytosis of E. coli cells by polymorphonuclear leukocytes and also degrades the phospholipids of E. coli cells treated with neutrophil-derived bactericidal, permeability-increasing protein (46).
The remarkable susceptibility of L. monocytogenes to human secretory PLA2 (Fig. 4) has an ironic aspect, since this organism uses two secreted phospholipases—a phosphatidylinositol-specific phospholipase C and a broad-range phospholipase C—to escape from vacuoles of the host’s phagocytes and spread from cell to cell (2, 39).
The development of molecules that can inhibit PLA2 activity is a major area of pharmaceutical research (11, 42), in part stimulated by the belief that the elevated concentrations of sPLA2 in the inflammatory fluids, plasma, and infected tissues are noxious. The present report and other recent demonstrations (14, 45) that sPLA2 has potent microbicidal properties suggest that its induction and release may be beneficial to hosts with infections caused by gram-positive bacteria. Should sPLA2 inhibitors enter into routine clinical use, it will be important to be watchful for evidence of impaired host resistance or of increased infections caused by gram-positive organisms.
ACKNOWLEDGMENTS
These studies were supported, in part, by research grant AI-29839 from the National Institutes of Health. Xiaodan Qu was supported by a fellowship from the Cystic Fibrosis Foundation. The UCLA Protein Microsequencing Facility is supported by grant CA 16042 from the National Cancer Institute.
We thank Guorong Xu for assisting with phospholipase purification, Kym Faull for performing the mass spectrometry, and Audree Fowler for performing the microsequencing and quantitative amino acid analysis.
REFERENCES
- 1.Berman E R. Biochemistry of the eye. N.Y: Plenum Press; 1991. pp. 63–88. [Google Scholar]
- 2.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis E A. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 4.Donaldson D M, Tew J G. Beta-lysin of platelet origin. Bacteriol Rev. 1977;41:501–513. doi: 10.1128/br.41.2.501-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsbach P, Weiss J. Utilization of labeled Escherichia coli as phospholipase substrate. Methods Enzymol. 1991;197:24–31. doi: 10.1016/0076-6879(91)97130-q. [DOI] [PubMed] [Google Scholar]
- 6.Endo S, Inada K, Nakae H, Takakuwa T, Yamada Y, Suzuki T, Taniguchi S, Yoshida M, Ogawa M, Teraoka H. Plasma levels of type II phospholipase A2 and cytokines in patients with sepsis. Res Commun Mol Pathol Pharmacol. 1995;90:413–421. [PubMed] [Google Scholar]
- 7.Fleming A. On a remarkable bacteriolytic ferment found in tissues and secretions. Proc R Soc Ser B. 1922;93:306–317. [Google Scholar]
- 8.Fleming A, Allison V D. Observations on a bacteriolytic substance (“lysozyme”) found in secretions and tissues. Br J Exp Pathol. 1922;3:252–260. [Google Scholar]
- 9.Ford L C, DeLange R J, Petty R W. Identification of a non-lysozymal bactericidal factor (beta-lysin) in human tears and aqueous humor. Am J Ophthalmol. 1976;81:30–33. doi: 10.1016/0002-9394(76)90188-4. [DOI] [PubMed] [Google Scholar]
- 10.Friedland B R, Anderson M D, Forster R K. Non-lysozyme antibacterial factor in human tears. Am J Ophthalmol. 1972;74:52–59. doi: 10.1016/0002-9394(72)91125-7. [DOI] [PubMed] [Google Scholar]
- 11.Glaser K B. Regulation of phospholipase A2 enzymes: selective inhibitors and their pharmacological potential. Adv Pharmacol. 1995;32:31–66. doi: 10.1016/s1054-3589(08)61011-x. [DOI] [PubMed] [Google Scholar]
- 12.Guidet B, Piot O, Masliah J, Barakett V, Maury E, Bereziat G, Offenstadt G. Secretory non-pancreatic phospholipase A2 in severe sepsis: relation to endotoxin, cytokines and thromboxane B2. Infection. 1996;24:103–108. doi: 10.1007/BF01713312. [DOI] [PubMed] [Google Scholar]
- 13.Harwig S S L, Chen N P, Park A S K, Lehrer R I. Purification of cysteine-rich bioactive peptides from leukocytes by continuous acid-urea polyacrylamide gel electrophoresis (CAU-PAGE) Anal Biochem. 1993;208:382–386. doi: 10.1006/abio.1993.1065. [DOI] [PubMed] [Google Scholar]
- 14.Harwig S S L, Tan L, Qu X-D, Cho Y, Eisenhauer P B, Lehrer R I. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95:603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hästbacka J, de la Chapelle A, Mahtani M M, Clines G, Reeve-Daly M P, Daly M, Hamilton B A, Kusumi K, Trivedi B, Weaver A, Coloma A, Lovett M, Buckler A, Kaitila I, Lander E S. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78:1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 16.Heinrikson R L, Krueger E T, Keim P S. Amino acid sequence of phospholipase A2-α from the venom of Crotalus adamanteus. J Biol Chem. 1977;252:4913–4921. [PubMed] [Google Scholar]
- 17.Janssen P T, Muytjens H L, van Bijsterveld P. Nonlysozyme antibacterial factor in human tears. Fact or fiction? Invest Ophthalmol Vis Sci. 1984;25:1156–1160. [PubMed] [Google Scholar]
- 18.Johnson L K, Frank S, Vades P, Pruzanski W, Lusis A J, Seilhamer J J. Localization and evolution of two human phospholipase A2 genes and two related genetic elements. Adv Exp Biol Med. 1990;275:17–34. doi: 10.1007/978-1-4684-5805-3_2. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy B P, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt D E, Cromlish W A. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J Biol Chem. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- 20.Kijlstra A, Kuizenga A. Analysis and function of the human tear proteins. Adv Exp Biol Med. 1994;350:299–308. doi: 10.1007/978-1-4615-2417-5_51. [DOI] [PubMed] [Google Scholar]
- 21.Kramer R M, Hession C, Johansen B, Hayes G, McGray P, Chow E P, Tizard R, Pepinsky R B. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989;264:5768–5775. [PubMed] [Google Scholar]
- 22.Manjunath P, Soubeyrand S, Chandonnet L, Roberts K D. Major proteins of bovine seminal plasma inhibit phospholipase A2. Biochem J. 1994;303:121–128. doi: 10.1042/bj3030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannis M J, Smolin G. Natural defense mechanisms of the ocular surface. In: Pepose J S, Holland G N, Wilhelmus K R, editors. Ocular infection and immunity. St. Louis, Mo: Mosby; 1996. pp. 185–190. [Google Scholar]
- 24.Mansbach C M, Pieroni G, Verger R. Intestinal phospholipase, a novel enzyme. J Clin Invest. 1982;69:368–376. doi: 10.1172/JCI110460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minami T, Tojo H, Shinomura Y, Matsuzawa Y, Okamoto M. Purification and characterization of a phospholipase A2 from human ileal mucosa. Biochim Biophys Acta. 1993;1170:125–130. [PubMed] [Google Scholar]
- 26.Nevalainen T J, Aho H J, Peuravuori H. Secretion of group 2 phospholipase A2 by lacrimal glands. Invest Ophthalmol Vis Sci. 1994;35:417–421. [PubMed] [Google Scholar]
- 27.Nevalainen T J, Gronroos J M, Kallajoki M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab Invest. 1995;72:201–208. [PubMed] [Google Scholar]
- 28.Nevalainen T J, Kortesuo P T, Rintala E, Marki F. Immunochemical detection of group I and group II phospholipases A2 in human serum. Clin Chem. 1993;38:1824–1829. [PubMed] [Google Scholar]
- 29.Nevalainen T J, Meri K M, Niemi M. Synovial-type (group II) phospholipase A2 human seminal plasma. Andrologia. 1993;25:355–358. doi: 10.1111/j.1439-0272.1993.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 30.Osserman E F, Lawlor D P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966;124:921–951. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padgett G A, Hirsch J G. Lysozyme: its absence in tears and leukocytes of cattle. Aust J Exp Biol Med Sci. 1967;45:569–570. doi: 10.1038/icb.1967.56. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal M D, Gordon M N, Buescher E S, Slusser J H, Harris L K, Franson R C. Human neutrophils store type II 14-kDa phospholipase A2 in granules and secrete active enzyme in response to soluble stimuli. Biochem Biophys Res Commun. 1995;208:650–656. doi: 10.1006/bbrc.1995.1388. [DOI] [PubMed] [Google Scholar]
- 33.Sapirstein A, Spech R A, Witzgall R, Bonventre J V. Cytosolic phospholipase A2 (PLA2) but not secretory PLA2 potentiates hydrogen peroxide cytotoxicity in kidney epithelial cells. J Biol Chem. 1996;271:21505–21513. doi: 10.1074/jbc.271.35.21505. [DOI] [PubMed] [Google Scholar]
- 34.Scott D L, Sigler P B. Structure and catalytic mechanism of secretory phospholipases A2. Adv Protein Chem. 1994;45:53–88. doi: 10.1016/s0065-3233(08)60638-5. [DOI] [PubMed] [Google Scholar]
- 35.Scott D L, Sigler P B. The structural and functional roles of calcium ion in secretory phospholipases A2. Adv Inorg Biochem. 1994;10:139–155. [PubMed] [Google Scholar]
- 36.Scott D L, White S P, Browning J L, Rosa J J, Gelb M H, Sigler P B. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science. 1991;254:1007–1010. doi: 10.1126/science.1948070. [DOI] [PubMed] [Google Scholar]
- 37.Seilhamer J J, Pruzanski W, Vadas P, Plant S, Miller J A, Kloss J, Johnson L K. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem. 1989;264:5335–5338. [PubMed] [Google Scholar]
- 38.Selsted M E, Martinez R J. Isolation and purification of bactericides from human tears. Exp Eye Res. 1982;34:305–318. doi: 10.1016/0014-4835(82)90079-3. [DOI] [PubMed] [Google Scholar]
- 39.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinberg D, Lehrer R I. Designer assays for antimicrobial peptides: disputing the “one size fits all” theory. Methods Mol Biol. 1997;78:169–187. doi: 10.1385/0-89603-408-9:169. [DOI] [PubMed] [Google Scholar]
- 41.Takayama K, Hara S, Kudo I, Inoue K. Detection of 14-kDa group II phospholipase A2 in human seminal plasma. Biochem Biophys Res Commun. 1991;178:1505–1511. doi: 10.1016/0006-291x(91)91064-j. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka K, Arita H. Secretory phospholipase A2 inhibitors. Possible new anti-inflammatory agents. Agents Actions Suppl. 1995;46:51–64. [PubMed] [Google Scholar]
- 43.Thompson R, Gallardo E. The antibacterial action of tears on staphylococci. Am J Ophthalmol. 1941;24:635–640. [Google Scholar]
- 44.Verger R, Ferrato F, Mansbach C M, Pieroni G. Novel intestinal phospholipase A2: purification and some molecular characteristics. Biochemistry. 1982;21:6883–6889. doi: 10.1021/bi00269a040. [DOI] [PubMed] [Google Scholar]
- 45.Weinrauch Y, Elsbach P, Madsen L M, Foreman A, Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss J, Inada M, Elsbach P, Crowl R M. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J Biol Chem. 1994;269:26331–26337. [PubMed] [Google Scholar]